Abstract
Fusarium is a cosmopolitan and highly diversified genus of saprophytic, phytopathogenic and toxigenic fungi. However, the existence and diversity of a few species of Fusarium are restricted to a certain area or climatic condition. The present study was conducted to determine the occurrence and diversity of Fusarium species in tropical highland areas in Malaysia and to compare with those in temperate and subtropical regions. A series of sampling was carried out in 2005 to 2009 at several tropical highland areas in Malaysia that is: Cameron Highlands, Fraser Hills and Genting Highlands in Pahang; Penang Hill in Penang; Gunung Jerai in Kedah; Kundasang and Kinabalu Park in Sabah; Kubah National Park and Begunan Hill in Sarawak. Sampling was done randomly from various hosts and substrates. Isolation of Fusarium isolates was done by using pentachloronitrobenzene (PCNB) agar and 1449 isolates of Fusarium were successfully recovered. Based on morphological characteristics, 20 species of Fusarium were identified. The most prevalent species occurring on the highlands areas was F. solani (66.1%) followed by F. graminearum (8.5%), F. oxysporum (7.8%), F. semitectum (5.7%), F. subglutinans (3.5%) and F. proliferatum (3.4%). Other Fusarium species, namely F. avenaceum, F. camptoceras, F. chlamydosporum, F. compactum, F. crookwellense, F. culmorum, F. decemcellulare, F. equiseti, F. nygamai, F. poae, F. proliferatum, F. sacchari, F. sporotrichioides, F. sterilihyphosum and F. verticillioides accounted for 1% recoveries. The present study was the first report on the occurrences of Fusarium species on highland areas in Malaysia.
Keywords: Diversity, Fusarium, Highland Areas, Malaysia
Abstract
Fusarium merupakan genus kosmopolitan dan sangat pelbagai, terdiri daripada kulat yang saprofitik, fitopatogenik dan toxigenik. Walaupun demikian, kewujudan dan kepelbagaian beberapa spesies Fusarium terhad kepada beberapa kawasan tertentu dan keadaan iklim. Kajian ini dijalankan untuk menentukan kewujudan dan kepelbagaian spesies Fusarium dari kawasan tanah tinggi tropika di Malaysia, dan untuk membandingkannya dengan kawasan temperat dan sub-tropika. Persampelan berperingkat dilakukan secara rawak daripada pelbagai perumah dan substrat dari tahun 2005 hingga 2009 di beberapa kawasan tanah tinggi di Malaysia iaitu: Cameron Highlands, Bukit Fraser dan Genting Highlands di Pahang; Bukit Bendera di Pulau Pinang; Gunung Jerai di Kedah; Kundasang dan Taman Kinabalu di Sabah; Taman Negara Kubah dan Bukit Begunan di Sarawak. Pemencilan Fusarium dilakukan menggunakan media agar pentakloronitrobenzena (PCNB) dan sebanyak 1449 pencilan Fusarium telah berjaya diperolehi. Berdasarkan ciri-ciri morfologi, 20 spesies Fusarium telah dikenal pasti. Spesies yang paling banyak diperolehi di kawasan tanah tinggi tersebut adalah F. solani (66.1%) diikuti dengan F. graminearum (8.5%), F. oxysporum (7.8%), F. semitectum (5.7%), F. subglutinans (3.5%) dan F. proliferatum (3.4%). Spesies Fusarium yang lain iaitu F. avenaceum, F. camptoceras, F. chlamydosporum, F. compactum, F. crookwellense, F. culmorum, F. decemcellulare, F. equiseti, F. nygamai, F. poae, F. proliferatum, F. sacchari, F. sporotrichioides, F. sterilihyphosum dan F. verticillioides, masing-masing terdiri daripada 1% pencilan. Kajian ini merupakan kajian yang pertama melaporkan kewujudan spesies Fusarium di kawasan tanah tinggi di Malaysia.
INTRODUCTION
The genus Fusarium is one of the most economically important group of fungi infecting some very important agricultural and horticultural crops worldwide. The fungus can be found in most bioclimatic regions of the world including tropical and temperate grasslands, shrub lands, forests as well as harsh desert and alpine environment, soils associated with plants, organic debris and any part of plants from deepest root to highest flowers (Leslie & Summerell 2006). Therefore, Fusarium occurs in almost all ecosystems worldwide (Young et al. 1978; Nelson et al. 1994; Arney et al. 1997).
Fusarium species have been reported as plant pathogenic fungus causing various plant diseases on a variety of tropical plant parts such as root, fruits, seeds, storage tissues, stem and stalk rots, vascular wilt, canker, die-back, gall and leaf diseases (Stover 1981; Leslie & Summerell 2006). Moreover, isolates of Fusarium can spread through air, soils and from infected plant debris (Summerell et al. 2010).
