Significance
Studies of diabetic retinopathy have focused especially on the retinal vasculature, but recent studies suggest that the neural retina also is involved. Oxidative stress and local inflammatory changes have been shown to play important roles in the pathogenesis of this retinopathy, but the source of reactive oxygen species has been less clear. We now show that most of the oxidative stress in retinas of diabetic mice emanates from neural photoreceptor cells, and elimination of these cells in diabetes inhibits both the oxidative stress and inflammatory changes shown to cause the vascular lesions of diabetic retinopathy. These studies suggest a mechanism by which neural cells can initiate the vascular injury characteristic of diabetic retinopathy.
Abstract
Accumulating evidence suggests that photoreceptor cells play a previously unappreciated role in the development of early stages of diabetic retinopathy, but the mechanism by which this occurs is not clear. Inhibition of oxidative stress is known to inhibit the vascular lesions of early diabetic retinopathy, and we investigated whether the diabetes-induced oxidative stress in the retina emanates from photoreceptors. Superoxide generation was assessed in retinas of male C57BL/6J mice made diabetic for 2 mo (4 mo of age when killed) using histochemical (dichlorofluorescein and dihydroethidine) and bioluminescence (lucigenin) methods. Photoreceptors were eliminated in vivo by genetic (opsin−/−) and chemical (iodoacetic acid) techniques. Immunoblots were used to measure expression of intercellular adhesion molecule 1 and the inducible form of nitric oxide synthase. Diabetes increased the generation of superoxide by diabetic mouse retina more at night than during the day. Photoreceptors were the major source of reactive oxygen species in the retina, and their deletion (either genetically in opsin−/− mice or acutely with iodoacetic acid) inhibited the expected diabetes-induced increase in superoxide and inflammatory proteins in the remaining retina. Both mitochondria and NADPH oxidase contributed to the observed retinal superoxide generation, which could be inhibited in vivo with either methylene blue or apocynin. Photoreceptors are the major source of superoxide generated by retinas of diabetic mice. Pharmaceuticals targeting photoreceptor oxidative stress could offer a unique therapy for diabetic retinopathy.
The pathogenesis of diabetic retinopathy remains unclear, but prior work by us and others has provided strong evidence in animal models that oxidative stress and inflammatory processes play important roles in the development of the vascular lesions characteristic of early stages of this retinopathy (1, 2). Inhibition of oxidative stress by feeding antioxidants or overexpressing antioxidant enzymes has reduced diabetes-induced degeneration of retinal capillaries (3–7). Moreover, oxidative stress has been reported to regulate expression of proinflammatory proteins (8–10), which also have been shown to play a critical role in the pathogenesis of this early retinopathy (2). However, which cells of the retina are the major sources of such oxidative stress in diabetes is unclear. In vitro studies of retinal endothelial cells or Müller cells incubated in elevated levels of glucose have demonstrated that these cells can contribute to oxidative stress (11, 12), but the contribution of other cell types and their relative importance has not been studied.
Rod and cone photoreceptor cells are the most prevalent cells in the retina. These postmitotic cells have a very high metabolic rate, using more oxygen than other cells throughout the body. They are also considered as likely contributors to the eventual development of retinal hypoxia and subsequent neovascularization in advanced stages of diabetic retinopathy (13, 14). The contribution of photoreceptors to early stages of diabetic retinopathy, however, has been less well investigated. Several recent reports have raised the possibility that photoreceptors could play an important role in the initiation of diabetic retinopathy. A survey of diabetic patients with photoreceptor degeneration and concomitant retinitis pigmentosa suggested that patients whose photoreceptors degenerated had less retinopathy than did diabetics with intact photoreceptors (13). Moreover, diabetic mice lacking photoreceptor cells (resulting from opsin deficiency) featured a lower density of retinal vessels than those with photoreceptors (15), suggesting that photoreceptors influence diabetes-induced degeneration of retinal capillaries.
Because oxidative stress and inflammation are strongly implicated in the development of early vascular lesions of diabetic retinopathy, we investigated the role of photoreceptors and their response to light/dark in this process. We provide evidence in mouse models that photoreceptors play a critical role in the pathogenesis of early diabetic retinopathy by inducing superoxide and inflammatory proteins.
Results
Status of Diabetic Mice.
All diabetic mice were hyperglycemic and failed to gain weight at a normal rate. Serum glucose levels did not differ significantly among diabetic members of different experimental groups (356 ± 58 and 344 ± 62 mg/dL in diabetic wild-type and opsin−/− groups, respectively, compared with nondiabetic values of 135 ± 62 mg/dL). Deletion of opsin or treatment with iodoacetic acid (IAA) had no apparent effect on the glycemia of any animals.
Photoreceptors Account for Most of the Diabetes-Induced Increase in Superoxide Generation by Retina.
Histochemical evidence.
