Development of postmenopausal osteoporosis
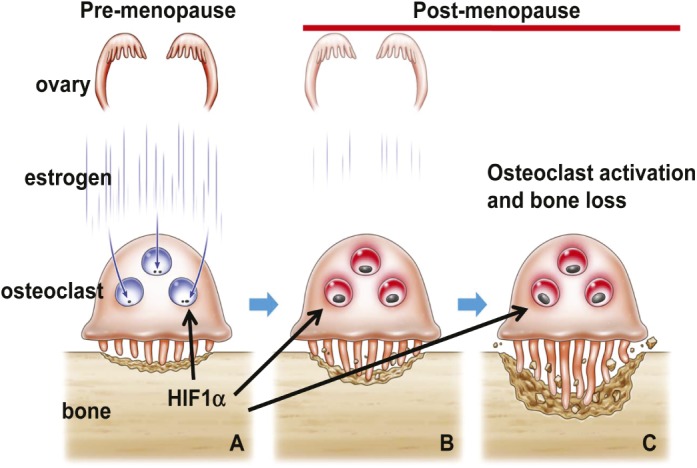
Estrogen regulates HIF1α stability and osteoclast activity.
The decline in estrogen production following menopause frequently accelerates the pace of bone resorption by cells called osteoclasts, a process that can lead to osteoporosis. However, the molecular mechanisms underlying the development of postmenopausal osteoporosis remain unclear. Yoshiteru Miyauchi et al. (pp. 16568–16573) found that the levels of the protein hypoxia-inducible factor 1 alpha (HIF1α), which is stabilized by hypoxic conditions, were low or undetectable in the osteoclasts of mice in vivo, even though these cells are located in hypoxic regions. By contrast, the authors detected HIF1α in osteoclasts from mice that had undergone ovariectomy, which results in estrogen deficiency. Loss of estrogen also promoted the activation of osteoclastic bone resorption and resulted in bone loss in vivo. When the authors treated mouse osteoclasts with estrogen in vitro, HIF1α levels decreased, even in hypoxic conditions; however, Hif1α mRNA levels remained unchanged, suggesting that estrogen destabilizes HIF1α protein. Further analysis revealed that genetic deletion or pharmacologic inhibition of HIF1α in the osteoclasts of mice lacking ovaries prevented osteoclast activation and protected the animals from osteoporosis, suggesting that estrogen depletion promotes bone loss via HIF1α-mediated activation of osteoclast activity. The findings suggest that HIF1α may be a promising therapeutic target for the treatment of postmenopausal osteoporosis, according to the authors. — N.Z.
Parsing the insoluble proteome of the Alzheimer’s brain
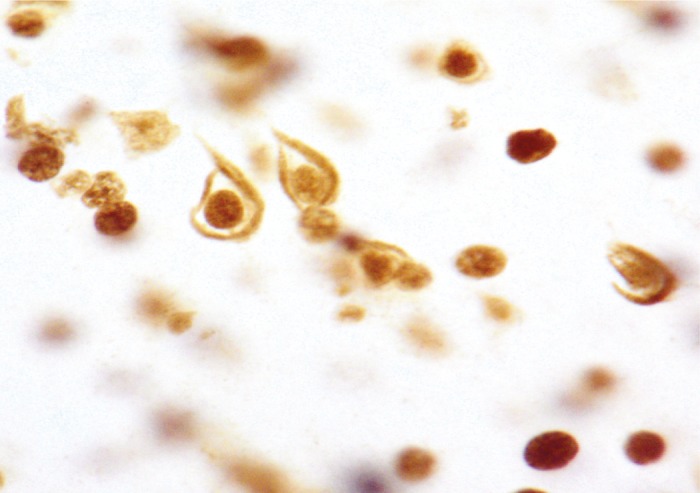
Splicing alternation in Alzheimer’s disease.
Though Alzheimer’s disease (AD) is known to manifest with the accumulation of insoluble amyloid plaques and tau aggregates, researchers remain unclear about the underlying etiology of the disease. Bing Bai et al. (pp. 16562–16567) have uncovered part of the insoluble proteome of the brain of patients with AD. Using mass spectrometry, the authors identified 4,216 insoluble proteins, of which 36—including U1-70K and other U1 small nuclear ribonucleoprotein (U1 snRNP) spliceosome components—accumulate in the brain of patients with AD. A comparison of RNA from the brain of patients and healthy individuals revealed that patients with AD undergo unique changes to snRNP and experience dysregulated RNA processing, with the accumulation of unspliced RNA species in the brain. Interestingly, the authors also found that patients with mild cognitive impairment undergo similar protein accumulation early in the progression to AD. According to the authors, the findings provide insight into the molecular mechanisms that contribute to AD, beyond factors related to β-amyloid and tau. — A.G.
