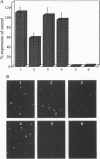
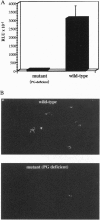
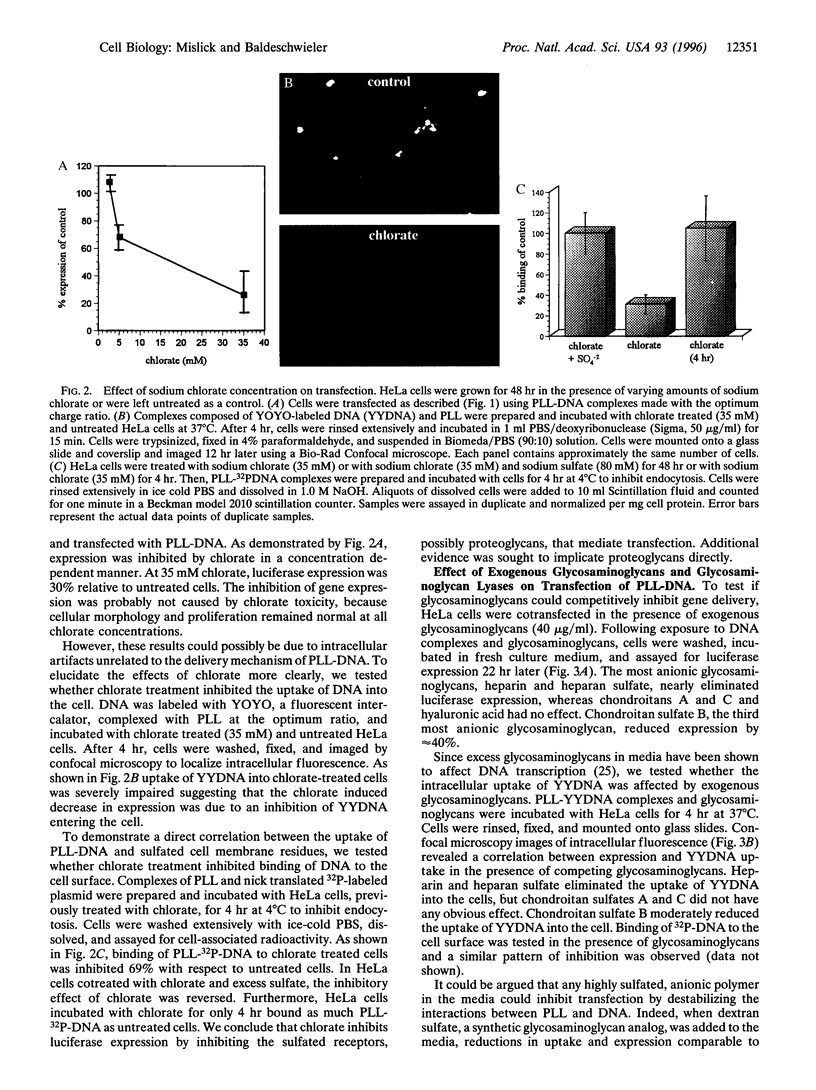
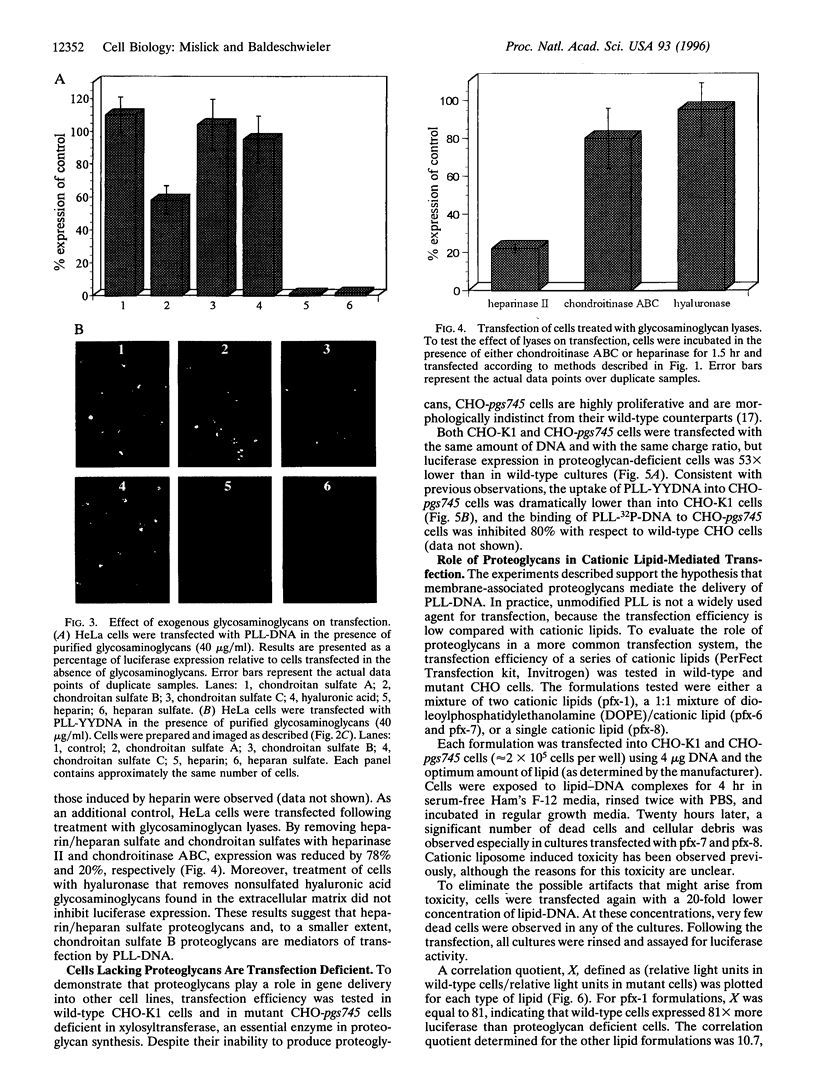
Abstract
We report evidence that gene complexes, consisting of polycations and plasmid DNA enter cells via binding to membrane-associated proteoglycans. Treatment of HeLa cells with sodium chlorate, a potent inhibitor of proteoglycan sulfation, reduced luciferase expression by 69%. Cellular treatment with heparinase and chondroitinase ABC inhibited expression by 78% and 20% with respect to control cells. Transfection was dramatically inhibited by heparin and heparan sulfate and to a smaller extent by chondroitan sulfate B. Transfection of mutant, proteoglycan deficient Chinese hamster ovary cells was 53 x lower than of wild-type cells. For each of these assays, the intracellular uptake of DNA at 37 degrees C and the binding of DNA to the cell membrane at 4 degrees C was impaired. Preliminary transfection experiments conducted in mutant and wild-type Chinese hamster ovary cells suggest that transfection by some cationic lipids is also proteoglycan dependent. The variable distribution of proteoglycans among tissues may explain why some cell types are more susceptible to transfection than others.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Huttner W. B. Chlorate--a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986 Dec 15;141(2):870–877. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- Behr J. P. Gene transfer with synthetic cationic amphiphiles: prospects for gene therapy. Bioconjug Chem. 1994 Sep-Oct;5(5):382–389. doi: 10.1021/bc00029a002. [DOI] [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Busch S. J., Martin G. A., Barnhart R. L., Mano M., Cardin A. D., Jackson R. L. Trans-repressor activity of nuclear glycosaminoglycans on Fos and Jun/AP-1 oncoprotein-mediated transcription. J Cell Biol. 1992 Jan;116(1):31–42. doi: 10.1083/jcb.116.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conget P., Minguell J. J. IL-3 increases surface proteoglycan synthesis in haemopoietic progenitors and their adhesiveness to the heparin-binding domain of fibronectin. Br J Haematol. 1995 Jan;89(1):1–7. doi: 10.1111/j.1365-2141.1995.tb08918.x. [DOI] [PubMed] [Google Scholar]
