Significance
Estrogen deficiency after menopause frequently accelerates osteoclastic bone resorption, leading to osteoporosis, the most common skeletal disorder in women. However, mechanisms underlying osteoporosis resulting from estrogen deficiency remain largely unknown. We report a unique mechanism underlying postmenopausal osteoporosis, and a therapeutic target, hypoxia-inducible factor 1 alpha (HIF1α), to treat this condition. HIF1α is unstable in the presence of oxygen, but is stabilized under hypoxic conditions. However, we found that HIF1α is destabilized by estrogen even under hypoxic conditions. Following estrogen deficiency due to menopause, HIF1α is stabilized in osteoclasts, leading to osteoclast activation. Oral administration of a HIF1α inhibitor protected postmenopausal osteoporosis model mice from osteoclast activation and bone loss. Thus, HIF1α represents a promising therapeutic target in osteoporosis.
Abstract
In women, estrogen deficiency after menopause frequently accelerates osteoclastic bone resorption, leading to osteoporosis, the most common skeletal disorder. However, mechanisms underlying osteoporosis resulting from estrogen deficiency remain largely unknown. Here we show that in bone-resorbing osteoclasts, estrogen-dependent destabilization of hypoxia-inducible factor 1 alpha (HIF1α), which is unstable in the presence of oxygen, plays a pivotal role in promoting bone loss in estrogen-deficient conditions. In vitro, HIF1α was destabilized by estrogen treatment even in hypoxic conditions, and estrogen loss in ovariectomized (Ovx) mice stabilized HIF1α in osteoclasts and promoted their activation and subsequent bone loss in vivo. Osteoclast-specific HIF1α inactivation antagonized bone loss in Ovx mice and osteoclast-specific estrogen receptor alpha deficient mice, both models of estrogen-deficient osteoporosis. Oral administration of a HIF1α inhibitor protected Ovx mice from osteoclast activation and bone loss. Thus, HIF1α represents a promising therapeutic target in osteoporosis.
Bone mass is tightly regulated by a delicate balance between osteoblastic bone formation and osteoclastic bone resorption. Estrogen loss in women after menopause frequently promotes activation of osteoclastic bone resorption, causing osteoporosis. Osteoporotic bone phenotypes are seen in ovariectomized female mice, and estrogen deficiency-induced bone loss in both mouse models and women is ameliorated by estrogen treatment (1, 2). However, estrogen administration reportedly increases risk of cardiovascular events and carcinogenesis of the mammary gland and uterus (3). Bioavailable estrogens including selective estrogen receptor modulators (SERMs) also protect bone from estrogen deficiency-induced osteoporosis (4), and estrogen and SERMs primarily act via estrogen receptors (ER), ERα and ERβ (5, 6). However, how SERMs act on bone remains largely unknown. Thus, understanding of osteoclast activation following estrogen loss is crucial for development of safe therapeutic reagents.
Both the endosteal zone of bone marrow cavities and epiphyseal growth plates are hypoxic areas, and the hypoxia-inducible factor (HIF) signaling pathway governs chondrocyte and osteoblast function in these respective areas (7, 8). The HIF1 transcription factor consists of an oxygen-regulated alpha subunit, HIF1α, and a constitutively expressed beta subunit, HIF1β. Under normoxia, HIF1α is posttranslationally modified by prolyl hydroxylases, which catalyze hydroxylation of proline residues in the presence of O2 and Fe2+. Recognition of hydroxylated HIF1α by the von Hippel-Lindau tumor suppressor protein recruits an E3 ubiquitin ligase complex targeting HIF1α for proteasomal degradation. Conversely, under hypoxia, proline hydroxylation is inhibited by substrate (O2) deprivation, allowing HIF1α accumulation and formation of an active transcriptional complex with HIF1β (9). Recently, regulation of HIF1α protein levels by factors other than O2, including reactive oxygen species and growth factors, has been reported (10), although hormonal HIF1α regulation has not been characterized.
HIF1α plays specific roles in vasculogenesis and tumorigenesis (11). Stabilization and activation of HIF1α in an avascular area due to hypoxia promotes vascularization by inducing typical HIF1α target genes, such as vascular endothelial growth factor (VEGF) (12). Such neovascularization through HIF1α-induced VEGF is activated in tumor growth, ischemic collateral formation, and wound healing (12–14). HIF1α is also reportedly activated and functions in tumor development in renal cell carcinoma (15).
Here we show that HIF1α is suppressed by estrogen in osteoclasts and that loss of estrogen stabilizes HIF1α in those cells, which in turn activates bone resorption and promotes bone loss. Inactivating HIF1α, either genetically or pharmacologically, protected mice from estrogen depletion and concomitant osteoclast activation and bone loss. Our observations confirm HIF1α as a potent therapeutic target for osteoporosis.
Results
Estrogen Depletion Stabilizes HIF1α in Osteoclasts in Vivo.
