Significance
There are a number of pathogens for which the immunity acquired postinfection does not fully protect against reinfection and disease. Therefore, vaccines offering superior protection compared with the protection following natural infection are needed. Due to a unique pattern of immune response induced by cytomegaloviruses (CMVs), live attenuated CMVs are attractive candidates for vaccine vectors. Here we have demonstrated that a recombinant CMV vector expressing RAE-1γ, a cellular ligand for activating NKG2D receptor expressed on several types of immune cells, has tremendous potential for subverting viral immunoevasion and enhancing the efficiency of the CD8 T-cell response against vectored antigens. This study demonstrates a significant new approach in designing T-cell–based vaccine vectors.
Keywords: RAE-1 gamma, CD8 T cell vaccine, vaccine vector
Abstract
Due to a unique pattern of CD8 T-cell response induced by cytomegaloviruses (CMVs), live attenuated CMVs are attractive candidates for vaccine vectors for a number of clinically relevant infections and tumors. NKG2D is one of the most important activating NK cell receptors that plays a role in costimulation of CD8 T cells. Here we demonstrate that the expression of CD8 T-cell epitope of Listeria monocytogenes by a recombinant mouse CMV (MCMV) expressing the NKG2D ligand retinoic acid early-inducible protein 1-gamma (RAE-1γ) dramatically enhanced the effectiveness and longevity of epitope-specific CD8 T-cell response and conferred protection against a subsequent challenge infection with Listeria monocytogenes. Unexpectedly, the attenuated growth in vivo of the CMV vector expressing RAE-1γ and its capacity to enhance specific CD8 T-cell response were preserved even in mice lacking NKG2D, implying additional immune function for RAE-1γ beyond engagement of NKG2D. Thus, vectors expressing RAE-1γ represent a promising approach in the development of CD8 T-cell–based vaccines.
Although vaccination plays a tremendous role in protection against infectious diseases, there are many pathogens for which even the immunity acquired after natural infection does not fully protect against reinfection and disease. Therefore, vaccines which offer superior protection compared with the protection following natural infection are needed. Most of the current vaccines induce protective antibodies but often fail to confer sufficient protection. An alternative approach is to develop vaccines which are based on the induction of cellular immunity in general and cytotoxic CD8 T cells in particular (1).
Cytomegaloviruses (CMVs) are excellent inducers of the CD8 T-cell response, despite having numerous immunoevasion strategies aimed at compromising antigen presentation by MHC class I molecules (2). Preferentially, CMVs induce the effector arm of memory CD8 T cells (3). This, together with a large genome allowing the insertion of multiple foreign genes, makes CMVs attractive vaccine vectors. The outstanding capacity of specific CD8 T cells induced by CMV vectors was proven by studies demonstrating the role of tissue-resident effector memory CD8 T cells in the protection against challenge infection (4, 5). The suitability of CMV as a vaccine vector was further emphasized in a recent study by Hansen et al. (6).
NKG2D is a receptor expressed on several lymphocyte subsets, with a predominant role in activation of NK cells. In addition, NKG2D has a costimulatory role on CD8 T cells (7). The ligands for the NKG2D receptor are several molecules induced by stress or cell transformation. In mice, NKG2D ligands comprise the RAE-1 family (RAE-1α–ε), H60 family (H60a–c), and MULT-1 proteins (8). The significance of NKG2D signaling in immune response to CMV infection is best illustrated by numerous strategies used by CMVs to evade the function of this receptor (9). We have recently shown that infection of mice with mouse CMV (MCMV) expressing RAE-1γ elicits a strong and long-lasting MCMV-specific CD8 T-cell response, despite a dramatic virus attenuation as a consequence of efficient NK cell control (10).
Here we demonstrate that MCMV expressing RAE-1γ has a tremendous potential for boosting the efficiency of CD8 T cells directed against a vectored antigenic peptide. We show that CMV expressing RAE-1γ and immunodominant CD8 T-cell epitope of Listeria monocytogenes listeriolysin O (LLO) or ovalbumin-derived SIINFEKL induces a superior epitope-specific and durable protective CD8 T-cell response. Moreover, our study indicates the existence of another, so far unknown, immune function of RAE-1γ beyond engagement of NKG2D. Altogether, our data set the stage for a powerful, unique approach in designing T-cell–based vaccines.
Results
CMV Expressing RAE-1γ and Listeriolysin Epitope Induces a Strong Listeriolysin-Specific CD8 T-Cell Response.