Climate is one of the important factors which can determine the occurrence of fungi on a broad, regional scale (Money 1972). Malaysia is located in the tropical region which has a hot wet equatorial climate. The mean daily temperature in lowlands throughout the year ranges from 21°C to 32°C whereas in the tropic highlands, the temperature is slightly cooler ranging from 16°C to 26°C.
The highland of Malaysia located in the centre of Peninsular Malaysia (at about 1200 m) consists of granite masses whereas at the interior of Sabah and Sarawak (at about 1200 m to 1800 m) it is densely forested mountainous area with alluvial and swampy coastal plains (Andrews & Freestone 1972; Ooi 1976). The vegetation at the highland areas is mainly oaks, laurels, conifers, myrtles and plants from the family Theaceae. Mount Kinabalu is the highest mountain in Malaysia with different kinds of vegetation. The forest of oaks and conifers at the middle level altitudes are not so tall and they become more dwarfed at higher level where there is an association of Himalayan and temperate region plants such as from the genus Rhododendron (Andrews & Freestone 1972).
Previous studies have shown that mycogeography of Fusarium species was influenced by climatic conditions (Burgess 1981; Burgess et al. 1988; Marasas et al. 1988; Burgess & Summerell 1992). The climatic factor which includes temperature, rainfall and season could influence the distribution of Fusarium species (Sangalang et al. 1995a). In temperate and tropical regions, Fusarium species are diverse in terms of the number of species, distribution, host range and virulence (Gordon 1960; Summerell et al. 2003; Leslie & Summerell 2006). There are some Fusarium species which appear to be limited in certain climatic region while some species were not influenced by climatic factor (Burgess et al. 1988; Summerell et al. 1993). F. compactum had only been recovered in warmer areas while F. solani and F. oxysporum can be found in all climatic regions, and these two species are commonly found in the soil (Burgess et al. 1988; Kommedahl et al. 1988; Jeschke et al. 1990; Leslie et al. 1990). Sangalang et al. (1995b) also reported that climate contributes to the distribution of many Fusarium species but the mechanism is unknown.
Occurrence of Fusarium species in lowland areas in Malaysia have been conducted by Latiffah et al. (2007, 2009, 2010) however, there is no report on the occurrence of Fusarium species in tropical highland areas in Malaysia. Therefore, the present study was carried out to determine the occurrences and diversity of Fusarium species at several tropical highlands areas in Malaysia and to compare with those in temperate and subtropical regions.
MATERIALS AND METHODS
Sampling Site
The samples were collected from 19 sampling sites in the Malaysian tropical highland areas which were located between 400 to 2030 m above sea level from 2005 to 2009. The highland tropical areas were: Cameron Highlands, Fraser’s Hill, Genting Highlands, Penang Hill and Gunung Jerai in Peninsular Malaysia; Kinabalu Park, Kundasang, Begunan Hill and Kubah National Park in Sabah and Sarawak (East Malaysia). Tropical highlands has a more parallel climate than the temperate region with a minimum average temperature of 16.5°C and a maximum average temperature of about 24.8°C (Table 1).
Table 1:
Location of sampling sites of tropical highland areas in Malaysia.
| Site no. | Location | Altitude (m) | Rainfalla (mm) | Mean temperaturea (°C)
|
|
|---|---|---|---|---|---|
| Min | Max | ||||
| C1 | Pine forest reserve, Cameron Highlands | 1829 | 2500 | 15.2 | 21.9 |
| C2 | Gunung Irau (mossy forest), Cameron Highlands | 1828 | 2500 | 15.2 | 21.9 |
| C3 | Gunung Brinchang, Cameron Highlands | 2031 | 2500 | 15.2 | 21.9 |
| C4 | Tringkap’s forest reserve, Cameron Highlands | 1545 | 2500 | 15.2 | 21.9 |
| C5 | Grass, Cameron Highlands | 1545 | 2500 | 15.2 | 21.9 |
| C6 | Boh Tea Plantation, Cameron Highlands | 1829 | 2500 | 15.2 | 21.9 |
| C7 | Ulu Bertam forest reserve, Cameron Highlands | 1500 | 2500 | 15.2 | 21.9 |
| C8 | Waterfall, Cameron Highlands | 1829 | 2500 | 15.2 | 21.9 |
| C9 | Asparagus farm, Cameron Highlands | 1829 | 2500 | 15.2 | 21.9 |
| C10 | Sugarcane plantation, Cameron Highlands | 1829 | 2500 | 15.2 | 21.9 |
| C11 | