Thirty human and 10 animal proteins form amyloid fibrils associated with codeposition of P-component (SAP), heparatan sulfate proteoglycans (HSPGs), certain apolipoproteins (e.g., ApoE), as well as other less well-defined “ground substances” that may mitigate fibrillogenesis and tissue-specific deposition in association with aging or disease. Once deposited, fibrils may take on a “tombstone” configuration, one of the oldest isolation techniques being to let tissue stand on the benchtop for long periods of time. For all forms of amyloidosis, soluble precursors exist, which may be synthesized locally or distributed systemically in forms of amyloid affecting multiple organs (1). For the systemic amyloidoses, the precursor-fibril conversion is only partially understood, progressing in some instances through oligomers with enhanced cytotoxic, oxidant, or apoptotic effects (2). In addition, clearance mechanisms exist for seemingly stable amyloid, as witnessed by the presence of surrounding macrophages and giant cells in some localized forms, clinical regression of systemic disease if the precursor protein is reduced or eliminated, or when amyloid is depleted immunologically (3).
Amyloid A (AA), as the lettering implies, was one of the first forms of amyloid to be characterized biochemically, and 100 y ago was the most prevalent form of systemic amyloid found in man. Although the incidence of AA amyloid has decreased pari passu with chronic infectious diseases, it persists in association with certain chronic rheumatic and inflammatory bowel diseases, and with hereditary autoinflammatory disorders; in addition, some 10–20% of cases seen in large referral centers remain “idiopathic” (4). The precursor of AA is serum amyloid A (SAA), an HDL-associated apolipoprotein. In PNAS, Simons et al. mine a unique transgenic model for AA amyloidosis to consider the role of inflammation in defining this form of amyloid in the mouse (5).
Spontaneous AA amyloid has been identified in mammals, as well as birds and fish. This type of AA is prevalent among certain domesticated or bred species of bird, dog, cat, hamster, or mouse, but may also occur in the wild, in some instances linked to transmissibility between animals (6). Similar to man, the murine SAA gene family includes two SAA acute-phase reactants (APRs), which have developed by gene conversion, synthesized in the liver: SAA3, which is only minimally increased by proinflammatory stimuli, synthesized in the liver and peripherally; and SAA4, which is expressed constitutively by the liver as a minor component of HDL (1, 7). The biochemistry, as well as the organ tropism, of human AA is replicated to a large extent in murine amyloid induced by a number of agents. The initial model was that of feeding mice a diet rich in casein, which includes a k fraction that is amyloidogenic; later, serial injections of azocasein were used; however, these were often unreliable between laboratories, evened somewhat by allowing bacterial growth in the preparations. Similar models used Freund’s adjuvant, LPS, and certain infectious agents (e.g., Mycobacterium butyricum) (8). These models were noted to be “biphasic,” with an induction-phase notable for the dominance of PAS-positive glycoprotein-rich macrophage preceding the actual appearance of amyloid, initially in a splenic periportal distribution, followed by periportal hepatic deposits, and finally glomerular amyloid. This induction period can be abrogated by the injection of splenic extracts obtained from amyloidotic mice, linked to an activity initially called amyloid enhancing factor, more recently replicated by fibrils inducing amyloid deposition by a nucleation mechanism. Amyloid enhancing factor, which has also been observed with oligomer-encapsulated exosomes, shortens the lag time for amyloid deposition in response to inflammatory stimuli, such as silver nitrate-induced sterile abscesses (9). Potential modulatory roles for ApoE, HSPGs, and SAP have been observed using standard protocols in mice with targeted deletions of SAP or ApoE, or overexpressing heparanase, which retard AA deposition, possibly by modulating fibrillogenesis, abrogating inflammatory effects, or rendering the fibril more susceptible to proteolysis (10, 11). For both spontaneous and induced murine amyloidoses, striking strain differences in susceptibility were noted, with some (e.g., CE/J) being absolutely resistant because of lack of the amyloidogenic isoform SAA2 (subsequently named SAA1.1). In contrast, AA amyloid is not found among rats, which lack functional APR SAA (although not SAA3) because of a deletion distal to the promoter. Thus, rats do perfectly well without APR SAA, arguing that knock-out of these genes in other species may not be deleterious. In contrast, insertion of amyloidogenic SAA via an adenovirus vector into CE/J mice and rats permitted the development of amyloid using standard induction protocols (12).