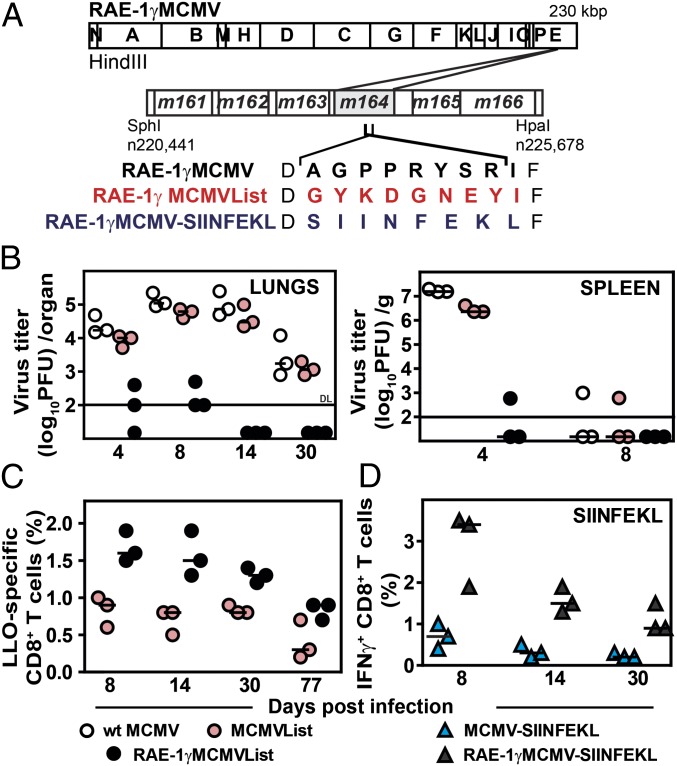
To test the potential of using MCMV expressing RAE-1γ as a vector, we have constructed a virus expressing immunodominant CD8 T-cell epitope of L. monocytogenes listeriolysin O91–99 (LLO) (11) on the backbone of RAE-1γMCMV (10), where RAE-1γ was introduced in place of its viral inhibitor m152 (RAE-1γMCMVList). The LLO epitope was introduced in place of the MCMV immunodominant CD8 T-cell epitope m164167–175 (Fig. 1A). MCMV expressing the LLO epitope only (MCMVList) was used as control. Neither of the genetic modifications affected viral growth kinetics in vitro (Fig. S1 A and B). As shown by the viral load in spleen and lungs of infected mice, RAE-1γMCMVList, but not MCMVList, was severely attenuated in BALB/c (H-2d) mice (Fig. 1B). Despite the striking attenuation, RAE-1γMCMVList induced a stronger LLO-specific CD8 T-cell response compared with MCMVList (Fig. 1C and Fig. S1C). To exclude the possibility that the robustness of the CD8 T-cell response after infection with RAE-1γMCMVList vector was restricted to a single MHC I haplotype, C57BL/6 (H-2b) mice were infected with RAE-1γMCMV expressing the H-2Kb–restricted peptide SIINFEKL (RAE-1γMCMV-SIINFEKL) (12) (Fig. 1A). The infection with RAE-1γMCMV-SIINFEKL resulted in a higher frequency of SIINFEKL-specific CD8 T cells compared with the infection with MCMV expressing SIINFEKL only (Fig. 1D). The CD8 T-cell response to some, but not all, MCMV immunodominant epitopes was also superior in RAE-1γMCMV-SIINFEKL–infected mice (Fig. S1D). Altogether, the results demonstrated the capacity of MCMV expressing RAE-1γ to potentiate the CD8 T-cell response directed against vectored CD8 T-cell epitopes.
Fig. 1.
Expression of RAE-1γ by an MCMV vector improves the specific CD8 T-cell response to a vectored antigen. (A) RAE-1γMCMVList and RAE-1γMCMV-SIINFEKL were constructed on the RAE-1γMCMV backbone by replacing the Dd-restricted antigenic m164 peptide 167AGPPRYSRI175 with either the Kd-restricted listeriolysin O (LLO)-derived peptide 91GYKDGNEYI99 or the H-2Kb–restricted ovalbumin-derived peptide SIINFEKL. (B) BALB/c mice were infected i.v. with 2 × 105 pfu per mouse of the indicated viruses, and viral titers were determined by plaque assay. DL, detection limit. (C) Splenocytes of MCMVList- or RAE-1γMCMVList–infected BALB/c mice (105 pfu per mouse, via f.p.) were stained for LLO tetramer-specific CD8 T cells. (D) C57BL/6 mice were f.p. infected with 105 pfu per mouse of MCMV-SIINFEKL or RAE-1γMCMV-SIINFEKL. At indicated time points, splenocytes were isolated, stimulated with SIINFEKL-peptide, and stained for the intracellular IFN-γ production. For panels B–D, individual animals (circles or triangles) and median values are shown.
Superior Protection Against a Challenge Infection in Mice Immunized with RAE-1γMCMV Expressing Antigenic Peptides.
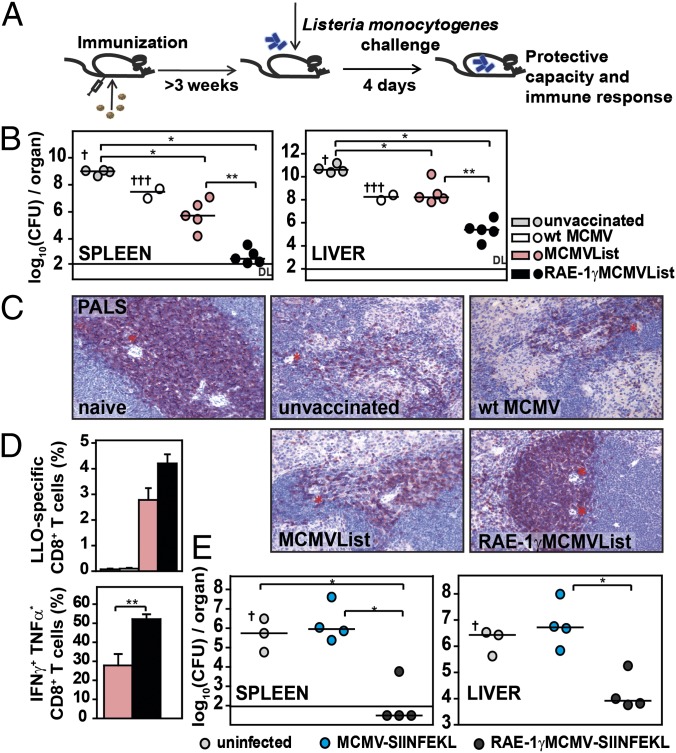
LLO-specific CD8 T cells play a major role in the protection against L. monocytogenes in BALB/c mice (11, 13). To prove the efficiency of RAE-1γMCMVList as a vaccine, BALB/c mice were immunized with RAE-1γMCMVList, MCMVList, or WT MCMV and tested for protective capacity against a challenge infection with L. monocytogenes 3 wk later (Fig. 2A). Mice were killed 4 d postchallenge and analyzed for bacterial load in the spleen and liver (Fig. 2B). Naive mice and mice immunized with WT MCMV showed higher bacterial loads in both organs, and a significant proportion of them succumbed to the infection. The vaccination with MCMVList resulted in a modest reduction of the bacterial load in the spleen but not in the liver compared with WT MCMV. In contrast, RAE-1γMCMVList induced efficient protection that was demonstrated by a dramatic reduction of the bacterial load in both organs. An important consequence of L. monocytogenes infection is the depletion of T cells in the periarteriolar lymphoid sheath of infected spleens (14) (Fig. 2C). Whereas mice infected with MCMVList showed only a low level of protection, periarteriolar T cells were completely preserved upon immunization with RAE-1γMCMVList. The superior property of RAE-1γMCMV as a vaccine vector was further illustrated by the higher frequency of LLO-specific CD8 T cells and their capacity for IFN-γ and TNF-α production after challenge infection with L. monocytogenes (Fig. 2D).
