Significance
We characterize a mechanism by which 14-3-3σ directs cell migration and tumor invasion through regulating cytoskeletal solubility and dynamics. Our data suggest that 14-3-3σ expression, rather than being a tumor suppressor, in fact, aids in breast tumor invasion at least in a subset of carcinomas. Our findings warrant further investigation into the role of this molecule in normal mammary gland and breast tumors and, indeed, in epithelial tissues and tumors where 14-3-3σ is expressed.
Keywords: cytoskeleton, motility, 14-3-3 family, triple-negative, basal breast cancer
Abstract
The protein 14-3-3σ (stratifin) is frequently described as a tumor suppressor silenced in about 80% of breast tumors. Intriguingly, we show that 14-3-3σ expression, which in normal breast is localized to the myoepithelial cells, tracks with malignant phenotype in two models of basal-like breast cancer progression, and in patients, it is associated with basal-like subtype and poor clinical outcome. We characterized a mechanism by which 14-3-3σ guides breast tumor invasion by integrating cytoskeletal dynamics: it stabilizes a complex of solubilized actin and intermediate filaments to maintain a pool of “bioavailable” complexes for polarized assembly during migration. We show that formation of the actin/cytokeratin/14-3-3σ complex and cellular migration are regulated by PKCζ-dependent phosphorylation, a finding that could form the basis for intervention in aggressive breast carcinomas expressing 14-3-3σ. Our data suggest that the biology of this protein is important in cellular movement and is contingent on breast cancer subtype.
The majority of breast tumors can be classified into at least five transcriptionally defined intrinsic subtypes that have distinct outcome and predict response to systemic therapy (1–3). Among these groups, the basal-like breast cancer (BLBC) subtype accounts for 15–20% of invasive breast cancers. BLBCs are often aggressive tumors of high histological grade that lack the expression of “druggable” targets, such as hormone receptors and human epidermal growth factor receptor 2 (HER2). Accordingly, major effort has been placed on understanding the molecular basis governing BLBCs.
Previously, we have characterized an isogenic culture model of human breast malignant progression HMT-3522 (4) (originally described in refs. 5 and 6), encompassing nonmalignant (S1), premalignant (S2), preinvasive (S3), and tumorigenic (T4-2) cell populations, and have found this model recapitulates a number of features of BLBC progression. Xenograft tumors formed from T4-2, a cell line that became malignant without activation of exogenous oncogenes and after prolong cultivation in culture (5, 6), display many cardinal features of human BLBCs, including high histological grade; positivity for epidermal growth factor receptor (EGFR) and basal cytokeratins K5/K14; negativity for HER2, estrogen receptor (ER), and progesterone receptor (PR); a high Ki-67–proliferative index; and aggressive invasion into surrounding tissues (4). Another isogenic cell culture model of breast cancer progression, the MCF10 series, is thought also to recapitulate features of basal-like malignant progression albeit with exogenous oncogene addition (7–9). As such, both models are useful tools to dissect molecular events related to breast cancer progression in general and to BLBC progression specifically.
The 14-3-3 family of molecular scaffolds is comprised of seven known mammalian isoforms (β, γ, ε, η, σ, θ/τ, and ζ) that regulate pathways involved in growth regulation and cell cycle progression through binding and sequestering the subcellular distribution of many ligands (10–12). Proteomic profiling of the HMT-3522 progression series (ref. 13 and the current study) identified 14-3-3σ (also known as stratifin, SFN) as a protein expressed at higher levels in malignant T4-2 cells relative to its nonmalignant S1 counterpart. The finding that 14-3-3σ expression followed HMT-3522 malignant progression was surprising, given that this molecule is described broadly as a tumor suppressor; in the breast, it is frequently down-modulated through promoter methylation or proteolysis (the latter particularly in ER-positive tumors) (14–16), and it has been shown also to regulate cell polarity through Par3 association and suppress metastasis in mouse models of HER2-positive mammary cancer (17, 18). Additionally, 14-3-3σ functions as an enforcer of the G2/M checkpoint in HCT116 colorectal cancer cells by sequestering cyclin B1 and cdc2 from the nucleus (19, 20) and facilitates mitotic translation in HeLa and U2OS cells (21). Despite the high frequency of promoter methylation, immunohistochemical analysis, nevertheless, demonstrated that 14-3-3σ silencing at the protein level does not occur as frequently as initially proposed (22), and in a pilot study, 14-3-3σ was found to be expressed highly in 10 of 12 cases of BLBCs (23). We, thus, hypothesized that 14-3-3σ may be a useful marker for progression of basal-like breast cancers and may functionally contribute to the aggressive clinical features of this subtype.
Here, we describe a surprising function of 14-3-3σ as a key integrator of the cytoskeletal dynamics necessary for optimal BLBC tumor invasion. We show that 14-3-3σ expression in both the HMT-3522 and the MCF10 BLBC models follows malignant progression, and in patients it correlates with basal immunohistochemical subtype and poor clinical outcome. Using multiple BLBC cell lines, we observed that 14-3-3σ regulates migration and invasion independently of proliferation; instead, it appears to function as a cytoskeletal solubility cofactor that stabilizes and maintains a soluble, bioavailable complex of actin and K5/K17 intermediate filaments for directional assembly into the cytoskeleton as needed for migration. We also show that formation of the actin/keratin/14-3-3σ complex and cell migration is regulated by PKCζ-dependent phosphorylation that can be targeted by pharmacological inhibitors. We propose that 14-3-3σ regulates tumor invasion directly by modulating cytoskeletal dynamics and suggest that patients with high 14-3-3σ expression, the majority of whom have basal-like subtype, may benefit from targeted therapies against 14-3-3σ and/or PKCζ.
Results
14-3-3σ Correlates with Malignant Progression in Two Models of Basal-Like Breast Cancer.
To identify and validate markers of BLBC, we performed 2D gel mass spectrometry of the HMT-3522 progression series (S1 and T4-2). We identified 14-3-3σ as being elevated in the tumorigenic T4-2 cells relative to their nontumorigenic counterpart, S1, which we confirmed by Western blot analysis (Fig. 1A). Protein levels of 14-3-3σ increased with HMT-3522 progression (5, 6) from S1 (nonmalignant) to S2 (premalignant and EGF-independent) to S3C [preinvasive and highly proliferative (4)], and were highest in T4-2 (tumorigenic and invasive). The σ isoform was the only 14-3-3 family member whose expression followed BLBC malignant progression in this model (Fig. S1). Similarly, in the MCF10 series, 14-3-3σ expression was found to follow tumor progression (7, 8) from MCF10A (nonmalignant) to MCF10neoT (premalignant and hyperproliferative) and to MCF10DCIS.com [which form comedo-type ductal carcinoma in situ (DCIS) in xenografts] (Fig. S1).
