Significance
This paper provides previously undescribed evidence that STING, an endoplasmic reticulum (ER)-resident protein involved in DNA sensing, couples ER stress with apoptotic signaling in alcoholic liver disease. The proapoptotic role of STING is mediated by the interferon regulatory factor 3 (IRF3), and this is independent of inflammation or Type-I interferons. Activation of STING and IRF3, originally reported in the context of antiviral response, determines survival of hepatocytes in early alcoholic liver disease suggesting that innate immunity regulates hepatocyte pathophysiology independent of inflammation.
Keywords: steatohepatitis, Kupffer cells, stimulator of interferon genes, interferon regulatory factor 3
Abstract
Emerging evidence suggests that innate immunity drives alcoholic liver disease (ALD) and that the interferon regulatory factor 3 (IRF3),a transcription factor regulating innate immune responses, is indispensable for the development of ALD. Here we report that IRF3 mediates ALD via linking endoplasmic reticulum (ER) stress with apoptotic signaling in hepatocytes. We found that ethanol induced ER stress and triggered the association of IRF3 with the ER adaptor, stimulator of interferon genes (STING), as well as subsequent phosphorylation of IRF3. Activated IRF3 associated with the proapoptotic molecule Bax [B-cell lymphoma 2 (Bcl2)-associated X protein] and contributed to hepatocyte apoptosis. Deficiency of STING prevented IRF3 phosphorylation by ethanol or ER stress, and absence of IRF3 prevented hepatocyte apoptosis. The pathogenic role of IRF3 in ALD was independent of inflammation or Type-I interferons. Thus, STING and IRF3 are key determinants of ALD, linking ER stress signaling with the mitochondrial pathway of hepatocyte apoptosis.
Alcoholic liver disease (ALD) affects over 140 million people worldwide, and currently there is no effective treatment. Acute alcohol consumption induces fatty liver and excessive alcohol use causes progression to steatohepatitis, cirrhosis, and hepatocellular carcinoma. Dysregulation of innate immunity and liver inflammation, triggered by the translocation of gut-derived endotoxin [lipopolysaccharide (LPS)] to the liver, represent major contributors to ALD (1). The recognition of gut-derived LPS by Kupffer cells (KC) requires the Toll-like receptor 4 (TLR4), which triggers two downstream pathways. The TLR4/myeloid differentiation primary response gene 88 (MyD88) pathway activates transcription of inflammatory cytokines, whereas TLR4/TRAM/TRIF [TIR domain-containing adaptor inducing interferon-beta (TRIF)–related adaptor molecule (TRAM)] triggers Type-I interferons (IFN) regulatory factor 3 (IRF3) to induce IFN (2). The essential role of the TLR4 signaling in ALD was demonstrated in mice lacking functional TLR4 that showed attenuation of alcoholic steatohepatitis (3, 4). In previous studies, we reported that the MyD88-dependent pathway was dispensable for ALD (4) and observed complete protection from alcohol-induced inflammation, steatosis, and injury in mice deficient in IRF3 (5), suggesting that the pathogenic effects of TLR4 in ALD were mediated via the TRAM/TRIF-dependent pathway. However, the mechanisms by which IRF3 causes ALD remain obscure.
IRF3 is a constitutively expressed transcription factor that resides in the cytoplasm and dimerizes and translocates to the nucleus upon phosphorylation (6). Phosphorylated IRF3 induces IFN-β during viral infection, but may also contribute to inflammatory cytokine response to LPS (7). IRF3 also promotes apoptosis in virus-infected cells through association with proapoptotic molecule Bax [B-cell lymphoma 2 (Bcl2)-associated X protein] (8). To elucidate the mechanism by which IRF3 determines ALD, we asked three fundamental questions. First, is the pathogenic effect of IRF3 in the liver mediated by inflammatory cytokines, Type-I IFNs, or other signals? Second, is IRF3 activated by TLR4 or by other mechanisms in ALD? Third, is the pathogenic role of IRF3 linked to KC or hepatocytes?
Here we show that IRF3 is activated early in ALD by endoplasmic reticulum (ER) stress, and this is mediated by the ER adaptor, stimulator of interferon genes (STING). We also demonstrate that IRF3 exerts its pathogenic role in ALD by inducing apoptosis of hepatocytes. Although IRF3 is a key component of the innate immune response, our data demonstrate that IRF3 in ALD acts independently of inflammatory cytokines and Type-I IFNs. Our results identify a unique role of IRF3 as a signaling molecule mediating a cross-talk between the ER stress and hepatocyte apoptosis and demonstrate an important role for STING in IRF3 phosphorylation in the liver.
Results
Pathogenic Role of IRF3 in ALD Is Independent of Inflammation and Type-I IFN Signaling.
