Significance
Skeletal muscle exhibits a remarkable ability to regenerate, a process that has been demonstrated to be dependent on satellite cells. Satellite cells uniformly express the transcription factor paired box 7 (Pax7), and extensive analysis of Pax7-deficient mice has documented the progressive loss of the satellite cell lineage likely due to a failure to proliferate together with precocious differentiation. In contrast to these findings, a recent provocative study suggested that Pax7 was entirely dispensable in adult life. Here, we demonstrate that Pax7 expression is an absolute prerequisite for the normal function of satellite cells during regenerative myogenesis at any age. Our experiments therefore demonstrate that Pax7 plays an essential role in regulating the myogenic potential and function of satellite cells.
Keywords: CreERT2, stem cell
Abstract
Extensive analyses of mice carrying null mutations in paired box 7 (Pax7) have confirmed the progressive loss of the satellite cell lineage in skeletal muscle, resulting in severe muscle atrophy and death. A recent study using floxed alleles and tamoxifen-induced inactivation concluded that after 3 wk of age, Pax7 was entirely dispensable for satellite cell function. Here, we demonstrate that Pax7 is an absolute requirement for satellite cell function in adult skeletal muscle. Following Pax7 deletion, satellite cells and myoblasts exhibit cell-cycle arrest and dysregulation of myogenic regulatory factors. Maintenance of Pax7 deletion through continuous tamoxifen administration prevented regrowth of Pax7-expressing satellite cells and a profound muscle regeneration deficit that resembles the phenotype of skeletal muscle following genetically engineered ablation of satellite cells. Therefore, we conclude that Pax7 is essential for regulating the expansion and differentiation of satellite cells during both neonatal and adult myogenesis.
Skeletal muscle exhibits a remarkable ability to regenerate, a process that has been demonstrated to be dependent on satellite cells (1–5). In resting muscle, satellite cells remain quiescent, whereas in response to growth or trauma, satellite cells become activated, enter the cell cycle, and give rise to proliferating myogenic precursor cells that either differentiate by either fusing with existing myofibers or forming new myofibers (6, 7). This process is highly regulated by growth factors and the composition of the niche, and by expression of key transcriptional regulators such as paired box 7 (Pax7) and myogenic regulatory factors, which control specification and differentiation analogously to embryonic development (6, 8–10).
Satellite cells uniformly express the transcription factor Pax7, and extensive analysis of Pax7−/− mice has thoroughly documented the progressive loss of the satellite cell lineage in multiple muscle groups likely due to a failure to proliferate together with precocious differentiation (1, 5, 11, 12). Pax7−/− muscles are reduced in size, the myofibers contain ∼50% the normal number of nuclei, and fiber diameters are significantly reduced. The mice exhibit poor survivability and typically die within the first 3 wk of life.
Consistent with a central role for Pax7 in satellite cell function, siRNA-mediated knockdown of Pax7 in any age of cultured myoblasts or satellite cells results in growth arrest and loss of Myf5 expression (13, 14). Indeed, Pax7 has been shown to inhibit differentiation by inhibiting MyoD-dependent activation of myogenin (15, 16). Recently, ChIP-seq analysis indicates that Pax7 binds to distinct DNA motifs to activate genes involved in specifying myogenic identity, promoting proliferation, and inhibiting differentiation (17). Together, these data support an essential role for Pax7 in regulating the myogenic potential and function of satellite cells. In contrast to these findings, a provocative study by Lepper et al. (18) suggested that Pax7 was entirely dispensable in adult life. Tamoxifen-induced Pax7 deletion in satellite cells after 3 wk of age (P21) was reported to not lead to any deficiency in muscle regeneration or satellite cell number (18). In this report, we demonstrate that Pax7 expression is an absolute prerequisite for the normal function of satellite cells during regenerative myogenesis at any age.
Results
Pax7 Deficiency Results in Cell-Cycle Arrest and Precocious Differentiation.