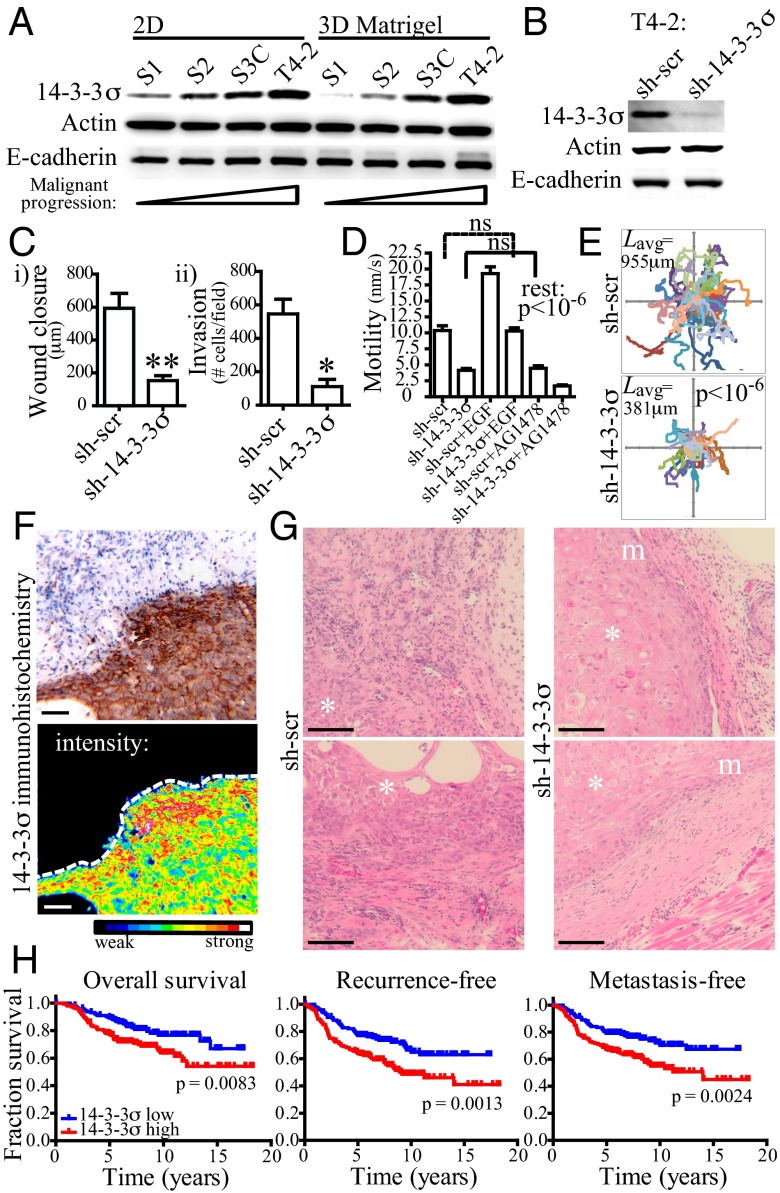
Fig. 1.
Protein 14-3-3σ increases during HMT-3522 basal-like breast tumor progression and contributes to motility and invasiveness in vivo. (A) Western blot analysis of lysates from the HMT-3522 series when grown on 2D tissue culture plastic (2D) or on top of 3D (3D) basement membrane extract (Matrigel). Actin and E-cadherin are provided as loading controls. (B) T4-2 cells expressing shRNA hairpins targeting 14-3-3σ (sh-14-3-3σ) have greater than 80% reduction on the protein level relative to cells expressing scrambled shRNAs (sh-scr). (C) T4-2 sh-14-3-3σ cells have decreased motility, as measured in a wound healing assay (B, i), and have decreased invasion through Matrigel-coated transwell inserts (B, ii). Error bars represent the SEM, and statistical significance was calculated using a two-tailed Student t test (*P < 0.05; **P < 0.01). (D) T4-2 sh-14-3-3σ cells have decreased motility on the single-cell scale. Mean individual cell velocities for 25 cells over a 24-h time lapse were calculated using particle tracking analysis. Cells were additionally treated with EGF (10ng/mL) or AG1478 (tyrphostin; 100 nM) in adjacent wells. Error bars represent the SEM, and statistical significance was calculated using a one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison posttest. (E) Individual tracks of T4-2 sh-14-3-3σ (n = 25) and control cells (n = 25) over a 24-h time lapse showing the influence of 14-3-3σ knockdown on path length. Lavg indicates the mean track length. Statistical significance was calculated using a two-tailed Student t test. (F) Immunohistochemical staining of 14-3-3σ in a T4-2 xenograft. The surrounding mouse stroma is negative for 14-3-3σ staining (Upper). A heat-map representation of the same tumor section emphasizes the 14-3-3σ distribution at the tumor invasive front (Lower). (Scale bar: 100 μm.) (G) Representative H&E stains of tumors derived from T4-2 sh-scr and sh-14-3-3σ xenografts. Tumors formed by injecting T4-2 sh-scr cells have poor demarcation and frequently disrupt adjacent normal tissues, whereas T4-2 sh-14-3-3σ tumors have much clearer margins and do not disrupt surrounding normal tissue architecture as robustly. Additional micrographs can be found in Fig. S2. Asterisk indicates tumor; m, margin. (Scale bar: 100 μm.) (H) Expression of 14-3-3σ correlates with decreased overall, recurrence-free, and metastasis-free survival in a series of 295 patients grouped by 14-3-3σ expression. P values were calculated using the log-rank test; 14-3-3σ low or high indicates 14-3-3σ mRNA expression levels lower or higher than the median expression in the cohort.
To evaluate whether 14-3-3σ functionally contributes to BLBC pathogenesis, we used shRNA silencing to perturb its expression in three basal-like cell lines: T4-2, MCF10DCIS.com, and MDA-MB-231 (Fig. 1B and Fig. S1). Whereas 14-3-3σ knockdown did not influence proliferation or cell–cell adhesion (Fig. S1), it decreased both cell motility and invasion through Matrigel in all three cell lines tested (Fig. 1 C–E and Fig. S1), providing robust evidence that 14-3-3σ regulates BLBC cell motility and invasion. To test the validity of these findings in vivo, we generated xenografts in mice using the parental T4-2 cells and the shRNA sublines. We observed robust 14-3-3σ immunohistochemical staining that was most prominent at the invasive front of the T4-2 tumors (Fig. 1F), providing circumstantial evidence that 14-3-3σ may promote tumor invasion into the surrounding stroma. Indeed, tumor xenografts formed from T4-2 cells expressing shRNAs targeting 14-3-3σ (sh-14-3-3σ) showed more distinct margins and less perturbation to the adjacent normal tissue in comparison with control tumors (Fig. 1G and Fig. S2) again independent of tumor proliferation (Fig. S1). These data suggest a role for 14-3-3σ in regulating a growth-independent transition toward invasion in two independent, isogenic models of BLBC progression.
14-3-3σ Is Associated with BLBC and Is an Independent Prognostic Marker.
Given the conflicting literature regarding 14-3-3σ expression in breast cancer (see details in the Introduction) and our data obtained using BLBC cell lines, we performed additional immunohistochemical analysis on a tissue microarray containing replicate tumor cores of invasive breast cancers from 245 patients to assess the expression of 14-3-3σ, and its associations with clinicopathological features and the expression of additional breast cancer phenotypic markers. Specifically, we investigated whether tumors that are positive for 14-3-3σ have an increased or decreased frequency of positivity for established tumor markers than expected by chance. We observed strong cytoplasmic in 16.2% of patients (Table 1), which correlated with high histological grade, with high Ki-67–proliferative index, and with several other BLBC markers (K5/6, K14, K17, EGFR, and caveolins 1 and 2). In contrast, 14-3-3σ immunoreactivity correlated inversely with luminal tumor markers (ER, PR, and FOXA1). When tumors were subclassified using an immunohistochemical surrogate of intrinsic subtypes (24), 14-3-3σ immunoreactivity was observed in 70% (16/23) of BLBC tumors and in 9% (15/164) of nonbasal tumors. Furthermore, using a breast cancer cohort comprised of 295 patients with well-documented follow-up (25), we found that 14-3-3σ expression correlated with shortened overall, recurrence-free, and metastasis-free survival (Fig. 1H) and was prognostic independently of other biomarkers (Table S1), such as patient age, tumor size, tumor grade, ER status, node status, adjuvant therapy received, intrinsic subtypes, wound-healing signature (26), and 70-gene signature (27). We have, thus, validated 14-3-3σ as a marker correlating with poor clinical outcome and aggressive breast cancer subtypes and concurrently validated the culture models as useful surrogates for investigating how 14-3-3σ may be linked to the aggressive clinical features of BLBCs.
Table 1.