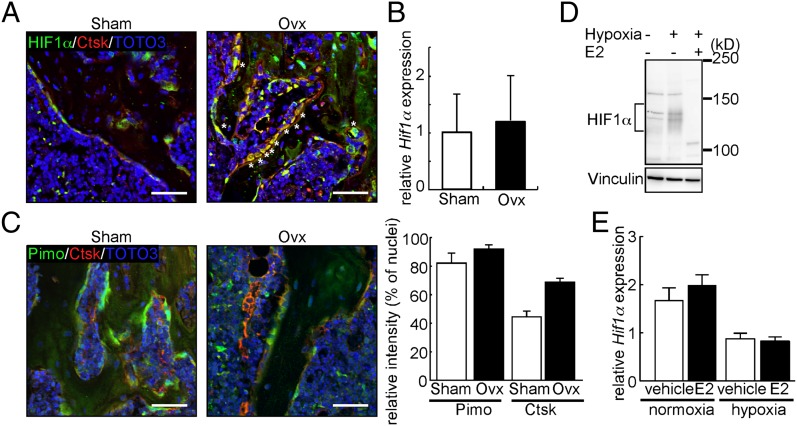
HIF1α is reportedly stabilized in hypoxic regions such as the endosteal zone of bone marrow cavities and growth plates (16). Indeed, immunofluorescence analysis indicated that HIF1α was stabilized in chondrocytes of the mouse growth plate (Fig. S1). By contrast, we detected lower levels of HIF1α protein in Cathepsin K (Ctsk)-positive osteoclasts localized to the hypoxic endosteal zone of sham-operated (Sham) mice compared with growth plate chondrocytes (Fig. 1A). However, ovariectomy (Ovx), which results in estrogen depletion, induced HIF1α stabilization in osteoclasts (Fig. 1A). Osteoclasts are reportedly c-Fms+Mac1high (17); interestingly, Hif1α expression levels in sorted c-Fms+Mac1high osteoclasts were comparable between Sham and Ovx mice (Fig. 1B), further suggesting that estrogen depletion stabilizes HIF1α protein rather than inducing Hif1α mRNA in osteoclasts. Assessment of hypoxia using pimonidazole (Pimo) (18), which detects low oxygen tension areas, showed that the hypoxic state was comparable in the endosteal zone of Sham and Ovx mouse bones (Fig. 1C). These results suggest that estrogen deficiency stabilizes HIF1α in osteoclasts without altering hypoxic conditions in the endosteal zone. In contrast, the uterus is an estrogen-responsive tissue; however, HIF1α protein was not stabilized in the uterus following Ovx (Fig. S2), suggesting that HIF1α protein stabilization following estrogen deficiency is tissue-specific.
Fig. 1.
Estrogen destabilizes HIF1α in vivo and in vitro. (A) Immunofluorescence for HIF1α (green) and Ctsk (red) in bone sections from Sham or Ovx mice. Nuclei are stained with TOTO-3 (blue). *, HIF1α/Ctsk double-positive cells. (B) Hif1α mRNA levels in c-Fms+Mac1high osteoclasts sorted from Sham or Ovx mouse bone marrow as determined by quantitative PCR. Cells were prepared 5 wk after surgery. (C, Left) Immunofluorescent detection of hypoxic areas (Pimo, green) and Ctsk (red) on bone sections from Sham or Ovx mice. Nuclei are stained with TOTO-3 (blue). (Scale bar, 100 μm.) (C, Right) Signal intensities of Pimo and Ctsk shown as mean values relative to TOTO-3 ± SD (n = 3). (D) Western blot analysis of HIF1α in Raw264.7 cells cultured with or without E2. (E) Hif1α mRNA levels in Raw264.7 cells as determined by quantitative PCR in the presence or absence of E2 under normoxic or hypoxic conditions. Data in B and E represent mean Hif1α expression relative to Actb ± SD (n = 3).
To confirm estrogen-dependent HIF1α destabilization in osteoclasts, we used immunoblotting to detect HIF1α in an osteoclast progenitor cell line, Raw264.7 cell, and primary osteoclasts derived from wild-type mouse bone marrow cells treated with or without the β-estradiol (E2) in vitro (Fig. 1D and Fig. S3). Under hypoxic conditions, HIF1α protein was stabilized in the absence of E2 but destabilized by treatment with E2 (Fig. 1D and Fig. S3). Quantitative PCR analysis showed that Hif1α was induced in osteoclasts over the course of differentiation stimulated by receptor activator of nuclear factor kappa B ligand (RANKL), while the expression of Hif2α, a different hypoxia-inducible transcription factor, was not detected (Fig. S4). Hif1α mRNA levels were unchanged in osteoclasts treated with E2 in both hypoxia and normoxia (Fig. 1E), strongly suggesting that it is HIF1α protein levels that are destabilized by E2, even in hypoxic conditions.
Osteoclast-Specific HIF1α Deletion Protects Bones from Ovx-Induced Osteoporosis.