Fig. 2.
RAE-1γMCMV expressing antigenic peptides protects mice against L. monocytogenes. (A) Mice were immunized via f.p. with 105 pfu per mouse of WT MCMV or MCMV vector with or without RAE-1γ or were left nonimmunized. At 3 wk p.i. or later, mice were challenged with L. monocytogenes. On day 4 postchallenge, mice were sacrified and analyzed for an immune response and protective capacity to L. monocytogenes. (B) BALB/c mice were challenged 4 wk postimmunization with 2 × 104 cfu per mouse of L. monocytogenes. Bacterial load was determined in spleen and liver. Individual animals (circles) and median values are shown. † indicates the death of a mouse. (C) Three weeks postimmunization, BALB/c mice were challenged with 104 cfu per mouse of L. monocytogenes. Paraffin-embedded spleen sections were stained for CD3ε expression. * indicates central artery. PALS, periarteriolar lymphoid sheath. (Magnification: 20×.) (D) Splenocytes were isolated and either stained with LLO-tetramers or stimulated with LLO-peptides and intracellularly stained for cytokine production (mean ± SEM, n = 4–5). (E) Three weeks postvaccination, C57BL/6 mice were challenged with 5 × 104 cfu per mouse of OVA-Listeria. Individual animals and median values are shown.
To test the efficiency of RAE-1γ expressing MCMV vectors in a second model, C57BL/6 mice were primed with viruses expressing SIINFEKL either with or without RAE-1γ coexpression and challenged with L. monocytogenes expressing ovalbumin (OVA-Listeria) (Fig. 2E). The results demonstrated superior resistance of RAE-1γMCMV-SIINFEKL–immunized mice to challenge with OVA-Listeria.
CD8 T Cells Represent a Dominant Protective Mechanism Against Challenge Infection in RAE-1γMCMVList–Vaccinated Mice.
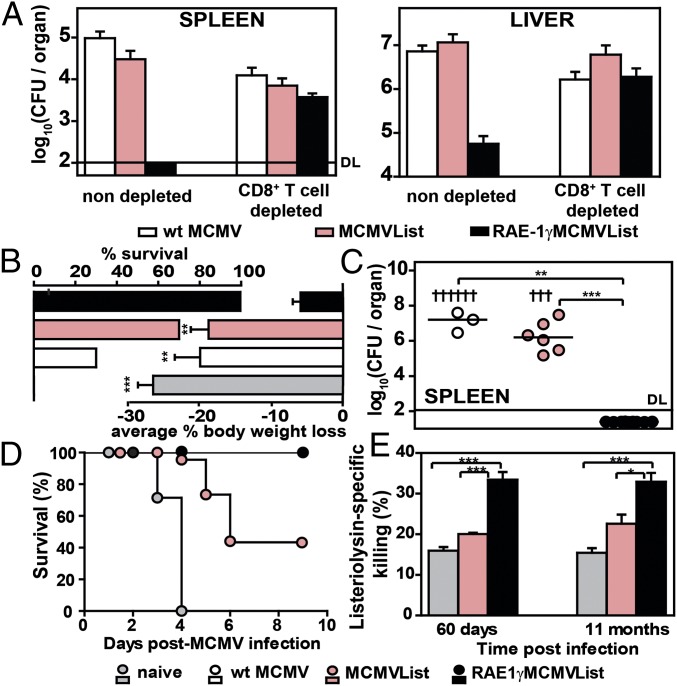
To demonstrate that CD8 T cells are responsible for the durable protective effect of RAE-1γMCMVList vaccination, a group of immunized BALB/c mice was depleted of CD8 T cells before the challenge infection. The depletion of CD8 T cells abolished the protection of RAE-1γMCMVList immunization against L. monocytogenes challenge (Fig. 3A). This was also confirmed by histopathological analysis of the livers of immunized mice (Fig. S2). Although RAE-1γMCMVList immunization significantly prevented the formation of lesions, the CD8 T-cell depletion resulted in a pathology that was similar to the one observed in other groups of mice. The immunization with MCMVList was insufficient to limit liver pathology caused by infection, indicating that CD8 T cells expressed as a result of immunization with RAE-1γ expresing vector were dominant in the protection against L. monocytogenes challenge.
Fig. 3.
Long-term protection against L. monocytogenes challenge in RAE-1γMCMVList–immunized mice. BALB/c mice were f.p. immunized with 105 pfu per mouse of indicated viruses. (A) Three weeks postimmunization, mice were challenged with 104 cfu per mouse of L. monocytogenes. One day before the challenge, mice were depleted of CD8 T cells or left undepleted. Four days later, the bacterial loads were determined (mean ± SEM, n = 4–5). (B) Challenge with 2 × 104 cfu per mouse of L. monocytogenes was performed 60 d postimmunization. Survival and body weight loss (mean ± SEM) on day 3 (n = 9–12) is shown. (C) Bacterial load of survived mice on day 4 postchallenge in spleen is shown (individual animals and median values, n = 9; † indicates the death of a mouse). (D) Challenge with 4 × 104 cfu per mouse of L. monocytogenes was performed 60 d postimmunization, and the survival rate was followed (n = 9). (E) Mice were injected with an equal ratio of unstimulated and LLO-peptide–stimulated CSFE-stained splenocytes 2 and 11 mo postimmunization. Percentage of listeriolysin peptide-specific killing is shown (mean ± SEM, n = 3–4).
Longevity of Memory CD8 T-Cell Response Induced by RAE-1γMCMVList.