Correlations between 14-3-3σ–staining intensity, clinicopathological parameters, and other immunohistochemical markers in 245 cases of invasive breast carcinoma
| Parameter | N | 14-3-3σ− | 14-3-3σ+ | Correlation | P |
| Size–TNM | 198 | None | NS* | ||
| T1 | 84 (50.6%) | 17 (53.1%) | |||
| T2 | 70 (42.2%) | 13 (40.6%) | |||
| T3 | 12 (7.2%) | 2 (6.3%) | |||
| Grade | 194 | Direct | 0.0010* | ||
| 1 | 18 (11.1%) | 0 (0.0%) | |||
| 2 | 53 (32.7%) | 3 (9.4%) | |||
| 3 | 91 (56.2%) | 29 (90.6%) | |||
| LVI | 197 | None | NS† | ||
| − | 53 (32.1%) | 11 (34.4%) | |||
| + | 112 (67.9%) | 21 (65.6%) | |||
| ER | 195 | Inverse | <0.0001† | ||
| − | 19 (11.7%) | 17 (53.1%) | |||
| + | 144 (88.3%) | 15 (46.9%) | |||
| PR | 195 | Inverse | <0.0001† | ||
| − | 33 (20.2%) | 19 (59.4%) | |||
| + | 130 (79.8%) | 13 (40.6%) | |||
| HER2 | 193 | None | NS† | ||
| − | 135 (83.9%) | 30 (93.8%) | |||
| + | 26 (16.1%) | 2 (6.3%) | |||
| Ki-67 | 186 | Direct | 0.0001* | ||
| <10% | 69 (44.5%) | 8 (25.1%) | |||
| 10–30% | 71 (45.8%) | 11 (35.5%) | |||
| >30% | 15 (9.7%) | 12 (38.7%) | |||
| EGFR | 198 | Direct | <0.0001† | ||
| − | 160 (96.4%) | 21 (65.6%) | |||
| + | 6 (3.6%) | 11 (34.4%) | |||
| K14 | 197 | Direct | <0.0001† | ||
| − | 160 (97.0%) | 22 (65.6%) | |||
| + | 5 (3.0%) | 10 (34.4%) | |||
| K5/6 | 191 | Direct | <0.0001† | ||
| − | 155 (95.7%) | 17 (58.6%) | |||
| + | 7 (4.3%) | 12 (41.4%) | |||
| K17 | 195 | Direct | <0.0001† | ||
| − | 153 (93.9%) | 20 (62.5%) | |||
| + | 10 (6.1%) | 12 (37.5%) | |||
| Caveolin 1 | 198 | Direct | <0.0001† | ||
| − | 158 (95.2%) | 22 (68.8%) | |||
| + | 8 (4.8%) | 10 (31.3%) | |||
| Caveolin 2 | 179 | Direct | 0.0023† | ||
| − | 146 (96.7%) | 22 (78.6%) | |||
| + | 5 (3.3%) | 6 (21.4%) | |||
| FOXA1 | 158 | Inverse | 0.0318† | ||
| − | 28 (20.7%) | 10 (43.5%) | |||
| + | 107 (79.3%) | 13 (56.5%) | |||
| Subtype‡ | 187 | Basal | <0.0001* | ||
| Basal | 7 (4.5%) | 16 (51.6%) | |||
| Luminal | 122 (78.2%) | 14 (45.2%) | |||
| HER2 | 27 (17.3%) | 1 (3.2%) |
K, cytokeratin; LVI, lymphovascular invasion; NS, not significant.
χ2 P values (two-tailed; 95% confidence interval).
Fisher’s exact test P values (two-tailed; 95% confidence interval).
Immunophenotypic groups are defined based upon the expression of ER, HER2, K5/6, and EGFR (24).
14-3-3σ Regulates Actin Dynamics in a Ligand-Binding Mechanism.
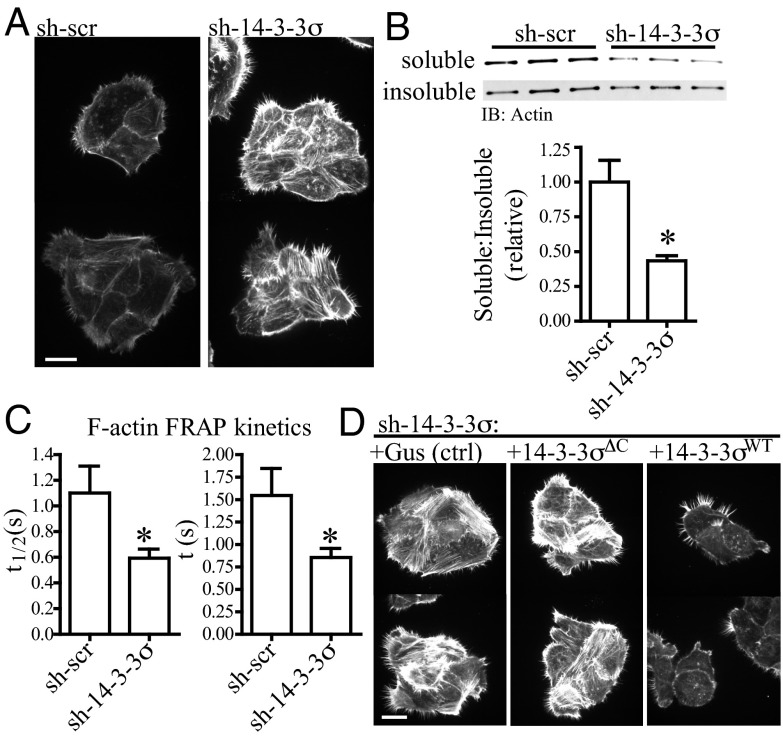
How does 14-3-3σ contribute to cell migration and invasion in culture and in vivo? Cell migration requires dynamic remodeling of the cytoskeleton at both the leading and the trailing edges of a cell or a group of cells in response to biochemical and mechanical stimuli; as a consequence, cytoskeletal dynamics often become deregulated during tumor invasion (28, 29). We investigated whether the motility and invasion phenotypes observed in sh-14-3-3σ cells are coupled to altered cytoskeletal dynamics. Comparing to control cells, T4-2 sh-14-3-3σ cells had a ∼twofold increase in filamentous (F)-actin staining intensity and a ∼twofold decrease in the level of Triton X-100 soluble actin (Fig. 2 A and B), suggesting the dynamics of actin turnover and/or assembly are perturbed. To test this, we performed fluorescence recovery after photobleaching (FRAP) experiments using an F-actin live-cell probe (30) and observed a ∼twofold increase in the rate of F-actin fluorescence recovery in sh-14-3-3σ cells (Fig. 2C), consistent with an increase in actin-polymerization kinetics rather than a suppression of filament turnover (31). By performing Western blot on whole cell lysates, we observed no difference in the total actin levels between cells (Fig.1B and Fig. S3A), indicating that actin polymerization is more rapid in sh-14-3-3σ cells independent of changes in total protein level. These data provide multiple lines of plausible evidence implicating 14-3-3σ as a regulator of actin dynamics and solubility.
Fig. 2.
Protein 14-3-3σ regulates actin-polymerization kinetics in a ligand-binding mechanism. (A) Phalloidin immunofluorescence, showing T4-2 cells with reduced 14-3-3σ (sh-14-3-3σ) have deregulated F-actin polymerization relative to control cells (sh-scr). Images were acquired in the same focal plane using cells grown in adjacent wells of a chamber slide. (Scale bar: 20 μm.) (B) sh-14-3-3σ cells have decreased actin solubility. Cells were fractionated into 1% Triton X-100–soluble and –insoluble pools, and the ratio of soluble to insoluble actin was measured and quantified by Western blot. Error bars represent the SEM, and statistical significance was calculated using a two-tailed Student t test (*P < 0.05). (C) sh-14-3-3σ cells have increased actin turnover kinetics. F-actin dynamics in cells expressing LifeAct-mCherry were measured using FRAP. The kinetic parameter describing the rate of half (t1/2) or full (t) plateau fluorescence recovery was collected for n > 30 individual cells; lower values in sh-14-3-3 cells indicates a more rapid rate of fluorescence recovery and, thus, increased kinetics. Error bars represent the SEM, and statistical significance was calculated using a two-tailed Student t test (*P < 0.05). (D) Phalloidin immunofluorescence, showing the increased actin polymerization in sh-14-3-3σ cells can be rescued with forced expression of 14-3-3σWT but not an irrelevant gene (Gus) or the mutant 14-3-3σΔC. Images were acquired in the same focal plane using cells grown in adjacent wells of a chamber slide. (Scale bar: 20 μm.)