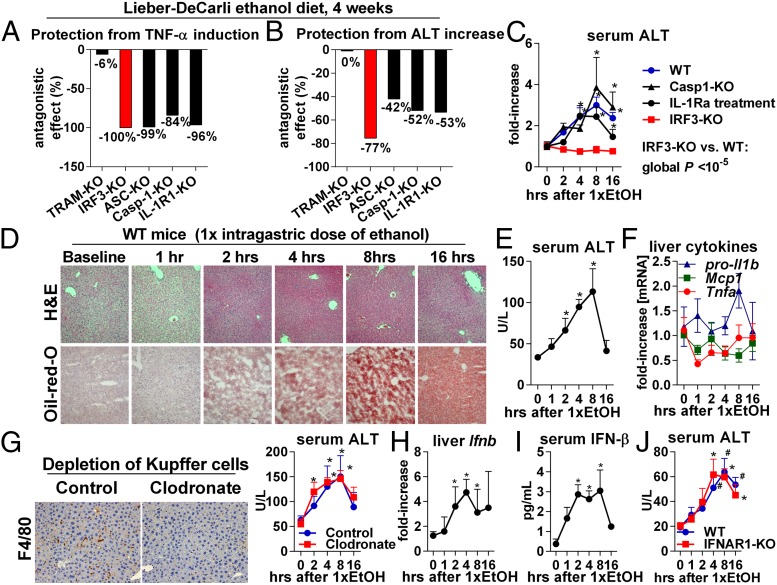
IRF3 is indispensable for the development of ALD (5), but the mechanisms underlying its pathogenic role are not known. Here we asked whether the effects of IRF3 in ALD are mediated by inflammatory signaling. Consistent with its role in induction of TNF-α (7), deficiency of IRF3 completely prevented liver inflammation induced by a 4-wk ethanol feeding in mice (Fig. 1A, SI Appendix, Fig. S2E, and ref. 5). In addition, IRF3-KO mice showed full protection from alcohol-induced liver damage (Fig. 1B, SI Appendix, Fig. S2C, and ref. 5). The extent of protection from liver damage in IRF3-KO mice was superior to that observed in other strains deficient in innate immune signaling. For example, interleukin (IL)-1 receptor (IL-1R1) deficiency or absence of inflammasome components, ASC or Caspase 1 (Casp-1), which are required for IL-1β maturation, protected from liver inflammation (Fig. 1A and ref. 9) but only partially attenuated alcoholic liver damage (Fig. 1B and ref. 9). Interestingly, no protection from alcohol-induced liver inflammation or acute or chronic liver injury was observed in mice deficient in TRAM (Fig. 1 A and B and SI Appendix, Fig. S1), which bridges TLR4 with downstream signaling that induces phosphorylation of IRF3 in immune cells (2).
Fig. 1.
The pathogenic role of IRF3 in ALD is independent of inflammation and Type-I IFN signaling. Mice of indicated genotypes were fed an alcohol diet and killed 4 wk later. Liver inflammation was assessed by TNF-α mRNA (A), and liver injury was evaluated by serum alanine aminotransferase (ALT) (B). The numbers indicate the extent by which deficiency of a specific gene protects from alcohol-induced increase in TNF-α or ALT; −100% indicates full protection. Source data are shown in SI Appendix, Figs. S1 A and C and S2 C and E, and ref. 9. (C) A single dose of ethanol (5 g/kg) was administered to WT, Casp1-KO, IRF3-KO, or IL-1R1-KO mice or to WT mice pretreated i.p. with IL-1 receptor antagonist (IL-1Ra), and serum ALT was measured. (D–F) WT mice received a single dose of ethanol, and liver injury, steatosis, and inflammation were evaluated using histology (D), ALT (E), and expression of Tnfa, pro-Il-1b, and Mcp1 (F). WT mice received i.v. clodronate. Two days later, depletion of KC in the liver was verified by F4/80, ethanol was administered, and serum ALT was measured (G). WT mice received a single dose of ethanol, and liver Ifnb and serum IFN-β were evaluated (H and I). WT or IFNAR1-KO mice received a single dose of ethanol. Liver injury was evaluated by ALT (J). n = 5–8 mice per group (C–J). *P < 0.05 vs. baseline. (Magnification, 200×.)
The 4-wk model of ALD represents a compound outcome of acute and chronic events. As IRF3 deficiency protected from ALD after 4 wk (Fig. 1 A and B), we next sought to evaluate the early phase of liver injury using a single intragastric dose of ethanol. We observed that IRF3-KO mice were fully protected from acute alcohol-induced liver injury (Fig. 1C), suggesting early involvement of IRF3 in ALD. In contrast, blockade of IL-1R1 or deficiency of Casp-1 provided little protection from acute alcoholic liver injury (Fig. 1C). Notably, acute liver injury and steatosis induced by a single dose of ethanol were not accompanied by inflammation (Fig. 1 D–F) and developed independent of KC (Fig. 1G), a major source of inflammatory cytokines in the liver. This data suggested that acute alcoholic liver injury and steatosis require IRF3 but are independent of inflammation.