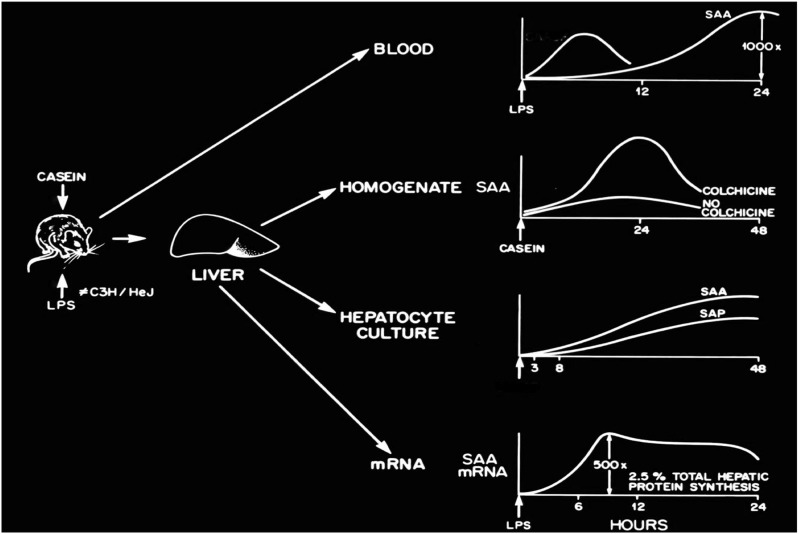
APR SAAs increase up to 1000-fold after proinflammatory stimuli, such as LPS (Fig. 1); sustained elevations may also be seen in humans developing AA amyloid and mice during azocasein induction. SAA stimulating factor was one of the activities shown to be identical to IL-1, with later studies also implicating TNF-α and IL-6. The SAA promoter includes C/EBPb, NF-κB, YY1, AP-2, SAF, and Sp1 (7), but not STAT3, response elements, with IL-6 assuming a dominant role, assessed by stimulation with individual and combination proinflammatory cytokines, as well as by specific cytokine blockade (13). Export of SAA from the liver can be abrogated by colchicine, providing a mechanism for treatment and prevention of AA amyloid. Simons et al. use a double-transgenic model in which both a doxycycline-inducible SAA2 transgene and a liver-specific reverse tet transactivator transgene are inserted, achieving peak SAA2 levels of 2–5 mg/dL and higher (5). This result is remarkable in the sustained levels of SAA attained, and with regard to the insertion of multiple copies of a specific amyloidogenic SAA isoform bypassing cytokine induction. Massive AA amyloid developed at a median of 5 wk in the absence of any influx of macrophages or induction of SAP, which is known to be a major APR in the mouse. Thus, sustained levels of an amyloidogenic SAA, induced via the transactivator gene instead of proinflammatory cytokines, may lead to AA amyloid in the apparent absence of inflammation. Lack of organ dysfunction or derivative “preamyloid” pathology call into question indirect effects of APR SAA, which have been assumed from in vitro studies that have used delipidated SAA to implicate chemoattractant activity for monocytes and lymphocytes, induction of the release of proinflammatory cytokines and G-GSF from neutrophils, and platelet effects (14, 15). Quantitation of other murine APR, interrogation of the effect of in vivo HDL-associated transgene on resident macrophage, neutrophils, and adipocytes, as well as quantitation of endogenous SAA3 in the transgene would add dimension to these conclusions.
Fig. 1.
LPS, casein, and proinflammatory cytokines trigger dramatic increases in the synthesis of APR SAA in the liver by both translational and posttranslational mechanisms. The export of SAA from the liver may be inhibited by colchicine, a therapeutic for AA amyloid. SAA rapidly associates with HDL and has an ∼90-min half-life in the circulation.
The double-transgenic model and the authors’ considerable experience with in vivo imaging of amyloid deposits have allowed Simons et al. to address the kinetics of SAA/AA interconversion with regard to the latency of deposition, priming, and regression induced by reducing the precursor or immunological clearance (3, 5). Probes for determining the in vivo amyloid load, and for monitoring treatment, include SAP, anti-AA antibodies, and HPSG ligands, in each instance controlling for possible cross-reactivity with circulating and soluble precursors in normal tissue and contiguous to fibrillar deposits (3, 5, 16, 17). Studies carried out during SAA triggering by inflammatory stimuli and amyloid induction have shown an ∼90-min half-life and a large HDL APR SAA pool as a result of increased hepatic synthesis, only 0.01% of which is deposited in the mouse spleen over a 24-h period (18). In contrast, in the double-transgenic mouse, a remarkable ∼90% sequestration of SAA was observed, correlating with increased organ weight caused by amyloid deposition over the ensuing 3 wk. The same kinetic analysis can now be applied to analyzing the effect of varying levels of SAA on the slow regression observed after withdrawal of doxycycline, the effect of residual microdeposits in priming rapid and uniform recurrence of amyloid with prominent glomerular deposits after transgene triggering, and the effect on turnover of therapeutic strategies that interfere with synthesis, modulate fibrillogenesis, and clear existing deposits (3, 19, 20).