To address whether 14-3-3σ influences cytoskeletal dynamics by a ligand binding mechanism and to rule out the possibility that the actin phenotype is simply an off-target effect of shRNA silencing, rescue constructs were generated to enable forced expression of wild-type 14-3-3σ (14-3-3σWT) or a C-terminal truncated 14-3-3σ (14-3-3σΔC). The latter is the human equivalent of the 14-3-3σ mutation responsible for the repeated-epilation mouse phenotype (32), and the truncation eliminates two helices and an acidic region thought to be important for 14-3-3 ligand interaction (33). When introduced into sh-14-3-3σ cells, only 14-3-3σWT was able to rescue the deregulated actin polymerization in comparison with an unrelated protein, Gus, and to 14-3-3σΔC (Fig. 2D). These data indicate that 14-3-3σ regulates both actin dynamics and motility in cells, that this occurs in a ligand-binding mechanism, and that the actin phenotype is not an off-target effect of shRNA expression.
Stimuli from the cell microenvironment promote localized activation of Rho family GTPases (Rac, Rho, and cdc42), and depending on the activity of these and other factors, such as cofilin, Src, Erk, and LIM Kinase (LIMK), results in the formation of discreet actin structures to promote or inhibit cell migration (29, 34–36). We performed Western blot analysis on the sh-14-3-3σ and control cells but were surprised to find that there were no differences in the activity of any canonical actin regulatory molecules tested (Fig. S3A), indicating that 14-3-3σ regulates cytoskeletal dynamics most probably downstream of these signaling pathways in BLBC cells.
14-3-3σ Interacts Directly with Soluble Actin and Inhibits Its Polymerization in Vitro.
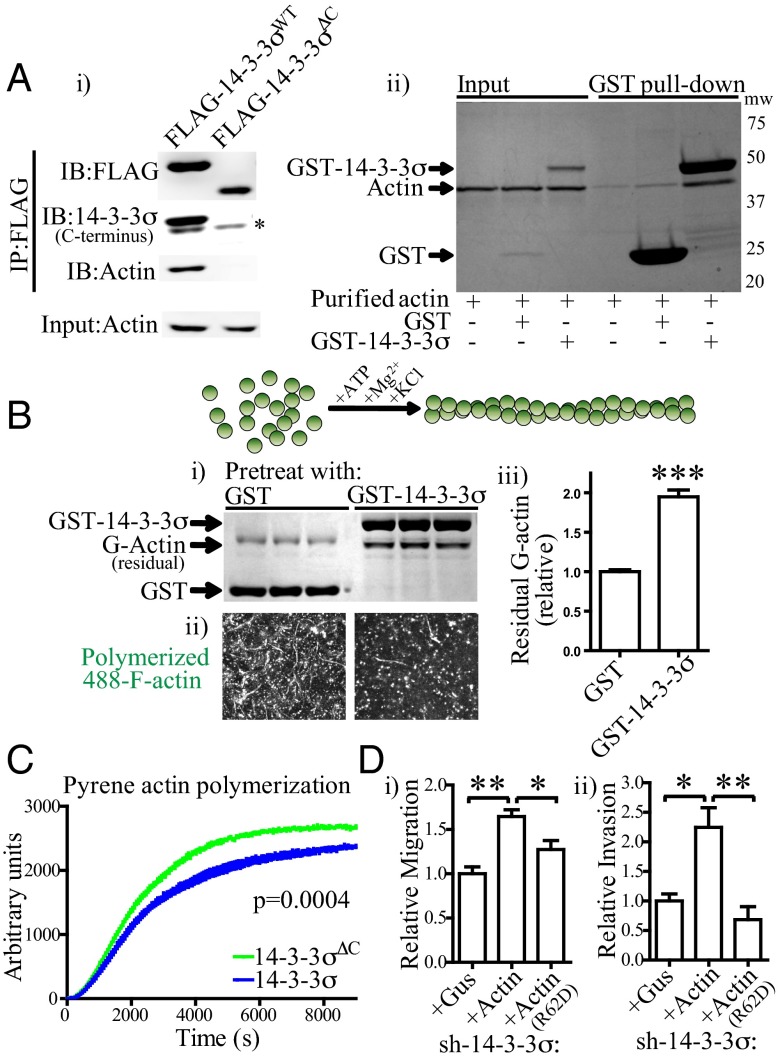
Given that 14-3-3σ appears to regulate actin dynamics (Fig. 2) downstream of small GTPases and other actin-reorganizing factors (Fig. S3A), and that actin is one of many ligands known to interact with 14-3-3σ by mass spectrometry-based analysis (21), we asked whether it may interact directly with actin in BLBC cells and, if so, whether this influences actin dynamics. Using FLAG-affinity immunoprecipitation from cell lines and GST-pull down experiments with purified proteins, we were able to measure direct interaction between 14-3-3σ and actin and, furthermore, show that the C terminus of 14-3-3σ is required for this interaction (Fig. 3A and Fig. S3B). Using F-actin sedimentation assays, we were unable to observe interaction between 14-3-3σ and F-actin, suggesting that the actin that coimmunoprecipitated with 14-3-3σ was globular (G) actin (Fig. S3D). We then tested whether actin dynamics are influenced through interaction with 14-3-3σ. By performing a series of in vitro actin-polymerization assays, we observed that pretreating G-actin with GST–14-3-3σ attenuated subsequent actin polymerization: there was a consistent twofold increase in the amount of G-actin remaining in solution following polymerization relative to pretreatment with GST alone and a modest, but highly reproducible, inhibition of pyrene actin-polymerization kinetics (Fig. 3 B and C). Similar data were obtained using AlexaFluor488-conjugated actin (488-actin), where fewer actin fibers were visible following 488–G-actin polymerization in the presence of GST–14-3-3σ (Fig. 3B). The use of an actin barbed-end polymerization assay revealed that 14-3-3σ inhibited 488–G-actin in situ in cell lines that contained low endogenous 14-3-3σ (Fig. S3C). Thus, 14-3-3σ interacts directly with G-actin, and this interaction is sufficient to modestly inhibit polymerization of G-actin to F-actin in the absence of other cofactors.
Fig. 3.
Protein 14-3-3σ interacts with actin and inhibits its polymerization, maintaining a pool of soluble actin for optimal F-actin remodeling during cell migration. (A) 14-3-3σ directly interacts with actin in cells and in vitro. (A, i) FLAG immunoprecipitation, showing that both 14-3-3σWT and 14-3-3σΔC are able to interact with endogenous 14-3-3σ (shown by the asterisk), but only full-length 14-3-3σ forms stable complexes with actin. (A, ii) GST pull-down experiment, showing that GST–14-3-3σ, but not GST, interacts directly with purified actin in vitro. IB, Western blot; IP, immunoprecipitation. (B) G-actin pretreatment with GST–14-3-3σ inhibits subsequent polymerization into F-actin. This was observed by fractionating the residual G-actin from F-actin following polymerization (B, i) and by polymerizing AlexaFluor488-conjugated G-actin (488–G-actin) and imaging F-actin fibrils by confocal microscopy (B, ii). Depicted is the maximum intensity projection of 20 superimposed fields. The quantification (B, iii) is the residual G-actin pool following polymerization of actin from B, i. Error bars represent the SEM, and statistical significance was calculated using a two-tailed Student t test (***P < 0.001). (C) Protein 14-3-3σ modestly inhibits pyrene actin polymerization in vitro. Pyrene G-actin was pretreated with 14-3-3σ and subsequently polymerized, with pyrene F-actin fluorescence measured in 60-s intervals. Note the decrease in Vmax (slope of linear portion of curve before the plateau) and the decline in plateau height (shift toward more soluble actin) in the presence of 14-3-3σ. Statistical significance was calculated using two-way ANOVA. (D) Forced expression of wild-type actin, but not a mutant actin that is unable to polymerize (R62D), rescues the decreased motility (D, i) and invasion (D, ii) phenotypes in sh-14-3-3σ cells. Error bars represent the SEM, and statistical significance was calculated using a two-tailed Student t test (*P < 0.05; **P < 0.01).
14-3-3σ Stabilizes a Soluble Complex of Actin, Keratin 5, and Keratin 17 to Concomitantly Regulate Actin and Intermediate Filament Dynamics.
