Significance
Converging lines of evidence suggest that increase in α-syn levels plays a central role in synucleinopathies pathogenesis. Therefore, lowering α-syn levels represents a viable therapeutic strategy for these disorders. Here, we describe a previously uncharacterized cellular mechanism controlling the selective autophagic degradation of α-syn. This effect is governed by PLK2 kinase activity, phosphorylation of α-syn, and PLK2/α-syn complex formation. In a rat model of Parkinson disease, PLK2 overexpression reduces α-syn levels and suppresses α-syn–induced toxicity and motor deficits. Collectively, our data support the neuroprotective role of PLK2 and suggest that modulating its activity is a viable therapeutic strategy for the treatment of Parkinson disease and related synucleinopathies.
Keywords: adeno-associated virus, animal model, serum inducible kinase
Abstract
An increase in α-synuclein levels due to gene duplications/triplications or impaired degradation is sufficient to trigger its aggregation and cause familial Parkinson disease (PD). Therefore, lowering α-synuclein levels represents a viable therapeutic strategy for the treatment of PD and related synucleinopathies. Here, we report that Polo-like kinase 2 (PLK2), an enzyme up-regulated in synucleinopathy-diseased brains, interacts with, phosphorylates and enhances α-synuclein autophagic degradation in a kinase activity-dependent manner. PLK2-mediated degradation of α-synuclein requires both phosphorylation at S129 and PLK2/α-synuclein complex formation. In a rat genetic model of PD, PLK2 overexpression reduces intraneuronal human α-synuclein accumulation, suppresses dopaminergic neurodegeneration, and reverses hemiparkinsonian motor impairments induced by α-synuclein overexpression. This PLK2-mediated neuroprotective effect is also dependent on PLK2 activity and α-synuclein phosphorylation. Collectively, our findings demonstrate that PLK2 is a previously undescribed regulator of α-synuclein turnover and that modulating its kinase activity could be a viable target for the treatment of synucleinopathies.
Parkinson disease (PD) is a neurodegenerative disorder characterized by the progressive neuronal loss in different brain regions, including the dopaminergic (DA) neurons of the substantia nigra pars compacta (SNc) (1, 2). Pathologically, PD is characterized by the presence, in surviving DA neurons, of intracellular inclusions called Lewy Bodies (LBs) and Lewy Neurites (LNs) (3). These fibrillar aggregates are mainly composed of the presynaptic protein α-synuclein (α-syn) (4). Converging evidence from pathologic, genetic, biochemical, and biophysical studies supports the hypothesis that α-syn accumulation and misfolding play a central role in the pathogenesis of PD and related disorders, which are altogether referred to as synucleinopathies (5).
Although α-syn is constitutively phosphorylated at low levels (<4%) at S129 (pS129) in normal brains (6–8), a clear accumulation of pS129 (>90%) is found in LBs (8, 9) and in the brain of animal models of synucleinopathies (7, 10–12). The roles of this major posttranslational modification in regulating α-syn physiology and LB formation and/or neurodegeneration in PD remain elusive.
Recently, our group and others (13–15) demonstrated that Polo-like kinase 2 (PLK2), a serine/threonine kinase playing a central role in cell division, oncogenesis, and synaptic regulation in the adult brain (16–19), efficiently phosphorylates α-syn, with an exclusive preference for the S129 site. Unlike other kinases that were reported to partially phosphorylate α-syn at S129, PLK2 phosphorylates α-syn with quantitative conversion in vitro (>95%) and in mammalian cell lines (13–15).
Interestingly, PLK2 expression levels are significantly up-regulated in the midbrain of aged monkeys and correlate with increased pS129 levels in dopaminergic neurons (20). Furthermore, neuropathological analysis of diseased brains and tissues from α-syn transgenic animal models of synucleinopathies also showed a significant up-regulation of PLK2 protein levels, and colocalization with pS129 in neuronal cell bodies and terminals (13). Together, these observations suggest that PLK2 might play an important role in the modulation of α-syn physiology in normal and diseased conditions. However, whether this increase in PLK2 contributes to α-syn aggregation and neurodegeneration in PD or reflects an active cellular response to prevent these processes remains unknown. Motivated by these findings and the desire to learn more about the interplay between α-syn phosphorylation, PLK2, and neurodegeneration, we sought to investigate the effect of PLK2 expression and PLK2-induced phosphorylation on α-syn physiology and toxicity in vitro and in vivo.
In the present study, we provide evidence that PLK2 binds directly to α-syn in an ATP-dependent manner and regulates α-syn selective clearance via the lysosome–autophagic degradation pathway. PLK2-mediated α-syn degradation is dependent on both PLK2 kinase activity and PLK2/α-syn protein–protein interaction. Moreover, we show that PLK2 WT, but not the kinase dead mutant, decreases α-syn protein levels in a rat genetic model of PD, reverses α-syn–induced toxicity, and prevents the motor deficits caused by the overexpression of human α-syn. Consistent with the role of PLK2 on α-syn degradation found in vitro, this effect depends on S129 phosphorylation. Collectively, our data support the neuroprotective role of PLK2 against PD pathology, which seems to be mediated by selective autophagy clearance of human α-syn, and suggest that modulating PLK2 activity is a viable therapeutic strategy for the treatment of PD and related disorders.
Results
PLK2 Promotes Selective Autophagic α-Syn Degradation.
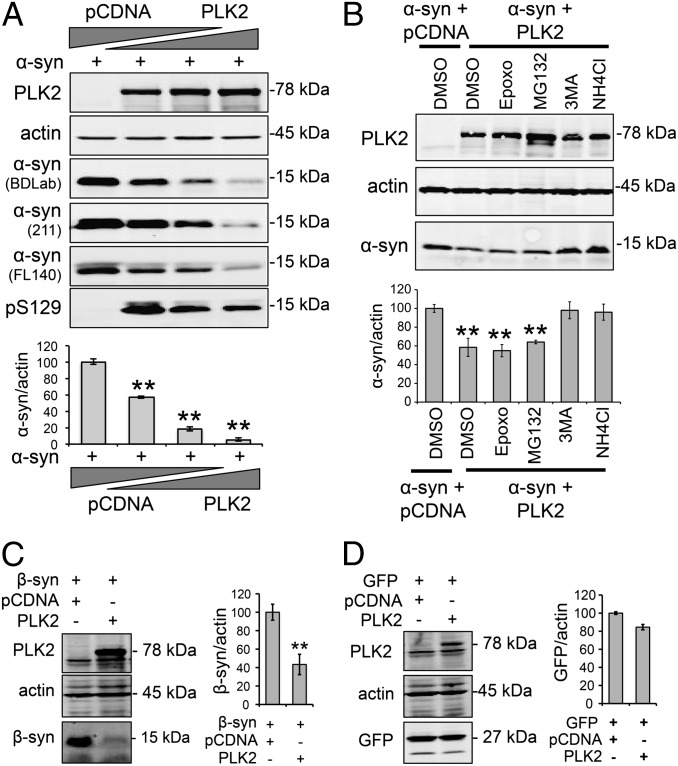
In our cell-culture experiments, we consistently observed that PLK2 WT overexpression results in a decrease in α-syn levels (Fig. S1). To confirm this observation and further investigate the effect of PLK2 overexpression on α-syn protein levels, we used a cell-based assay, in which α-syn expression plasmid was cotransfected with increasing amounts of the PLK2 plasmid. As shown in Fig. 1A, increasing the expression of PLK2 induced a significant decrease in α-syn protein levels, which is detected at an apparent molecular weight of ∼15 kDa. At the highest levels of PLK2 expression, only a small amount of α-syn remained detectable, suggesting that PLK2 significantly reduced total α-syn protein levels (Fig. 1A). Using different α-syn antibodies directed against N- or C-terminal epitopes, we ruled out the possibility that the decrease of α-syn signal could be due to loss of antibody epitope recognition (Fig. 1A). Using a pS129-specific antibody, we also observed a decrease of pS129 levels. This decrease of pS129 levels, inversely correlating with increasing levels of PLK2 kinase expression, indicates that pS129 α-syn is likely to be a major target for PLK2-mediated degradation (Fig. 1A).
Fig. 1.
PLK2 overexpression enhances α-syn autophagy-mediated degradation in a cell-based assay. (A) Western blot and quantification of whole-cell lysate from HEK cells transfected with human α-syn (1 µg) and increasing amounts of PLK2 WT (0.5, 1, and 2 µg). For DNA titrations, total DNA per transfection was equalized by addition of pcDNA empty vector. The overexpression of PLK2 induced a concentration-dependent decrease of α-syn protein levels. The detection of α-syn using antibodies targeting different epitopes on α-syn ruled out the possibility of loss of epitope recognition (**P < 0.01). (B) Whole-cell lysate form HEK cells transfected with α-syn (1 µg) and PLK2 WT (0.5 µg) and treated with proteasome inhibitors (50 nM Epoxomicin and 10 µM MG132) and autophagy–lysosome inhibitors (10 mM 3MA and 25 mM NH4Cl). Western blot and optical density quantification showed that only autophagy–lysosome inhibitors reverse PLK2-induced reduction of α-syn (**P < 0.01). (C) Whole-cell lysate analysis and optical-density quantification showing that β-synuclein (β-syn) is degraded after PLK2 overexpression (**P < 0.01). (D) Whole-cell lysate analysis and optical density quantification showing that GFP levels are not affected by PLK2 overexpression.
To determine the mechanism by which α-syn protein is degraded, we used a pharmacological approach to selectively block the different protein-degradation pathways. Interestingly, treatment of cells with the proteasome inhibitors epoxomicin (50 nM) or MG132 (10 µM) had no effect on PLK2-mediated degradation of α-syn (Fig. 1B). However, blocking the early and late stages of the autophagy–lysosomal pathway, using 3MA (10 mM) and NH4Cl (25 mM), respectively, restored α-syn amount to the same level as the condition without PLK2 (Fig. 1B). Together, these data demonstrate that PLK2 overexpression enhances α-syn clearance via the lysosome–autophagy pathway.
To assess whether other members of the synuclein family could also be targeted by PLK2 for protein degradation, we coexpressed PLK2 with β-synuclein (β-syn), which we showed previously to be a substrate of PLK2 (13). Interestingly, PLK2 overexpression significantly decreased the levels of β-syn (Fig. 1C) but did not affect the expression levels of a nonrelated cytosolic protein Green Fluorescent Protein (GFP) (Fig. 1D). This observation suggests that PLK2 can mediate the degradation of phosphorylated synuclein substrates.
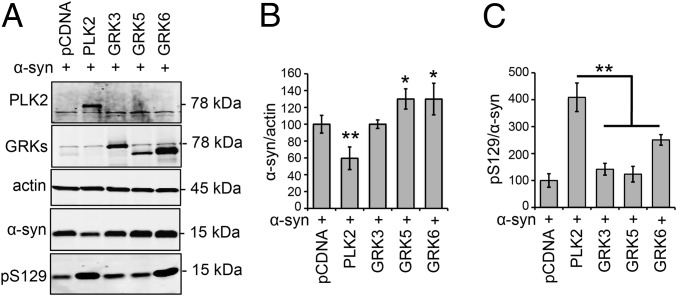
Next, we sought to determine whether enhancing autophagic degradation of α-syn is a specific feature of PLK2, or could be induced by any of the enzymes that are able to catalyze α-syn phosphorylation at S129. In particular, we assessed the effect of members of the G protein-coupled receptor kinase (GRK) family, which have been reported to phosphorylate human α-syn at S129 (21, 22). The results shown in Fig. 2 A and B demonstrate that the overexpression of PLK2 only, but not GRK3, -5, or -6, induced a significant decrease of α-syn protein levels. In particular, GRK5 and -6 even induced a significant increase in the level of human α-syn (Fig. 2 A and B). Evaluation of pS129 levels confirmed the superiority of PLK2 in catalyzing α-syn phosphorylation (Fig. 2C). These results suggest that, although various kinases can phosphorylate α-syn at S129, only PLK2 activity increases the turnover of synuclein substrates via autophagy.
Fig. 2.
PLK2 overexpression, but not the GRKs, mediated α-syn degradation in HEK239T cells. (A) Whole-cell analysis and Western blot illustrating the levels of total α-syn and pS129 in HEK cells transfected with α-syn and PLK2 or GRKs (GRK3, GRK5, and GRK6). (B) Optical-density quantification of α-syn signal showing that only the overexpression of PLK2 induced a decrease of α-syn levels. Interestingly, GRK5 and GRK6 induced an increase of α-syn signal (*P < 0.05; **P < 0.01). (C) Optical-density quantification of pS129 levels, relative to total α-syn signal, showing the superiority of PLK2 in phosphorylating α-syn in cell culture (**P < 0.01).
PLK2/α-Syn Complex Formation and PLK2 Kinase Activity Are Essential for PLK2-Mediated α-Syn Degradation.
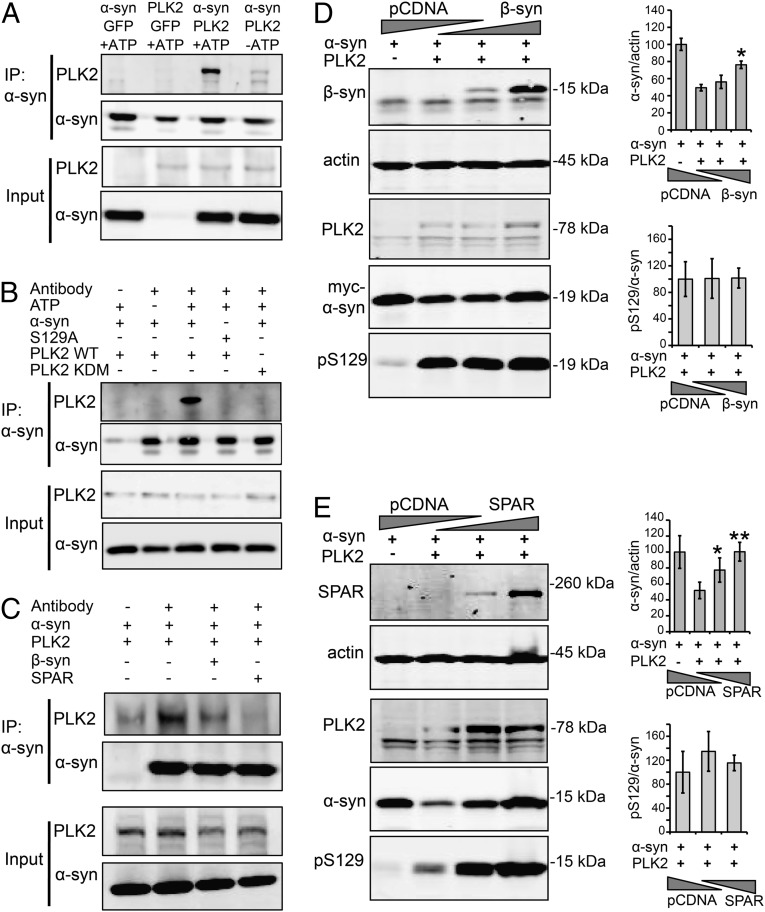
To investigate whether a protein–protein interaction exists between PLK2 and α-syn and modulates PLK2-mediated α-syn degradation, we first assessed the direct binding between the two proteins in a cell-based assay. Because PLK2 interaction with several substrates has been reported to be ATP-dependent (23), coimmunoprecipitation was performed in the presence or absence of ATP. We observed that PLK2 immunoprecipitated with α-syn only in the presence of ATP (Fig. 3A), indicating that a direct ATP-dependent interaction exists between the two proteins.
Fig. 3.
PLK2/α-syn complex formation is required for PLK2-mediated α-syn turnover. (A) Coimmunoprecipitation of α-syn and PLK2 in the presence or absence of ATP, showing that the presence of ATP is required for the stabilization of PLK2 and α-syn protein–protein interaction. (B) Coimmunoprecipitation of α-syn (WT and S129A) with PLK2 (WT and KDM), showing that the integrity of α-syn phosphorylation site S129 and PLK2 kinase domain are important for PLK2/α-syn interaction. (C) Coimmunoprecipitation of α-syn and PLK2 in the presence of β-syn or SPAR, showing that the presence of one of these two PLK2 substrates competes with α-syn for the interaction with PLK2 and reduces PLK2/α-syn coimmunoprecipitation. (D) Whole-cell lysate form HEK cells transfected with human α-syn (1 µg), PLK2 WT (0.5 µg), and increasing amounts of β-syn (1.5 and 3 µg). For DNA titrations, total DNA per transfection was equalized by addition of pcDNA empty vector. The Western blot revealed that the overexpression of β-syn reverses α-syn turnover without affecting pS129/α-syn levels (*P < 0.05). (E) Whole-cell lysate form HEK cells transfected with human α-syn (1 µg), PLK2 WT (0.5 µg), and increasing amounts of SPAR (1.5 and 3 µg). For DNA titrations, total DNA per transfection was equalized by addition of empty pcDNA empty vector. The Western blot revealed that the overexpression of SPAR reverses α-syn turnover without affecting pS129/α-syn levels (*P < 0.05; **P < 0.01).
Given that ATP is also important for α-syn phosphorylation by PLK2, we investigated whether α-syn phosphorylation and the integrity of PLK2 kinase domain are important for PLK2/α-syn interaction. A pull-down assay showed that the substitution of Serine 129 to Alanine in α-syn structure abolished the PLK2/α-syn protein–protein interaction (Fig. 3B). Using the kinase dead mutant form of PLK2 (PLK2 KDM) (Fig. S1), we also observed a loss of interaction between α-syn and PLK2 KDM (Fig. 3B). Collectively, these results indicate that α-syn binds directly to PLK2 in an ATP-dependent manner and that the integrity of α-syn phosphorylation site S129 and PLK2 kinase domain are important for this interaction.
To evaluate whether α-syn and PLK2 interaction is essential for α-syn clearance, we decided to expose the α-syn/PLK2 complex to competitive interactors, namely, the alternative PLK2 substrates β-syn (13) and spine-associated Rap guanosine triphosphatase-activating protein (SPAR) (24). First, using a pull-down assay, we verified that PLK2/α-syn complex formation was reduced in the presence of β-syn or SPAR (Fig. 3C), confirming that the presence of β-syn or SPAR can effectively compete with the PLK2/α-syn interaction. Next, cells were cotransfected with α-syn, PLK2, and increasing concentrations of plasmids encoding either β-syn (Fig. 3D) or SPAR (Fig. 3E). Interestingly, competitive inhibition reversed PLK2-mediated α-syn degradation in a concentration-dependent manner (Fig. 3 D and E). Collectively, these data indicate that PLK2/α-syn complex formation is essential for PLK2-mediated α-syn degradation.