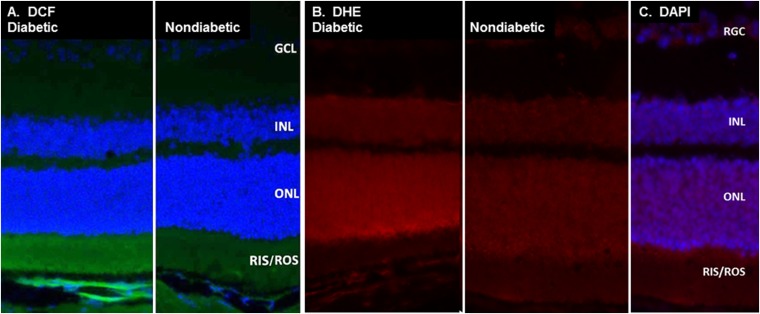
Cryosections of retinas from dark-adapted 2-mo-old diabetic and nondiabetic C57BL/6 mice were stained with dichlorofluorescein (DCF) and dihydroethidium (DHE) (Fig. 1). Neither dye is highly fluorescent unless it reacts with reactive oxygen species (DCF) or superoxide (DHE). The images demonstrate that the sites of reactive oxygen species (bright green) and superoxide (bright red) are greater than normal in retinas of diabetic animals and that these reactive species are located mainly in the photoreceptor layers (based on their costaining with nuclear stain). There was much less staining for superoxide in the retinal pigmented epithelium, inner nuclear (INL), or ganglion cell layers (GCL). DHE stains DNA in the presence of superoxide, so its localization in photoreceptors is restricted to nuclei close to the site of superoxide generation. In contrast, DCF becomes fluorescent upon direct exposure to reactive oxygen species, indicating that those reactive species are especially prevalent in the photoreceptor inner and outer segments (RIS/ROS).
Fig. 1.
Photoreceptors largely account for the diabetes-induced increase of reactive oxygen species in mouse retina. (A) DCF stain (green) demonstrates that diabetic mice generated more reactive oxygen species than did nondiabetic animals, and that most of those reactive species in the diabetic mice originate in the inner and/or outer segments of photoreceptors. (B) DHE stain (red) likewise indicates that retinas from diabetic animals generated more superoxide than did nondiabetic animals. The DHE stain localized primarily in the nuclei of photoreceptors because it stains DNA red in the presence of superoxide. The blue (nuclear) stain is DAPI. Micrographs from representative animals (n > 3 per group) are placed next to each other. GCL, retinal ganglion cell layer; ONL, outer nuclear layer; RGC, retinal ganglion cells.
Superoxide generation by retina in diabetic mice is greater in darkness.
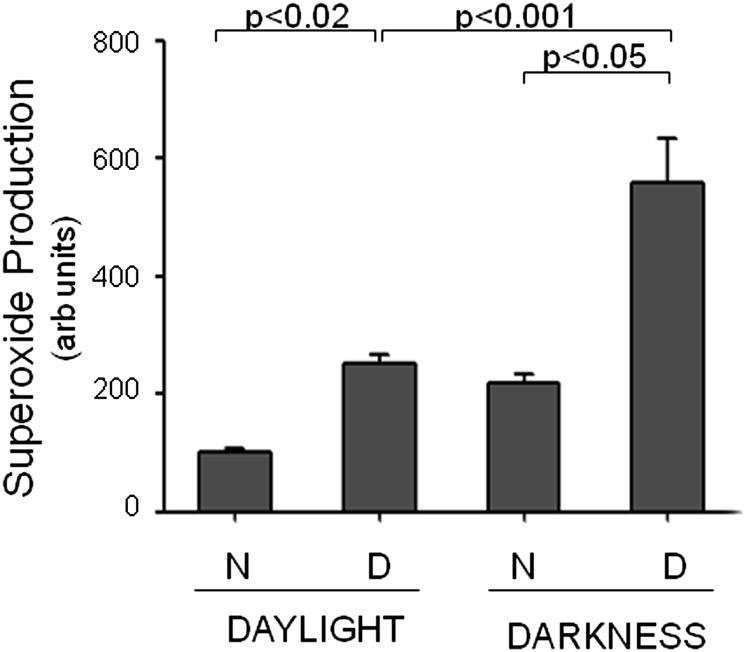
Diabetic (D; 2-mo duration) and age-matched nondiabetic (N) mice (C57BL/6) were killed at 1:00 PM (daylight; 7 h after lights came on) and 1:00 AM (darkness; 7 h after onset of darkness), and superoxide generated in whole retinas was quantitated by reaction with lucigenin (11, 16). Sample collection and assays for the darkness data point were carried out in darkness under a dim red light. Fig. 2 shows that diabetes significantly increased retinal superoxide generation both day and night, and that the increase in superoxide generation was greatest during the period of darkness in retinas from diabetic mice. These studies strongly support the hypothesis that photoreceptors are a major source of retinal oxidative stress in diabetes, and provide evidence that superoxide production changes with daylight versus darkness.
Fig. 2.
Dark exacerbates superoxide generation by retina in diabetic mice. Superoxide was measured (lucigenin method) 7 h after lights came on (daylight) and 7 h after lights went off (darkness) in N and D wild-type mice. Samples collected during the night were collected under dim red light. Duration of diabetes was 2 mo at the time of this assay (n = 5 per group, 4 mo of age when killed).