The above data suggest a model by which 14-3-3σ coordinates cell motility and invasion by regulating actin bioavailability and solubility. In sh-14-3-3σ cells, actin-polymerization rates are elevated and the pool of soluble actin declines, possibly making the availability of soluble actin (G-actin) a rate-limiting factor for cell motility. In accordance with predictions from this model, forced expression of actin, but not a mutated actin (R62D) that is unable to polymerize (37), rescued the deficient motility and invasion in cells devoid of 14-3-3σ (Fig. 3D). However, the modest influence on pyrene actin-polymerization kinetics suggested that the regulation of cytoskeletal dynamics by 14-3-3σ may be more elaborate than a simple actin binding and sequestration mechanism.
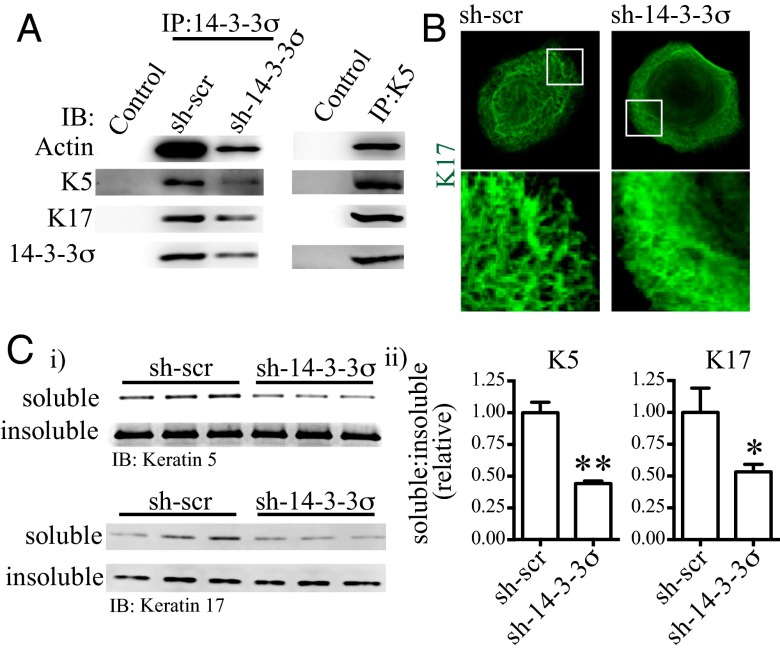
To probe further, we performed quadrupole time-of-flight mass spectrometry analysis on endogenous coimmunoprecipitated 14-3-3σ ligands to further characterize the mechanism and identified K5 and K17 as the predominant proteins also present in the complex; these results were verified by Western blot analysis and K5 coimmunoprecipitation (Fig. 4A). K5 and K17 are frequently coexpressed in myoepithelial cells and in BLBC (38), and their expression predicts poor breast cancer clinical outcome independent of tumor grade and size in node-negative disease (39). To our knowledge, there are no previous reports of direct interaction between K5 and K17, nor between either of these cytokeratins and actin, in myoepithelial cells or in BLBC cells.
Fig. 4.
Protein 14-3-3σ concomitantly regulates actin and intermediate filament dynamics by sequestering keratin 5, keratin 17, and G-actin in a soluble complex. (A) 14-3-3σ, keratin 5 (K5), keratin 17 (K17), and actin form a soluble complex in cells. This was observed by immunoprecipitation of either endogenous 14-3-3σ (Left) or K5 (Right). Control, nonspecific IgG; IB, Western blot; IP, immunoprecipitation. (B) Keratin 17 immunofluorescence, showing T4-2 sh-14-3-3σ cells have a disproportionately high number of intermediate filaments, which are disorganized and lack the mesh-like geometry observed in control (sh-scr) cells. (C) sh-14-3-3σ cells have decreased keratin 5 and keratin 17 solubility. Cells were fractionated into 1% Triton X-100–soluble and –insoluble pools, and then the ratio of soluble to insoluble actin was measured and quantified by Western blot. Error bars represent the SEM, and statistical significance was calculated using a two-tailed Student t test (*P < 0.05; **P < 0.01).
Knock down of 14-3-3σ led to excessive polymerization and disorganized intermediate filaments similar to our observation on actin dynamics; sh-14-3-3σ cells also had less soluble K5 and K17 relative to control cells (Fig. 4 B and C). Furthermore, there was a decrease in the amount of actin that coimmunoprecipitated with K5 in sh-14-3-3σ cells, indicating that 14-3-3σ, in fact, stabilizes the formation of the actin/K5/K17 complex (Fig. 5A). These data suggest that 14-3-3σ influences cell motility and invasion by stabilizing the formation of a soluble, bioavailable cytoskeletal complex, thus ensuring cells have a ready reservoir when needed to respond to promigratory cues from their microenvironment.
Fig. 5.
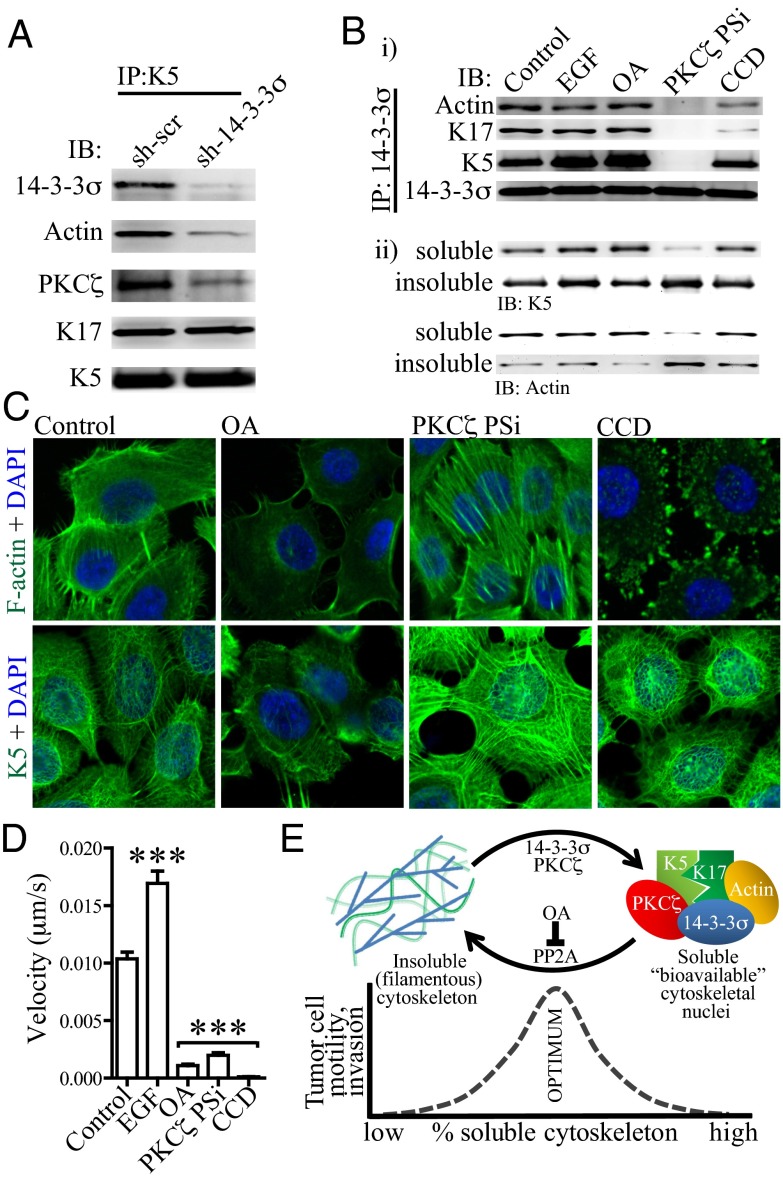
Cytoskeletal turnover is regulated by PKCζ- and PP2A-dependent phosphorylation and dephosphorylation, respectively, with 14-3-3σ stabilizing the solubilized actin/keratin complex to coordinate cell motility. (A) Protein 14-3-3σ stabilizes formation of the soluble actin/keratin/14-3-3σ complex. Less actin and PKCζ coimmunoprecipitated with keratin 5 in sh-14-3-3σ cell lysate relative to control cells, suggesting that 14-3-3σ is required for stable interaction between soluble actin and keratin 5/17. IB, Western blot; IP, immunoprecipitation. (B) Formation of the soluble actin/keratin/14-3-3σ complex requires PKCζ-dependent phosphorylation. Treatment with a myristoylated PKCζ pseudosubstrate peptide inhibitor (PKCζ PSi) eliminates interactions between 14-3-3σ, actin, and keratin 5/17 (B, i) and concomitantly decreases the pool of soluble actin and keratins (B, ii). Cells were treated with EGF, okadaic acid (OA), PKCζ PSi, or cytochalasin D (CCD) before immunoprecipitation or fractionation into 1% Triton X-100–soluble and –insoluble pools. IB, Western blot; IP, immunoprecipitation. (C) The influence of pharmacological inhibitors on the cytoskeletal architecture. In agreement with B, treating cells with OA leads to rapid solubilization of F-actin and intermediate filaments; conversely, treating cells with PKCζ PSi dramatically promotes filament formation. (D) All cytoskeletal pharmacological inhibitors terminate cell migration, indicating that the correct balance of soluble to insoluble cytoskeleton is necessary for optimal cell migration. Velocities for 25 individual cells were calculated using particle tracking analysis. Error bars represent the SEM, and statistical significance was calculated using a one-way analysis of variance with Bonferroni's multiple comparison posttest. (E) A proposed model by which cytoskeleton dynamic equilibrium and cell motility are integrated by 14-3-3σ; 14-3-3σ maintains a soluble pool of nonfilamentous cytoskeletal nuclei to be assimilated into filaments as needed during motility. Formation of this soluble pool depends on PKCζ and is antagonized by PP2A. Disrupting cytoskeletal homeostasis, either by using pharmacological inhibitors that shift the equilibrium away from the optimal ratio of soluble to filamentous cytoskeleton or by using shRNAs targeting 14-3-3σ, leads to decreased tumor cell motility and invasion.