Strikingly, evaluation of the pS129/α-syn ratio revealed similar levels of the phosphorylated form in the presence or absence of competitors β-syn or SPAR (Fig. 3 D and E). This observation indicates that, despite the disruption of the PLK2/α-syn complex and the suppression of α-syn degradation, the extent of α-syn phosphorylation at S129 remained unchanged, demonstrating that α-syn phosphorylation at S129 is essential but not sufficient for PLK2-mediated α-syn clearance.
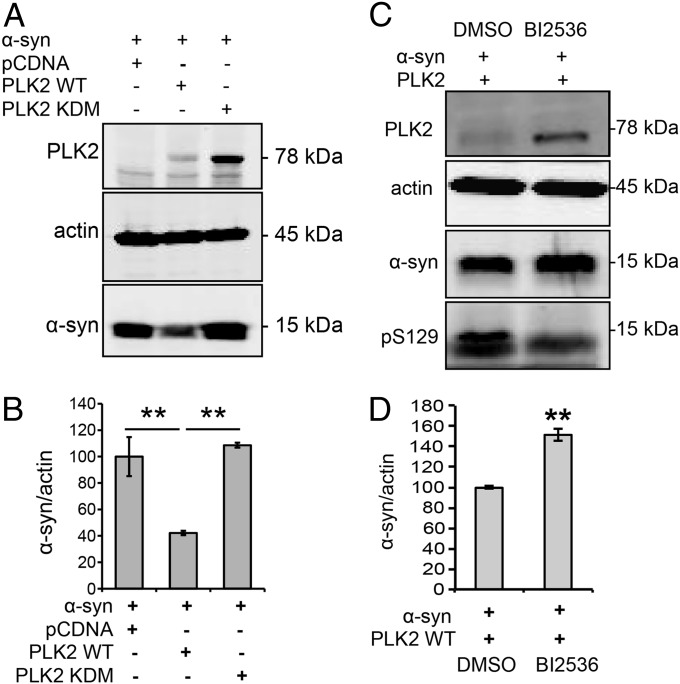
Then, to investigate whether the kinase activity is required for PLK2-mediated α-syn degradation, we used two approaches: (i) a genetic approach by using the kinase dead mutant (PLK2 KDM) and (ii) a pharmacological approach by using a potent and selective ATP competitor, BI2536, which inhibits PLK2 activity (14). After overexpression of the kinase dead mutant form of PLK2 (PLK2 KDM), which failed to interact with α-syn, α-syn protein levels remained unchanged, in contrast to the reduced α-syn levels observed with PLK2 WT (Fig. 4 A and B). Moreover, when cells overexpressing PLK2 were treated with BI2536, we observed a reduction of pS129 levels, demonstrating the inhibition of PLK2 kinase activity (Fig. 4C). Remarkably, BI2536 also reversed PLK2-mediated α-syn degradation (Fig. 4C). These data indicate that PLK2 kinase activity is essential for PLK2-mediated α-syn clearance. It is worth noting that the genetic and the pharmacological inhibition of PLK2 activity induced an accumulation of PLK2 protein levels (Fig. 4 A and C), confirming that PLK2 autophosphorylation controls its self-degradation (19). Collectively, our data suggest that the formation of the PLK2/α-syn complex and PLK2 kinase activity are essential for PLK2-mediated α-syn degradation.
Fig. 4.
PLK2 kinase activity is required for PLK2-mediated α-syn turnover. (A) Whole-cell lysate Western blot and (B) optical-density analysis showing that the overexpression of PLK2 KDM did not induce α-syn turnover. (C) Whole-cell lysate Western blot and (D) optical-density analysis showing that inhibition of PLK2 kinase activity, using the ATP-competitive inhibitor BI2536 (10 µM), decreases pS129 levels and suppresses PLK2-mediated α-syn turnover (**P < 0.01).
PLK2 WT, but Not the Kinase-Dead Mutant, Induces the Phosphorylation of Human α-Syn and Reduces Its Accumulation in Vivo.
To investigate whether PLK2 catalyzes α-syn phosphorylation at S129 and modulates its expression levels in the nigral dopaminergic neurons, we used a genetic rat model of PD, in which human α-syn was cooverexpressed with either PLK2 WT or KDM, using an adeno-associated virus (AAV) gene-delivery system. To keep the total amount of injected viral particles constant in all conditions, we coinjected the vector encoding α-syn with an empty vector (Stuffer) in the control group.
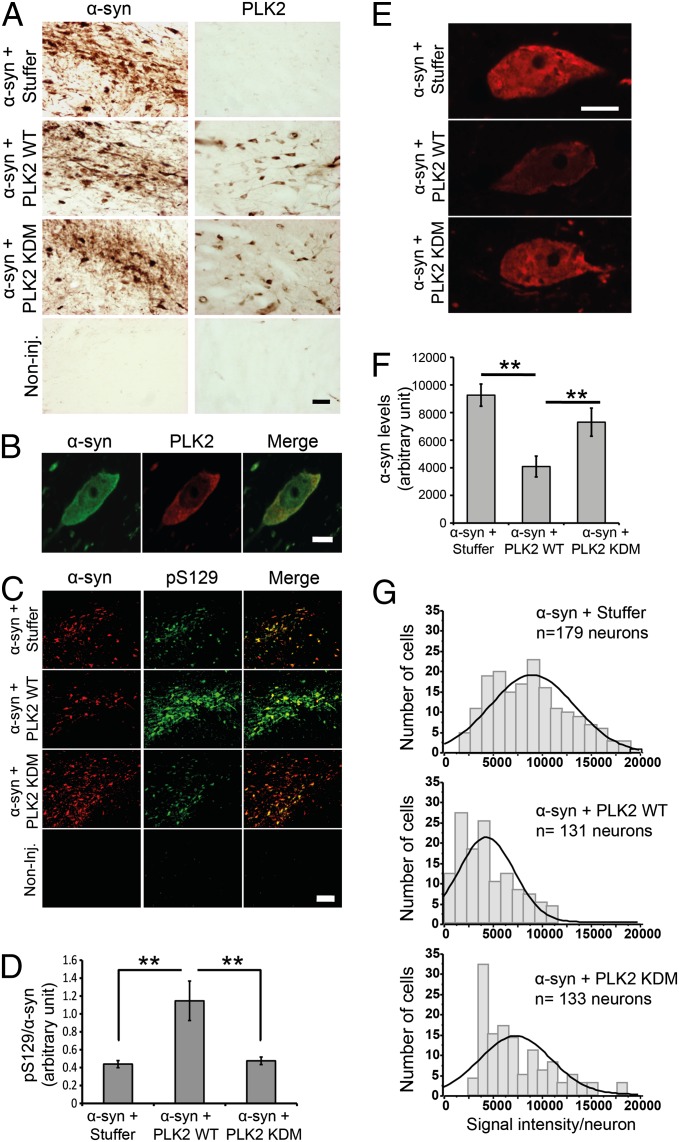
Four months postinjection, histological evaluation of transgene expression confirmed the detection of human α-syn or PLK2 immunoreactivity exclusively in the correspondent injected midbrain (Fig. 5A). Coimmunofluorescence analysis revealed that, in animals injected with both vectors, the infected PLK2-positive neurons coexpress α-syn (Fig. S2). At high magnification, the analysis revealed α-syn and PLK2 colocalization in the cytoplasmic compartment (Fig. 5B).
Fig. 5.
PLK2 WT overexpression, but not the KDM, induces α-syn phosphorylation at S129 and reduces intraneuronal α-syn protein levels. (A) Photomicrographs illustrating the expression of α-syn and PLK2 in the injected brains. (Scale bar: 50 µm.) (B) A high-magnification illustration of α-syn and PLK2 colocalization in a coinfected dopaminergic neuron. (Scale bar: 5 µm.) (C) Immunofluorescence of α-syn and pS129 showing the increase of pS129 levels after cooverexpression with PLK2 WT. (Scale bar: 100 µm.) (D) Optical-density quantification of pS129 levels, compared with total α-syn, estimating threefold increase of pS129 levels after cooverexpression of α-syn with PLK2 WT and histogram illustrating pS129 levels, relative to total α-syn. (E) High-magnification confocal images illustrating the population of neurons exhibiting α-syn intensity around the median line of the histograms of frequency distribution (Scale bar: 5μm). (F) Quantification, using ImageJ software, of α-syn protein levels per neuron showing that α-syn levels are significantly decreased when α-syn is cooverexpressed with PLK2 WT, compared with α-syn + Stuffer or α-syn + PLK2 KDM. (G) Histograms of frequency distribution of labeling, showing the distribution of α-syn signal intensity in the dopaminergic neuronal population (**P < 0.01).
Then, using antibodies directed against pS129 and tyrosine hydroxylase (TH), a marker of the dopaminergic neurons, costaining revealed that the majority of pS129-positive neurons were dopaminergic and that the levels of pS129 were dramatically increased when α-syn was cooverexpressed with PLK2 WT, but not with PLK2 KDM or the empty vector (Fig. S3). Semiquantitative estimation of pS129 levels, by the evaluation of pS129 relative fluorescence intensity in individual neurons, with respect to total α-syn, revealed threefold increase when PLK2 WT is coexpressed with α-syn, indicating that PLK2 phosphorylates α-syn efficiently in rat dopaminergic neurons (Fig. 5 C and D).
Then we investigated whether PLK2 overexpression could induce a decrease of α-syn protein levels, similarly to what we observed in cell culture assays. Quantification of fluorescent signal intensity of human α-syn in the injected midbrains showed that PLK2 WT overexpression reduced intraneuronal α-syn protein levels by 55.8%, in contrast to PLK2 KDM, which did not affect α-syn signal (Fig. 5 E and F). These results demonstrate that PLK2 kinase activity is required for regulating human α-syn protein levels in rat midbrains, consistent with our cell culture results. The distribution of α-syn signal intensity per neuron demonstrates that the decrease of α-syn levels is homogenous among the population of dopaminergic neurons, as reflected by the shift of the entire cell population toward lower signal intensity, indicating that α-syn protein levels decreased in the majority of the coinfected neurons (Fig. 5G).