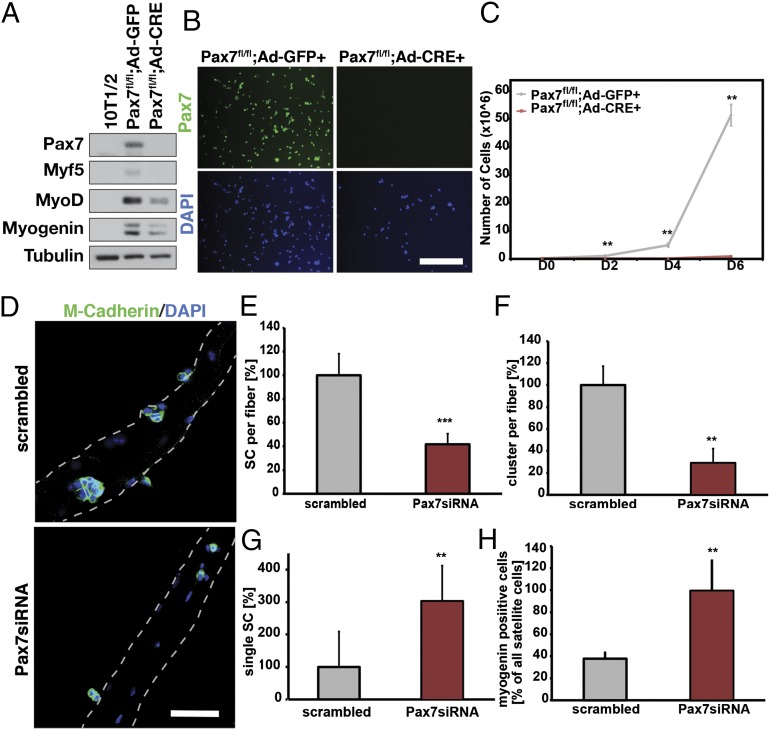
To investigate the growth and differentiation of Pax7-deficient primary myoblasts, we used adenovirus expressing Cre recombinase (Ad-Cre) to efficiently delete Pax7 in primary myoblasts derived from 6-wk-old Pax7fl/fl mice. Primary myoblasts (Pax7fl/fl) infected with Ad-Cre clearly showed a complete loss of Pax7 expression in 100% of the cells (Fig. 1 A and B). Strikingly, Pax7 deletion resulted in an immediate and complete growth arrest, with no appreciable proliferation after 6 d in culture or increased apoptosis (Fig. 1C and Fig. S1 E and F). By contrast, primary myoblasts infected with a control adenovirus (Ad-GFP) underwent a 50-fold expansion during the same time period (Fig. 1C). We also noted that infection with Ad-Cre did not have a discernable effect on proliferation using primary myoblasts from WT mice (Fig. S1G).
Fig. 1.
Pax7 deletion results in cell-cycle arrest and precocious differentiation. (A) Infection of Pax7fl/fl myoblasts with an adenovirus encoding the Cre gene leads to depletion of Pax7 expression and loss of Myf5 protein expression. (B) Infection of Pax7fl/fl myoblasts with an adenovirus encoding the Cre gene leads to depletion of Pax7 expression. Pax7 immunostaining is depicted in green. Nuclei are counterstained with DAPI. (Scale bar: 100 µm.) (C) Ad-Cre-mediated Pax7 deletion results in growth arrest in primary myoblasts, relative to control (Ad-GFP) myoblasts; n = 3, **P < 0.01. (D) The siRNA-mediated depletion of Pax7 expression in satellite cells leads to reduced numbers of satellite cells (marked by M-Cadherin staining in green) after 72 h of culture. Nuclei are counterstained with DAPI. (Scale bar: 100 µm.) (E) Following depletion of Pax7 in satellite cells on single myofibers, the number of satellite cells per fiber is significantly reduced; n = 4, ***P < 0.001. (F) Numbers of clusters (three or more satellite cells on a fiber attached to each other) are reduced following treatment with Pax7 siRNA compared with scrambled control; n = 4, ***P < 0.01. (G) Increase in the number of single satellite cells on myofibers following 72 h in culture and treatment with Pax7siRNA; n = 4, ***P < 0.01. (H) Numbers of myogenin-positive satellite cells increase after 72 h in culture following depletion of Pax7 expression with Pax7 siRNA; n = 4, ***P < 0.01.
We have previously documented that Myf5 is a direct target gene of Pax7, and that Myf5 transcription varies directly with Pax7 levels (13, 17). Primary myoblasts where Pax7 was deleted with Ad-Cre exhibited an almost 75% reduction in the levels of Myf5 mRNA, a 25% reduction in MyoD expression, and no change in myogenin expression levels (Fig. S1A). Notably, Pax7-deficient myoblasts plated at high density were nevertheless able to undergo normal differentiation, as evidenced by the diameter and fusion index of the myotubes (Fig. S1 B−D).