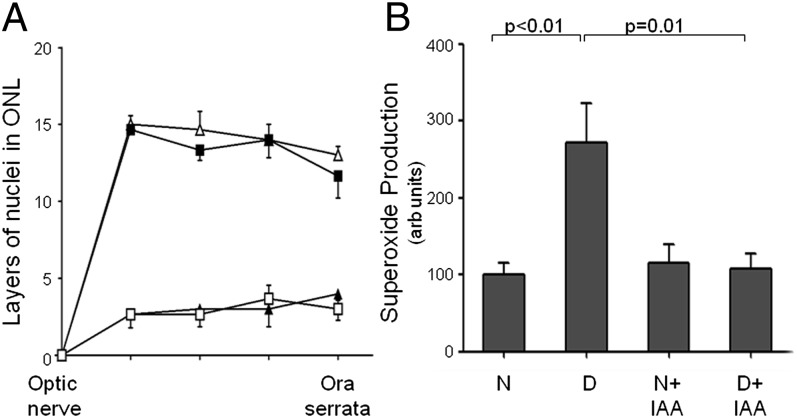
Degeneration of photoreceptors in opsin−/− mice or experimental destruction of photoreceptor cells with IAA significantly inhibits diabetes-induced generation of superoxide in the retina.
opsin−/− and wild-type mice were made diabetic for 2 mo (4 mo of age at autopsy). To acutely cause photoreceptor degeneration, we injected some wild-type diabetic and nondiabetic mice intraperitoneally with IAA (at 7 wk of diabetes, and 1 wk before killing the animals). At 2 mo of diabetes, essentially all photoreceptors were missing from retinas of opsin−/− mice and from retinas of mice injected with IAA 1 wk before (Figs. 3A and 4A) compared with wild-type controls. As expected, diabetes significantly increased superoxide in wild-type mice (Figs. 3B and 4B), but this increase in superoxide generation was significantly inhibited in diabetic mice lacking photoreceptors because either of the deficiency of opsin or the IAA injection. Interestingly, superoxide generation tended to be greater than normal in retinas from nondiabetic (not significant) and diabetic (P < 0.05) animals lacking photoreceptors resulting from opsin deficiency.
Fig. 3.
Genetic degeneration of photoreceptors prevents diabetes-induced generation of superoxide in mouse retina. Photoreceptor degeneration was induced by genetic deficiency of opsin (opsin−/−) in C57BL/6 mice. (A) Compared with wild-type control and diabetic mice, essentially all photoreceptors were missing from the retinas of opsin−/− mice at 4 mo of age. ∆, N wild-type mice; ■, D wild-type mice; ▲, N opsin−/−; □, D opsin−/−. (B) The diabetes-induced increase in retinal superoxide production seen in wild-type mice was largely absent in diabetic mice lacking photoreceptors. Data are expressed as a percent of the value of nondiabetic wild-type controls. The duration of diabetes was 2 mo at the time of this assay (n = 3–5 per group).
Fig. 4.
Rapid degeneration of photoreceptors prevents diabetes-induced generation of superoxide by mouse retina. Photoreceptor degeneration was induced with IAA. (A) Compared with wild-type control N and D mice, essentially all photoreceptors were missing from mouse retina 7 d after i.p. injection of IAA. ∆, N wild-type; ■, D wild-type; ▲, N+IAA; □, D+IAA. (B) Likewise, the diabetes-induced increase in retinal superoxide production seen in control mice was essentially prevented in diabetic mice lacking photoreceptors as a result of IAA treatment. Data are expressed as a percent of the value of nondiabetic wild-type controls. Duration of diabetes was 2 mo at the time of this assay (n = 3–5 per group, 4 mo of age when killed).
Cellular Sources of Retinal Superoxide in Diabetes.
We studied mitochondria and NADPH oxidase for their contribution to diabetes-induced increase in retinal generation of superoxide in mice.
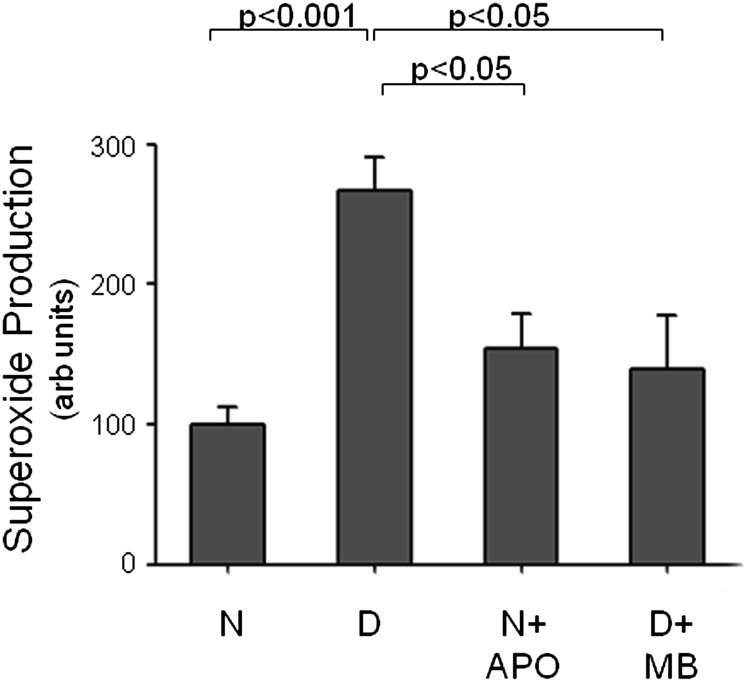
Mitochondria are known to contribute to superoxide generation in diabetes (11, 17). Because most mitochondria in the retina are located in photoreceptors, we tested the possibility that enhancing delivery of electrons to complex IV of the electron transport system would inhibit superoxide generation in diabetic mouse retina. Methylene blue has been reported to act as an alternate electron transporter that can suppress mitochondrial-induced oxidative stress (18). In our hands, daily administration of methylene blue over the 2 mo of diabetes significantly inhibited the diabetes-induced generation of superoxide by the retina (Fig. 5). This finding is consistent with the postulate that impaired mitochondrial electron transport is an important contributor to retinal superoxide production in diabetes.