- De Luca S., Caplan A. I., Hascall V. C. Biosynthesis of proteoglycans by chick limb bud chondrocytes. J Biol Chem. 1978 Jul 10;253(13):4713–4720. [PubMed] [Google Scholar]
- Esko J. D., Stewart T. E., Taylor W. H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faassen A. E., Schrager J. A., Klein D. J., Oegema T. R., Couchman J. R., McCarthy J. B. A cell surface chondroitin sulfate proteoglycan, immunologically related to CD44, is involved in type I collagen-mediated melanoma cell motility and invasion. J Cell Biol. 1992 Jan;116(2):521–531. doi: 10.1083/jcb.116.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Ringold G. M. Cationic liposome-mediated transfection. Nature. 1989 Jan 26;337(6205):387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Rye H. S. Stable dye-DNA intercalation complexes as reagents for high-sensitivity fluorescence detection. Nature. 1992 Oct 29;359(6398):859–861. doi: 10.1038/359859a0. [DOI] [PubMed] [Google Scholar]
- Hortin G. L., Schilling M., Graham J. P. Inhibitors of the sulfation of proteins, glycoproteins, and proteoglycans. Biochem Biophys Res Commun. 1988 Jan 15;150(1):342–348. doi: 10.1016/0006-291x(88)90526-8. [DOI] [PubMed] [Google Scholar]
- Kabanov A. V., Kabanov V. A. DNA complexes with polycations for the delivery of genetic material into cells. Bioconjug Chem. 1995 Jan-Feb;6(1):7–20. doi: 10.1021/bc00031a002. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Reiland J., Rapraeger A. C. Heparan sulfate proteoglycan and FGF receptor target basic FGF to different intracellular destinations. J Cell Sci. 1993 Aug;105(Pt 4):1085–1093. doi: 10.1242/jcs.105.4.1085. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Rye H. S., Yue S., Wemmer D. E., Quesada M. A., Haugland R. P., Mathies R. A., Glazer A. N. Stable fluorescent complexes of double-stranded DNA with bis-intercalating asymmetric cyanine dyes: properties and applications. Nucleic Acids Res. 1992 Jun 11;20(11):2803–2812. doi: 10.1093/nar/20.