Knockdown of Pax7 prevents the activation of Myf5 following an asymmetric satellite stem cell division on cultured myofibers from adult skeletal muscle (14). Therefore, we investigated the ability of Pax7-deficient satellite cells on cultured myofibers to undergo proliferation and differentiation. Following siRNA-mediated knockdown of Pax7, the numbers of satellite cells were decreased 2.4-fold after 72 h of culture (Fig. 1E, n = 4, P < 0.001; Pax7 siRNA knockdown efficiency: 90 ± 15%, n = 4). Furthermore, numbers of multicell clusters were reduced 3.5-fold (Fig. 1F, n = 4, P < 0.01), and numbers of single satellite cells were increased threefold (Fig. 1G, n = 4, P < 0.01). We also observed a 2.6-fold increase in the numbers of satellite cells expressing myogenin, suggesting precocious differentiation (Fig. 1H, n = 4, P < 0.01). Similar results were obtained by comparing single myofiber cultures from tamoxifen-induced Pax7fl/CreERT2 and Pax7fl/+ mice (Fig. S2). Therefore, we conclude that satellite cells and primary myoblasts lacking Pax7 undergo cell cycle arrest and precocious differentiation.
Inactivation of Pax7 in Adult Satellite Cells Markedly Impairs Muscle Regeneration.
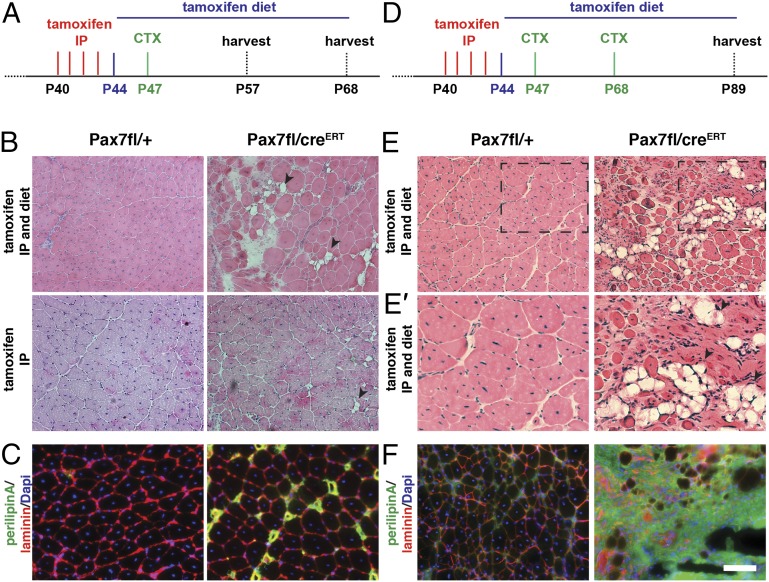
By using the same alleles generated by Lepper et al. (18), we next set out to examine the adult phenotype of Pax7-deficient satellite cells. Pax7fl/CreERT2 and Pax7fl/+ mice were injected four times intraperitonally (IP) with tamoxifen between P40 and P44 to induce the excision of exon 2, partially encoding the paired domain, and generate an out-of-frame mutant Pax7 transcript in satellite cells (Fig. 2A). One week later, muscle regeneration was induced by injection of cardiotoxin (CTX) into the tibialis anterior (TA) muscle in the hind limb. The TA muscles in control Pax7fl/+ mice were efficiently regenerated 21 d after acute injury (Fig. 2A). Notably, regenerated muscle in Pax7fl/CreERT2 mice following tamoxifen IP injection exhibited evidence of accumulation of cells in interstitial spaces and ectopic adipogenesis (Fig. 2B) at 21 d following CTX injection.
Fig. 2.
Inactivation of Pax7 in adult satellite cells markedly impairs muscle regeneration. (A) Schematic showing the experimental regime for single injury used in this study. (B) H&E staining of tibialis anterior muscle 21 d after acute injury induced by CTX injection. Arrowheads point to fat depositions. (C) Immunostaining for perilipin (in green) demonstrates increased adipogenesis in Pax7fl/CreERT2 mice, laminin staining is shown in red, and nuclei are counterstained with DAPI (in blue). (D) Schematic showing the experimental regime for double CTX injury used in this study. (E) H&E staining of tibialis anterior muscle 21 d after the second injury induced by CTX injection. Arrowheads point to fat depositions. (E’) A magnification of E. (F) Immunostaining for perilipin (in green) demonstrates increased adipogenesis in Pax7fl/CreERT2 mice, laminin staining is shown in red, and nuclei are counterstained with DAPI (in blue). (Scale bar: 100 µm.)