PLK2 WT, but Not the Kinase-Dead Mutant, Suppresses α-Syn–Induced Toxicity and Motor Deficits in Vivo.
To assess whether the PLK2-mediated reduction of α-syn protein levels, in the midbrain neurons, is associated with a modulation of α-syn–induced neuronal toxicity, we estimated the total number of the TH-positive neurons per SNc, using unbiased stereological quantification. We first sought to evaluate the toxic effect induced by α-syn and PLK2 (WT and KDM) overexpressed separately in rat midbrain. Unbiased cell quantification revealed an extensive loss of TH-positive neurons in the injected SNc after α-syn overexpression (36.2% ± 2.7%, versus the noninjected hemisphere; n = 8) (Fig. S4). This neuronal loss was accompanied by the induction of hemiparkinsonian motor impairment (Fig. S4). In contrast, neither the vector overexpressing PLK2 WT, nor the KDM, caused any comparable neuron loss (13.3% ± 2.7%, n = 8 and 10.3% ± 1.9%, n = 8, respectively) or motor dysfunction (Fig. S4). In addition, we found no difference between the active and kinase-dead forms, indicating that kinase activity had no significant role in the observed minor neuron loss. These data confirm the toxicity of overexpressed α-syn (25, 26) and demonstrate that PLK2 overexpression is not toxic under our experimental conditions.
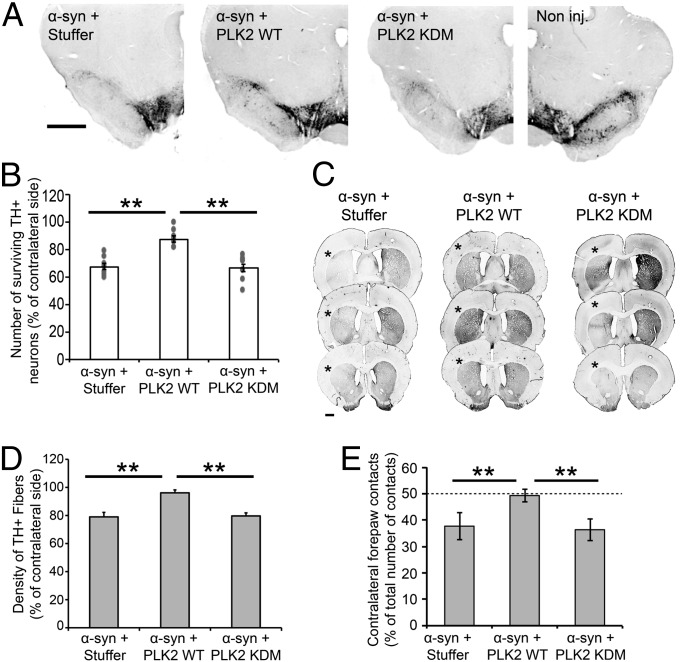
Then, we evaluated the toxicity induced by the cooverexpression of α-syn with PLK2. Interestingly, when injected in combination with the noncoding vector or PLK2 KDM, α-syn overexpression induced a significant neuronal loss (32.5% ± 2.1%, n = 9 and 33.5% ± 3%, n = 9, of TH-positive neurons loss, respectively) (Fig. 6 A and B). However, the coexpression with PLK2 WT significantly reduced the α-syn–induced loss of DA neurons to 12.6% ± 2.3% (n = 8) (P < 0.01, versus the two other conditions) (Fig. 6 A and B). Quantification of TH-positive fibers in the striatum confirmed this result, as striatal projections were almost completely preserved in the PLK2 WT/α-syn group, compared with the coinjection of the α-syn vector with the stuffer and PLK2 KDM vectors (P < 0.01) (Fig. 6 C and D). These data demonstrate that the kinase activity of PLK2 suppresses human α-syn toxicity in vivo. Previous studies from our group and others have established that the decrease in TH+ neuron number, induced by α-syn overexpression in this rat model, is due to neuronal loss rather than the loss of dopaminergic phenotype. In these studies, quantification of Nissl-positive or cresyl violet-positive cells in the SNc revealed a reduction of the number of positive cells, after α-syn overexpression, compared with the noninjected side, confirming that the neurons had indeed degenerated (25, 27).
Fig. 6.
PLK2 WT overexpression, but not the KDM, suppresses α-syn toxicity in vivo and alleviates hemiparkinsonian motor deficits. (A) Photomicrographs and (B) stereological quantification of the dopaminergic neurons in the injected SNc. (Scale bar: 1 mm.) (B) Quantification revealed that α-syn overexpression, alone or in combination with PLK2 KDM, induced a significant dopaminergic cell loss. However, PLK2 WT overexpression reverses α-syn toxicity (**P < 0.01). (C) Illustration of TH innervation density in the striatum and (D) optical-density quantification showed that only the overexpression of α-syn, but not PLK2 WT or KDM, induced a TH fiber loss in the ipsilateral striatum (*). (**P < 0.01) (Scale bar: 1 mm.) (E) Histograms illustrating the induction of hemiparkinsonian motor impairment after the overexpression of human α-syn. Before injection, all of the animals showed equal motor performances; however, the overexpression of α-syn, with the empty vector or PLK2 KDM, induced a significant decrease of the use of the contralateral forepaw, reflected by the forelimb asymmetry, whereas the overexpression of PLK2 WT reverses α-syn–induced motor impairment. **P < 0.01.
To determine whether PLK2-mediated protection against α-syn toxicity is associated with improvement of α-syn–induced motor impairments, we assessed the symmetry of forelimb activity in each group using the cylinder test. Four months postinjection, rats overexpressing human α-syn in combination with the noncoding vector or PLK2 KDM exhibited a significant reduction in the use of contralateral forepaws, as compared with their performance measured before vector injection (Fig. 6E). In contrast, the coexpression of PLK2 WT fully rescued contralateral forelimb motor activity, as animals in the group PLK2 WT/α-syn remained symmetrical over the entire experiment time course (P < 0.01; compared with Stuffer/α-syn and PLK2 KDM/α-syn) (Fig. 6E). Together, these data demonstrate that PLK2 WT overexpression, but not the kinase dead form, reduces significantly α-syn protein levels in a PLK2 activity and an α-syn phosphorylation-dependent manner and suppresses neuronal degeneration and hemiparkinsonian motor symptoms induced by α-syn overexpression.
PLK2 WT Does Not Rescue the Toxic Effects of S129A α-Syn.
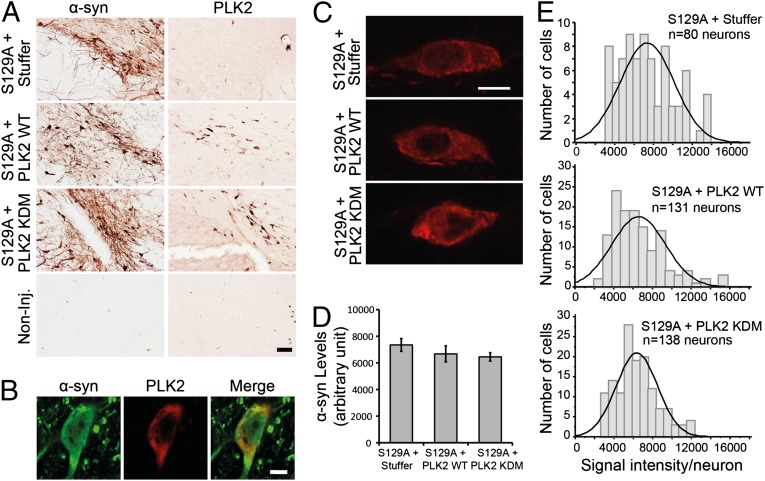
To determine whether α-syn phosphorylation at S129 is required for the neuroprotective effects of PLK2 and the reduction of intraneuronal levels of α-syn, we overexpressed the S129A mutant form of human α-syn (to block α-syn phosphorylation at serine 129), in combination with PLK2 WT or PLK2 KDM. Four months postinjection in the rat SNc, we detected by immunohistochemistry midbrain expression of human α-syn and PLK2 only in injected hemispheres (Fig. 7A). Immunofluorescence revealed that a significant subpopulation of neurons in the SNc coexpressed both human α-syn S129A and PLK2 (Fig. S5), which were colocalized in the cytoplasm (Fig. 7B).
Fig. 7.
PLK2 WT overexpression did not reduce α-syn protein levels in rat midbrain. (A) Photomicrographs illustrating the expression of α-syn S129A and PLK2 in the injected brains. (Scale bar: 50 µm.) (B) High-magnification illustrations of α-syn S129A and PLK2 colocalization in a coinfected dopaminergic neuron. (Scale bar: 5 µm.) (C) High-magnification confocal images illustrating the population of neurons exhibiting α-syn S129A intensity around the median line of the histograms of frequency distribution (Scale bar: 5μm). (D) Quantification of α-syn S129A protein levels per neurons showing that the protein levels are similar in all of the experimental conditions. (E) Histograms of frequency distribution of labeling, showing the distribution of α-syn S129A signal intensity in the dopaminergic neuronal population.