Based on our observations that both acute administration of ethanol and chronic ethanol feeding significantly induced IFN-β in the liver and in the serum (Fig. 1 H and I and SI Appendix, Fig. S2A), we asked whether the pathogenic role of IRF3 in ALD was mediated by Type-I IFNs. We found that deficiency of the Type-I IFN receptor (IFNAR1) failed to provide protection from acute alcohol-induced liver injury (Fig. 1J) and from inflammation, steatosis, or damage elicited by a chronic ethanol diet (SI Appendix, Fig. S2 I–K). There was no protection from ALD in mice deficient in IRF7 (SI Appendix, Fig. S2 F–H), a Type-I IFN-inducing transcription factor distinct from IRF3 (2). Collectively, these findings demonstrated that IRF3 determines pathology in early in ALD, independently of inflammation or Type-I IFNs. Our data also suggested that the signal for activation of IRF3 in the early phase of alcohol-induced liver injury was not mediated by TLR4/TRAM.
IRF3 Is Required for Apoptosis of Hepatocytes.
The unexpected finding of inflammation- and Type-I IFN-independent role of IRF3 in alcohol-induced liver damage directed our attention to the recently described proapoptotic role of IRF3 (8). We hypothesized that IRF3 partners with Bax to translocate to mitochondria and initiate hepatocyte apoptosis. We further postulated that should this mechanism occur in the liver, IRF3 would be present in hepatocytes and interact with components of apoptotic signaling. Further, we predicted that IRF3 in hepatocytes themselves would drive development of ALD.
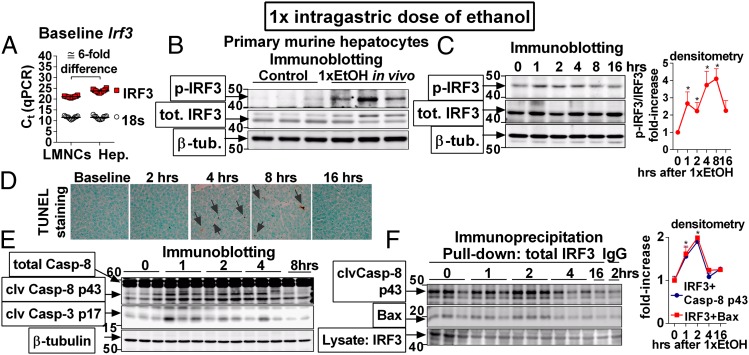
We found Irf3 mRNA both in liver mononuclear cells (LMNCs) and in hepatocytes (Fig. 2A) and observed that administration of a single dose of ethanol to WT mice resulted in phosphorylation of IRF3 in hepatocytes (Fig. 2B), indicating activation of hepatocyte-specific IRF3 in ALD. Phosphorylation of IRF3 in the liver occurred early after a single dose of alcohol (Fig. 2C), which preceded the development of significant liver injury and steatosis (Fig. 1D), and was accompanied by cleavage of Casp-3 and Casp-8 and by apoptosis in the liver (Fig. 2 D and E). Notably, we found that following ethanol administration, IRF3 associated with the apoptosis-initiating Casp-8 and with the mitochondria-permeabilizing protein Bax in the liver (Fig. 2F). Together, these data demonstrated that early after administration of ethanol, IRF3 is activated and interacts with the apoptotic signaling pathway in the liver, leading us to hypothesize that IRF3 regulates apoptosis in hepatocytes.
Fig. 2.
IRF3 is activated in hepatocytes and associates with components of apoptotic signaling in ALD. (A) Mononuclear cells (LMNCs) or hepatocytes (Hep.) were isolated from the livers of WT mice, and expression of the Irf3 was evaluated. (B) WT mice were administered a single dose of ethanol. Two hours afterward, hepatocytes were isolated, and phospho-IRF3 was analyzed. (C–F) WT mice were administered a single dose of ethanol and phosphorylation of IRF3 (C), apoptosis (D), and cleavage of Casp-8 and Casp-3 (E), and association of IRF3 with Casp-8 or Bax (F) was analyzed in the liver. n = 11 mice per cell type (A); n = 3 mice per treatment (B); and n = 6 mice per time point (C–F). Arrows in D indicate TUNEL+ nuclei. *P < 0.05 vs. baseline. (Original magnification, 400×.)