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh M. T., WuDunn D., Montgomery R. I., Esko J. D., Spear P. G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992 Mar;116(5):1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellrecht C. M., Mars W. M., Miwa H., Beran M., Saunders G. F. Expression pattern of a hematopoietic proteoglycan core protein gene during human hematopoiesis. Differentiation. 1991 Nov;48(2):127–135. doi: 10.1111/j.1432-0436.1991.tb00251.x. [DOI] [PubMed] [Google Scholar]
- Sugumaran G., Silbert J. E. Relationship of sulfation to ongoing chondroitin polymerization during biosynthesis of chondroitin 4-sulfate by microsomal preparations from cultured mouse mastocytoma cells. J Biol Chem. 1990 Oct 25;265(30):18284–18288. [PubMed] [Google Scholar]
- Timpl R. Proteoglycans of basement membranes. Experientia. 1993 May 15;49(5):417–428. doi: 10.1007/BF01923586. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Vertel B. M., Dorfman A. Translation and characterization of messenger RNAs in differentiating chicken cartilage. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4847–4851. doi: 10.1073/pnas.76.10.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertel B. M., Walters L. M., Flay N., Kearns A. E., Schwartz N. B. Xylosylation is an endoplasmic reticulum to Golgi event. J Biol Chem. 1993 May 25;268(15):11105–11112. [PubMed] [Google Scholar]
- Xu Y., Szoka F. C., Jr Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996 May 7;35(18):5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- Zenke M., Steinlein P., Wagner E., Cotten M., Beug H., Birnstiel M. L. Receptor-mediated endocytosis of transferrin-polycation conjugates: an efficient way to introduce DNA into hematopoietic cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3655–3659. doi: 10.1073/pnas.87.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]