To assess whether the vaccination with MCMV vector expressing RAE-1γ provides a long-lasting protection against L. monocytogenes, we have challenged the mice by infecting them 2 mo postvaccination. Groups of vaccinated BALB/c mice challenged with 2 × 104 cfu per mouse of L. monocytogenes were monitored for survival and body weight loss (Fig. 3B). All unvaccinated mice and most of the mice infected with WT MCMV succumbed to infection by day 4, which was accompanied by a dramatic weight loss by day 3 postchallenge. Vaccination with MCMVList protected a substantial fraction of the immunized mice, but nevertheless, these mice exhibited a significant weight loss. All RAE-1γMCMVList–vaccinated mice survived the infection with minimal weight loss by day 4 postchallenge. Mice which survived 4 d postinfection were killed, and the bacterial loads in spleen were determined (Fig. 3C). When mice were challenged with higher inoculums (4 × 104 cfu per mouse), it was demonstrated that RAE-1γMCMVList–vaccinated mice could still resist the challenge (Fig. 3D). The efficient and long-lasting protective capacity of the LLO-specific CD8 T-cell response in mice vaccinated with RAE-1γMCMVList was confirmed by assessing the LLO-specific CD8 T-cell–mediated cytotoxicity in vivo (Fig. 3E). Listeriolysin-specific killing was significantly higher for at least 11 mo postvaccination in mice vaccinated with RAE-1γMCMVList. Altogether, it can be concluded that the herpesviral vector engineered to express the NKG2D ligand RAE-1γ provided highly efficient long-term CD8 T-cell–mediated protection against L. monocytogenes infection.
Better Priming of CD8 T Cells and Attenuation of RAE-1γMCMVList Are Consequences of Ectopic Expression of RAE-1γ.
Although it has been previously shown that the deletion of MCMV inhibitors of MHC class I presentation does not enhance the CD8 T-cell response (3), this possibility could not be completely excluded, particularly because m152 is also a viral inhibitor of RAE-1 expression (9). To investigate whether RAE-1γ alone or accompanied by deletion of m152 enhances the potency of our viral vector, a virus expressing the LLO epitope on the backbone of the m152-deficient virus (15) (Δm152MCMVList) was used. Δm152MCMVList showed attenuated growth in vivo, but the level of attenuation was much more pronounced with the RAE-1γMCMVList (Fig. S3A). Furthermore, the frequency of LLO-specific CD8 T cells induced by Δm152MCMVList at day 8 postinfection was inferior compared with the level induced by the RAE-1γMCMVList (Fig. S3B). However, at later time points we have observed a high frequency of epitope-specific CD8 T cells also in mice infected with Δm152MCMVList, suggesting an impact of m152 deletion. These data led us to conclude that the major mechanism behind attenuation and improved CD8 T-cell response in mice infected with virus expressing RAE-1γ does not result from endogenous RAE-1γ or improved MHC-I presentation due to deletion of m152 but rather from the ectopic expression of RAE-1γ.
NKG2D-Independent Immune Function of RAE-1γ.
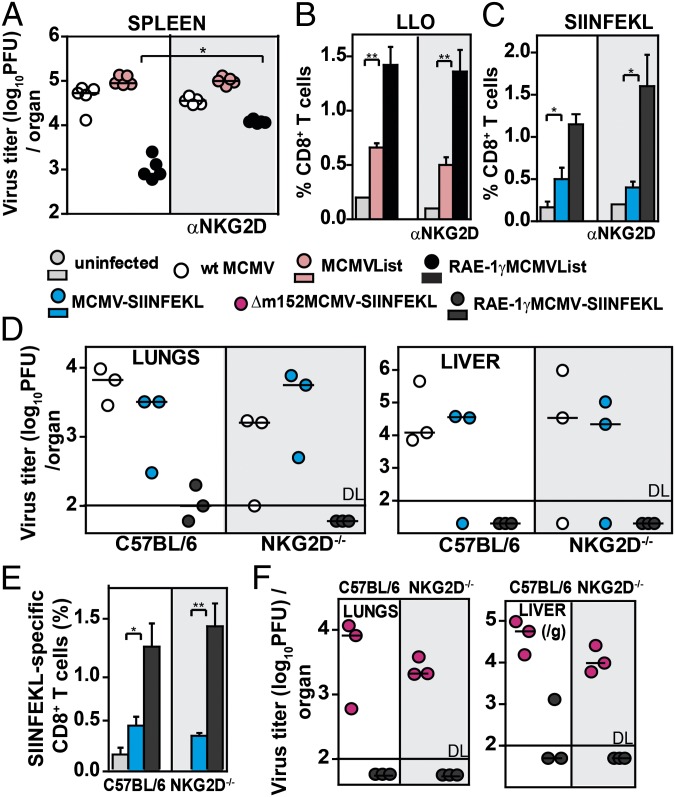
Next, we wanted to investigate how RAE-1γ, in the context of MCMV vector, mediates its immune-stimulatory effects. It has been well established that costimulation via NKG2D plays an important role in shaping of the CD8 T-cell response (7, 16). This function may be crucial for efficient priming of CD8 T cells by RAE-1γMCMV because MCMV down-regulates costimulatory molecules on antigen-presenting cells as does HCMV (17, 18). Therefore, we investigated the capacity of RAE-1γMCMVList to prime CD8 T cells in the presence of blocking NKG2D antibodies. Blocking of NKG2D significantly reduced but did not completely abolish the control of RAE-1γMCMVList at day 3 postinfection (p.i.) (Fig. 4A). Surprisingly, we observed that CD8 T-cell responses were not diminished in the presence of blocking antibodies in either BALB/c or C57BL/6 mice (Fig. 4 B and C). These observations suggested an NKG2D-independent role for RAE-1γ. To ensure that our observations were not the result of insufficient blocking of RAE-1γ–NKG2D interactions, we took advantage of mice lacking the NKG2D receptor (NKG2D−/− mice) (19). C57BL/6 mice and NKG2D−/− mice were i.v. infected with either WT MCMV, MCMV-SIINFEKL, or RAE-1γMCMV-SIINFEKL, and the virus titers in organs were determined at day 7 p.i. (Fig. 4D). MCMV expressing RAE-1γ was attenuated not only in control C57BL/6 mice but also in NKG2D−/− mice. Moreover, in accordance with our findings after the application of blocking antibodies, we also found a higher frequency of SIINFEKL-specific CD8 T cells in NKG2D−/− mice after infection with RAE-1γMCMV-SIINFEKL compared with MCMV-SIINFEKL (Fig. 4E). This difference did not depend on the absence of m152 because RAE-1γMCMV-SIINFEKL was much more attenuated than Δm152MCMV-SIINFEKL in both WT and NKG2D−/− mice (Fig. 4F). These findings suggested that RAE-1γ has an immune-stimulatory role beyond the engagement of NKG2D.