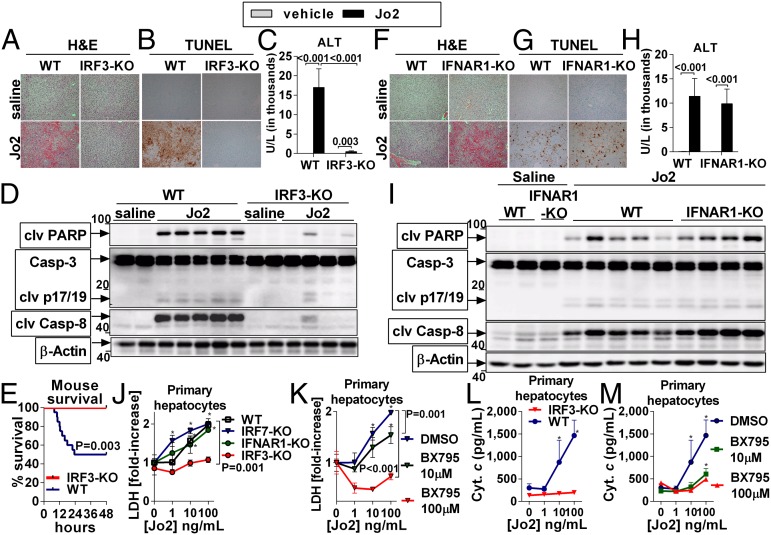
We further investigated whether IRF3 was required for hepatocyte apoptosis using the Fas cell surface death receptor (Fas)-activating anti-CD95 antibody, clone Jo2 (Jo2). Hepatocytes have a pronounced susceptibility to Fas-mediated apoptosis, and we observed a significant increase of the Fas ligand (FasL) in the livers and serum of alcohol-treated mice (SI Appendix, Fig. S3A). Administration of Jo2 in WT mice resulted in early phosphorylation of IRF3 in the liver (SI Appendix, Fig. S3B), followed by apoptosis and liver injury (SI Appendix, Fig. S3 B–D). Importantly, we found that IRF3-KO mice were fully protected from Jo2-induced liver damage (Fig. 3 A and C), hepatocyte apoptosis (Fig. 3B), and mortality (Fig. 3E), and this protection was accompanied by the lack of cleavage of Casp-8, Casp-3, and poly (ADP-ribose) polymerase 1 (PARP) in the liver (Fig. 3D). In contrast to IRF3-KO animals, no protection from Jo2-induced liver damage or apoptosis was observed in mice deficient in IFNAR1 (Fig. 3 F–I). Finally, we observed complete protection from Jo2-mediated apoptosis in primary hepatocytes isolated from IRF3-KO mice (Fig. 3 J and L) and in WT hepatocytes treated with BX795 (Fig. 3 K and M), an inhibitor of IRF3 phosphorylation (10). Consistent with these findings, induction of IRF3 phosphorylation accelerated hepatocyte apoptosis triggered by Jo2 (SI Appendix, Fig. S4C). In contrast, primary hepatocytes isolated from IFNAR1- or IRF7-KO mice were susceptible to Jo2-induced cell death to an extent comparable to WT hepatocytes (Fig. 3J).
Fig. 3.
IRF3 is required for apoptosis of hepatocytes. WT or IRF3-KO mice received a single i.p. dose of Jo2 (0.5 mg/kg) or saline and were killed after 8 h (A –D) or were evaluated for survival (E). In another experiment, 0.5 mg/kg Jo2 or saline was administered to WT or IFNAR1-KO mice (F–I). Liver injury was evaluated by H&E (A and F) and by serum ALT (C and H). Apoptosis was evaluated by TUNEL (B and G) and by cleavage of PARP, Casp-3, and Casp-8 in the liver (D and I). Primary hepatocytes were isolated from WT, IRF7-KO, IFNAR1-KO, or IRF3-KO mice and ex vivo treated with Jo2 for 8 h (J–M). Some WT hepatocytes were pretreated for 30 min with BX795 (K and M). Hepatocyte death was evaluated by lactate dehydrogenase (LDH) (J and K) or cytochrome c in the supernatant (L and M). n = 7 (Jo2-treated, per genotype), and n = 3 (saline-treated, per genotype) in A–D; n = 5–6 (Jo2-treated, per genotype), and n = 3 (saline treated, per genotype) in F–I; and n = 24 (WT), and n = 13 (IRF3-KO) in E. Experiments in J–M were performed in triplicate. Numbers in the graphs indicate P values. *P < 0.05 vs. baseline. Densitometric analysis for Fig. 3 D and I is shown in SI Appendix, Fig. S4 A and B. (Original magnification, 200×.)
Collectively, these data demonstrated that IRF3 is expressed in hepatocytes and is required for hepatocyte apoptosis in response to alcohol. Our results demonstrate that the pathogenic effect of IRF3 in ALD is mediated by intrinsic IRF3-dependent hepatocyte apoptosis and is independent of the role of IRF3 in inflammatory or Type-I IFN responses and provide explanation for the superior protection from ALD in IRF3-KO mice compared with strains deficient in other components of innate immune signaling.
IRF3 Associates with the Endoplasmic Reticulum Adaptor, STING, in the Liver.