Fig. 5.
Both NADPH oxidase and mitochondria in mouse retina contribute to diabetes-induced generation of superoxide. Apocynin (APO), an inhibitor of NADPH oxidase, was administered daily (i.p. injections at a dose of 36 mg/kg). Methylene blue (MB), which donates electrons directly to mitochondrial cytochrome oxidase and thereby bypasses the leaky electron transport system in diabetes, was administered daily in drinking water at a dose of 5 mg/day. Both therapies significantly inhibited the diabetes-induced generation of superoxide by the retina. Data are expressed as a percent of the value of N wild-type controls. Duration of diabetes was 2 mo at the time of this assay, and administration of both agents began 1 wk after the onset of diabetes (n = 5 mice per group, 4 mo of age when killed).
NADPH oxidase is another potential source of superoxide in photoreceptors (19). We administered apocynin, a potent inhibitor of NADPH oxidase (20) for the 2 mo of diabetes to investigate the contribution of NADPH oxidase to retinal superoxide generation in diabetes. As with the previously mentioned mitochondrial therapy, inhibition of NADPH oxidase significantly inhibited the diabetes-induced increase in retinal generation of superoxide (Fig. 5). Thus, both mitochondria and NAPDH oxidase apparently contribute to superoxide generation by retina in diabetes. Interestingly, both therapeutic approaches inhibited the diabetes-induced increase in retinal superoxide generation to a similar extent.
Consequences of Diabetes-Induced Oxidative Stress in Photoreceptors; Induction of Proinflammatory Proteins.
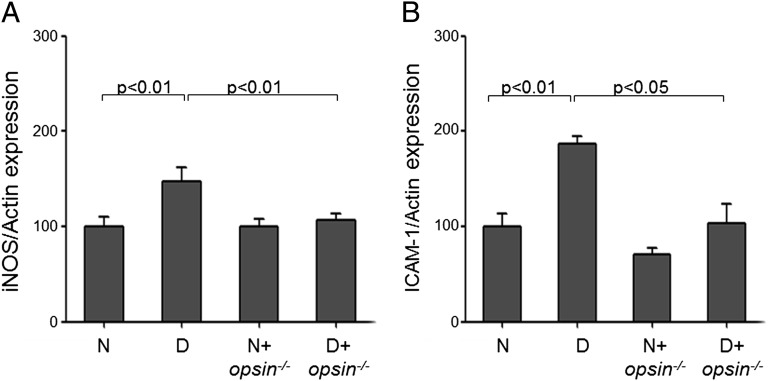
Several proinflammatory proteins, including the inducible form of nitric oxide synthase (iNOS) and intercellular adhesion molecule 1 (ICAM-1), are induced in the retina in diabetes and have been shown to play a critical role in the pathogenesis of the vascular degeneration that occurs in diabetic retinopathy (5, 21). Thus, we investigated if the increased expression of iNOS and ICAM-1 are related to changes occurring in photoreceptors in diabetes. Retinas from wild-type mice diabetic for 2 mo showed the expected induction of iNOS and ICAM-1 in diabetes (Fig. 6), but these abnormalities were significantly reduced in retinas from diabetic animals whose photoreceptors had degenerated (opsin−/− mice).
Fig. 6.
Degeneration of photoreceptor cells prevents the diabetes-induced production of proinflammatory enzymes in retina. Expression of iNOS and ICAM-1 were measured by Western blots in retinal tissue of wild-type control and diabetic mice, and in opsin−/− N and D mice. Data are expressed relative to the expression of actin, a housekeeping protein in the same lanes, and are expressed as a percent of the value of nondiabetic wild-type controls. Duration of diabetes was 2 mo (n = 3–5 animals per group, 4 mo of age when killed).
Discussion
Photoreceptors have long been recognized as playing an important role in the development of retinal hypoxia in advanced stages of diabetic retinopathy when vascular perfusion of the retina is impaired. Recent reports, however, have raised the possibility that photoreceptors or outer retina could play a role in the development of even the early stages of diabetic retinopathy. Stitt and colleagues (15) reported that diabetes-induced reduction in retinal vasculature density was less in mice lacking opsin, a model that leads to photoreceptor degeneration. Moreover, expected increases in the expression of VEGF and TNF-α in diabetes also were significantly lower in diabetic opsin−/− mice. The authors concluded that loss of the outer retina reduced the severity of diabetic retinopathy in that model. In addition, two studies involving diabetic patients produced results consistent with a role of photoreceptors in the development of diabetic retinopathy. Results of a survey sent to a small group of diabetic patients who also had retinitis pigmentosa suggested that their diabetic retinopathy was less severe, presumably because of photoreceptor degeneration (13). In addition, Arden et al. (22) conducted a study of 40 patients to determine if sleeping with one eye weakly illuminated with light (505 nm) to reduce the rod dark current could improve diabetes-induced macular edema of the retina. The authors reported that treatment with dim light kept rods light-adapted, and speculated that inhibition of the dark current was responsible for the reduction of defects in contrast sensitivity, tritan thresholds, and retinal edema in treated versus untreated eyes (23). We recently reported (24) that illuminating diabetic animals with far-red light for only 4 min per day also partially inhibited electrophysiologic and molecular abnormalities that have been implicated in the pathogenesis of the retinopathy. Together, these findings are consistent with a possible role for photoreceptors in the development of certain aspects of diabetic retinopathy.
How photoreceptors actually could contribute to retinal pathology in diabetes has not been clarified. Possible mechanisms include: (i) hypoxia resulting from rapid metabolism by photoreceptors; (ii) excessive generation of reactive oxidative species by photoreceptors, perhaps from hyperglycemia-induced defects in mitochondrial electron transport; (iii) altered metabolism or function of other neurons in the retina secondary to abnormalities in the photoreceptors; and/or (iv) defects caused by visual processes (phototransduction or visual cycle activity) within these specialized cells. These possibilities are not mutually exclusive.
Retinal hypoxia has long been postulated to be important, at least in advanced diabetic retinopathy. It is well known that photoreceptor cells account for much of the oxygen consumed by the retina, and that such metabolism is increased in the dark (14, 25) when the rod dark current becomes maximal (26–28). However, the evidence is less clear about the presence of hypoxia in early stages of diabetic retinopathy. Some investigators have reported semiquantitative histochemical evidence that retinal O2 tension was subnormal in diabetic animals (15, 29), an observation not confirmed by others (30–33). Additional work is needed to resolve this controversy.
The present studies demonstrate that photoreceptors are a major source of superoxide in the retina of diabetic animals. This generation of superoxide by photoreceptors is not a side effect of using streptozotocin to induce diabetes, because staining for superoxide also has been found in photoreceptors of spontaneously diabetic animals (34). Because photoreceptors are the major cell type in the retina and possess more mitochondria than do other retinal cells, they are likely to be the major source of the retinal superoxide in diabetes. Elimination of photoreceptor cells (either from birth as a result of opsin deficiency or acutely secondary to IAA injection) results in significantly less of the diabetes-induced generation of superoxide than characteristically observed in retinas from wild-type diabetic mice. We recognize that IAA exerts broad toxic effects because of alkylation, but the similar effects of opsin deletion strongly suggest that photoreceptor cells are a major source of retinal superoxide in diabetes. Our histochemical studies confirm this. Moreover, retinal superoxide generation in both diabetic and nondiabetic animals is greater at night, a time when energy demands and O2 consumption are far greater than in daylight (35–37). We postulate that this light/dark difference in rod energy demand accounts for the exacerbation of superoxide generation at night. Whether or not diurnal rhythm contributes to this phenomenon remains to be determined.
Although superoxide generation in retinas from diabetic opsin-deficient animals was significantly less than in diabetic wild-type controls, both nondiabetic and diabetic opsin−/− mice tended to generate more superoxide than did nondiabetic wild-type controls. Because essentially all photoreceptors had degenerated in these animals, the observed superoxide in opsin-deficient animals seems contrary to our hypothesis that photoreceptors are responsible for the superoxide. In contrast, IAA-induced photoreceptor degeneration did not result in an elevated superoxide level. A possible explanation is that glia and perhaps other retinal cells become activated (and generate superoxide) during retinal remodeling after photoreceptor degeneration. This activation is likely to be substantial in the progressive degeneration that has occurred since birth in opsin deficiency, whereas rapid (7 d) degeneration of photoreceptors with IAA likely did not provide adequate time for appreciable remodeling. Additional studies are needed to investigate this.
Diabetes results in mitochondrial defects in multiple tissues including the retina, and prominent among these abnormalities is the increased generation of superoxide (1, 4, 5, 11, 17, 38–40). We previously reported that mitochondria are major contributors to diabetes-induced generation of superoxide by retina (11). Because most of the mitochondria in the retina are present in photoreceptor cells, it seemed likely that these cells are primarily responsible for the mitochondrial contribution to superoxide generation in diabetes. Consistent with this hypothesis, we now present direct evidence that much of the superoxide generated by the retina in diabetes emanates from photoreceptor cells, and that superoxide production by the retina is essentially abolished in their absence. Because mitochondria are packed within photoreceptors to support visual cycle activity, we postulate that either modest inhibition of visual cycle activity that has no discernible effect on normal vision or normalization of superoxide release from mitochondria in diabetes could decrease oxidative stress and the subsequent development of retinopathy. Methylene blue potently inhibits superoxide generation by the retina in diabetes, although it has multiple other reported effects as well (41).
Another important source of superoxide generation by photoreceptor cells is NADPH oxidase (19). Interestingly, pharmacologic inhibition of this enzyme also decreased the diabetes-induced generation of superoxide by mouse retina. In a previous ex vivo study of the retina (11), we failed to identify NADPH oxidase as a major contributor to retinal superoxide production in diabetes, but the brief 30-min exposure of retina to the NADPH oxidase inhibitor in that study could have been insufficient. In the present study, chronic administration of apocynin in vivo did significantly inhibit the diabetes-induced increase in superoxide generation. Thus, results of the present study suggest that both mitochondria and NADPH oxidase contribute to the diabetes-induced increase in retinal superoxide. That pharmacologic inhibition of either pathway inhibited the abnormal increase in retinal superoxide generation in diabetes suggests that these two mechanisms for superoxide generation are interrelated.
Elimination of photoreceptor cells also reduced the diabetes-induced induction of inflammatory proteins such as iNOS and ICAM-1. Whether these inflammatory proteins are induced in photoreceptors themselves or in other retinal cells is not yet clear, but there is evidence that iNOS is induced within photoreceptors in uveoretinitis (42). Inhibition of NADPH oxidase with apocynin also was noted to decrease diabetes-induced increases in ICAM-1, leukostasis, and breakdown of the blood–retinal barrier in diabetic retina (43). Superoxide generated by photoreceptors could be the mediator for induction of those inflammatory proteins (in photoreceptor or other retinal cells), but the high reactivity and thus the short migration distance of superoxide needs to be considered.
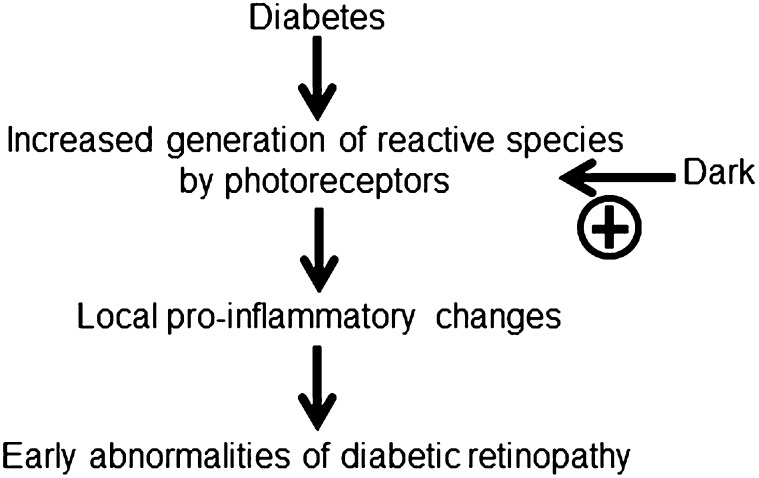
In summary, we provide evidence in mice that: (i) photoreceptor cells are the primary source of oxidative stress that develops in the retina of diabetic animals, (ii) retinal oxidative stress caused by diabetes is significantly worse in the dark, and (iii) diabetes-induced induction of the proinflammatory molecules iNOS and ICAM-1 does not occur in retina in the absence of photoreceptor cells. Thus, photoreceptor cells play an important role in the diabetes-induced increase in retinal superoxide and local inflammation in mice. Because prior studies show that therapies that inhibit oxidative stress or expression of iNOS and ICAM-1 also ameliorate the development of vascular lesions in early stages of diabetic retinopathy (1, 2), we postulate that oxidative stress in photoreceptors plays a critical role in the development of this condition, as summarized in Fig. 7. Precisely what molecular abnormalities in diabetes lead to the activation of superoxide generation by photoreceptor cells, and the intermediate steps between photoreceptor oxidative stress (which develops over a few weeks) and vascular pathology (which develops over months to years), remain to be clarified.
Fig. 7.
Postulated schematic relationship between oxidative stress in photoreceptors, the induction of proinflammatory proteins, and development of early diabetic retinopathy in mice.
Methods
Experimental Animals.
opsin+/− and WT C57BL/6 mice were obtained from J. Lem (Tufts University, Boston, MA) and the Jackson Laboratory, respectively. Male mice (2 mo old) were randomly assigned to become diabetic or remain as a nondiabetic control group. Diabetes was induced by five sequential daily i.p. injections of a freshly prepared solution of streptozotocin in citrate buffer (pH 4.5) at 60 mg/kg of body weight. Hyperglycemia was verified at least three times during the second week after streptozotocin administration, and mice having three consecutive measurements of blood glucose >275 mg/dL were classified as being diabetic. Insulin was given as needed to prevent weight loss without preventing hyperglycemia and glucosuria (0–0.2 units of NPH insulin s.c., 0–3 times per week). Glycohemoglobin was measured by the Bio-Rad Total Glycated Hemoglobin Assay (Bio-Rad) just before the animals were killed. Food consumption (Harlan Teklad; 7004 diet) and body weight were measured weekly. Treatment of animals conformed to the Association for Research in Vision and Ophthalmology Resolution on Treatment of Animals in Research as well as to the Case Western Reserve University Institutional Animal Care and Use Committee. At 2 mo of diabetes, eyes were collected from anesthetized animals in the morning (except for those killed at 1:00 PM and 1:00 AM to test effects of light and dark on superoxide generation by the retina), and the animals were then killed. All animals were 4 mo of age when they were killed.
IAA-Induced Degeneration of Retinal Photoreceptors.
IAA has been reported to induce acute photoreceptor degeneration (44). After 7 wk of diabetes (and in age-matched nondiabetic controls), IAA was injected intraperitoneally (two doses of 30 mg/kg, 2 h apart). Two diabetic animals died within 24 h of this injection. Seven days later, all remaining animals were killed. Cryosections were prepared from one eye and stained to count cells in the outer nuclear layer; superoxide was measured in the other retina with lucigenin.
Molecular Causes of Superoxide Generation.
Two approaches were used to assess the contributions of mitochondria and NADPH oxidase to superoxide generation in diabetic animals: administration of methylene blue or the NADPH oxidase inhibitor, apocynin. Methylene blue functions as an alternative electron carrier that accepts electrons from NADH and transfers them to cytochrome c, bypassing complex I/III blockage in mitochondria (18). Methylene blue was administered daily at a dose of 5 mg/day in the drinking water for 2 mo. Retinal tissue then was isolated, and superoxide generation was measured with lucigenin.
Other animals were injected intraperitoneally daily for 2 mo with 36 mg/kg of apocynin, an NADPH oxidase inhibitor in DMSO as previously reported (19). NADPH oxidase is known to exist in photoreceptor cells, where it has been linked to retinal degeneration (45–47). Administration of both agents commenced 1 wk after the onset of diabetes to assure that drug treatment did not influence the severity of diabetes. Retinal tissue then was isolated and superoxide generation was measured with lucigenin.
Counting of Photoreceptor Cell Layers in Histological Sections.
Posterior eyecups were fixed for 6–8 h in 4% (wt/vol) paraformaldehyde/PBS at 4 °C. They were then incubated for 30 min each in successive 5–20% concentrations of sucrose in PBS. Tissues were then frozen in a 1:1 mix of 20% sucrose:optimal cutting temperature solution. Sections were cut at 12 µm on a cryostat at or adjacent to the optic nerve. Nuclei were stained with DAPI and the number of layers of nuclei in the outer nuclear layer were counted at five positions along the retina from the disk to the ora serrata with a fluorescent microscope (Nikon Eclipse 80i) at 358/461 nm.
Histochemical Assays for Reactive Oxygen Species and Superoxide.
Cryosections (12 µm) of posterior eyecups were stained with DCF (final concentration, 10 μM) or DHE (final concentration, 0.625 μM) at 37 °C in a dark chamber for 60 min or 20 min, respectively. Sections were immediately photographed using a fluorescence microscope.
Lucigenin Assay of Superoxide.
Superoxide levels were measured chemically with lucigenin (bis-N-methylacridinium nitrate), as reported previously (6, 11, 16, 48, 49).
Immunoblots.
Retinas were isolated, sonicated, and centrifuged, and the supernatants were used for immunoblotting. Samples (50 µg) were fractionated by SDS/PAGE, electroblotted onto nitrocellulose membranes, and membranes were blocked in Tris-buffered saline, pH 7.6, containing 0.02% Tween 20 and 5% nonfat milk. Primary antibodies for ICAM-1 and iNOS (both at a 1:200 dilution; Santa Cruz Biotechnology) were applied for 1 h followed by a secondary antibody, also for 1 h. After washing, immunoblots were visualized for enhanced chemiluminescence (Amersham). Results are normalized to actin in the same band and to appropriate nondiabetic controls.
Sample Sizes and Statistics.
Data were analyzed by two-tailed ANOVA followed by the Fisher post hoc test. Differences were considered statistically significant at P < 0.05.
Acknowledgments
This work was supported by grants from the National Eye Institute of the National Institutes of Health under Awards R01EY00300, R01EY022938, and R24EY021126; and the Medical Research Service of the Department of Veteran Affairs, Veterans Health Administration, Office of Research and Development. The services of the Case Western Reserve University Visual Science Research Center Core Facilities are acknowledged (P30EY11373). K.P. is John H. Hord Professor of Pharmacology.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Zheng L, Kern T. Role of nitric oxide, superoxide, peroxynitrite and poly(ADP-ribose) polymerase in diabetic retinopathy. Front Biosci. 2009;14:3974–3987. doi: 10.2741/3505. [DOI] [PubMed] [Google Scholar]
- 2.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50(8):1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- 4.Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: Possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48(8):3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 5.Zheng L, et al. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 2007;50(9):1987–1996. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]
- 6.Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes. 2008;57(5):1387–1393. doi: 10.2337/db07-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkowitz BA, Gradianu M, Bissig D, Kern TS, Roberts R. Retinal ion regulation in a mouse model of diabetic retinopathy: Natural history and the effect of Cu/Zn superoxide dismutase overexpression. Invest Ophthalmol Vis Sci. 2009;50(5):2351–2358. doi: 10.1167/iovs.08-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji LL, Gomez-Cabrera MC, Vina J. Exercise and hormesis: Activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci. 2006;1067:425–435. doi: 10.1196/annals.1354.061. [DOI] [PubMed] [Google Scholar]
- 9.Esposito E, et al. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging. 2002;23(5):719–735. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 10.Lee JI, Burckart GJ. Nuclear factor kappa B: Important transcription factor and therapeutic target. J Clin Pharmacol. 1998;38(11):981–993. doi: 10.1177/009127009803801101. [DOI] [PubMed] [Google Scholar]
- 11.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med. 2003;35(11):1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Du Y, Kowluru A, Kern TS. PP2A contributes to endothelial death in high glucose: Inhibition by benfotiamine. Am J Physiol Regul Integr Comp Physiol. 2010;299(6):R1610–R1617. doi: 10.1152/ajpregu.00676.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arden GB. The absence of diabetic retinopathy in patients with retinitis pigmentosa: Implications for pathophysiology and possible treatment. Br J Ophthalmol. 2001;85(3):366–370. doi: 10.1136/bjo.85.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arden GB, Sidman RL, Arap W, Schlingemann RO. Spare the rod and spoil the eye. Br J Ophthalmol. 2005;89(6):764–769. doi: 10.1136/bjo.2004.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Gooyer TE, et al. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci. 2006;47(12):5561–5568. doi: 10.1167/iovs.06-0647. [DOI] [PubMed] [Google Scholar]
- 16.Du Y, et al. Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest Ophthalmol Vis Sci. 2010;51(4):2158–2164. doi: 10.1167/iovs.09-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishikawa T, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 18.Wen Y, et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011;286(18):16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, et al. Mechanism of all-trans-retinal toxicity with implications for Stargardt disease and age-related macular degeneration. J Biol Chem. 2012;287(7):5059–5069. doi: 10.1074/jbc.M111.315432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefanska J, Pawliczak R. Apocynin: Molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joussen AM, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 22.Arden GB, et al. A preliminary trial to determine whether prevention of dark adaptation affects the course of early diabetic retinopathy. Eye (Lond) 2010;24(7):1149–1155. doi: 10.1038/eye.2009.328. [DOI] [PubMed] [Google Scholar]
- 23.Arden GB, Jyothi S, Hogg CH, Lee YF, Sivaprasad S. Regression of early diabetic macular oedema is associated with prevention of dark adaptation. Eye (Lond) 2011;25(12):1546–1554. doi: 10.1038/eye.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang J, et al. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: In vivo and in vitro. Invest Ophthalmol Vis Sci. 2013;54(5):3681–3690. doi: 10.1167/iovs.12-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arden GB, Wolf JE, Tsang Y. Does dark adaptation exacerbate diabetic retinopathy? Evidence and a linking hypothesis. Vision Res. 1998;38(11):1723–1729. doi: 10.1016/s0042-6989(98)00004-2. [DOI] [PubMed] [Google Scholar]
- 26.Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986;88(4):521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haugh LM, Linsenmeier RA, Goldstick TK. Mathematical models of the spatial distribution of retinal oxygen tension and consumption, including changes upon illumination. Ann Biomed Eng. 1990;18(1):19–36. doi: 10.1007/BF02368415. [DOI] [PubMed] [Google Scholar]
- 28.Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20(2):175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 29.Ly A, et al. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci. 2011;52(13):9316–9326. doi: 10.1167/iovs.11-7879. [DOI] [PubMed] [Google Scholar]
- 30.Diederen RM, Starnes CA, Berkowitz BA, Winkler BS. Reexamining the hyperglycemic pseudohypoxia hypothesis of diabetic oculopathy. Invest Ophthalmol Vis Sci. 2006;47(6):2726–2731. doi: 10.1167/iovs.06-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau JC, Linsenmeier RA. Oxygen consumption and distribution in the diabetic rat retina. Invest Ophthalmol Vis Sci. 2010;51:5644 (ARVO abstract). [Google Scholar]
- 32.Wright WS, McElhatten RM, Messina JE, Harris NR. Hypoxia and the expression of HIF-1alpha and HIF-2alpha in the retina of streptozotocin-injected mice and rats. Exp Eye Res. 2010;90(3):405–412. doi: 10.1016/j.exer.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright WS, McElhatten RM, Harris NR. Increase in retinal hypoxia-inducible factor-2α, but not hypoxia, early in the progression of diabetes in the rat. Exp Eye Res. 2011;93(4):437–441. doi: 10.1016/j.exer.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao C, et al. Physiological effects of superoxide dismutase on altered visual function of retinal ganglion cells in db/db mice. PLoS ONE. 2012;7(1):e30343. doi: 10.1371/journal.pone.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu DY, Cringle SJ. Outer retinal anoxia during dark adaptation is not a general property of mammalian retinas. Comp Biochem Physiol A Mol Integr Physiol. 2002;132(1):47–52. doi: 10.1016/s1095-6433(01)00528-1. [DOI] [PubMed] [Google Scholar]
- 36.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: Fundamental and clinical aspects. Arch Ophthalmol. 2003;121(4):547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 37.Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18(24):1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishikawa T, Edelstein D, Brownlee M. The missing link: A single unifying mechanism for diabetic complications. Kidney Int Suppl. 2000;77:S26–S30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- 39.Sakai K, et al. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun. 2003;300(1):216–222. doi: 10.1016/s0006-291x(02)02832-2. [DOI] [PubMed] [Google Scholar]
- 40.Kiritoshi S, et al. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: Potential role in diabetic nephropathy. Diabetes. 2003;52(10):2570–2577. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- 41.Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of Methylene Blue in the nervous system. Med Res Rev. 2011;31(1):93–117. doi: 10.1002/med.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajendram R, Saraswathy S, Rao NA. Photoreceptor mitochondrial oxidative stress in early experimental autoimmune uveoretinitis. Br J Ophthalmol. 2007;91(4):531–537. doi: 10.1136/bjo.2006.101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Shabrawey M, et al. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2008;49(7):3239–3244. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang L, et al. Long-term cellular and regional specificity of the photoreceptor toxin, iodoacetic acid (IAA), in the rabbit retina. Vis Neurosci. 2008;25(2):167–177. doi: 10.1017/S0952523808080401. [DOI] [PubMed] [Google Scholar]
- 45.Haruta M, et al. Depleting Rac1 in mouse rod photoreceptors protects them from photo-oxidative stress without affecting their structure or function. Proc Natl Acad Sci USA. 2009;106(23):9397–9402. doi: 10.1073/pnas.0808940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhatt L, Groeger G, McDermott K, Cotter TG. Rod and cone photoreceptor cells produce ROS in response to stress in a live retinal explant system. Mol Vis. 2010;16:283–293. [PMC free article] [PubMed] [Google Scholar]
- 47.Usui S, et al. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J Neurochem. 2009;110(3):1028–1037. doi: 10.1111/j.1471-4159.2009.06195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kern TS, Du Y, Miller CM, Hatala DA, Levin LA. Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am J Pathol. 2010;176(5):2550–2558. doi: 10.2353/ajpath.2010.091062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kern TS, et al. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes. 2007;56(2):373–379. doi: 10.2337/db05-1621. [DOI] [PubMed] [Google Scholar]