Fig. 4.
NKG2D-independent attenuation and immune function of RAE-1γ. (A) BALB/c mice were infected i.v. with 2 × 105 pfu per mouse of the indicated viruses. One day before infection and on days 2 and 5 p.i., mice were treated with αNKG2D antibody. Virus titer was determined on day 8 p.i. (B) Mice were infected with 105 pfu per mouse via f.p. and treated with αNKG2D as in A. LLO-specific CD8 T-cell response was determined on day 8 p.i. (n = 5). (C) C57BL/6 mice were infected with 2 × 105 pfu per mouse i.v. and treated with αNKG2D. The frequency of SIINFEKL-specific CD8 T cells was determined on day 8 p.i. (n = 3–4). (D) C57BL/6 and NKG2D−/− mice were infected i.v. with 2 × 105 pfu per mouse. On day 7 p.i., virus titer is shown. (E) The frequency of SIINFEKL-specific CD8 T cells was determined on day 7 p.i. (n = 3–4). (F) Virus titer in organs was determined on day 7 p.i. For A, D, and F, individual animals and median values are shown. For B, C, and E, mean ± SEM is shown.
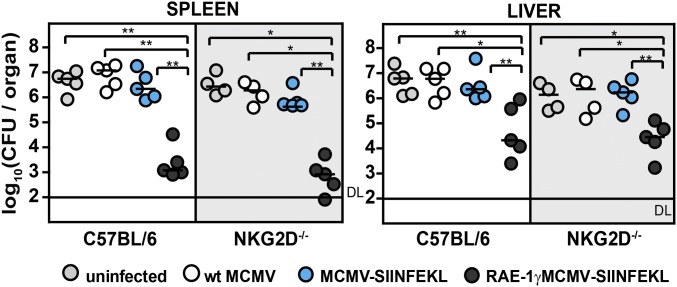
Next, we tested whether NKG2D-independent triggering of CD8 T cells in RAE-1γMCMV-SIINFEKL infection of NKG2D−/− mice influenced their protective capacity as well. C57BL/6 and NKG2D−/− mice were immunized with either WT MCMV, MCMV-SIINFEKL, or RAE-1γMCMV-SIINFEKL, and 3 wk later, mice were challenged with 5 × 104 cfu per mouse of OVA-Listeria (Fig. 5). NKG2D−/− mice immunized with RAE-1γMCMV-SIINFEKL were equally as protected against infection as C57BL/6 mice, once again demonstrating the capacity of RAE-1γ expressed in context of MCMV vector to induce robust protective immune response even in mice lacking NKG2D signaling.
Fig. 5.
RAE-1γMCMV expressing SIINFEKL-peptide protects mice against OVA-Listeria in NKG2D−/− mice. C57BL/6 and NKG2D−/− mice were immunized with 105 pfu per mouse via f.p. of indicated viruses or left nonimmunized. Three weeks postimmunization, mice were challenged with 5 × 104 cfu per mouse of OVA-Listeria. The bacterial load was determined. Individual animals (circles) and median values are shown.
MCMV Expressing RAE-1γ Preserves Dendritic Cell Subsets and Enables Priming of CD8 T Cells.
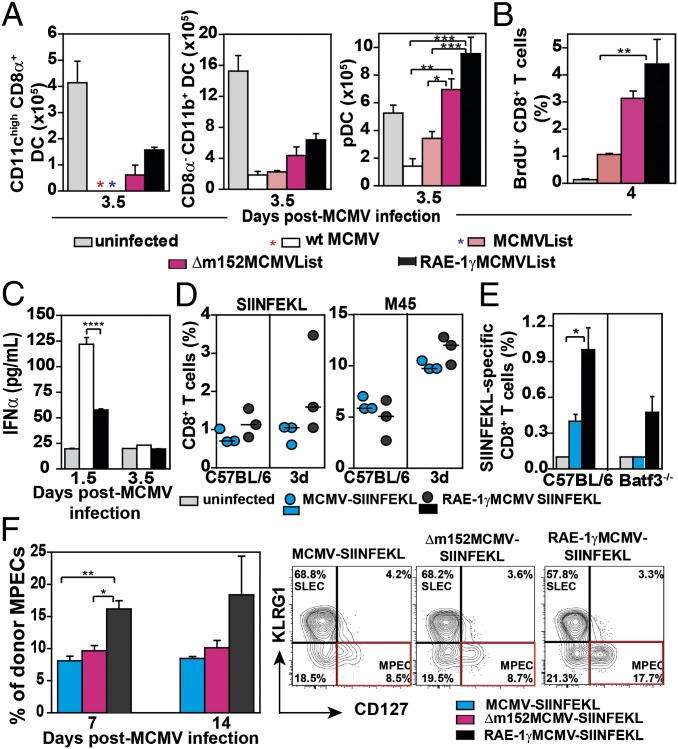
Dendritic cells (DCs) are known targets of MCMV, and infection with WT MCMV results in a dramatic reduction of splenic DCs in the early days p.i (20, 21). However, in comparison with WT MCMV, the virus expressing RAE-1γ affects the frequency of DCs in the spleen to a much smaller extent (10) (Fig. 6A). Although the deletion of m152 on its own prevented the loss of DCs in spleen compared with WT MCMV, both DC subsets were preserved to a much higher level in mice infected with RAE-1γMCMVList. The preserved frequency of DCs in RAE-1γMCMVList–infected mice was not a consequence of NK cell disfunction because we showed that their functional capacity was completely preserved (Fig. S4). The preservation of DCs during early days p.i. corresponded to the enhanced priming of CD8 T cells in RAE-1γMCMVList–infected mice, which was illustrated by the higher frequencies of LLO-specific CD8 T cells (Fig. 1C and Fig. S1C) and a higher proliferation capacity of CD8 T cells (Fig. 6B). This was in inverse correlation with the level of IFN-α in the sera of RAE-1γMCMVList–infected mice (Fig. 6C). Thus, we can conclude that the expression of RAE-1γ by MCMV promotes the priming of CD8 T cells by preserving antigen-presenting cells.