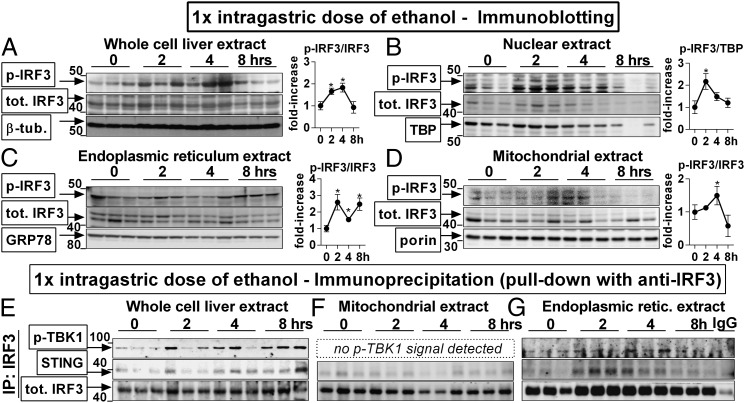
Next, we sought to identify the mechanism of IRF3 activation in ALD. Given our initial findings (Fig. 1A and SI Appendix, Fig. S1), we hypothesized that a TRAM-independent mechanism was responsible for IRF3 activation. Although administration of Jo2 induced phosphorylation of IRF3 in the liver (SI Appendix, Fig. S3B), we rejected the hypothesis that Fas signaling would be a major source of early IRF3 activation in ALD because up-regulation of FasL in the liver occurred late after ethanol administration (4 h, SI Appendix, Fig. S3A). Analysis of the baseline distribution of IRF3 in mouse livers revealed that IRF3 was present not only in the cytoplasm but also in mitochondrial and ER extracts (SI Appendix, Fig. S5A). To study the functional significance of this finding, we analyzed the subcellular localization of IRF3 after a single dose of ethanol and found that phosphorylation of IRF3 was detected in the whole-cell liver extract (Fig. 4A), cytoplasm (SI Appendix, Fig. S5C), nucleus (Fig. 4B), ER (Fig. 4C), and mitochondria (Fig. 4D). The presence of phospho-IRF3 in the nucleus (Fig. 4B) was consistent with the transcriptional role of activated IRF3 (7) and the early alcohol-induced Ifnb (Fig. 1H). The presence of phospho-IRF3 in the mitochondria (Fig. 4D) further supported the involvement of IRF3 in the mitochondrial pathway of hepatocyte apoptosis (Figs. 2 and 3).
Fig. 4.
IRF3 associates with the endoplasmic reticulum adaptor, STING, in the liver. WT mice received a single dose of ethanol. At indicated time points, mice were killed, and phosphorylation of IRF3 was assessed by immunoblotting in liver whole-cell (A), nuclear (B), ER (C), and mitochondrial (D) extracts. Using immunoprecipitation with antitotal IRF3 antibody for protein pull-down, we evaluated association between total IRF3 and STING or phosphorylated TBK1 in the whole-cell (E), mitochondrial (F), and ER (G) extracts. n = 3 per time point. *P < 0.05 vs. baseline. Densitometric analysis for Fig. 4 E–G is shown in SI Appendix, Fig. S4B.
The finding of phospho-IRF3 in the liver ER (Fig. 4C) was unexpected and prompted us to search for implications of this phenomenon. It has been reported that ER serves as a platform for activation of IRF3 by cytosolic RNA or DNA of viral origin and that during certain viral infections, IRF3 associates with ER via binding to Stimulator of IFN genes (STING, also known as TMEM173 or MPYS), an adaptor molecule residing in the ER membrane (11). To explore whether interaction between IRF3 and STING occurred in the liver, we performed immunoprecipitation and found that following administration of ethanol, IRF3 associated with STING in the whole-cell liver lysate (Fig. 4E) and in the ER extract (Fig. 4G). IRF3 also bound to phospho-TBK1 [TRAF family member-associated NFκB activator (TANK)-binding kinase] (Fig. 4 E and G), which is a kinase required for IRF3 phosphorylation (11). Notably, the association of IRF3 with STING or p-TBK1 in the ER occurred rapidly after alcohol administration (Fig. 4G), at the same time point as the binding of IRF3 to Bax (Fig. 2F). Interestingly, although STING is also present in the outer mitochondrial membrane (13), we did not see any association between IRF3 and STING or TBK1 in the liver mitochondrial fraction (Fig. 4F). These findings demonstrated that early after ethanol administration, IRF3 interacts with the adaptor, STING, in the ER and raised the question of whether ER stress was a trigger for IRF3 phosphorylation in ALD.
ER Stress Activates IRF3 in the Liver via the Adaptor, STING.
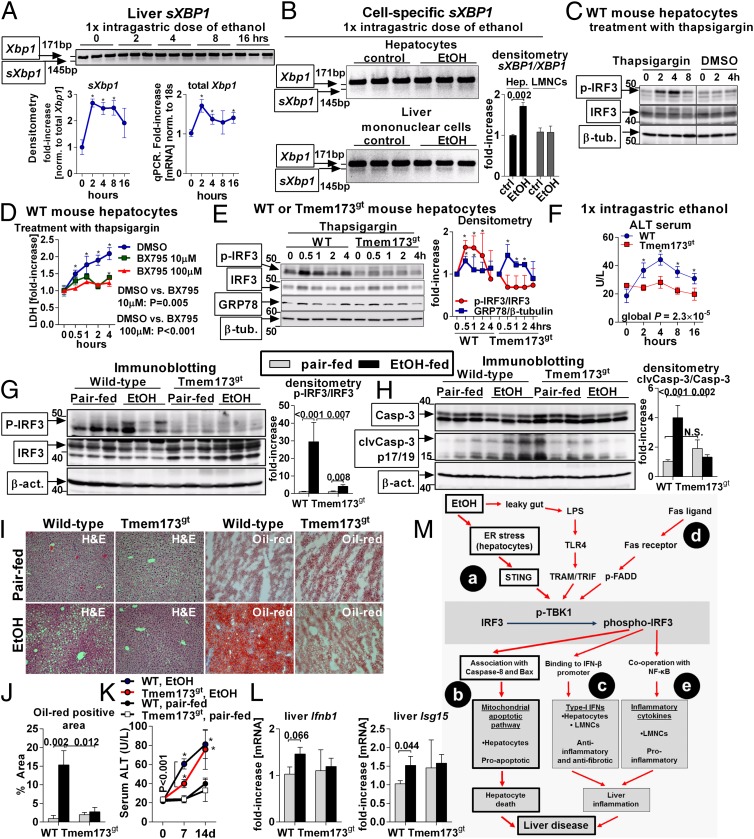
A variety of stressors perturb ER function in ALD, including oxygen deprivation, dysregulation of calcium signaling, and accumulation of misfolded proteins (12). These events, collectively labeled as ER stress, initiate homeostatic responses but also contribute to liver pathology. We found that administration of a single dose of ethanol induced early ER stress in the liver, as demonstrated by splicing of Xbp1 mRNA (sXbp1) (Fig. 5A), a component of the ER stress-associated response (12). Analysis of cell populations in the liver demonstrated that increased splicing of Xbp1 occurred in hepatocytes but not in LMNC of mice exposed to acute alcohol (Fig. 5B), which was consistent with the dominant role of hepatocytes in ethanol metabolism. Further, we found that treatment of primary hepatocytes with ethanol resulted in ER stress independently of acetaldehyde (SI Appendix, Fig. S6), which is consistent with previously published evidence indicating that the pathogenic effects of ethanol are mediated by cytochrome P450 independently of acetaldehyde (14).
Fig. 5.
ER stress activates IRF3 in the liver via the adaptor, STING. WT mice received a single dose of ethanol. Splicing of Xbp1 mRNA (sXBP1) in the liver was evaluated by PCR, and expression of total Xbp1 in the liver was measured by qPCR (A). WT mice received a single dose of ethanol. After 2 h, hepatocytes or LMNCs were isolated and analyzed for sXBP1 (B). Primary hepatocytes isolated from WT mice were treated with thapsigargin and analyzed for phospho-IRF3 (C). Some hepatocytes were pretreated with BX795 and analyzed for cytotoxicity by measuring LDH in supernatants (D). Primary hepatocytes isolated from WT or Tmem173gt mice were stimulated with thapsigargin and analyzed for phospho-IRF3 and for GRP78 indicating ER stress (E). WT or Tmem173gt mice received a single dose of ethanol, and liver injury was evaluated by serum ALT (F). WT or Tmem173gt mice were fed a control or alcohol diet. After 2 wk, we evaluated phospho-IRF3 (G), cleavage of Casp-3 in the liver (H), lipid accumulation (I and J), histology (I), and serum ALT (K). Expression of Ifnb1 and Isg15 was evaluated by qPCR (L). n = 3 mice per time point and genotype in A–E; and n = 10–15 mice (ethanol-fed, per genotype), and n = 3–6 mice (pair-fed, per genotype) in F–L. Numbers in the graphs indicate P values. *P < 0.05 vs. baseline. (Original magnification, 200×.) Vertical line in C divides two noncontiguous parts of the same blot. (M) The role of IRF3 in the pathogenesis of ALD. In the acute setting, alcohol-induced ER stress in hepatocytes triggers association of IRF3 with the ER adaptor, STING. Serving as a molecular scaffold, STING facilitates phosphorylation of IRF3 by phospho-TBK1 (a). Subsequently, phospho-IRF3 associates with Bax and triggers mitochondrial pathway of hepatocyte apoptosis (b). This pathway represents the major mechanism of IRF3-induced pathology in ALD and is independent of inflammation or Type-I IFNs. In chronic ALD, phospho-IRF3 also induces Type-I IFNs in hepatocytes, which have antifibrotic and anti-inflammatory effects (c). Phosphorylation of IRF3 is augmented by FasL (d). Induction of inflammatory cytokines by phospho-IRF3 does not play a role in acute ALD. In the chronic ALD, phospho-IRF3 in KC is required for induction of TNF-α, but only marginally contributes to liver pathology (e).
We observed that induction of ER stress by thapsigargin elicited early phoshorylation of IRF3 (Fig. 5 C and E) and death (Fig. 5D) in primary mouse hepatocytes. We confirmed the requirement of IRF3 activation for ER stress-induced cytotoxicity using BX795, an inhibitor of IRF3 phosphorylation, which inhibited hepatocyte death (Fig. 5D). Next, we observed that thapsigargin failed to induce IRF3 phosphorylation in hepatocytes isolated from the transmembrane protein 173 (Tmem173)gt mice (Fig. 5E), which lack the STING protein. This finding indicated that activation of IRF3 in hepatocytes by ER stress is dependent on STING.