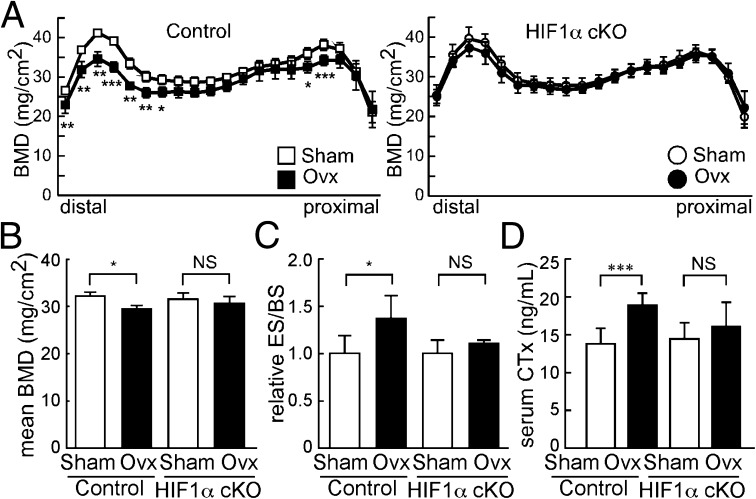
To analyze the role of E2-dependent HIF1α protein destabilization in osteoclasts in vivo, we generated osteoclast-specific HIF1α conditional knockout mice (HIF1α cKO: CtskCre/+; Hif1αf/f) by crossing Ctsk-Cre mice, in which Cre recombinase was knocked into Ctsk gene locus, with Hif1α-floxed mice (Fig. 2). HIF1α cKO mice exhibit little change in bone mineral density (BMD), as measured by dual energy X-ray absorptiometry (DEXA) compared with control (Sham) mice (Fig. 2 A and B). Osteoclast formation detected by tartrate resistance acid phosphatase (TRAP) staining was normal in HIF1α cKO mice (Fig. S5A). Bone morphometric analysis demonstrated that parameters such as bone volume per tissue volume (BV/TV), osteoblast surface per bone surface (Ob.S/BS), osteoclast surface per bone surface (Oc.S/BS), and bone formation rate per bone surface (BFR/BS) did not differ between HIF1α cKO and control mice (Fig. S5B). However, although Sham HIF1α cKO mice exhibited few bone phenotypes in vivo (Fig. 2A and Fig. S5 A and B), they were protected from Ovx-induced bone loss (Fig. 2 A and B). Osteoclast progenitor cells were isolated from HIF1α cKO and control mouse bone marrow and cultured in the presence of M-CSF and RANKL (Fig. S5C). Under normoxic conditions, we observed no difference in osteoclastogenesis between HIF1α cKO and control progenitors in vitro (Fig. S5C). However, under hypoxic culture conditions, expression of osteoclastic marker Ctsk was inhibited in HIF1α cKO compared with control osteoclasts (Fig. S5D). The elevated expression level was down-regulated by E2 in control but not in HIF1α cKO osteoclasts cultured in hypoxic conditions (Fig. S5D). Bone morphometric analysis demonstrated that accelerated osteoclastogenesis seen in control mice following Ovx was not evident in HIF1α cKO mice in vivo (Fig. 2C). Serum levels of CTx, a marker of bone resorption, were elevated in control but not in HIF1α cKO mice following Ovx (Fig. 2D). These results suggest that HIF1α promotes bone loss under estrogen-depleted conditions and its suppression by estrogen protects bones by inhibiting osteoclast activation.
Fig. 2.
HIF1α cKO mice are resistant to ovariectomy-induced bone loss and osteoclast activation. (A and B) BMD of femurs divided equally longitudinally from Sham or Ovx control (Hif1αf/f) and HIF1α cKO (CtskCre/+; Hif1αf/f) mice. (C) ES/BS of femurs from sham-operated or ovariectomized control and HIF1α cKO mice. (D) Levels of serum CTx, a marker of bone resorption, in control and HIF1α cKO mice. Analysis was undertaken 5 wk after Ovx or Sham surgery. All data are means ± SD (n = 5). *P < 0.05; **P < 0.01; ***P < 0.005; NS, not significant.
Estrogen-Dependent Osteoprotection Requires ERα in Vitro and in Vivo.