This partial phenotype led us to hypothesize that tamoxifen injection was not 100% effective in deleting the floxed Pax7 allele in satellite cells. To address this possibility, mice that were IP-injected with tamoxifen were additionally maintained on chow containing tamoxifen throughout the experimental time course (Fig. 2A). Importantly, control Pax7fl/+ and Pax7CreERT2/+ mice subjected to continuous tamoxifen treatment exhibited efficient regeneration of the TA muscle 21 d after acute injury as evidenced by centrally located nuclei and no appreciable deposition of fibrotic tissue, adipose tissue, or deposition of calcium (Fig. 2 B and C and Fig. S3 A−C and E). By contrast, the TA muscles of Pax7fl/CreERT2 mice continuously treated with tamoxifen exhibited a severe regeneration deficit characterized by smaller numbers of regenerating fibers, extensive deposition of adipose tissue, fibrotic tissue, and calcium deposits (Fig. 2 B and C, Fig. S3 A−C, and Tables S1 and S2). When Pax7fl/CreERT mice were injured a second time with CTX (Fig. 2D), the regeneration deficit was even more striking, as evidenced by massive adipogenesis, fibrosis, and significantly reduced muscle weights at 21 d after the second injury (2.3-fold). Total mouse weights were not significantly different between Pax7fl/CreERT and Pax7fl/+ mice (Fig. 2 D−F and Fig. S4 D−H). Together, these experiments unequivocally demonstrate the importance of Pax7 expression for satellite cell function in adult skeletal muscle.
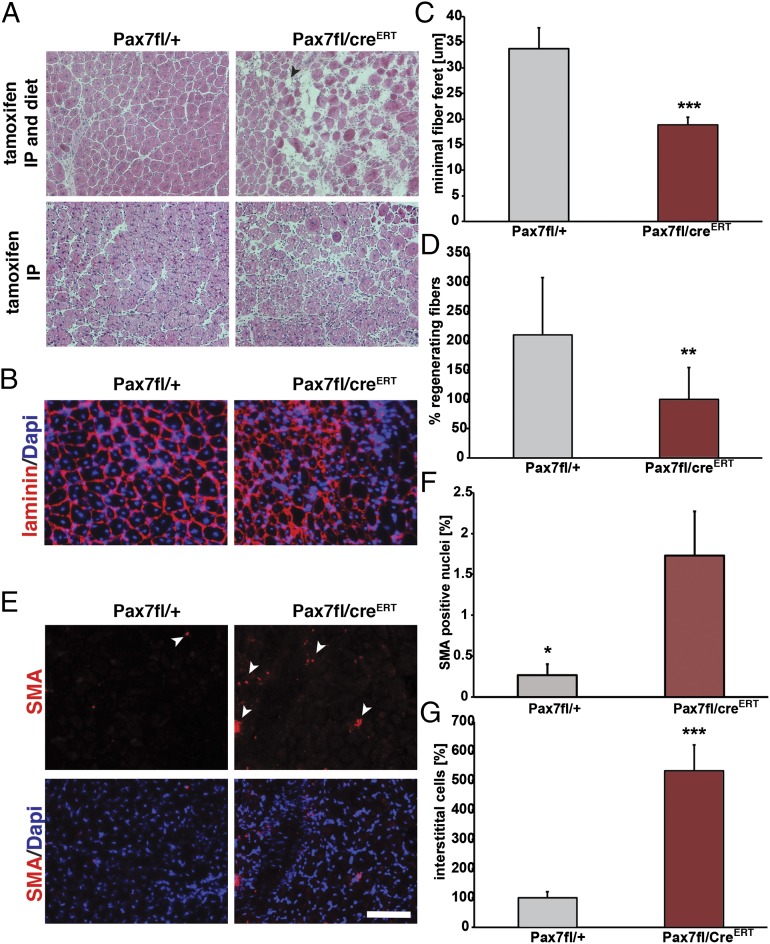
To quantitatively evaluate muscle regeneration in Pax7fl/+ versus Pax7fl/CreERT2 mice, we examined histological sections of TA muscles at 10 d after acute injury through CTX injection in mice where CreERT2 activity was maintained with tamoxifen-containing chow. In comparison with mice treated only by tamoxifen IP injection, muscle regeneration was dramatically impaired in Pax7fl/CreERT2 mice continuously treated with tamoxifen (Fig. 3A and Fig. S4 A−C). We observed a 2.1-fold reduction in the number of regenerated myofibers (Fig. 3 B and D) together with a marked increase in the numbers of interstitial cells relative to Pax7fl/+ TA muscle (Fig. 3 A and G). Moreover, muscle weight was reduced by 1.3-fold (Fig. S4A), myofibers were 1.8-fold smaller as measured by the minimal fiber feret, and the overall numbers of myonuclei were reduced 3.3-fold (Fig. 3C and Fig. S5 E and F).