Fig. 6.
RAE-1γ expression promotes epitope-specific CD8 T cells priming. BALB/c mice were i.v. infected with 2 × 105 pfu, and the following parameters were analyzed: (A) the frequency of DC subsets in spleen, (B) the frequency of proliferating CD8 T cells, and (C) IFN-α level in the sera. For A–C, mean ± SEM is shown; n = 3–5. (D) C57BL/6 and 3d mice were i.p. infected with 2 × 105 pfu of indicated viruses and the specific CD8 T-cell response in spleen was determined on day 7 p.i. (E) C57BL/6 and Batf3−/− mice were infected i.v. with 2 × 105 pfu of indicated viruses and SIINFEKL-specific CD8 T cells in spleen were determined on day 8 p.i. Mean ± SEM is shown. (F) Naive recipients (CD45.1) were transferred with 104 NKG2DΔ/ΔOT1 cells (CD45.2) and infected with 104 pfu per mouse of indicated viruses 24 h later. On days 7 and 14 p.i., donor CD8 T cells (CD45.1) were analyzed for the frequency of MPECs (KLRG1−CD127+ CD8 T cells) in blood (n = 3). Representative FACS plots of donor MPECs expansion are shown; n = 3–4.
Although it is generally assumed that cross-presentation plays a dominant role in the priming of CD8 T cells during MCMV infection (3, 22, 23), we proposed that priming in mice infected with MCMV expressing RAE-1γ would not be dependent on cross-presentation because a lowered antigenic load should, at least in theory, reduce the cross-priming capacity of such a virus. To test this hypothesis, we used 3d mice defective in TLR3, TLR7, and TLR9 signaling which are unable to cross-present foreign antigens (24). The 3d mice were infected with either MCMV-SIINFEKL or RAE-1γMCMV-SIINFEKL, and the frequency of SIINFEKL-specific and MCMV-specific (M45) CD8 T cells was determined 7 d later (Fig. 6D). Although both viruses induced an epitope-specific CD8 T-cell response, the one induced by RAE-1γMCMV-SIINFEKL was slightly stronger than the one in mice infected with MCMV-SIINFEKL. Additionally, the priming capacity of RAE-1γMCMV vector was assessed in Batf3−/− mice deficient for CD8α+ DCs, which can present antigenic peptides through direct or cross-presentation mechanisms (25, 26). In contrast to control C57BL/6 mice and 3d mice, the priming capacity of both RAE-1γMCMV-SIINFEKL and MCMV-SIINFEKL was dramatically reduced in Batf3−/− mice, suggesting the crucial role of this specific DC subset rather than cross-presentation as such (Fig. 6E). However, contrary to the mice infected with MCMV-SIINFEKL, the CD8 T-cell response to RAE-1γMCMV-SIINFEKL was not completely abolished in Batf3−/− mice, suggesting independent priming of some CD8α+ DCs.
To investigate more directly the effect of RAE-1γ on memory CD8 T-cell differentiation, naive CD8 T cells from MHC class I-restricted TCR transgenic mice (27) with a high specificity for the MCMV-derived epitope M38 were i.v. injected into C57BL/6 mice. One day later, recipient C57BL/6 mice were i.v. injected with either WT MCMV, Δm152MCMV, or RAE-1γMCMV. On days 7 and 14 p.i., CD8 T cells from spleens (Fig. S5) were analyzed for the frequency of short-lived effector cells (KLRG1+CD127−) and memory precursor effector cells (MPECs) (KLRG1−CD127+) (28). A significant shift in favor of MPECs was observed in mice infected with RAE-1γMCMV virus.
To investigate whether this effect of RAE-1γ on the pattern of memory CD8 T-cell differentiation is NKG2D-dependent, NKG2DΔ/Δ mice, a second NKG2D-deficient mouse line generated in our laboratory (29), were crossed on OT-1 TCR transgenic C57BL/6 mice (30). NKG2DΔ/ΔOT-1 cells were transferred into syngeneic naive C57BL/6 recipients infected with either MCMV-SIINFEKL, Δm152MCMV-SIINFEKL, or RAE-1γMCMV-SIINFEKL (Fig. 6F). The frequency of MPEC NKG2DΔ/ΔOT-1 CD8 T cells within donor CD8 T cells in blood on days 7 and 14 p.i. was tested. Similarly to the results obtained with M38 TCR transgenic mice, the frequency of MPECs within donor cells was the highest in mice infected with RAE-1γMCMV-SIINFEKL. To rule out the possibility that ectopically expressed RAE-1γ (recognized by host’s cells expressing NKG2D) was influencing the transition of transferred NKG2DΔ/ΔOT-1 toward MPECs, we have transferred NKG2DΔ/ΔOT-1 cells and NKG2D+/−OT-1 into NKG2D-deficient recipients and analyzed their expansion and MPEC formation on day 7 p.i. As shown in Fig. S6, the frequency of SIINFEKL-specific MPECs transferred from either NKG2DΔ/ΔOT-1 or control OT-1 mice was higher in mice infected with RAE-1γMCMV vector. Altogether, the results indicate that the ectopic expression of RAE-1γ in the MCMV vector accelerates the transition of the CD8 T-cell response toward memory cell differentiation in a NKG2D-independent manner.