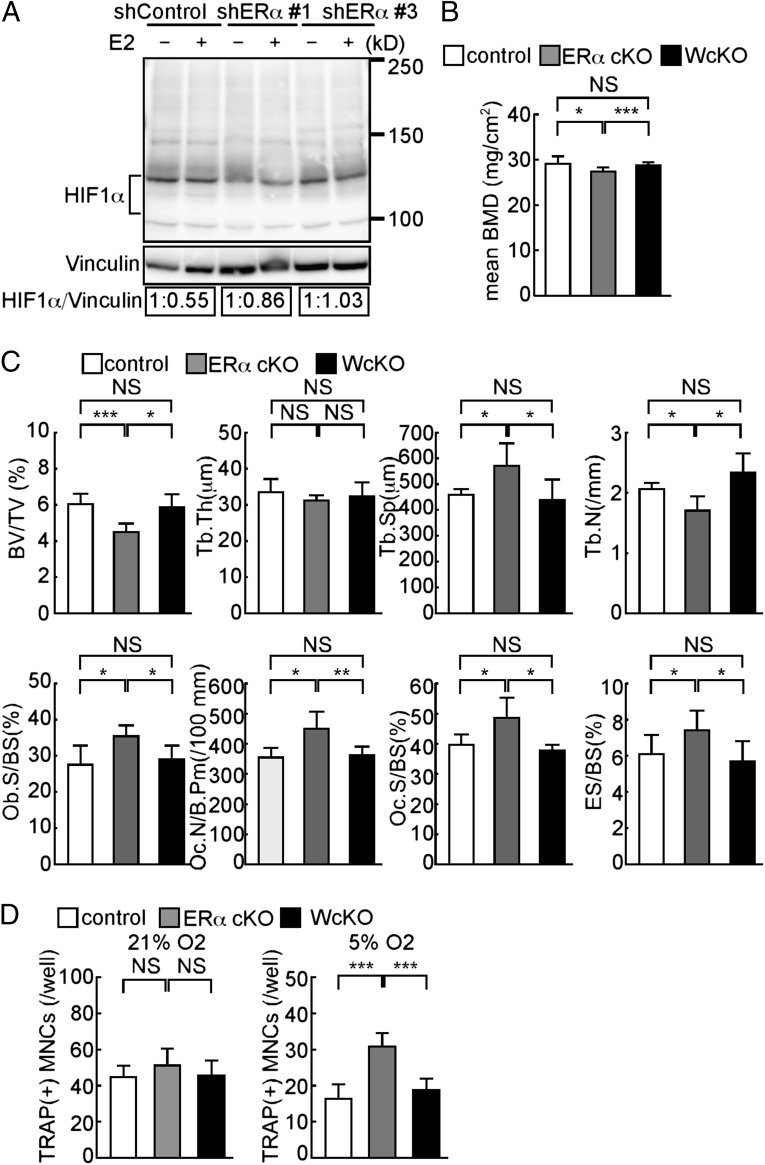
To determine whether ERs were required for HIF1α destabilization in osteoclasts, we obtained two independent shRNAs that effectively targeted ERα (Fig. S6). ERα knockdown in osteoclasts prevented E2-dependent HIF1α suppression, as detected by immunoblotting of extracts from ERα knockdown cell lines (Fig. 3A), indicating that ERα in osteoclasts is required for E2-induced HIF1α decrease.
Fig. 3.
HIF1α deletion rescues bone phenotypes seen in ERα cKO mice in vivo. (A) Raw264.7 cells transfected with shRNA targeting ERα (shERα) or nontarget control shRNA (shControl) and treated with (+) or without (−) E2. HIF1α protein levels determined by immunoblot were quantified by densitometry and are shown as values relative to E2(−). (B) BMD of femurs divided equally longitudinally from control (CtskCre/+), ERα cKO (CtskCre/+; ERαf/f), and WcKO (CtskCre/+; Hif1αf/f; ERαf/f) mice. (C) Bone histomorphometrical analysis of femurs from control, ERα cKO, and WcKO female mice. (D) Osteoclast formation, as evidenced by TRAP positivity, of osteoclast progenitor cells from control, ERα cKO, and WcKO mouse bone marrow under normoxic (21%) or hypoxic (5%) conditions during the course of RANKL-stimulated differentiation. Data (B–D) represent the mean value of the indicated parameter ± SD (*P < 0.05; ***P < 0.005; NS, not significant; n = 5). White, gray, and black bars represent control, ERα cKO, and WcKO mice, respectively.
Osteoclast-specific ERα conditional knockout mice (ERα cKO: CtskCre/+; ERαf/f), which mimic the estrogen-depleted conditions in osteoclasts, are reportedly osteoporotic without Ovx (19). To further assess HIF1α function in estrogen loss-induced osteoporosis, we analyzed bone density by DEXA in osteoclast-specific HIF1α and ERα double conditional knockout mice (WcKO: CtskCre/+; Hif1αf/f; ERαf/f). Significantly, decreased BMD seen in ERα cKO mice relative to controls was rescued in WcKO mice (Fig. 3B). In addition, bone morphometric analysis demonstrated that reduced BV/TV, trabecular number (Tb.N), and increased trabecular separation (Tb.Sp) seen in ERα cKO were restored to basal levels in WcKO mice (Fig. 3C). Indicators of increased osteoclastogenesis seen in ERα cKO mice, such as osteoclast number per bone perimeter (Oc.N/B.Pm), Oc.S/BS, and erosion surface per bone surface (ES/BS), were negated in WcKO mice (Fig. 3C), indicating that estrogen/ERα-dependent HIF1α destabilization antagonizes acceleration of osteoclast activation and bone loss. In ERα cKO cells grown in vitro, osteoclastogenesis was not facilitated in normoxia but was significantly stimulated in hypoxic conditions, as evidenced by TRAP staining (Fig. 3D). Furthermore, marker analysis indicated that accelerated osteoclastogenesis seen in ERα cKO cells under hypoxia was inhibited in WcKO cells (Fig. 3D). These results indicate that ERα is required in osteoclasts for HIF1α stability shifts seen in the presence of estrogen. HIF1α protein is reportedly targeted by the ubiquitin-proteasome system (9). To elucidate molecular mechanisms underlying estrogen-induced HIF1α destabilization, we treated Raw264.7 cells with inhibitors of serine proteases, cysteine proteases, calpains, or the proteasome in the presence of E2 under hypoxic conditions. However, none of these inhibitors blocked estrogen-induced destabilization of HIF1α (Fig. S7). These results suggest that estrogen-induced HIF1α destabilization is mediated via a unique mechanism.
Administration of a HIF1α-Inhibitor Protects Bone from Ovx-Induced Osteoporosis.
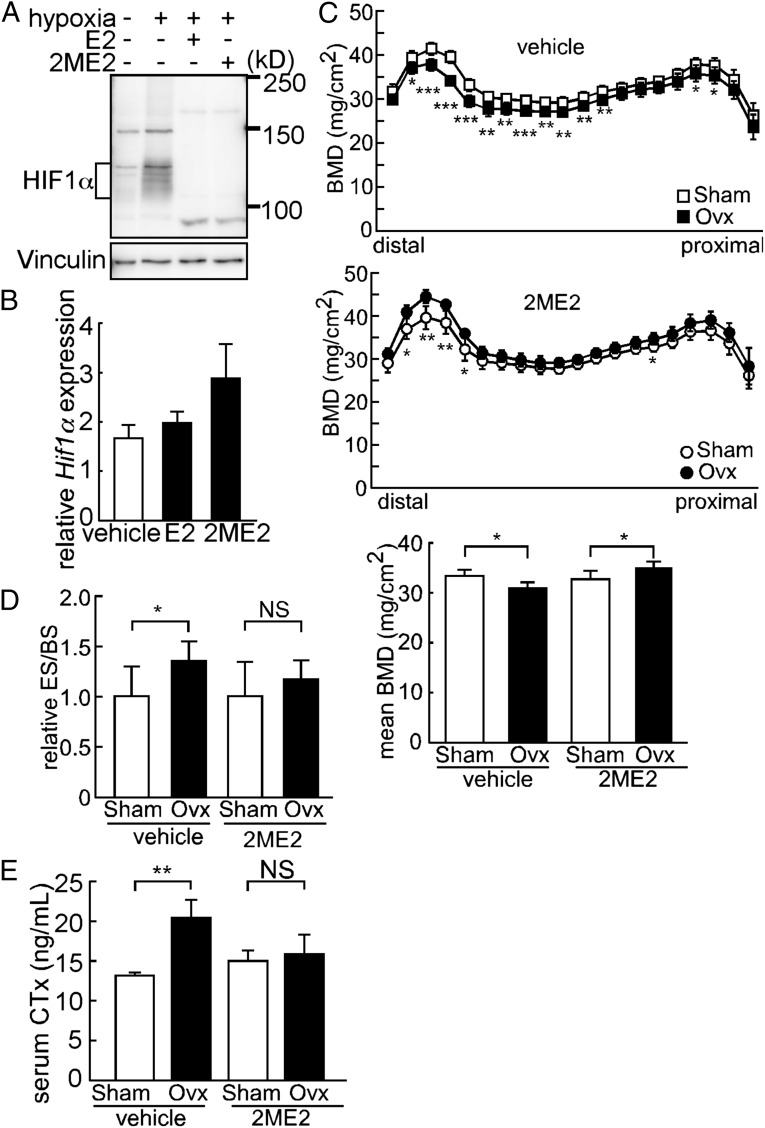
Because HIF1α stabilization following Ovx-induced estrogen deficiency is predicted to promote bone loss, we evaluated phenotypes seen in cultured cells and Ovx mice following treatment with 2-methoxyestradiol (2ME2), a specific inhibitor of HIF1α and an antitumor chemotherapeutic agent (Fig. 4). The 2ME2 reportedly down-regulates HIF1α posttranscriptionally (20), and indeed, immunoblotting indicated that 2ME2 treatment of cultured osteoclasts decreased HIF1α protein in hypoxic conditions similarly to E2 (Fig. 4A) without altering Hif1α mRNA expression (Fig. 4B). In vivo, pharmacological inhibition of HIF1α following oral administration of 2ME2 did not increase BMD in Sham mice; interestingly, however, BMD was greater in 2ME2-treated Ovx mice compared with similarly treated Sham mice (Fig. 4C). Significantly increased osteoclast activation, as evidenced by elevated ES/BS and serum CTx in Ovx compared with Sham mice, was suppressed to basal levels by 2ME2 administration (Fig. 4 D and E). The 2ME2 treatment of Ovx mice resulted in increased numbers of apoptotic cells, as detected by a TUNEL assay, suggesting that stabilization of HIF1α in osteoclasts following estrogen deficiency functions to block osteoclast apoptosis (Fig. S8).
Fig. 4.
Pharmacological HIF1α ablation antagonizes Ovx-induced bone loss and osteoclast activation. (A) Suppression of HIF1α protein expression in Raw264.7 cells by E2 or 2ME2 in hypoxic conditions. (B) Effects of E2 or 2ME2 on Hif1α mRNA levels in Raw264.7 cells. Data represent mean Hif1α expression relative to that of Actb ± SD (n = 5). (C, Top, Middle, and Bottom) BMD of femurs from Sham or Ovx treated with vehicle or 2ME2. (D) ES/BS of femurs from Sham or Ovx treated with vehicle or 2ME2. (E) Serum CTx of Sham or Ovx mice treated with vehicle or 2ME2. Error bars indicate means ± SD (n = 5). *P < 0.05; **P < 0.01; ***P < 0.005; NS, not significant.
Overall, results obtained in vitro and in vivo indicate that HIF1α protein is stabilized by deficiencies in estrogen signaling and position HIF1α as a promising potential therapeutic target for osteoporosis.
Discussion
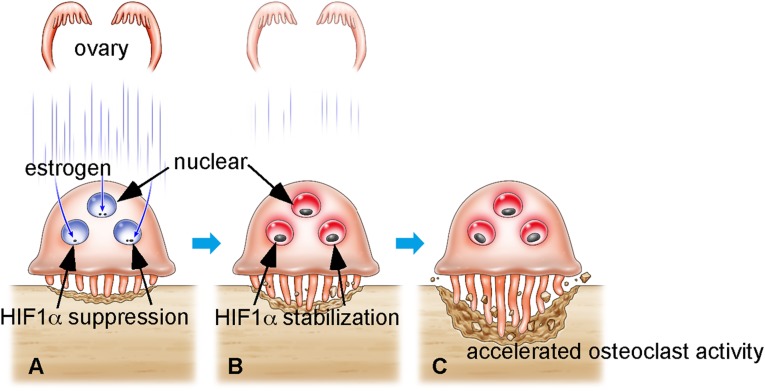
This study demonstrates that HIF1α stabilization in osteoclasts by estrogen loss promotes osteoclast activation followed by increased bone resorption and decreased bone mass (Fig. 5). HIF1α reportedly potentiates bone formation in vivo by promoting osteoblast activation, as demonstrated by an osteoblast-specific HIF1α knockout (8). However, in our study, global HIF1α inhibition in mice following administration of a HIF1α inhibitor does not reduce bone mass. HIF1α cKO mice exhibited maintenance of bone mass under estrogen-depleted conditions by Ovx. These findings reveal that HIF1α can be used as a target for osteoprotection in postmenopausal osteoporosis. Small molecule screens to identify antiosteoporosis drugs have been carried out using Ovx animals. However, now, in vitro screening based on assessment of HIF1α stabilization in osteoclasts under hypoxia could serve as a method to screen effective compounds.
Fig. 5.
Schematic model of postmenopausal osteoporosis. (A) Osteoclasts are localized in the endosteal zone, where oxygen levels are low. Even in hypoxic conditions, HIFα protein is continuously destabilized by estrogen in osteoclasts. (B) After menopause or Ovx, HIFα is stabilized in osteoclasts due to estrogen depletion. (C) HIFα stabilization in osteoclasts promotes osteoclast activation, which promotes reduced bone mass and osteoporosis.
HIF1α stabilization in cells is tightly controlled by oxygen levels. Although osteoclasts localize in the endosteal zone where it is hypoxic, HIF1α in osteoclasts is continuously destabilized by estrogen in an ERα-dependent manner (Fig. 5). HIF1α destabilization is reportedly mediated by the ubiquitin-proteasome system under normoxic conditions (9). Our results demonstrate that ERα plays a crucial role in decreasing HIF1α protein levels in osteoclasts, even in hypoxic conditions. Because various protease inhibitors were ineffective in inhibiting HIF1α destabilization by estrogen (Fig. S7), ERα-mediated HIF1α destabilization likely occurs via a unique mechanism regulating HIF1α posttranscriptionally.
HIF1α transcriptionally regulates a variety of hypoxia-responsive genes, including VEGF (21). We found that osteoclast formation was stimulated in ERα cKO cells (a condition in which HIF1α is stabilized), and is blocked by Hif1α-deficiency in hypoxic conditions, indicating that HIF1α functions in osteoclast formation likely through various hypoxia-responsive genes.
In bone, SERMs act as ERα agonists and mimic estrogen activity (6), and HIF1α in osteoclasts is likely a target of SERMs. In other tissues, HIF1α functions in a wide variety of biological activities such as vasculogenesis and tumorigenesis (11–14). Estrogen is also implicated in cardiovascular events such as infarction and arteriosclerosis, and prolonged estrogen exposure promotes carcinogenesis in the mammary gland and uterus (3). Taken together, our findings indicate that HIF1α is closely regulated by the estrogen signaling in osteoclasts, and represents a promising therapeutic target to treat postmenopausal osteoporosis. Identification of estrogen-mediated HIF1α regulation will contribute to our future understanding of molecular mechanisms underlying diseases involving estrogen and HIF1α.