To further evaluate the contribution of STING to IRF3 phosphorylation in ALD, we administered a single dose of ethanol to WT or Tmem173gt mice and found that deficiency of STING completely protected mice from acute liver injury (Fig. 5F), similar to the protection observed in IRF3-KO animals (Fig. 1C). To define the importance of STING in chronic ALD, we fed an ethanol or control diet to WT and Tmem173gt mice. In contrast to WT mice, Tmem173gt mice failed to phosphorylate IRF3 in the liver (Fig. 5G) and did not cleave Casp-3 indicating absence of alcohol-induced apoptosis (Fig. 5H). Further, Tmem173gt mice showed normalization of the morphological features of ALD and absence of steatosis upon histological analysis (Fig. 5 I and J). Consistent with the absent phosphorylation of IRF3 in the liver, alcohol-fed Tmem173gt mice failed to up-regulate Ifnb and did not show induction of Isg15, a marker of Type-I IFN signaling (Fig. 5L) after ethanol feeding. We observed that Tmem173gt mice were significantly protected from alcohol-induced liver injury only at early time points (Fig. 5 F and K) but not at the conclusion of the experiment (day 14 in Fig. 5K). We hypothesize that the lack of protection from late alcohol liver damage in Tmem173gt mice was attributable to the up-regulation of inflammatory cytokines (SI Appendix, Fig. S7), which have not been reported to be controlled by STING-dependent signaling. Importantly, our data demonstrated that activation of IRF3 in ALD is triggered by the ER stress in hepatocytes via the adaptor, STING. Thus, STING and IRF3 are key determinants of ALD, linking ER stress signaling with the mitochondrial pathway of hepatocyte apoptosis.
Discussion
Innate immunity and inflammation are involved in alcoholic steatohepatitis, cirrhosis, and hepatocellular cancer. These entities represent advanced stages of ALD and develop only in a subset of alcohol-drinking individuals (1). However, it is not known whether innate immune signaling is also involved in the early stage of ALD which is characterized by simple steatosis and mild hepatocyte injury. Here we demonstrated that innate immune signaling is a major determinant of early ALD, even before the onset of inflammation. We provide unique evidence that IRF3, an essential component of innate immunity, is activated by ER stress via the adaptor molecule STING and that IRF3 is required for apoptosis of hepatocytes. Thus, we have identified a unique role of STING and IRF3 in coupling ER stress with mitochondrial death signaling in ALD.
Our study provides several lines of evidence that IRF3 determines early ALD without the involvement of inflammation. First, IRF3 was present in hepatocytes where it underwent phosphorylation shortly after a single exposure to ethanol, before the development of inflammation. Second, IRF3 was activated by the ER stress in hepatocytes via the adaptor, STING. Third, IRF3 was required for hepatocyte apoptosis. Finally, IRF3 determined the early stage of ALD independent of inflammatory cytokine-producing KC or Type-I IFN signaling. Although KC are required for alcoholic steatohepatitis (1), our results demonstrate that the early phase of ALD is driven by hepatocyte-specific IRF3 rather than KC-dependent inflammation. Phosphorylation of IRF3 in hepatocytes occurred within 30 min after induction of ER stress, and IRF3 associated with STING, phospho-TBK1, and Bax in less than 2 h after administration of ethanol in the absence of liver inflammation. The dispensability of inflammation for these early events was further supported by the development of acute alcohol-induced liver injury in mice depleted of KC. Our data are consistent with findings that liver inflammation develops late after prolonged exposure to alcohol (15). Hepatocyte-specific IRF3 is key to ALD, whereas the contribution of myeloid cell-specific IRF3 to ALD is minor, as demonstrated by our previous data showing that deficiency of IRF3 in KC prevented up-regulation of TNF-α in the chronic model of ALD but provided only marginal protection from liver damage (5).