Discussion
A better understanding of subset diversification of antigen-specific CD8 T cells during immune responses is key for designing T-cell–based vaccines. The strength and the quality of the CD8 T-cell response is determined by various factors including antigenic stimulation through the TCR, various costimulation signals, and stimulation via inflammatory cytokines (31, 32). These signals shape the CD8 T cells’ differentiation toward short-living effector cells and long-living memory T cells (28, 33, 34). Here we demonstrate that an MCMV vector expressing RAE-1γ, despite its dramatically attenuated growth in vivo, boosts the quality of the specific CD8 T-cell response against vectored antigenic epitopes. Moreover, the CD8 T-cell response to LLO and SIINFEKL epitopes induced by MCMV vector expressing RAE-1γ demonstrated a superior and long-lasting protection against a challenge infection with L. monocytogenes and L. monocytogenes expressing OVA. In addition, the phenotype of MCMV expressing RAE-1γ, with regard to its attenuation and the quality of protective CD8 T-cell response to vectored antigens, was also preserved in NKG2D−/− mice, demonstrating the NKG2D-independent immune function of RAE-1γ.
MCMV efficiently down-regulates costimmulatory molecules in infected cells which may result in deficient priming of CD8 T cells (17). Because NKG2D can also serve as a costimulatory receptor, we designed our RAE-1γMCMV vector to circumvent viral evasins of costimmulatory signaling. Our goal was to attenuate in vivo replication of our vector, while simultaneously enhancing its capacity to prime CD8 T cells. The molecular communication between DCs and CD8 T cells regulates both the magnitude and the quality of the resulting CD8 T-cell response. Thus, improved antigen presentation with MCMV expressing RAE-1γ can, at least in part, be explained by a preserved frequency of DCs and by rescuing their costimmulatory capacity through RAE-1γ. It is worth emphasizing that preserved CD8α+ DC subset rather than cross-presentation mechanism is the dominant factor for CD8 T-cell priming in mice infected with the RAE-1γMCMV vector. This is in line with more recent findings demonstrating that MCMV-specific CD8 T-cell priming is dependent on the CD8α+ DC subset rather than on cross-presentation (25).
The promoting effect of NKG2D ligands on specific CD8 T-cell responses is not without precedent in the literature: it has been shown that NKG2D ligands RAE-1 and H60 ectopically expressed on tumor cell lines strongly enhance the generation of tumor-specific CD8 T-cell response and are able to protect an immunized host against a challenge with the original tumor lines lacking the NKG2D ligands (35). Here we demonstrated that MCMV expressing RAE-1γ has improved priming potential also in NKG2D−/− mice, suggesting an NKG2D-independent function of RAE-1γ. Further studies are needed to define the molecular basis of this NKG2D-independent function of RAE-1γ. It is also possible that this NKG2D-independent immune function is expressed or even induced only in NKG2D-deficient mice. However, the inability of NKG2D blocking to abolish the effect of RAE-1γ on CD8 T-cell priming argues in favor of a constitutive existance of NKG2D-independent function for RAE-1γ.
Priming in the absence of a systemic inflammation accelerates the transition of early-memory to late-memory CD8 T cells (36). In contrast, immunization accompanied with a strong induction of proinflamatory cytokines prevents the transition of memory CD8 T cells, while only minimally altering their expansion. Our results with RAE-1γMCMV vector are in line with this scenario because the level of type I IFN was much lower in RAE-1γMCMV vector-immunized mice compared with the mice infected with WT MCMV. Together with the observation that CD8 T cells in RAE-1γMCMV vector-primed mice secrete higher levels of IFNγ and TNFα, these findings indicate a qualitative difference of memory response after immunization with MCMV vector expressing RAE-1γ.
Stimulation of CD8 T cells has been widely recognized as a method of choice for the development of new vaccines against various pathogens and tumors. RAE-1γMCMV is unique among CD8 T-cell experimental vaccine vectors for several reasons: (i) it is strongly attenuated in vivo; (ii) it possesses deletion of viral immunoevasin, whose product negatively regulates not only the expression of RAE-1γ but also MHC-I presentation; and (iii) it promotes a CD8 T-cell response superior to the response obtained by a vector lacking RAE-1γ expression. Moreover, it generates and maintaines lifelong protective immunity without the need for prime-boost immunization protocols. Bearing in mind that ULBP2 is a homolog of RAE-1γ (37), the translation of these results into a HCMV vector system may be critical for the design of HCMV-based vaccine vectors for various infections and tumors.
Materials and Methods
Mice.
BALB/c, C57BL/6, NKG2D−/− (19), OT-1 and Klrk1Δ/ΔOT1 (NKG2DΔ/ΔOT1) (29), M38 TCR transgenic (27), and C57BL/6 Ly5.1 mice were bred under specific pathogen free conditions at the Faculty of Medicine, University of Rijeka. The 3d mice (24) were bred and maintained at the Helmholtz Centre for Infection Research. Batf3−/− mice were kindly provided by Thomas Brocker (Ludwig Maximilian University, Munich, Germany). All experimental procedures were approved by the Ethics Committee of the University of Rijeka.
Infection.
Mice were infected with MCMV strains at the age of 6–12 wk by injection of the virus in 50 μL of the diluent when administered via footpad (f.p.) or 500 μL of the diluent when administered i.v. The hemolytic EGD strain (serovar1/2a) of L.monocytogenes and recombinant L. monocytogenes that stably express the chicken ovalbumin (aa134–387) (38) were cultured to an exponential growth phase. Infection was performed in 500 μL of pyrogen-free saline i.v.
Flow Cytometry and Immune Assays.
For CD8 T-cell in vitro cytokine production, splenocytes were stimulated with 1 μg of MHC class I restricted peptides, followed by incubation in the presence of Brefeldin A (eBioscience).
Statistics.