Controlling HIV-1 infection in mice
While monoclonal antibodies have proved valuable in the treatment of various cancers and immune disorders, they have been rarely used to treat infections, and early efforts to control HIV by passive immunization have proved disappointing. However, interest in antibody therapy for HIV has been renewed by the discovery of broadly neutralizing antibodies (bNAbs), which contain multiple somatic mutations, have far more potent neutralizing activity than earlier clones, and protect humanized mice and nonhuman primates from an HIV or SHIV challenge. Building on their prior observation that a combination of bNAbs can suppress viremia in humanized mice infected with HIV-1, Joshua Horwitz et al. (pp. 16538–16543) sought to optimize the regimen by combining bNAbs with antiretroviral drug therapy (ART). The authors treated HIV-infected mice with daily ART for 3 weeks to reduce the viral load, added a twice-weekly cocktail of three bNAbs, and continued to administer bNAbs after ART was terminated. The authors report that triple-bNAb therapy alone was sufficient to control viremia following discontinuation of ART. Among HIV-infected mice injected with a recombinant adeno-associated virus expressing only a single bNAb, 86% maintained HIV viral loads at or near undetectable levels following termination of ART. Given the cost, side effects, and emergence of drug resistance associated with long-term ART, the therapeutic use of bNAbs bears clinical investigation, according to the authors. — C.B
Induced neurons reproduce phenotype linked to autism mutation
Previous studies have shown that nonneuronal cells, such as pluripotent stem cells or fibroblasts, can be converted into functional induced neuronal (iN) cells. Although these results suggest an approach for modeling neuropsychiatric diseases, researchers have yet to determine whether disease phenotypes observed in iN cells replicate the hallmark characteristics of endogenous neurons in the same organism. To address the question, Soham Chanda et al. (pp. 16622–16627) analyzed the phenotypes of iN cells derived from murine embryonic fibroblasts, which were cultured from wild-type and mutant mouse littermates carrying a neuroligin-3 point mutation, previously linked to autism. The neuroligin-3 mutant iN cells, the authors report, exhibited a large and selective decrease in AMPA-type glutamate receptor-mediated synaptic transmission. Furthermore, the decrease occurred without changes in NMDA-type glutamate receptor- or GABAA receptor-mediated synaptic transmission, thus replicating the signature characteristics of the known mouse model. The findings demonstrate that the effect of the mutation applies even to neurons differentiated from fibroblasts, and that iN cells could be used to model certain diseases, according to the authors. — T.J.
Epigenetic clock regulates seasonal reproduction in mammals
Seasonal timing of reproduction, a common feature among vertebrates, is marked by alterations in physiology and behavior in response to modulations in melatonin secretion, cued by changes in day length. In birds and mammals, signaling of photoperiod information to the reproductive machinery is mediated by the thyroid hormone T3, generated in either the active form or as its inactive enantiomer, rT3; seasonal regulation is achieved by altering the production of the enzymes that synthesize the two forms of the hormone. Tyler Stevenson and Brian Prendergast (pp. 16651–16656) attempted to identify the molecular mechanism by which expression of the deiodinase gene dio3, whose enzyme product helps synthesize rT3, is activated in response to short day photoperiods. The authors housed seasonally breeding Siberian hamsters in long days (LD) with 15 hours of light. Following transfer to 9 hours of light/day, hypothalamic DNA methyltransferase expression and methylation of the dio3 promoter were reduced, dio3 expression was increased, and reproduction was inhibited. Similar results were obtained when hamsters in LD received daily injections of melatonin. After prolonged exposure to SD, hamsters become refractory to melatonin and reproductive physiology is stimulated; in the study, the dio3 promoter was remethylated and dio3 expression fell to LD-like levels following 42 weeks of SD. According to the authors, the findings demonstrate a potential role for reversible DNA methylation in maintaining a seasonal calendar in mammals. — C.B.
Role of GABA in schizophrenia’s cognitive deficits
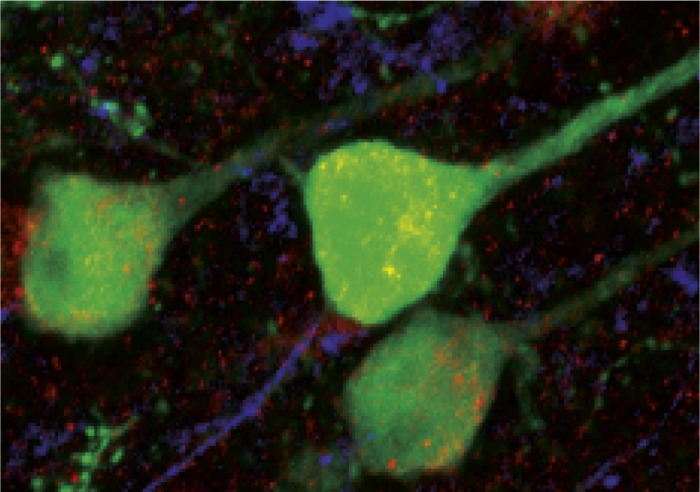
Adeno-associated virus infected neurons of the frontal cortex in the mouse.
Cognitive impairments in schizophrenia are highly predictive of poor patient outcomes and are not responsive to current medications. Recent studies suggest a role for the inhibitory neurotransmitter GABA in the cognitive defects underlying schizophrenia. To better understand the role of GABA in cognitive deficits, Rochelle Hines et al. (pp. 16628–16633) developed a mouse model of schizophrenia by altering the accumulation of specific types of GABA receptors. Specifically, the authors used a dominant negative virus that, when injected into the frontal cortex of mice, disrupted the α2 subunit of the GABAA receptors (GABAARs). The mice showed cognitive deficits, including working memory deficits, as well as prepulse inhibition deficits similar to those seen in patients with schizophrenia. The findings suggest that GABAergic synapses in the prefrontal cortex might directly contribute to cognition, and that selectively increasing their activity might improve the outcome of patients with schizophrenia. According to the authors, the animal model may provide a platform for screening potential cognition-improving treatments. — B.A.