Quantitative estimation of total α-syn levels per neuron in brain samples injected with the S129A α-syn vector, in combination with empty vector or PLK2, showed that the level of S129A α-syn remained similar across experimental conditions (Fig. 7 C and D), with a similar distribution of S129A α-syn expression levels among the dopaminergic neuronal population (Fig. 7E). Together, these data demonstrate that phosphorylation at S129 is essential for PLK2-mediated α-syn turnover in vivo.
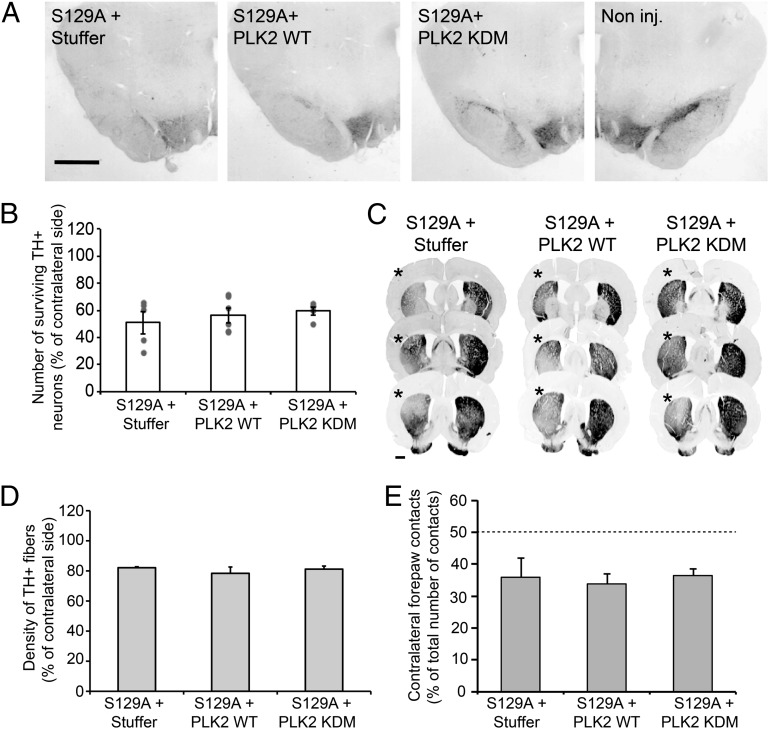
Assessment of dopaminergic neuron loss showed that coinjection of the S129A α-syn vector with the noncoding vector led to 49.1% ± 8.3% (n = 5) TH-positive neuron loss in the injected SNc (Fig. 8 A and B). Remarkably, when the mutated α-syn vector was injected in combination with PLK2 vectors (WT or KDM), the extent of neuronal loss was very similar (43.8% ± 7.2%, n = 6 and 40.3% ± 3.5%, n = 5, TH loss, respectively) (Fig. 8 A and B). Moreover, the analysis of TH-positive fiber loss in the striatum also showed similar and extensive dopaminergic degeneration in all experimental conditions (Fig. 8 C and D). Interestingly, the induction of motor deficits, expressed as the percentage of ipsilateral asymmetry in the use of forelimbs in the cylinder test, further confirmed the absence of any neuroprotective effect of PLK2 against S129A α-syn toxicity (Fig. 8E).
Fig. 8.
PLK2 WT overexpression did not rescue S129A-induced toxicity. (A) Photomicrographs and (B) stereological quantification of the dopaminergic neurons in the injected SNc. Quantification revealed that α-syn overexpression with empty vector or in combination with PLK2 induced extensive dopaminergic lesion. (Scale bar: 1 mm.) (C) Illustration of TH innervation density in the striatum. (D) Optical-density quantification showed that α-syn overexpression with empty vector or PLK2 induced comparable and extensive TH fiber loss in the ipsilateral striatum (*). (Scale bar: 1 mm.) (E) Histograms illustrating the induction of hemiparkinsonian motor impairment after the overexpression of human α-syn in combination with empty vector or PLK2. Four months postinjection, animals exhibited comparable forelimb asymmetry caused by the dopaminergic cell loss.
Collectively, our data confirmed the toxic effect of S129A α-syn overexpression in the rat SNc, as previously described (26, 28). Importantly, PLK2 overexpression did not reverse S129A toxicity, thereby indicating that the neuroprotective effects of PLK2 are mediated via α-syn phosphorylation at residue S129.
Discussion
Although phosphorylated α-syn at S129 accumulates in aged brains (20) and over the course of PD pathogenesis (29) and is localized within Lewy bodies in synucleinopathy-diseased brains (8, 9) and in transgenic mice models of PD (7, 10–12), its role in modulating α-syn physiology and toxicity in vivo remains elusive.
Recently, a growing body of evidence suggests that α-syn and its natural kinase, PLK2, could play synergistic roles in the modulation of α-syn physiology and toxicity during PD pathogenesis: (i) PLK2 is the most efficient kinase phosphorylating α-syn specifically at S129 in vitro (15), in cell culture (13), and in vivo (14); (ii) α-syn and PLK2 levels are up-regulated during aging (20) and in synucleinopathy-diseased brains and transgenic mice models of PD (13); and (iii) PLK2 is involved in the phosphorylation of aggregated α-syn in vitro (13) and in cell culture (30). Collectively, these observations suggest a synergic role of PLK2 and α-syn in health and disease states.
α-Syn Directly Binds to PLK2 in an ATP-Dependent Manner.
A recent study reported a weak and transient interaction between α-syn and PLK2 in a cell-based assay. Detection of this interaction was possible only in the presence of chemical cross-linkers (31). Here, we report a direct binding of these two proteins, which is stabilized in the presence of ATP. This type of protein interaction ATP-dependent has been also described for another PLK2 substrate in neurons, N-ethylmaleimide-sensitive fusion protein (NSF) (23), suggesting that PLK2 enzymatic activity could modulate its interaction with the substrates. Moreover, we observed that the integrity of α-syn phosphorylation site S129 and PLK2 ATP-binding pocket are important for driving PLK2/α-syn interaction, suggesting that phosphorylation and ATP binding induce structural and conformational changes in α-syn and PLK2, respectively (32, 33), and facilitate the physical interaction between these two partners.
PLK2 Activity Enhances α-Syn Autophagic Clearance in Cell Culture and in Vivo.
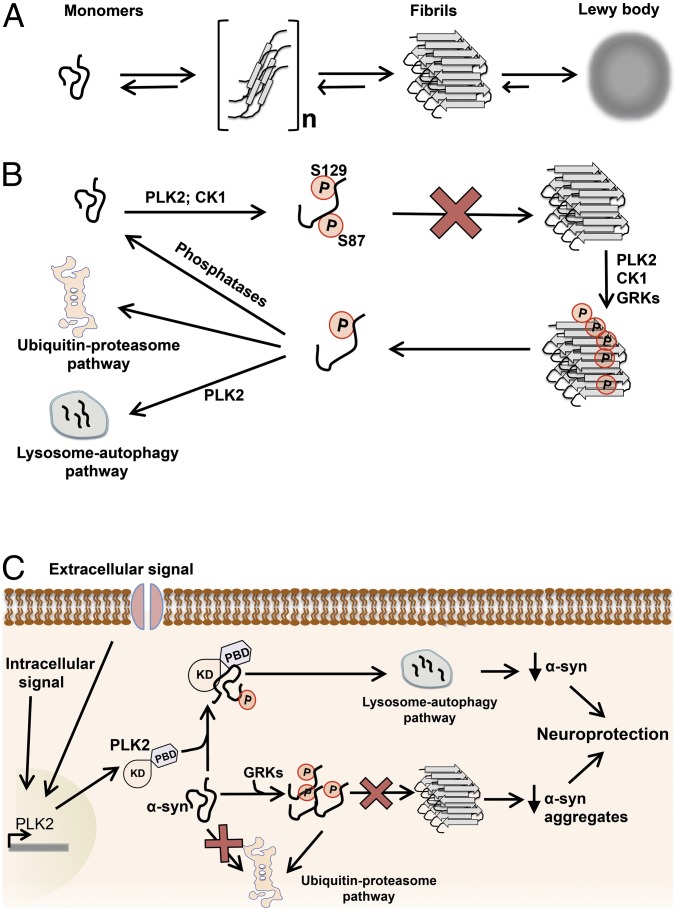
Although recent studies have suggested that phosphorylation at S129 targets α-syn for degradation by the proteasome (34, 35), we report that PLK2-phosphorylated α-syn is mainly degraded by the lysosome–autophagy pathway. This effect depends on PLK2 activity and PLK2/α-syn complex formation. Interestingly, kinase-mediated enhancement of substrate turnover appears to be a characteristic of PLK2 physiological function in vivo, notably the synuclein family. In dividing cells, PLK2 regulates centriole duplication through phosphorylation-mediated degradation of Fbxw7 (36). In nondividing cells and in response to neuronal overactivity, PLK2 interacts, phosphorylates, and targets SPAR for proteasome degradation, resulting in reduction of dendritic spines and preservation of the synaptic homeostasis (24, 37, 38). In a recent study, PLK2 overexpression in vivo promoted ubiquitin-mediated proteasomal degradation of RasGRF1, a member of the Ras superfamily of small GTPases implicated in neuronal plasticity, and induced spine remodeling at the postsynaptic level (39). Collectively, these observations suggest that PLK2 could regulate α-syn protein levels under nonphysiological conditions, such as a neuronal overactivity or abnormal intraneuronal increase of calcium concentration, two conditions where PLK2 expression is up-regulated (Fig. 9) (24, 37).
Fig. 9.