Fig. 3.
Significantly impaired muscle regeneration with continuous tamoxifen treatment. (A) H&E staining 10 d after acute injury reveals markedly impaired regeneration following continuous tamoxifen treatment. (Scale bar: 100 μm.) (B) Immunostaining for laminin (in red) showing decreased numbers of fibers and also reduced fiber feret. Nuclei are counterstained with DAPI (in blue). (Scale bar: 100 μm.) (C) Quantification of the minimal fiber feret of muscles at 10 d after acute injury; n= 6, ***P < 0.001. (D) Quantification of the amount of regenerating fibers at 10 d after CTX injury; n = 6, *P < 0.01. (E) Immunostaining for smooth muscle actin (SMA, in red) demonstrating increased numbers of SMA-positive nuclei in Pax7fl/CreERT2 animals compared with Pax7fl/+ animals at 10 d after acute injury. Nuclei are counterstained with DAPI (in blue). (Scale bar: 100 μm.) (F) Quantification of SMA-positive nuclei relative to the total number of nuclei; n = 6, *P < 0.05. (G) Quantification of the numbers of interstitial cells, n = 4, ***P < 0.001.
Consistent with a significant delay in regeneration, immunostaining for developmental myosin heavy chain revealed a 7.5-fold increase in the numbers of immature myofibers (Fig. S5 A and B), and a 1.8-fold increase in the numbers of myogenin-expressing cells (Fig. S5 C and D). Furthermore, we observed a 6.7-fold increase in the numbers of MyoD negative smooth muscle actin (SMA)-positive cells (Fig. 3 E and F), indicative of myofibroblasts. Therefore, we conclude that maintenance of CreERT2 activity by continuous exposure to tamoxifen results in a dramatic loss of a muscle regeneration capability due to a loss of satellite cell proliferative capacity.
Continuous Tamoxifen Treatment Prevents Grow-Back of Pax7-Expressing Satellite Cells.
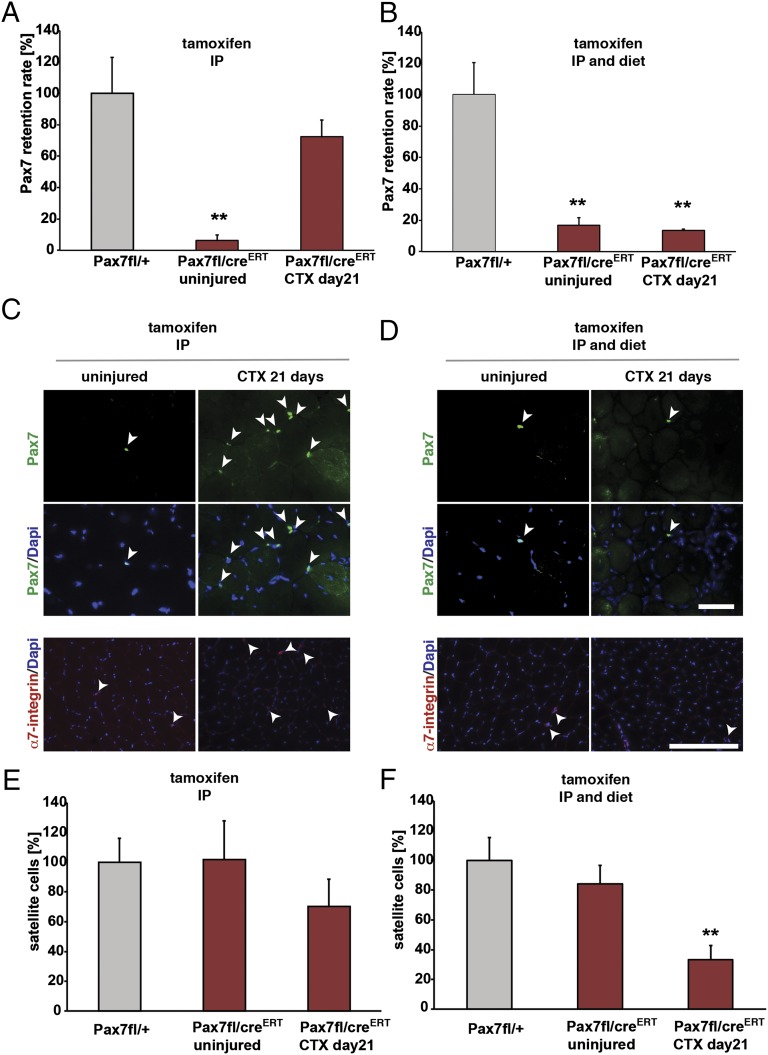
One explanation for the apparently normal regeneration following IP injection of tamoxifen (18) is that satellite cells that escape tamoxifen-induced deletion of the floxed Pax7 allele are capable of repopulating a regenerating muscle to mediate a partial regeneration phenotype. To test this hypothesis, we enumerated the numbers of Pax7-expressing satellite cells in Pax7fl/CreERT2 mice that had received continuous tamoxifen treatment (Fig. 2A) in the injured TA muscle relative to the uninjured contralateral TA muscle or mice that had only received IP injections of tamoxifen (Fig. 4). The uninjured muscle exhibited efficient excision of Pax7 in satellite cells with an over 80% reduction in the numbers of Pax7-expressing satellite cells (Fig. 4 A−D). Strikingly, in mice that only received tamoxifen through IP injections, the number of residual Pax7-expressing satellite cells and total number of satellite cells