Footnotes
The author declares no conflict of interest.
See companion article on page 16115 of issue 40 in volume 110.
References
- 1.Sipe JD, et al. Nomenclature Committee of the International Society of Amyloidosis Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2012;19(4):167–170. doi: 10.3109/13506129.2012.734345. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan S, et al. Pathogenic serum amyloid A 1.1 shows a long oligomer-rich fibrillation lag phase contrary to the highly amyloidogenic non-pathogenic SAA2.2. J Biol Chem. 2013;288(4):2744–2755. doi: 10.1074/jbc.M112.394155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodin K, et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468(7320):93–97. doi: 10.1038/nature09494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunker D, Gorevic P. AA amyloidosis: Mount Sinai experience, 1997-2012. Mt Sinai J Med. 2012;79(6):749–756. doi: 10.1002/msj.21342. [DOI] [PubMed] [Google Scholar]
- 5.Simons JP, et al. Pathogenetic mechanisms of amyloid A amyloidosis. Proc Natl Acad Sci USA. 2013;110(40):16115–16120. doi: 10.1073/pnas.1306621110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, et al. Fecal transmission of AA amyloidosis in the cheetah contributes to high incidence of disease. Proc Natl Acad Sci USA. 2008;105(20):7263–7268. doi: 10.1073/pnas.0800367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265(2):501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 8.Gruys E, Snel FWJJ. Animal models for reactive amyloidosis. Baillieres Clin Rheumatol. 1994;8(3):599–611. doi: 10.1016/s0950-3579(05)80117-7. [DOI] [PubMed] [Google Scholar]
- 9.Tasaki M, et al. Transmission of circulating cell-free AA amyloid oligomers in exosomes vectors via a prion-like mechanism. Biochem Biophys Res Commun. 2010;400(4):559–562. doi: 10.1016/j.bbrc.2010.08.101. [DOI] [PubMed] [Google Scholar]
- 10.Botto M, et al. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med. 1997;3(8):855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, et al. Accelerated resolution of AA amyloid in heparanase knockout mice is associated with matrix metalloproteases. PLoS ONE. 2012;7(7):e39899. doi: 10.1371/journal.pone.0039899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kindy MS, et al. Adenoviral expression of murine serum amyloid A proteins to study amyloid fibrillogenesis. Biochem J. 1998;332(Pt 3):721–728. doi: 10.1042/bj3320721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshizaki K. Pathogenic role of IL-6 combined with TNF-α or IL-1 in the induction of acute phase proteins SAA and CRP in chronic inflammatory diseases. Adv Exp Med Biol. 2011;691:141–150. doi: 10.1007/978-1-4419-6612-4_15. [DOI] [PubMed] [Google Scholar]
- 14.Christenson K, et al. Endogenous acute phase serum amyloid A lacks pro-inflammatory activity, contrasting the two recombinant variants that activate human neutrophils through different receptors. Front Immunol. 2013;4:92. doi: 10.3389/fimmu.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MH, de Beer MC, Wroblewski JM, Webb NR, de Beer FC. SAA does not induce cytokine production in physiological conditions. Cytokine. 2013;61(2):506–512. doi: 10.1016/j.cyto.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall J, et al. In vivo radioimmunodetection of amyloid deposits in experimental amyloidosis. In: Glenner GG, Costa PP, De Freitas F, editors. Amyloid and Amyloidosis. Amsterdam, The Netherlands: Excerpta Medica; 1980. pp. 163–174. [Google Scholar]
- 17.Wall JS, et al. In vivo molecular imaging of peripheral amyloidosis using heparin-binding peptides. Proc Natl Acad Sci USA. 2011;108(34):E586–E594. doi: 10.1073/pnas.1103247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tape C, Tan R, Nesheim M, Kisilevsky R. Direct evidence for circulating apoSAA as the precursor of tissue AA amyloid deposits. Scand J Immunol. 1988;28(3):317–324. doi: 10.1111/j.1365-3083.1988.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 19.Inoue S, Hultin PG, Szarek WA, Kisilevsky R. Effect of poly(vinylsulfonate) on murine AA amyloid: A high-resolution ultrastructural study. Lab Invest. 1996;74(6):1081–1090. [PubMed] [Google Scholar]
- 20.Kluve-Beckerman B, et al. Antisense oligonucleotide suppression of serum amyloid A reduces amyloid deposition in mice with AA amyloidosis. Amyloid. 2011;18(3):136–146. doi: 10.3109/13506129.2011.597464. [DOI] [PubMed] [Google Scholar]