Potential role of phosphorylation in the regulation of α-syn turnover, aggregation, and toxicity. (A) Schematic representation of the α-syn aggregation pathway. A dynamic equilibrium exists between monomeric and mutimeric forms of α-syn (dimers, trimmers, ...). Deregulation of this equilibrium promotes the formation of α-syn fibrils and then the development of abnormal proteinaceous cytoplasmic inclusions, called Lewy bodies. (B) Schematic diagram depicting the role of phosphorylation in regulating α-syn aggregation. PLK2- and CK1-mediated phosphorylation of α-syn, at S129 and S129 + S87, respectively, inhibits α-syn aggregation in vitro and in vivo (25, 32, 52). Moreover, α-syn fibrils could also be directly phosphorylated by PLKs and GRKs at S129 (13) and by CK1 at S87 and S129 (52). Phosphorylation of aggregated α-syn could result in monomer dissociation or stabilize dissociated monomers, and then the soluble phosphorylated α-syn is addressed for degradation via autophagy or proteasome degradation pathways or rapidly dephosphorylated by cytoplasmic phosphatases. (C) Scheme representing the effect of phosphorylation on α-syn aggregation and toxicity in neurons. Accumulated α-syn could be phosphorylated by endogenous kinases, notably GRKs (21, 22), inducing an inhibition of α-syn aggregation and proteasomal degradation of the soluble phosphorylated monomers (34, 35). Under stress conditions (e.g., excitotoxicity) and elevation of endogenous concentration of calcium, PLK2 expression is induced in neurons (24, 37, 38). Overexpressed PLK2 interacts, phosphorylates, and induces autophagic degradation of phosphorylated α-syn. All together, the two phosphorylation-mediated pathways result in a significant reduction of α-syn protein levels and inhibition of α-syn aggregation and consequently protect neurons against α-syn–induced toxicity.
Then, we confirmed that the kinase-mediated α-syn clearance is a unique feature of PLK2, among other α-syn natural kinases. Indeed, our data demonstrated that only PLK2 overexpression, but not GRKs (GRK3, GRK5, and GRK6), induces a decrease of α-syn protein levels in cell culture. Interestingly, analysis of pS129 levels confirmed the superiority of PLK2 in phosphorylating α-syn, suggesting that the specificity of PLK2 in regulating α-syn levels could be, in part, related to its ability to more efficiently phosphorylate α-syn in vivo. Together, these results describe a previously uncharacterized molecular mechanism controlling α-syn protein levels in which PLK2 interacts with α-syn and enhances its autophagic clearance. This mechanism could play an important role in regulating neuronal homeostasis.
PLK2 Overexpression Suppresses α-Syn Toxicity in Rat Midbrain.
The increased levels of PLK2, in aged brains and in Alzheimer’s disease and PD-diseased brains, suggests that up-regulation of this kinase may play an important role in the pathogenesis of synucleinopathies (13, 20). Interestingly, in the present study and under the experimental conditions used, we report that PLK2 (WT or KDM) overexpression, in rat midbrain, did not induce any significant toxicity on the dopaminergic neurons, suggesting that PLK2 accumulation is not toxic per se and may not be deleterious during PD pathogenesis. Previous studies have shown that PLK2 plays important roles in the regulation of postsynaptic homeostasis in response to neuronal overactivity, by remodeling dendritic spine morphology (19, 23, 24, 39), thus suggesting that the observed accumulation of PLK2 in diseased brain could be due to the induction of its expression in adult brain after age-related synaptic dysfunction (40) and/or by the impairment of protein degradation observed in synucleinopathy-diseased brains (41).
Interestingly, when overexpressed with α-syn, PLK2 induces a significant increase of pS129 levels (∼threefold) and protects against α-syn-induced toxicity and motor impairment in vivo. Our findings are in accordance with previous work by Gitler et al., where they identified CDC5, the yeast ortholog of PLK2, as suppressor of α-syn toxicity in a yeast model of PD (42). This observation was also confirmed in rat-midbrain neuronal culture and in a transgenic worm model of PD, in which the overexpression of human PLK2 rescues A53T α-syn–induced neurodegeneration (42). In our rat model, PLK2-mediated neuroprotective effect is correlated with the reduction of intraneuronal levels of human α-syn in the injected midbrains. Together, these data provide a molecular rational for PLK2-mediated suppression of α-syn toxicity and demonstrate that lowering α-syn protein levels under the threshold of neuronal toxicity could be a viable therapeutic strategy for the treatment of synucleinopathies. However, it remains also likely that phosphorylation at S129 further impacts on α-syn properties, notably by inhibiting α-syn aggregation (32), modulating its interaction with cellular membranes (43), and attenuating α-syn–induced trafficking defects between the endoplasmic reticulum and Golgi (44). In addition, we cannot rule out the possible implication of other PLK2 substrates, which could mediate PLK2 neuroprotective effects via α-syn–independent pathways.
Furthermore, our in vivo data may initially appear to contradict previous results, from animal models of PD in which α-syn was overexpressed with members of GRKs, reporting that enhancing pS129 levels exacerbates α-syn toxicity, but the unique interaction between PLK2 and α-syn and selective targeting of α-syn for degradation by PLK2 may explain the discrepancies. In transgenic Drosophila, the coexpression of α-syn with GRK2 enhances α-syn phosphorylation at S129 and accelerates neuronal loss compared with nonphosphorylated α-syn (45). Recently, Sato et al. reported that GRK6 overexpression moderately accelerates A53T α-syn toxicity in an AAV-based rat model of PD, in an α-syn phosphorylation-dependent manner (46). Therefore, clear discrepancies are found between different kinases with respect to their effect on α-syn toxicity in vivo. However, in our study, we also find that a clear difference exists between PLK2 and GRK kinases in the impact of these enzymes on α-syn protein levels in a cell-based assay. Indeed, our data demonstrate that, whereas PLK2 overexpression reduces α-syn protein levels, GRK3 did not affect α-syn protein levels and GRK5 and GRK6 induced further accumulation of α-syn in HEK239T cells (Fig. 2 A and B). This effect could partially explain the discrepancy of the effect of GRK overexpression on α-syn toxicity in vivo, compared with PLK2. Moreover, our data support the hypothesis that this effect could be related to the ability of PLK2 to more efficiently phosphorylate α-syn in vivo.
Potential Implications for the Physiological Function of α-Syn.
Although PLK2 has been reported as the most efficient kinase phosphorylating α-syn at S129 in vitro and in vivo (13–15), the physiological role of PLK2-mediated α-syn phosphorylation remains unknown. In neurons, PLK2 plays an important role in the regulation of postsynaptic density and the preservation of synaptic homeostasis (23, 24, 37–39) whereas α-syn is rather considered to regulate synaptic transmission at the presynaptic level, by modulating the recycling of synaptic vesicles (47) and neurotransmitter release (48, 49). Based on these observations, one could speculate that interactions between these two proteins may play a role in synaptic homeostasis at the presynaptic level by (i) reducing total α-syn level which in turn regulates synaptic vesicle trafficking and neurotransmitter release and/or (ii) modulation of α-syn interaction with other synaptic proteins (50) or its association with membranes (43).
Taken together, these observations suggest that the induction of PLK2 expression/activity, in response to stress conditions (i.e., excitotoxicity), may play an important role in orchestrating global neuronal synaptic homeostasis by modulating the levels and the function of α-syn at the presynaptic compartment and the modulation of postsynaptic scaffolding proteins (SPAR and PSD95) and receptors and remodeling the dendritic spine morphology. Further investigations, notably electrophysiological analysis, are required to confirm this hypothesis.
Implications for PD Pathogenesis and Therapeutic Strategies.
Recent studies suggest PLK2 and pS129 increased levels as age-related risk factors for synucleinopathies (20) and showed that PLK2 and pS129 colocalize in diseased brains. In this context, our data suggest that PLK2-induced enhancement of α-syn turnover could represent a protective strategy against α-syn toxicity by reducing and maintaining the total protein levels under a pathologic threshold. Nevertheless, the impairment of the degradation pathways observed during PD pathogenesis (51) could lead to the decrease of phosphorylated α-syn turnover and the accumulation of pS129 proteins over the disease time course (Fig. 9) (29). Moreover, despite the increased accumulation of pS129 within LBs, our results are consistent with the emerging evidence in support of the hypothesis that S129 phosphorylation is not required for LB formation and toxicity and may instead play an important role in regulating the turnover and physiological properties of the more soluble forms of α-syn (Fig. 9). Several lines of evidence support this hypothesis: (i) phosphorylation at S129 and S87 inhibits α-syn aggregation in vitro and in vivo (25, 32, 52), (ii) phosphorylation at S129 is not required for α-syn aggregation (53), (iii) α-syn fibrils are better substrates for kinases that phosphorylate α-syn at S129 (13, 30) (Fig. 9), and (iv) phosphorylation at S129 regulates α-syn turnover (34), subcellular localization (54), and protein–protein interactions (50). It is also plausible that the increased phosphorylation of α-syn within α-syn aggregates and LBs may reflect an active process whereby phosphorylation of aggregated α-syn enhances their clearance by promoting monomer disassociation and degradation (Fig. 9B).
Collectively, these data suggest that enhancing, rather than inhibiting, PLK2 activity may constitute a more viable and effective therapeutic strategy for the treatment of PD and related disorders, as enhancing S129 phosphorylation results in reduction of α-syn levels and inhibition of its aggregation and toxicity (Fig. 9C). The identification of upstream pathways that regulate PLK2 levels or activity could yield novel therapeutic targets to modulate PLK2 kinase activity and thereby inhibit or reverse the aggregation and toxic activity of α-syn.
Materials and Methods
Plasmid Construction and Production of Recombinant AAV2/6 Viral Vectors.
pEGFP-PLK2 WT, pEGFP-PLK2 pdm, pCMV-PLK2 WT, and pCMV-PLK2 1–334 were kindly provided by Ingrid Hoffmann (German Cancer Research Center, Heidelberg, Germany) (55–57). pCDNA-β-syn was kindly provided by Anita Sidhu (Laboratory of Molecular Neurochemistry, Washington, DC), and pCDNA-myc-SPAR plasmid was kindly provided by Christina Spilker (Leibniz Institute for Neurobiology, Magdeburg, Germany). Human α-syn cDNA was cloned in the pCDNA6 vector (Invitrogen). The primers used to generate the different mutant forms of human α-syn and PLK2 are summarized in Table S1.
To generate plasmids for the production of adeno-associated viral vectors, the cDNAs encoding human PLK2 WT and the kinase dead mutant (KDM) were subcloned in the AAV-CMV-MCS shuttle plasmid (Stratagene), using standard cloning procedures. The cDNA encoding human α-syn was similarly subcloned in the AAV-PGK-MCS plasmid, where the mouse PGK-1 promoter replaces the CMV promoter. The production of the recombinant pseudotyped AAV2/6 vectors (serotype 2 genome/serotype 6 capsid) was performed as described previously (58), and relative infectivity titers were determined as described in ref. 58. A noncoding AAV vector (stuffer) was used as a control. The final viral titers of individual vectors were as follows: Stuffer: 2.66E10 transducing units (TU)/mL; PLK2 WT: 3.79E11 TU/mL; PLK2 KDM: 3.82E11 TU/mL; α-syn WT: 4.49E12 TU/mL and α-syn S129A: 1.6E13 TU/mL.
Stereotaxic Injections.
All surgical and behavioral procedures were performed in accordance with the Swiss legislation and the European Community council directive (86/609/EEC) for the care and use of laboratory animals. The injections were performed under xylazine/ketamine anesthesia as described by Paleologou et al. (52). Female Sprague–Dawley rats (Charles River Laboratories), weighing 180–200 g at the time of surgery, were placed in the stereotaxic frame (David Kopf Instruments) and received a unilateral intranigral injection of 2 µL of viral suspension, which corresponds to a total viral load of 1.5E7 TU (human α-syn or S129A) and 1E7 TU of PLK2 (WT or KDM). In the condition of combined injection, 1.5E7 TU of α-syn (WT or S129A) was coinjected with 1E7 TU of PLK2 WT or KDM. As a control group, α-syn (WT or S129A) was coinjected with 1E7 TU of noncoding vector (Stuffer).
Motor Behavior: Cylinder Test.
The cylinder test was performed to evaluate the motor impairment induced by transgene overexpression by quantifying the akinesia of the contralateral forelimb as described previously in Oueslati et al. (25). Briefly, the rats were placed in a 20-cm Plexiglas cylinder and videotaped for 10–15 min to record their exploratory behavior. A total number of 30 forepaw contacts made on the cylinder wall with the ipsilateral or the contralateral (impaired) forelimbs were scored, and the asymmetry in forelimb activity was expressed as the percentage of contacts made by the left forepaw, contralateral to the site of vector injection, with respect to the total number of forepaw contacts.
Immunohistochemistry.
For immunohistochemistry, the animals were deeply anesthetized and transcardially perfused with 4% (wt/vol) paraformaldehyde (PFA; Fluka, Sigma-Aldrich). Rat brains were removed, postfixed for 2 h in PFA 4%, and then placed in 25% (wt/vol) sucrose for 2 d. Coronal sections (35 µm thick) were cut with a microtome (SM2400; Leica), and the slices were stored at −20 °C in cryoprotection medium. Immunohistochemical analysis was performed as described previously by Paleologou et al. (52). Briefly, slices were incubated with primary antibody, anti-tyrosine hydroxylase (1:500; AB152; Millipore), anti-human α-syn (1:500; LB509; Zymed Laboratories), anti-human α-syn (1:500; 211; Santa Cruz), or anti-human PLK2 (1:500; Santa Cruz) and subsequently incubated with secondary antibodies conjugated to Alexa Fluor-488 or Alexa Fluor-568 (1:1,000; Invitrogen) for immunofluorescence or with biotinylated secondary antibody (1:200; Vector Laboratories) for 3,3′-diaminobenzidine tetrahydrochloride (DAB) revelation (Pierce).
Imaging was performed on a Leica DMI 4000 microscope (Leica LAS software) equipped with an Olympus AX70 camera. The confocal acquisitions were obtained on a Zeiss LSM 700 upright confocal microscope (Zen software).
The α-syn fluorescence intensity within substantia nigra neurons was determined using NIH ImageJ software by analysis of the fluorescence signal at different sections throughout the entire midbrain region of each rat.
Unbiased Stereological Estimation of Dopaminergic Neurons in the SNc.
The number of nigral neurons was estimated using unbiased stereology according to the optical fractionator principle described by West (59). Briefly, the number of TH-immunoreactive neurons was determined every sixth section covering the entire SNc. The analysis was performed using MBF Stereo Investigator software (version 9.14.5; MBF Bioscience). The parameters used for the stereological analysis were as follows: grid size, 200 µm × 180 µm; counting frame, 75 µm × 75 µm; and 2-µm guard zones. The coefficient of error was <0.1.
Cell Culture, Transfection, and Small-Molecule Treatments.
HEK 293T cells were maintained at 37 °C and 5% CO2 in DMEM (Invitrogen) supplemented with 10% (vol/vol) FBS (Invitrogen) and 1% penicillin/streptomycin. Cells were transfected with 1 µg of α-syn plasmid and 0.5 µg or increasing plasmid amount (0.5, 1, and 2 µg) of PLK2 (WT or KDM), using calcium phosphate transient transfection. For DNA titrations, total DNA per transfection was equalized by addition of empty pCDNA vector. Four hours posttransfection, the media were replaced, and cells were incubated for 24 h.
To study the relative implication of the different protein-degradation pathways, 24 h posttransfection, cells were treated for 6 h with ubiquitin–proteasome inhibitors Epoxomicin (50 nM; Sigma-Aldrich) or MG132 (10 µM; Enzo Life Sciences) or with lysosome–autophagy inhibitors 3 Methyladenine (3MA, 10 mM; Sigma-Aldrich) or NH4Cl (25 mM; Sigma-Aldrich). To pharmacologically inhibit PLK2, cells were treated for 6 h with BI2536 (10µM; Sigma). Then, the total protein fraction was collected by harvesting the cells and lysing them in 1× sample loading buffer. After incubation at 95 °C for 15 min, the samples were electrophoresed on 12% SDS/PAGE.
Immunoprecipitation.
Twenty-four hours posttransfection, cells were lysed by sonication in PLK2 phosphorylation buffer (10 mM Tris⋅HCl, pH 7.4, 5 mM EGTA, 2 mM DTT, 25 mM beta-glycerophosphate, 10 mM MgCl2) complemented with Protease inhibitor (1:200), PMSF (1:300), and phosphatase inhibitor mixture 2 and 3 (1:200; Sigma), in the presence or absence of 2 mM ATP (Sigma). After centrifugation, cleared lysate was incubated overnight at 4 °C with anti–α-syn antibody (BD Laboratory). The day after, Deanabeads (Invitrogen) were washed and equilibrated in lysis buffer and then added to the mixture of cell lysate and antibody and incubated overnight at 4 °C under rotation. After three washes in PBS-tween 0.01, beads were incubated with 1× loading buffer at 95 °C for 10 min, and then the samples were electrophoresed on 4–16% SDS/PAGE.
Statistical Analysis.
Statistical analysis was performed using one-way ANOVA followed by the Newman–Keuls post hoc test. Separate analyses were performed between the injected and noninjected side for each group. P < 0.05 was required for rejection of the null hypothesis.
Supplementary Material
Acknowledgments
We thank the Histology Core Facility at the Ecole Polytechnique Fédérale de Lausanne (EPFL) for technical assistance, V. Padrun and F. Pidoux for virus production, and N. Jordan for assistance with plasmid construction. We also thank Prof. Darren Moore, Mr. Mohammed-Bilal Fares, and Dr. Margot Fournier for helpful discussions. This work was supported by the EPFL, Swiss National Science Foundation Grants 315230-125483 (to H.A.L. and A.O.) and 31003A_135696 (to P.A. and B.L.S.), the Michael J. Fox Foundation, and a Marie Curie post-doctoral fellowship (to A.O.). A for-profit corporation, Merck-Serono S.A. partly supported this work (H.A.L. and A.O.).
Footnotes
Conflict of interest statement: This work has been partly supported by Merck Serono S.A., a for-profit corporation.
*This Direct Submission article had a prearranged editor.
See Commentary on page 16293.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309991110/-/DCSupplemental.
References
- 1.Lang AE, Lozano AM. Parkinson’s disease: First of two parts. N Engl J Med. 1998;339(15):1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 2.Lang AE, Lozano AM. Parkinson’s disease: Second of two parts. N Engl J Med. 1998;339(16):1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 3.Obeso JA, et al. Missing pieces in the Parkinson’s disease puzzle. Nat Med. 2010;16(6):653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee VM, Trojanowski JQ. Mechanisms of Parkinson’s disease linked to pathological alpha-synuclein: New targets for drug discovery. Neuron. 2006;52(1):33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Muntané G, Ferrer I, Martinez-Vicente M. α-Synuclein phosphorylation and truncation are normal events in the adult human brain. Neuroscience. 2012;200:106–119. doi: 10.1016/j.neuroscience.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa M, et al. Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J Biol Chem. 2002;277(50):49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4(2):160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JP, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281(40):29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 10.Kahle PJ, et al. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3(6):583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann M, et al. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110(10):1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi M, et al. Phosphorylation of alpha-synuclein characteristic of synucleinopathy lesions is recapitulated in alpha-synuclein transgenic Drosophila. Neurosci Lett. 2003;336(3):155–158. doi: 10.1016/s0304-3940(02)01258-2. [DOI] [PubMed] [Google Scholar]
- 13.Mbefo MK, et al. Phosphorylation of synucleins by members of the Polo-like kinase family. J Biol Chem. 2010;285(4):2807–2822. doi: 10.1074/jbc.M109.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inglis KJ, et al. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem. 2009;284(5):2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvi M, et al. Superiority of PLK-2 as α-synuclein phosphorylating agent relies on unique specificity determinants. Biochem Biophys Res Commun. 2012;418(1):156–160. doi: 10.1016/j.bbrc.2011.12.152. [DOI] [PubMed] [Google Scholar]
- 16.Xie S, Xie B, Lee MY, Dai W. Regulation of cell cycle checkpoints by polo-like kinases. Oncogene. 2005;24(2):277–286. doi: 10.1038/sj.onc.1208218. [DOI] [PubMed] [Google Scholar]
- 17.Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24(2):287–291. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- 18.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24(2):267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 19.Seeburg DP, Pak D, Sheng M. Polo-like kinases in the nervous system. Oncogene. 2005;24(2):292–298. doi: 10.1038/sj.onc.1208277. [DOI] [PubMed] [Google Scholar]
- 20.McCormack AL, Mak SK, Di Monte DA. Increased α-synuclein phosphorylation and nitration in the aging primate substantia nigra. Cell Death Dis. 2012;3:e315. doi: 10.1038/cddis.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pronin AN, Morris AJ, Surguchov A, Benovic JL. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J Biol Chem. 2000;275(34):26515–26522. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto M, et al. Contribution of endogenous G-protein-coupled receptor kinases to Ser129 phosphorylation of alpha-synuclein in HEK293 cells. Biochem Biophys Res Commun. 2009;384(3):378–382. doi: 10.1016/j.bbrc.2009.04.130. [DOI] [PubMed] [Google Scholar]
- 23.Evers DM, et al. Plk2 attachment to NSF induces homeostatic removal of GluA2 during chronic overexcitation. Nat Neurosci. 2010;13(10):1199–1207. doi: 10.1038/nn.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pak DT, Sheng M. Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science. 2003;302(5649):1368–1373. doi: 10.1126/science.1082475. [DOI] [PubMed] [Google Scholar]
- 25.Oueslati A, Paleologou KE, Schneider BL, Aebischer P, Lashuel HA. Mimicking phosphorylation at serine 87 inhibits the aggregation of human α-synuclein and protects against its toxicity in a rat model of Parkinson’s disease. J Neurosci. 2012;32(5):1536–1544. doi: 10.1523/JNEUROSCI.3784-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azeredo da Silveira S, et al. Phosphorylation does not prompt, nor prevent, the formation of alpha-synuclein toxic species in a rat model of Parkinson’s disease. Hum Mol Genet. 2009;18(5):872–887. doi: 10.1093/hmg/ddn417. [DOI] [PubMed] [Google Scholar]
- 27.Gaugler MN, et al. Nigrostriatal overabundance of α-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol. 2012;123(5):653–669. doi: 10.1007/s00401-012-0963-y. [DOI] [PubMed] [Google Scholar]
- 28.McFarland NR, et al. Alpha-synuclein S129 phosphorylation mutants do not alter nigrostriatal toxicity in a rat model of Parkinson disease. J Neuropathol Exp Neurol. 2009;68(5):515–524. doi: 10.1097/NEN.0b013e3181a24b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, et al. Changes in the solubility and phosphorylation of α-synuclein over the course of Parkinson’s disease. Acta Neuropathol. 2011;121(6):695–704. doi: 10.1007/s00401-011-0815-1. [DOI] [PubMed] [Google Scholar]
- 30.Waxman EA, Giasson BI. Characterization of kinases involved in the phosphorylation of aggregated α-synuclein. J Neurosci Res. 2011;89(2):231–247. doi: 10.1002/jnr.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, et al. α-Synuclein disrupts stress signaling by inhibiting polo-like kinase Cdc5/Plk2. Proc Natl Acad Sci USA. 2012;109(40):16119–16124. doi: 10.1073/pnas.1206286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paleologou KE, et al. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008;283(24):16895–16905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson EF, Stewart KD, Woods KW, Giranda VL, Luo Y. Pharmacological and functional comparison of the polo-like kinase family: Insight into inhibitor and substrate specificity. Biochemistry. 2007;46(33):9551–9563. doi: 10.1021/bi7008745. [DOI] [PubMed] [Google Scholar]
- 34.Machiya Y, et al. Phosphorylated alpha-synuclein at Ser-129 is targeted to the proteasome pathway in a ubiquitin-independent manner. J Biol Chem. 2010;285(52):40732–40744. doi: 10.1074/jbc.M110.141952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chau KY, Ching HL, Schapira AH, Cooper JM. Relationship between alpha synuclein phosphorylation, proteasomal inhibition and cell death: Relevance to Parkinson’s disease pathogenesis. J Neurochem. 2009;110(3):1005–1013. doi: 10.1111/j.1471-4159.2009.06191.x. [DOI] [PubMed] [Google Scholar]
- 36.Cizmecioglu O, et al. Plk2 regulates centriole duplication through phosphorylation-mediated degradation of Fbxw7 (human Cdc4) J Cell Sci. 2012;125(Pt 4):981–992. doi: 10.1242/jcs.095075. [DOI] [PubMed] [Google Scholar]
- 37.Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58(4):571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeburg DP, Sheng M. Activity-induced Polo-like kinase 2 is required for homeostatic plasticity of hippocampal neurons during epileptiform activity. J Neurosci. 2008;28(26):6583–6591. doi: 10.1523/JNEUROSCI.1853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KJ, et al. Requirement for Plk2 in orchestrated ras and rap signaling, homeostatic structural plasticity, and memory. Neuron. 2011;69(5):957–973. doi: 10.1016/j.neuron.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 41.Picconi B, Piccoli G, Calabresi P. Synaptic dysfunction in Parkinson’s disease. Adv Exp Med Biol. 2012;970:553–572. doi: 10.1007/978-3-7091-0932-8_24. [DOI] [PubMed] [Google Scholar]
- 42.Gitler AD, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41(3):308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Visanji NP, et al. Effect of Ser-129 phosphorylation on interaction of α-synuclein with synaptic and cellular membranes. J Biol Chem. 2011;286(41):35863–35873. doi: 10.1074/jbc.M111.253450. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Sancenon V, et al. Suppression of α-synuclein toxicity and vesicle trafficking defects by phosphorylation at S129 in yeast depends on genetic context. Hum Mol Genet. 2012;21(11):2432–2449. doi: 10.1093/hmg/dds058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8(5):657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 46.Sato H, et al. Authentically phosphorylated α-synuclein at Ser129 accelerates neurodegeneration in a rat model of familial Parkinson’s disease. J Neurosci. 2011;31(46):16884–16894. doi: 10.1523/JNEUROSCI.3967-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nemani VM, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65(1):66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14(1):38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burré J, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McFarland MA, Ellis CE, Markey SP, Nussbaum RL. Proteomics analysis identifies phosphorylation-dependent alpha-synuclein protein interactions. Mol Cell Proteomics. 2008;7(11):2123–2137. doi: 10.1074/mcp.M800116-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebrahimi-Fakhari D, Wahlster L, McLean PJ. Protein degradation pathways in Parkinson's disease: Curse or blessing. Acta Neuropathol. 2012;124(2):153–172. doi: 10.1007/s00401-012-1004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paleologou KE, et al. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J Neurosci. 2010;30(9):3184–3198. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volpicelli-Daley LA, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schell H, Hasegawa T, Neumann M, Kahle PJ. Nuclear and neuritic distribution of serine-129 phosphorylated alpha-synuclein in transgenic mice. Neuroscience. 2009;160(4):796–804. doi: 10.1016/j.neuroscience.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Krause A, Hoffmann I. Polo-like kinase 2-dependent phosphorylation of NPM/B23 on serine 4 triggers centriole duplication. PLoS ONE. 2010;5(3):e9849. doi: 10.1371/journal.pone.0009849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warnke S, et al. Polo-like kinase-2 is required for centriole duplication in mammalian cells. Curr Biol. 2004;14(13):1200–1207. doi: 10.1016/j.cub.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 57.Cizmecioglu O, Warnke S, Arnold M, Duensing S, Hoffmann I. Plk2 regulated centriole duplication is dependent on its localization to the centrioles and a functional polo-box domain. Cell Cycle. 2008;7(22):3548–3555. doi: 10.4161/cc.7.22.7071. [DOI] [PubMed] [Google Scholar]
- 58.Dusonchet J, Bensadoun JC, Schneider BL, Aebischer P. Targeted overexpression of the parkin substrate Pael-R in the nigrostriatal system of adult rats to model Parkinson’s disease. Neurobiol Dis. 2009;35(1):32–41. doi: 10.1016/j.nbd.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 59.West MJ. Stereological methods for estimating the total number of neurons and synapses: Issues of precision and bias. Trends Neurosci. 1999;22(2):51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.