Given the increased gut permeability induced by ethanol (1), we initially hypothesized that LPS would activate IRF3 in ALD, but in kinetic studies we found that serum endotoxin peaked after the alcohol-induced phosphorylation of IRF3 in the liver had already been established (SI Appendix, Fig. S8 and Fig. 2C). Instead, our data demonstrate that the signal to trigger phosphorylation of IRF3 in early ALD comes from the ER and is dependent on STING. STING is predominantly associated with ER, where it enables IRF3 phosphorylation by TBK1 in the cytosolic dsDNA-signaling pathway, but it is also present on the outer mitochondrial membrane to facilitate the retinoic acid-inducible gene I pathway activated by viral RNA (10). We found that although IRF3 phosphorylated in ER and mitochondria, it associated with STING and phospho-TBK1 only in ER, but not in mitochondria. Therefore, we hypothesize that early in ALD, IRF3 is phosphorylated by the ER-associated STING aided by TBK1 and translocates to the mitochondria along with Bax to initiate hepatocyte apoptosis. Although our data demonstrate that the activation of IRF3 by STING is initiated by ER stress, we cannot rule out other potential activators of STING, in particular cytosolic dsDNA, which activates STING via cGMP-AMP synthase (cGAS) (16). As cGAS recognizes both bacterial and host dsDNA, we cannot exclude the possibility that DNA, released from the nuclei of damaged hepatocytes, activates STING and IRF3. We speculate that should this phenomenon occur in the liver, it would probably contribute to the later phase of ALD. Investigation of mechanisms other than ER stress that might be involved in STING activation in early ALD is beyond the scope of this study. Although deficiency of IRF3 or STING prevented lipid accumulation in hepatocytes in our model (SI Appendix, Fig. S2 C and D and Fig. 5 I and J), it has been shown that chemical induction of ER stress directly contributed to hepatocyte steatosis via a mechanism involving sXBP1 (17), which has not been reported to interact with STING or IRF3. Thus, it is likely that multiple pathways downstream of ER mediate alcohol-induced hepatocyte pathology, and their relative contribution to early ALD remains to be determined.
Based on our data, we propose that the major pathogenic effect of IRF3 in early ALD is hepatocyte-specific and that it occurs within the first few hours after alcohol ingestion in response to ER stress and STING and is mediated by hepatocyte apoptosis (Fig. 5M). Other mechanisms of IRF3 activation, e.g., IRF3 phosphorylation induced by the TLR4 pathway in KC or by Fas ligand upon hepatocytes, occur at a later stage and may contribute to amplification of liver damage and development of liver inflammation but are unlinked from STING-IRF3-Bax– driven liver injury. From the therapeutic perspective, a cautious approach should be used with inhibitors of IRF3. Although not required for the pathogenic role of IRF3 in ALD, Type-I IFNs are protective in liver inflammation induced by TLR9 and are required for induction of the anti-inflammatory IL-10 in chronic ALD, as we previously reported (5, 18). Also, Type-I IFNs have an inhibitory role in liver fibrosis (18). Therefore, it would be desirable to design molecules that would suppress the proapoptotic role and spare the Type-I IFN-inducing capability of IRF3. This could be theoretically possible, as the transcriptional and the proapoptotic domains of IRF3 are distinct and located on the opposite termini of the molecule (8).
Based on our data, activation of IRF3 and induction of hepatocyte apoptosis is a highly reproducible response to chronic and even a single ethanol exposure. Although human alcoholics consume alcohol chronically, alcohol-drinking patterns often include acute binges in the chronic alcohol use setting, suggesting that the phenomenon observed in our animal model may also occur in humans. However, testing for this in humans is technically difficult because there are no sufficient serum markers for IRF3 activation. We hypothesize that if the alcohol exposure events are sufficiently spaced out, hepatocyte apoptosis may be a self-limiting phenomenon. However, alcohol binges in close succession, as occurs in chronic alcoholics, may result in overlapping waves of hepatocyte death associated with the release of hepatocyte-derived damage-associated molecular patterns which are required for inflammation and advanced stages of ALD.
Taken together, our results identify a unique role of IRF3 and STING as signaling molecules mediating cross-talk between the ER stress and apoptosis in hepatocytes. To the best of our knowledge, we show for the first time that activation of IRF3 by STING and induction of cell death by IRF3, originally reported in the context of antiviral response (8, 10), occur in hepatocytes, an essentially metabolic cell type. Our data suggest that innate immune mechanisms regulate a response to acute, inflammation-independent derangements in hepatocyte biology induced by ethanol. The purpose of this biological principle awaits further elucidation.
Methods
Information on animal studies, Kupffer-cell depletion protocol, cytokine measurement, protein and RNA quantification, histological analysis, in vitro experiments, and statistics is provided in SI Appendix. All P-values are 2-sided. All animals received proper care in agreement with animal protocols approved by the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School.
Supplementary Material
Acknowledgments
This work was supported by Grant AA017729 from NIAAA (to G.S.). Core resources supported by Diabetes Endocrinology Research Core Grant DK32520 were also used.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.S.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308331110/-/DCSupplemental.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology. 2011;141(5):1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 3.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34(1):101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 4.Hritz I, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48(4):1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrasek J, et al. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology. 2011;53(2):649–660. doi: 10.1002/hep.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282(21):15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 7.Zhao XJ, et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181(5):3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chattopadhyay S, et al. Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J. 2010;29(10):1762–1773. doi: 10.1038/emboj.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrasek J, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122(10):3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5(214):ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplowitz N, Ji C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J Gastroenterol Hepatol. 2006;21(Suppl 3):S7–S9. doi: 10.1111/j.1440-1746.2006.04581.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29(4):538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44(5):723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roychowdhury S, et al. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology. 2009;49(4):1326–1334. doi: 10.1002/hep.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320(5882):1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrasek J, Dolganiuc A, Csak T, Kurt-Jones EA, Szabo G. Type I interferons protect from toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology. 2011;140(2):697–708. doi: 10.1053/j.gastro.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.