Materials and Methods
Mice.
Osteoclast-specific inactivation of Hif1α in mice (HIF1α cKO: CtskCre/+; Hif1αf/f) was carried out by intercrossing the mice harboring Cre in the Cathepsin K locus (CtskCre/+) with mice homozygous for a floxed Hif1α allele (7, 19). To generate mice lacking both Hif1α and ERα in osteoclasts (WcKO: CtskCre/+; Hif1αf/f; ERαf/f), we first crossed HIF1α cKO mice with mice homozygous for a floxed ERα allele and then interbred the offspring for three generations. Genotyping was performed as previously described (7, 22). Female HIF1α cKO and control littermates (Hif1αf/f) were ovariectomized or sham-operated at 12 wk of age, and subjected to bone analyses at 6 wk after operation. Animals were maintained under specific pathogen-free conditions in animal facilities certified by the Keio University School of Medicine Animal Care Committee. All procedures involving mice were approved by the Keio University School of Medicine Animal Care Committee.
Cell Culture.
For in vitro osteoclast formation, bone marrow cells isolated from femurs and tibias were cultured for 72 h in αMEM (Sigma-Aldrich Co.) containing 10% (vol/vol) heat-inactivated FBS (JRH Biosciences) and GlutaMax (Invitrogen Corp.) supplemented with M-CSF (50 ng/mL, Kyowa Hakko Kirin Co.). Subsequently, adherent cells were collected and cultured under indicated conditions containing M-CSF (50 ng/mL), recombinant soluble RANKL (25 ng/mL, PeproTech Ltd.) for 1 × 105 cells per well in 96 well plates. Osteoclastogenesis was evaluated by May-Grüenwald Giemsa and TRAP staining (17, 23). Raw264.7 cell was maintained in DMEM (Sigma-Aldrich Co.) containing 10% (vol/vol) heat-inactivated FBS (JRH Biosciences) and GlutaMax (Invitrogen Corp.). For chemical treatment, cells were cultured in phenol red-free media containing 10% (vol/vol) charcoal-stripped FBS (Thermo Fisher Scientific K.K.), and treated by 17β-estradiol (E2; Wako Pure Chemicals Industries, 10−6 M) or 2-methoxyestradiol (2ME2; EMD Millipore Corporation, 10−5 M). Hypoxic culture was performed at 5% O2 and 5% CO2 using an INVIVO2 hypoxia workstation (Ruskin Technology Ltd.) according to the manufacturer’s instructions. Protease inhibitor treatment was carried out under hypoxic conditions in the presence of E2 using 4-(2-aminoethyl)-benzenesulfonyl fluoride as a serine protease (AEBSF; Wako Pure Chemicals Industries, 10−4 M), E-64d as a cysteine protease (Wako Pure Chemicals Industries, 10−5 M), ALLN as a calpain (EMD Millipore Corporation, 10−5 M) inhibitor, or MG-132 as a proteasome (EMD Millipore Corporation, 10−5 M) inhibitor.
Quantitative PCR Analysis.
Total RNAs were isolated from bone marrow cultures by TRIzol reagent (Invitrogen Corp.), and cDNA synthesis was done by using oligo(dT) primer and reverse transcriptase (Wako Pure Chemicals Industries). Quantitative PCR was performed using SYBR Premix ExTaq II reagent and the DICE Thermal cycler (Takara Bio Inc.), according to the manufacturer’s instructions. Samples were matched to a standard curve generated by amplifying serially diluted products using the same PCR reactions. β-actin (Actb) expression served as an internal control. Primers for Nfatc1, Ctsk, Dc-stamp, and Actb were described previously (24). Other primers were purchased from Takara Bio Inc.
Immunoblotting.
Whole cell lysates were prepared using radio immunoprecipitation assay (RIPA) buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 5 mM EDTA, 1 mM DTT, 10 mM Tris-HCl, pH7.5) supplemented with a protease inhibitor mixture (Sigma-Aldrich Co.) and MG-132 (EMD Millipore Corporation), and the insoluble fraction was removed by centrifugation followed with Bradford protein analysis (Bio-Rad Laboratories Inc.). Equivalent amounts of protein were separated by SDS/PAGE and transferred to a PVDF membrane (EMD Millipore Corporation). To quantify immunoblotting and control for nonequivalent protein loading, we used densitometric analysis using ImageJ software. Proteins were detected using the following antibodies: anti-HIF1α (Novus Biologicals), anti-ERα (Santa Cruz Biotechnology, Inc), and anti-Vinculin and anti-Tubulin (Sigma-Aldrich Co.).
Immunohistochemical Analysis.