Integration of Cytoskeletal Homeostasis, Filament Solubility, and Tumor Invasion by 14-3-3σ Requires PKCζ-Dependent Phosphorylation.
It is known that cytokeratins are rapidly depolymerized in the presence of phosphatase inhibitors such as the protein phosphatase 2A (PP2A) inhibitor, okadaic acid, indicating that intermediate filament homeostasis is regulated by phosphorylation (40). Furthermore, it was shown previously that protein kinase Cζ (PKCζ) phosphorylates K18 at a 14-3-3–binding site, leading to K8/K18 solubilization through interaction with 14-3-3ζ (41, 42). We asked whether formation of the 14-3-3σ/K5/K17/actin complex, cytoskeletal solubility, and cell motility are promoted by PKCζ-dependent phosphorylation and/or antagonized by PP2A-dependent dephosphorylation. Treatment of cells with a myristoylated PKCζ pseudosubstrate peptide inhibitor ablated the interaction between the cytoskeletal proteins and 14-3-3σ, concomitantly shifting the cytoskeletal dynamics toward more filamentous actin and keratins (and, thus, less soluble proteins) and eliminated cell motility (Fig. 5 B–D). In contrast, treatment with okadaic acid led to rapid solubilization of both actin and intermediate filaments and yet eliminated cell motility analogous to the PKCζ inhibitor. Together, these data indicate that cell motility requires an optimal balance of actin and intermediate filament dynamics and solubility, in particular, rapid cycling of monomers between soluble and filamentous states. This cycling, in turn, is orchestrated by PKCζ-dependent phosphorylation, PP2A-dependent dephosphorylation, and direct interaction between solubilized cytoskeletal components and 14-3-3σ.
Discussion
How the different cytoskeletal components interact to bring about coordinated movement and function is a rich and active field of investigation. In this paper, we present data suggesting that 14-3-3σ functions as a “cytoskeletal rheostat” influencing cell motility and invasion by stabilizing the formation of a bioavailable cytoskeletal complex: these interactions maintain the dynamic cycling between soluble and filamentous cytoskeleton necessary for optimal cell migration (Fig. 5E). Other 14-3-3 family members were shown previously to regulate intermediate filament architecture by functioning as K8/K18 solubility cofactors in a PKCζ-dependent mechanism (40–42). In addition, a number of studies have highlighted different mechanisms by which 14-3-3 family members regulate actin dynamics either through stabilizing cofilin phosphorylation or by inhibiting signals through the AKT-RhoA pathway (12, 43–45).
Here, we elucidated a mechanism that unifies the previously reported models by which 14-3-3σ concomitantly regulates actin and intermediate filament homeostasis in breast cancer: it stabilizes a complex of soluble (nonfilamentous) actin and keratins to integrate cytoskeletal dynamics with cell motility (Figs. 2–5). We show further that regulation of cytoskeletal homeostasis by 14-3-3σ depends on PKCζ providing an opportunity (Fig. 5) to target 14-3-3σ in invasive breast cancers by pharmacological inhibitors. This is particularly important because unlike ER+ or HER2+ subtypes, BLBC currently lacks targeted therapeutics.
Cells expressing shRNAs targeting 14-3-3σ have decreased motility and tumor invasion (Fig. 1 and Fig. S1) and deregulated actin and intermediate filament polymerization (Figs. 2 and 4). Specifically, our F-actin FRAP experiments indicate that the rate of actin polymerization is higher in sh-14-3-3σ cells, perturbing the dynamic cycling between monomeric and filamentous cytoskeleton necessary for persistent cell migration. In such cells, the availability of soluble cytoskeletal proteins becomes a rate-limiting factor for migration (Figs. 3D and 5E). Surprisingly, however, we found no change in the overall activity of LIMK, AKT, cofilin, small GTPases, and other canonical cytoskeletal signaling molecules in T4-2 sh-14-3-3σ cells (Fig. S3A). However, we observed 14-3-3σ stabilizes a multimeric complex containing actin, K5 and K17 downstream of these signaling pathways, thus maintaining a soluble pool of cytoskeletal proteins for eventual incorporation into dynamic filaments during migration (Fig. 5A). These data are concordant with prior studies (40–42) and provide evidence that the 14-3-3 family members regulate global cytoskeletal homeostasis in a mechanism more elaborate than previously appreciated.
Our data suggest that 14-3-3σ provides a signaling switch bridging cytoskeletal dynamic equilibrium with cell motility. Loss of cytoskeletal homeostasis in sh-14-3-3σ cells or in cells grown in the presence of pharmacological inhibitors targeting PKCζ or PP2A results in suboptimal cytoskeletal plasticity, leading to loss of cell motility. Intriguingly, 14-3-3ζ has previously been shown to interact with keratins and with actin (46, 47), and, like 14-3-3σ (Fig. 1 and Table S1), 14-3-3ζ expression in breast cancer is associated with poor clinical outcome (48, 49). Given the body of literature linking actin-regulatory proteins and BLBC keratins with tumor cell invasion, metastasis, and poor clinical outcome (28, 29, 34, 39, 50), we suggest 14-3-3σ regulation of cytoskeletal dynamics may play a fundamental role in BLBC progression to metastatic disease. Intriguingly, Saccharomyces cerevisiae strains with dominant-negative or temperature-sensitive mutations to BMH1 or BMH2, genes encoding yeast 14-3-3 homologs, display disrupted actin cytoskeletal integrity and polarity (51, 52), suggesting that regulation of the cytoskeleton by 14-3-3 is conserved across evolution.
The protein 14-3-3σ is frequently described as a canonic tumor suppressor. Despite the evidence supporting this description (see details in the Introduction), it is surprising that there are no reports of its overexpression resulting in the suppression of tumors in vivo. Using a tissue microarray derived from 245 cases of invasive breast carcinoma, we showed clearly that robust 14-3-3σ expression is most frequent in patients with BLBC (Table 1). It was shown previously that 14-3-3σ expression in ER+ tumors is also associated with poor clinical outcome (23). Indeed, in the Netherlands Cancer Institute (NKI)295 cohort, 14-3-3σ is a predictor of poor clinical outcome independent of clinicopathological parameters and genomic predictors, including intrinsic subtypes and ER status (Table S1). Our data suggest that 14-3-3σ should not be regarded exclusively as a tumor suppressor in cancers of the breast and possibly other tissues. In addition to our studies, others also have questioned this generalization (for a review, see ref. 53). Given the previous reports linking 14-3-3σ with polarity and metastasis suppression in HER2+ mouse mammary gland cancer models (17, 18), our data suggest that the functions of this intriguing protein are contingent on the cancer subtype. In agreement with prior reports, we observed only 6% (2 of 28) of HER2+ tumors retained detectable 14-3-3σ expression (Table 1).