increased over 10-fold following acute injury (Fig. 4 A, C, and E). By contrast, continuous exposure to tamoxifen completely suppressed the regrowth of satellite cells as determined by Pax7 and α7-integrin immunostaining (Fig. 4 B, D, and F). Detection of satellite cells by α7-integrin immunostaining identifies both Pax7-expressing and Pax7-deleted satellite cells (Fig. 4 E and F). Comparison of Fig. 4 A and E reveals that before injury, deletion of Pax7 does not reduce the overall number of satellite cells (uninjured). We interpret these data to indicate that Pax7-deficient satellite cells are lost through terminal differentiation during the first round of repair and that satellite cells expressing Pax7 undergo expansion and are capable of repopulating the satellite cell niche (compare Fig. 4 A and B). Thus, without continuous tamoxifen exposure, virtually all of the satellite cells generated through regeneration express Pax7 (compare Fig. 4 A and E), whereas with continuous tamoxifen exposure, less than 40% of the small number of remaining satellite cells express Pax7 (compare Fig. 4 B and F). Taken together, these results underscore the need to perform control experiments when using conditional alleles in conditions where a small subpopulation that escapes deletion can repopulate the tissue due to a tremendous growth advantage.
Fig. 4.
Continuous tamoxifen treatment prevents grow-back of residual Pax7-expressing satellite cells. (A) Quantification of Pax7-expressing nuclei in Pax7fl/CreERT2 mice induced with tamoxifen before and after acute injury; n = 3, **P < 0.01. (B) Quantification of Pax7-expressing nuclei in Pax7fl/CreERT2 mice with continuous tamoxifen application before and after acute injury; n = 3, **P < 0.01. (C) Pax7 expression is lost after tamoxifen-induced excision in Pax7fl/CreERT2 mice (IP injection) but reoccurs after acute injury, Pax7 immunostaining is shown in green, and nuclei are counterstained with DAPI (in blue). Staining for α7-integrin is shown in red, and nuclei are counterstained with DAPI (in blue). (D) Pax7 deletion is only maintained when tamoxifen is continuously administered during regeneration of CTX-injured muscles, Pax7 immunostaining is shown in green, and nuclei are counterstained with DAPI (in blue). Staining for α7-integrin is shown in red, and nuclei are counterstained with DAPI (in blue). (E) Quantification of numbers of satellite cells (marked by expression of α7-integrin) in Pax7fl/CreERT2 mice induced with tamoxifen before and after acute injury; n = 3. (F) Quantification of numbers of satellite cells (marked by expression of α7-integrin) in Pax7fl/CreERT2 mice with continuous tamoxifen application before and after acute injury; n = 3, **P < 0.01. (Scale bar: 50 µm.)