Mouse femurs were dissected, fixed in 10% buffered formalin, decalcified in 8% Na2EDTA, paraffin-embedded, and subjected to immunohistochemistry. Antigen retrieval was performed by treatment with 0.4 mg/mL Proteinase K for 8 min at room temperature. Sections were incubated in 5% BSA-PBS and then incubated with antibodies against HIF1α (Novus Biologicals) and Ctsk (Daiichi Finechemical Co.). Nuclei were counterstained using TOTO-3 (Invitrogen Corp.). Detection of low oxygen tension areas in tissue was performed using pimonidazole (Hypoxyprobe-1 Kit; Hypoxyprobe Inc.) according to the manufacturer’s instructions. A confocal laser scanning microscope (FV1000; OLYMPUS Corp.) was used to examine immunostained sections. Quantification of signal intensity was performed by using operating software for FV1000 according to the manufacturer’s manual.
ERα Knockdown.
Raw264.7 transformants were generated using MISSION shRNA lentiviruses targeting ERα or nontarget control constructs (Sigma-Aldrich Co.) according to the manufacturer’s instructions.
2ME2 Treatment.
Wild-type C57BL/6 mice were obtained from CLEA Japan, Inc., and subjected to ovariectomy or sham operation at 12 wk old, immediately followed by 2ME2 (EMD Millipore Corporation) treatment. The 2ME2 was prepared at 15 mg/mL in a 0.5% methylcellulose solution (Wako Pure Chemicals Industries) and administered daily at 75 mg/kg via intragastric infusion for 4 wk. Vehicle groups were treated with solvent.
Analysis of Skeletal Morphology.
Sham or Ovx HIF1α cKO and control littermates were necropsied, and their hind limbs were removed, fixed with 70% ethanol, and subjected to DEXA analysis to measure bone mineral density and for bone histomorphometric analysis. Bones were collected from female HIF1α cKO, ERα cKO, and control littermates 8 wk of age. Mice treated with 2ME2 or vehicle were used for bone analysis at the end of drug administration. Mice were additionally administered 16 mg/kg calcein (Dojindo Co.) injected intraperitoneally at 6 and 1 d before sacrifice to evaluate bone formation rate.
TUNEL Staining.
Paraffin-embedded sections of decalcified femurs from Sham or Ovx mice were subjected to TUNEL staining. Experiments were carried out using a MEBSTAIN Apoptosis TUNEL Kit (MBL Co. Ltd.) following the manufacturer’s instructions. After staining, sections were reacted with an anti-Ctsk antibody and TOTO-3.
ELISA.
C-terminal telopeptide of type I collagen (CTx) serum levels were measured using a RatLaps EIA kit (Immunodiagnostic Systems Ltd.) based on the manufacturer’s instructions.
Statistical Analyses.
Statistical analyses were performed using the unpaired two-tailed Student’s t test (*P < 0.05; **P < 0.01; ***P < 0.005; NS, not significant, throughout the paper). All data are expressed as the mean ± SD.
Supplementary Material
Acknowledgments
We thank Prof. M. Suematsu and Dr. Y. A. Minamishima for critical reading of the manuscript. T. Miyamoto was supported by a Grant-in-Aid for Scientific Research, the Takeda Science Foundation. Y.M. was supported by a Grant-in-Aid for Scientific Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308755110/-/DCSupplemental.
References
- 1.Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002;359(9322):2018–2026. doi: 10.1016/S0140-6736(02)08827-X. [DOI] [PubMed] [Google Scholar]
- 2.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289(5484):1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 3.Nelson ER, Wardell SE, McDonnell DP. The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: Implications for the treatment and prevention of osteoporosis. Bone. 2013;53(1):42–50. doi: 10.1016/j.bone.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—Mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 5.Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 6.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295(5564):2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 7.Schipani E, et al. Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15(21):2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, et al. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117(6):1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh MY, Spivak-Kroizman TR, Powis G. HIF-1 regulation: Not so easy come, easy go. Trends Biochem Sci. 2008;33(11):526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Ryan HE, et al. Hypoxia-inducible factor-1α is a positive factor in solid tumor growth. Cancer Res. 2000;60(15):4010–4015. [PubMed] [Google Scholar]
- 12.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor—HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19(1):90–97. doi: 10.2174/092986712803413944. [DOI] [PubMed] [Google Scholar]
- 13.Liao D, Johnson RS. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26(2):281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 14.Andrikopoulou E, et al. Current insights into the role of HIF-1 in cutaneous wound healing. Curr Mol Med. 2011;11(3):218–235. doi: 10.2174/156652411795243414. [DOI] [PubMed] [Google Scholar]
- 15.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12(1):9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol. 2012;8(6):358–366. doi: 10.1038/nrrheum.2012.36. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto T, et al. An adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor kappa B ligand. Blood. 2000;96(13):4335–4343. [PubMed] [Google Scholar]
- 18.Fujita N, et al. Vascular endothelial growth factor-A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem Biophys Res Commun. 2008;372(2):367–372. doi: 10.1016/j.bbrc.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Mabjeesh NJ, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3(4):363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 21.Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors. Exp Eye Res. 2006;83(3):473–483. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 23.Yagi M, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202(3):345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyauchi Y, et al. The Blimp1-Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J Exp Med. 2010;207(4):751–762. doi: 10.1084/jem.20091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.