In summary, we show that 14-3-3σ is directly linked to cell migration and invasion independent of proliferation and that its expression in tumors is highest at the invasive front where soft tissue infiltration occurs. We elucidate a mechanism by which 14-3-3σ may regulate cell migration and tumor invasion in vivo by directly regulating cytoskeletal dynamics and provide evidence that targeting the 14-3-3σ/PKCζ/cytoskeleton pathway may benefit patients who present with high expression of 14-3-3σ.
Materials and Methods
Cell Culture and Inhibitors.
HMT-3522 S1, S2, S3C, and T4-2 were maintained in tissue culture monolayers or in 3D (Matrigel) cultures as described previously (4). The MCF10 series was kindly provided by Fred Miller (Karmanos Cancer Institute, Detroit, MI) and cultured as recommended (8). MDA-MB-231, BT549, and HCC1143 cell lines were obtained from ATCC and were maintained as recommended. Okadaic acid, myristoylated PKCζ pseudosubstrate and cytochalasin D were obtained from Calbiochem and were used at concentrations of 0.1 μg/mL, 1 μM, and 5 μM, respectively, for 1 h before analysis.
Animal Studies.
For xenografts, 5,000,000 cells in 50% (vol/vol) Matrigel/Media were injected s.c. into the rear flanks of 8 wk old female BALB/c (nu/nu) mice. Tumors were allowed to grow for 5–6 wk and subsequently formalin fixed and paraffin embedded for H&E staining (University of California, San Francisco Mouse Pathology Core). Experiments were performed using a protocol approved by the Lawrence Berkeley National Laboratory Animal Research and Welfare Committee.
Immunohistochemistry.
Immunohistochemistry was performed as previously described for 14-3-3σ (23) or Ki-67 (4). Tissue microarray sections were scored by two observers (F.C.G. and J.S.R.-F.), with observers blinded to the results of other immunohistochemical markers and patient outcome. For heat-map projection, 16-bit images were inverted and a 16-color lookup table was applied using ImageJ software.
Western Blot, Small-GTPase Assays, and Immunoprecipitation.
A complete list of antibodies and reagents used for Western blot, GTPase activity, cytoskeletal fractionation, and immunoprecipitation can be found in SI Materials and Methods.
Constructs.
Lentiviral expression constructs were kindly provided by Eric Campeau (Lawrence Berkeley National Laboratory) or purchased (Invitrogen). pGEX-2TK 14-3-3σ was kindly provided by Michael Yaffe (Massachusetts Institute of Technology, Cambridge, MA; Addgene plasmid 11944). The actin constructs were described previously (37). Detailed subcloning methods can be found in SI Materials and Methods.
Migration, Invasion, and Other Motility Assays.
Cell motility, chemotaxis, and invasion were measured using standard assays as described in SI Materials and Methods. Single-cell tracking movies were captured using a microscope fitted with an incubator over a period of 20 h in the presence or absence of EGF (10 nM; Sigma-Aldrich) or AG1478 (100 nM; Calbiochem). Cell tracking was performed on >25 cells per well using ImageJ software (Manual tracking macro).
Confocal Microscopy.
Immunofluorescence was performed as recommended by antibody suppliers (SI Materials and Methods). Cells were seeded in the same chamber slides to ensure equivalent antibody exposure and washing conditions between cell lines. Microscopy was performed with a spinning disk (Yokogawa) confocal based on a Zeiss Axiovert 200 or on a Zeiss 710 laser scanning microscope using a 63× Plan Apochromat (1.4 numeric aperture) oil immersion lens. For F-actin imaging, the basal-most focal plane was depicted to provide a convenient reference point. For FRAP experiments, cells were seeded on collagen-coated 1.0 chamber coverslips; only cells weakly expressing LifeAct-mCherry were analyzed to minimize the chance of disrupting endogenous actin dynamics. Bleaching and recovery kinetics were analyzed using the FRAP module within Zen 2008 software using a Zeiss 710 LSM.
In Vitro Assays.
Actin was purified from young rabbit muscle tissue (Pel-Freez) according to previous methods (54); 488-actin and biotinylated actin were generated by labeling F-actin with AlexaFluor488-TFP ester (Invitrogen) or Sulfo-NHS-Biotin (Pierce) according to the manufacturer’s protocol, followed by subsequent cycles of depolymerization, polymerization, and depolymerization to ensure retention of activity following bioconjugation. Pyrene-labeled actin was obtained commercially (Cytoskeleton). Recombinant GST–14-3-3σ and GST were purified from BL21(DE3)pLysS cells (Promega) using glutathione Sepharose 4B (GE Healthcare) as described (33). In vitro and in situ actin-polymerization assays were performed as described in SI Materials and Methods.
Microarray Expression-Profiling Survival Analysis.
Expression data for patients comprising the NKI295 cohort (25) and clinical data (26) were downloaded from the Stanford Microarray Database server (http://microarray-pubs.stanford.edu/wound_NKI/explore.html; currently available at http://changlab.stanford.edu/2005-PNAS-Data.html). Patients were sorted based on 14-3-3σ expression into high (above median) and low (below median) groups for Kaplan–Meier survival analysis.
Statistics.
All statistics reported were calculated using GraphPad Prism software (Version 5.01) and are the two-tailed, 95% confidence interval P values.
Supplementary Material
Acknowledgments
We thank Genee Lee, Connie Myers, Aylin Rizki, Paraic Kenny, Britta Weigelt, Jason Jung, Joe Gray, Marc Lenburg, Eric Collisson, Sanjay Kumar, Laura van ‘t Veer, and all members of the M.J.B. laboratory for either excellent technical advice and/or fruitful discussions. We also thank Roland Meier for generously helping with xenograft experiments and Kay Savage for help in scoring the tissue microarrays. This work was funded by Department of Defense Predoctoral Fellowship W81XWH-05-1-0339 and California Breast Cancer Research Program Dissertation Award 14GB-0007 (to A.B.). For this work, J.S.R.-F. and F.C.G. were funded, in part, by Breakthrough Breast Cancer. D.W. received funding support from the US Department of Energy Low Dose Radiation Research Program. The work of the M.J.B. laboratory is supported by grants from the US Department of Energy Office of Biological and Environmental Research and the Low Dose Radiation Program (Contract DE-AC02-05CH1123); by National Cancer Institute Grants R37CA064786, R01CA140663, U54CA112970, U01CA143233, and U54CA143836 awarded to the Bay Area Physical Sciences–Oncology Center (University of California, Berkeley); by US Department of Defense Grant W81XWH0810736; and, in part, by a grant from The Breast Cancer Research Foundation.
Footnotes
Conflict of interest statement: A.B., D.W. and M.J.B. have filed pending Patent Application 13/330,46 that is assigned to The Regents of the University of California and has not been licensed.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315022110/-/DCSupplemental.