Discussion
In adult muscle, satellite cells express Pax7, a paired-box transcription factor, and remain quiescent under normal physiological conditions. Satellite cells are readily responsive to molecular triggers from exercise, injuries, or disease, and have a remarkable ability to self-renew, expand, proliferate as myoblasts, or undergo myogenic differentiation to fuse and restore damaged muscle. Since the discovery of satellite cells, extensive evidence from multiple laboratories has accumulated to demonstrate that they are the primary contributors to postnatal growth, maintenance, and repair of skeletal muscle (6, 19).
Many studies have demonstrated that Pax7 plays a critical role in regulating the function of satellite cells. We and others have performed extensive analyses of Pax7−/− mice and have thoroughly documented the progressive loss of the satellite cell lineage in all muscle groups (1, 5, 11, 12). By contrast, the study by Lepper et al. (18) suggested that Pax7 was entirely dispensable for normal satellite cell function after a critical juvenile period.
Our experiments demonstrate that deletion of Pax7 in adult satellite cells results in markedly impaired regeneration as evidenced by extensive deposition of fibrotic and adipose tissue and reduced formation of myofibers. Notably, this prominent regeneration deficit was only observed when Pax7fl/CreERT2 mice were continuously treated with tamoxifen after receiving IP injections of tamoxifen (Figs. 2–4). Indeed, the regeneration phenotype in our experiments is highly similar to that observed following genetically engineered ablation of satellite cells in adult muscle (2, 3).
Our observations indicate that the observed difference in the severity of the regeneration phenotype is attributable to the regrowth of the small population of Pax7-expressing satellite cells when mice are not continuously treated with tamoxifen (Fig. 4). This argument is strongly supported by our observation that deletion of Pax7 in primary myoblasts and satellite cells results in growth arrest and down-regulation of the myogenic regulatory factors (Fig. 1). Our data strongly support the interpretation that the limited regeneration that occurs in adult muscle in the absence of Pax7 is mediated by the precocious differentiation of committed satellite cells without their normal proliferation. However, Pax7-deficient satellite cells are unable to self-renew, leading to their ultimate loss through terminal differentiation (Fig. 4). Consequently, repeated injury in presence of continuous tamoxifen treatment results in a profound regeneration deficit that is virtually identical to that observed in Pax7-null mice (Fig. 2F).
An interesting question is what is the fate of satellite stem cells that have yet to activate myogenic factor transcription. Satellite stem cells give rise to committed satellite myogenic cells through asymmetric cell divisions (20). Satellite stem cells express Pax7 but do not transcribe Myf5. Following an asymmetric cell division, Carm1-mediated arginine methylation of residues in the N-terminus of Pax7 allows for the binding of MLL1/2 and the activation of Myf5 transcription in the daughter satellite myogenic cell (14). We have recently demonstrated that satellite stem cells are multipotent stem cells capable of myogenic and brown adipogenic commitment (21). Thus, it is interesting to speculate that in the absence of Pax7, lineage switching to alternative fates would also contribute to the loss of the satellite cell pool.
In this manuscript, we report, using the same Pax7 alleles that were used by Lepper et al. (18), that continuous tamoxifen treatment results in a pronounced muscle regeneration deficit. Moreover, primary myoblasts in culture and satellite cells in myofiber culture exhibit cell-cycle arrest following Pax7 deletion. Our observations indicate that Pax7-deficient progenitors are incapable of expansion in vivo and undergo precocious differentiation. Therefore, we conclude that Pax7 is absolutely required for the normal function of satellite cells in regenerative myogenesis in both neonatal and adult skeletal muscle.
Materials and Methods
Mouse Procedures and Tissue Culture.