Statistical significance was determined by GraphPad Prism5 Software. The differences in viral titers and bacterial load were determined by a two-tailed Mann-Whitney u test. The differences in CD8 T-cell frequencies were determined by a two-tailed Mann-Whitney u test or two-tailed unpaired Student t test. The significant differences between tested groups are indicated with star symbols as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Differences between groups were determined with one-way ANOVA analysis assuming not-repeated measures, followed by Bonferroni posttest. P values of <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank D. Rumora, S. Slavic Stupac, G. Begic, K. Wagner, D. Rebic, and E. Razic for technical help. We also thank Dr. D. Zehn for providing OVA-Listeria and Dr. L. Traven for critical reading of the manuscript. This study was supported by National Institutes of Health Grants 1R01AI083201-01 and ERC-2012-AdG_20120314 (to S.J.) and by the Impuls- und Vernetzungsfonds of the Helmholtz Association, Grant VH-VI-424-4 (to S.J., M.M., and M.M.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310215110/-/DCSupplemental.
References
- 1.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddehase MJ. Antigens and immunoevasins: Opponents in cytomegalovirus immune surveillance. Nat Rev Immunol. 2002;2(11):831–844. doi: 10.1038/nri932. [DOI] [PubMed] [Google Scholar]
- 3.Lemmermann NA, Böhm V, Holtappels R, Reddehase MJ. In vivo impact of cytomegalovirus evasion of CD8 T-cell immunity: Facts and thoughts based on murine models. Virus Res. 2011;157(2):161–174. doi: 10.1016/j.virusres.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Hansen SG, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15(3):293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuda Y, et al. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis. 2011;5(8):e1275. doi: 10.1371/journal.pntd.0001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen SG, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340(6135):1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markiewicz MA, et al. Costimulation through NKG2D enhances murine CD8+ CTL function: Similarities and differences between NKG2D and CD28 costimulation. J Immunol. 2005;175(5):2825–2833. doi: 10.4049/jimmunol.175.5.2825. [DOI] [PubMed] [Google Scholar]
- 8.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235(1):267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonjić S, Babić M, Polić B, Krmpotić A. Immune evasion of natural killer cells by viruses. Curr Opin Immunol. 2008;20(1):30–38. doi: 10.1016/j.coi.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slavuljica I, et al. Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest. 2010;120(12):4532–4545. doi: 10.1172/JCI43961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harty JT, Bevan MJ. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175(6):1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rötzschke O, et al. Exact prediction of a natural T cell epitope. Eur J Immunol. 1991;21(11):2891–2894. doi: 10.1002/eji.1830211136. [DOI] [PubMed] [Google Scholar]
- 13.Bouwer HG, Nelson CS, Gibbins BL, Portnoy DA, Hinrichs DJ. Listeriolysin O is a target of the immune response to Listeria monocytogenes. J Exp Med. 1992;175(6):1467–1471. doi: 10.1084/jem.175.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrero JA, Calderon B, Unanue ER. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J Immunol. 2004;172(8):4866–4874. doi: 10.4049/jimmunol.172.8.4866. [DOI] [PubMed] [Google Scholar]
- 15.Wagner M, Gutermann A, Podlech J, Reddehase MJ, Koszinowski UH. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J Exp Med. 2002;196(6):805–816. doi: 10.1084/jem.20020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zloza A, et al. NKG2D signaling on CD8⁺ T cells represses T-bet and rescues CD4-unhelped CD8⁺ T cell memory recall but not effector responses. Nat Med. 2012;18(3):422–428. doi: 10.1038/nm.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mintern JD, et al. Viral interference with B7-1 costimulation: A new role for murine cytomegalovirus fc receptor-1. J Immunol. 2006;177(12):8422–8431. doi: 10.4049/jimmunol.177.12.8422. [DOI] [PubMed] [Google Scholar]
- 18.Arens R, et al. B7-mediated costimulation of CD4 T cells constrains cytomegalovirus persistence. J Virol. 2011;85(1):390–396. doi: 10.1128/JVI.01839-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zafirova B, et al. Altered NK cell development and enhanced NK cell-mediated resistance to mouse cytomegalovirus in NKG2D-deficient mice. Immunity. 2009;31(2):270–282. doi: 10.1016/j.immuni.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P, Degli-Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat Immunol. 2001;2(11):1077–1084. doi: 10.1038/ni724. [DOI] [PubMed] [Google Scholar]
- 21.Robbins SH, et al. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog. 2007;3(8):e123. doi: 10.1371/journal.ppat.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder CM, Allan JE, Bonnett EL, Doom CM, Hill AB. Cross-presentation of a spread-defective MCMV is sufficient to prime the majority of virus-specific CD8+ T cells. PLoS ONE. 2010;5(3):e9681. doi: 10.1371/journal.pone.0009681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busche A, et al. Priming of CD8+ T cells against cytomegalovirus-encoded antigens is dominated by cross-presentation. J Immunol. 2013;190(6):2767–2777. doi: 10.4049/jimmunol.1200966. [DOI] [PubMed] [Google Scholar]
- 24.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7(2):156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 25.Nopora K, et al. MHC class I cross-presentation by dendritic cells counteracts viral immune evasion. Front Immunol. 2012;3:348. doi: 10.3389/fimmu.2012.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torti N, Walton SM, Brocker T, Rülicke T, Oxenius A. Non-hematopoietic cells in lymph nodes drive memory CD8 T cell inflation during murine cytomegalovirus infection. PLoS Pathog. 2011;7(10):e1002313. doi: 10.1371/journal.ppat.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zafirova B, Wensveen FM, Gulin M, Polić B. Regulation of immune cell function and differentiation by the NKG2D receptor. Cell Mol Life Sci. 2011;68(21):3519–3529. doi: 10.1007/s00018-011-0797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 31.Mescher MF, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 32.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8(2):107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205(3):625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 35.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413(6852):165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11(7):748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson GW, et al. Modulation of natural killer cells by human cytomegalovirus. J Clin Virol. 2008;41(3):206–212. doi: 10.1016/j.jcv.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458(7235):211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.