References
- 1.Sørlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Børresen-Dale AL. Systems biology and genomics of breast cancer. Cold Spring Harb Perspect Biol. 2011;3(2):a003293. doi: 10.1101/cshperspect.a003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizki A, et al. A human breast cell model of preinvasive to invasive transition. Cancer Res. 2008;68(5):1378–1387. doi: 10.1158/0008-5472.CAN-07-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briand P, Nielsen KV, Madsen MW, Petersen OW. Trisomy 7p and malignant transformation of human breast epithelial cells following epidermal growth factor withdrawal. Cancer Res. 1996;56(9):2039–2044. [PubMed] [Google Scholar]
- 6.Briand P, Petersen OW, Van Deurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Dev Biol. 1987;23(3):181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- 7.Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst. 2000;92(14):1185–1186. doi: 10.1093/jnci/92.14.1185a. [DOI] [PubMed] [Google Scholar]
- 8.Miller FR, et al. Xenograft model of progressive human proliferative breast disease. J Natl Cancer Inst. 1993;85(21):1725–1732. doi: 10.1093/jnci/85.21.1725. [DOI] [PubMed] [Google Scholar]
- 9.Hu M, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13(5):394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aitken A. 14-3-3 proteins: A historic overview. Semin Cancer Biol. 2006;16(3):162–172. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Mhawech P. 14-3-3 proteins—an update. Cell Res. 2005;15(4):228–236. doi: 10.1038/sj.cr.7290291. [DOI] [PubMed] [Google Scholar]
- 12.Freeman AK, Morrison DK. 14-3-3 Proteins: Diverse functions in cell proliferation and cancer progression. Semin Cell Dev Biol. 2011;22(7):681–687. doi: 10.1016/j.semcdb.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Y, Weaver VM, Blair IA. Analysis of protein expression during oxidative stress in breast epithelial cells using a stable isotope labeled proteome internal standard. J Proteome Res. 2005;4(6):2007–2014. doi: 10.1021/pr050175d. [DOI] [PubMed] [Google Scholar]
- 14.Prasad GL, Valverius EM, McDuffie E, Cooper HL. Complementary DNA cloning of a novel epithelial cell marker protein, HME1, that may be down-regulated in neoplastic mammary cells. Cell Growth Differ. 1992;3(8):507–513. [PubMed] [Google Scholar]
- 15.Ferguson AT, et al. High frequency of hypermethylation at the 14-3-3 sigma locus leads to gene silencing in breast cancer. Proc Natl Acad Sci USA. 2000;97(11):6049–6054. doi: 10.1073/pnas.100566997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urano T, et al. Efp targets 14-3-3 sigma for proteolysis and promotes breast tumour growth. Nature. 2002;417(6891):871–875. doi: 10.1038/nature00826. [DOI] [PubMed] [Google Scholar]
- 17.Ling C, Zuo D, Xue B, Muthuswamy S, Muller WJ. A novel role for 14-3-3sigma in regulating epithelial cell polarity. Genes Dev. 2010;24(9):947–956. doi: 10.1101/gad.1896810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling C, Su VM, Zuo D, Muller WJ. Loss of the 14-3-3sigma tumor suppressor is a critical event in ErbB2-mediated tumor progression. Cancer Discov. 2012;2(1):68–81. doi: 10.1158/2159-8290.CD-11-0189. [DOI] [PubMed] [Google Scholar]
- 19.Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14-3-3Sigma is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401(6753):616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- 20.Hermeking H, et al. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1(1):3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 21.Wilker EW, et al. 14-3-3sigma controls mitotic translation to facilitate cytokinesis. Nature. 2007;446(7133):329–332. doi: 10.1038/nature05584. [DOI] [PubMed] [Google Scholar]
- 22.Moreira JM, Ohlsson G, Rank FE, Celis JE. Down-regulation of the tumor suppressor protein 14-3-3sigma is a sporadic event in cancer of the breast. Mol Cell Proteomics. 2005;4(4):555–569. doi: 10.1074/mcp.M400205-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Simpson PT, et al. Distribution and significance of 14-3-3sigma, a novel myoepithelial marker, in normal, benign, and malignant breast tissue. J Pathol. 2004;202(3):274–285. doi: 10.1002/path.1530. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen TO, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 25.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 26.Chang HY, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA. 2005;102(10):3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van ’t Veer LJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 28.Ridley AJ. Life at the leading edge. Cell. 2011;145(7):1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Nürnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nat Rev Cancer. 2011;11(3):177–187. doi: 10.1038/nrc3003. [DOI] [PubMed] [Google Scholar]
- 30.Tanner K, Boudreau A, Bissell MJ, Kumar S. Dissecting regional variations in stress fiber mechanics in living cells with laser nanosurgery. Biophys J. 2010;99(9):2775–2783. doi: 10.1016/j.bpj.2010.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai FP, et al. Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 2008;27(7):982–992. doi: 10.1038/emboj.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Lu Q, Estepa G, Verma IM. Identification of 14-3-3σ mutation causing cutaneous abnormality in repeated-epilation mutant mouse. Proc Natl Acad Sci USA. 2005;102(44):15977–15982. doi: 10.1073/pnas.0508310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaffe MB, et al. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell. 1997;91(7):961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Eddy R, Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer. 2007;7(6):429–440. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machacek M, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461(7260):99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009;28(1-2):5–14. doi: 10.1007/s10555-008-9166-3. [DOI] [PubMed] [Google Scholar]
- 37.Spencer VA, et al. Depletion of nuclear actin is a key mediator of quiescence in epithelial cells. J Cell Sci. 2011;124(Pt 1):123–132. doi: 10.1242/jcs.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badve S, et al. Basal-like and triple-negative breast cancers: A critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011;24(2):157–167. doi: 10.1038/modpathol.2010.200. [DOI] [PubMed] [Google Scholar]
- 39.van de Rijn M, et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol. 2002;161(6):1991–1996. doi: 10.1016/S0002-9440(10)64476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strnad P, Windoffer R, Leube RE. Induction of rapid and reversible cytokeratin filament network remodeling by inhibition of tyrosine phosphatases. J Cell Sci. 2002;115(Pt 21):4133–4148. doi: 10.1242/jcs.00096. [DOI] [PubMed] [Google Scholar]
- 41.Sivaramakrishnan S, Schneider JL, Sitikov A, Goldman RD, Ridge KM. Shear stress induced reorganization of the keratin intermediate filament network requires phosphorylation by protein kinase C zeta. Mol Biol Cell. 2009;20(11):2755–2765. doi: 10.1091/mbc.E08-10-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao J, Omary MB. 14-3-3 proteins associate with phosphorylated simple epithelial keratins during cell cycle progression and act as a solubility cofactor. J Cell Biol. 1996;133(2):345–357. doi: 10.1083/jcb.133.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gohla A, Bokoch GM. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr Biol. 2002;12(19):1704–1710. doi: 10.1016/s0960-9822(02)01184-3. [DOI] [PubMed] [Google Scholar]
- 44.Soosairajah J, et al. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J. 2005;24(3):473–486. doi: 10.1038/sj.emboj.7600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kakinuma N, Roy BC, Zhu Y, Wang Y, Kiyama R. Kank regulates RhoA-dependent formation of actin stress fibers and cell migration via 14-3-3 in PI3K-Akt signaling. J Cell Biol. 2008;181(3):537–549. doi: 10.1083/jcb.200707022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angrand PO, et al. Transgenic mouse proteomics identifies new 14-3-3-associated proteins involved in cytoskeletal rearrangements and cell signaling. Mol Cell Proteomics. 2006;5(12):2211–2227. doi: 10.1074/mcp.M600147-MCP200. [DOI] [PubMed] [Google Scholar]
- 47.Meek SE, Lane WS, Piwnica-Worms H. Comprehensive proteomic analysis of interphase and mitotic 14-3-3-binding proteins. J Biol Chem. 2004;279(31):32046–32054. doi: 10.1074/jbc.M403044200. [DOI] [PubMed] [Google Scholar]
- 48.Bergamaschi A, et al. 14-3-3ζ as a predictor of early time to recurrence and distant metastasis in hormone receptor-positive and -negative breast cancers. Breast Cancer Res Treat. 2013;137(3):689–696. doi: 10.1007/s10549-012-2390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neal CL, et al. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009;69(8):3425–3432. doi: 10.1158/0008-5472.CAN-08-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mouneimne G, et al. Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell. 2012;22(5):615–630. doi: 10.1016/j.ccr.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roth D, Birkenfeld J, Betz H. Dominant-negative alleles of 14-3-3 proteins cause defects in actin organization and vesicle targeting in the yeast Saccharomyces cerevisiae. FEBS Lett. 1999;460(3):411–416. doi: 10.1016/s0014-5793(99)01383-6. [DOI] [PubMed] [Google Scholar]
- 52.Lottersberger F, Panza A, Lucchini G, Piatti S, Longhese MP. The Saccharomyces cerevisiae 14-3-3 proteins are required for the G1/S transition, actin cytoskeleton organization and cell wall integrity. Genetics. 2006;173(2):661–675. doi: 10.1534/genetics.106.058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, Liu JY, Zhang JT. 14-3-3sigma, the double-edged sword of human cancers. Am J Transl Res. 2009;1(4):326–340. [PMC free article] [PubMed] [Google Scholar]
- 54.Pardee JD, Spudich JA. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.