All animal procedures conform with the Canadian Council on Animal Care's Guide to the Care and Use of Experimental Animals and the Animals for Research Act, and were approved by the Animal Care Committee at University of Ottawa. For the study, we used female Pax7fl/CreERT2 or Pax7fl/+ mice (18) that were kindly provided by Chen-Ming Fan, Carnegie Institution, Baltimore. Adult mice were injected intraperitoneally with tamoxifen (10 mg/mL in corn oil) and kept on tamoxifen-containing standard chow (1 mg tamoxifen per day/20 g body weight). The TA muscle was injured by injection of 50 μL cardiotoxin (Sigma, 10 μM). Single myofibers were isolated from the extensor digitorum longus muscle and cultured as described previously (8). Primary myoblasts were derived from lower hind limb skeletal muscle of 6-wk-old mice as described previously (22). For adenoviral infections, primary myoblasts were seeded and infected the following day with Ad-Cre or Ad-GFP at 5 multiplicity of infection for 1 h using established techniques (23).
Immunostaining, Protein, and RNA Analyses.
Western blot analyses, immunofluorescence analyses, RNA purification, and quantitative RT-PCR were carried out as previously described (24). Antibodies used in the study were as follows: Laminin (Sigma, 1:1,000), Pax7 (Developmental Studies Hybridoma Bank, undiluted), Myogenin (Sigma, 1:200), Tubulin (Sigma, 1:5,000), developmental Myosin (Leica, 1:50), Perilipin (Sigma, 1:200), smooth muscle actin (Sigma, 1:200), M-Cadherin (Millipore, 1:500), Myosin heavy chain (Developmental Hybridoma Bank, undiluted), and α7-integrin-Alexa647 (AbLab, 1:100). Tunel staining was performed using the In Situ Cell Death Detection Kit (Roche) following the instructions from the manufacturer.
Statistical Analyses.
Three or more replicates were analyzed for each experiment presented. Data are shown as SEM (Microsoft Excel); statistical significance was assessed by a Student t test (Microsoft Excel). Differences were considered significant with a P value < 0.05. Data presented as percentages are normalized either to the control or the experimental group.
Supplementary Material
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research, National Institutes of Health, the Muscular Dystrophy Association, and the Canada Research Chair Program. M.A.R. holds the Canada Research Chair in Molecular Genetics.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307680110/-/DCSupplemental.
References
- 1.Relaix F, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172(1):91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138(17):3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sambasivan R, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138(17):3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 4.Seale P, Ishibashi J, Scimè A, Rudnicki MA. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol. 2004;2(5):E130. doi: 10.1371/journal.pbio.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23(16):3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22(11):602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentzinger CF, et al. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12(1):75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. 2012;4(2):a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentzinger CF, Wang YX, von Maltzahn J, Rudnicki MA. The emerging biology of muscle stem cells: Implications for cell-based therapies. BioEssays. 2012;35(3):231–241. doi: 10.1002/bies.201200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seale P, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 12.Kuang S, Chargé SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172(1):103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinnell IW, et al. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2008;10(1):77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawabe Y, Wang YX, McKinnell IW, Bedford MT, Rudnicki MA. Carm1 regulates Pax7 transcriptional activity through MLL1/2 recruitment during asymmetric satellite stem cell divisions. Cell Stem Cell. 2012;11(3):333–345. doi: 10.1016/j.stem.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: A potential mechanism for self-renewal. Dev Biol. 2004;275(2):375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olguin HC, Yang Z, Tapscott SJ, Olwin BB. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol. 2007;177(5):769–779. doi: 10.1083/jcb.200608122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soleimani VD, et al. Transcriptional dominance of Pax7 in adult myogenesis is due to high-affinity recognition of homeodomain motifs. Dev Cell. 2012;22(6):1208–1220. doi: 10.1016/j.devcel.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460(7255):627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129(5):999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin H, et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013;17(2):210–224. doi: 10.1016/j.cmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seale P, Ishibashi J, Holterman C, Rudnicki MA. Muscle satellite cell-specific genes identified by genetic profiling of MyoD-deficient myogenic cell. Dev Biol. 2004;275(2):287–300. doi: 10.1016/j.ydbio.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 23.Huh MS, Parker MH, Scimè A, Parks R, Rudnicki MA. Rb is required for progression through myogenic differentiation but not maintenance of terminal differentiation. J Cell Biol. 2004;166(6):865–876. doi: 10.1083/jcb.200403004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Maltzahn J, Bentzinger CF, Rudnicki MA. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat Cell Biol. 2012;14(2):186–191. doi: 10.1038/ncb2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.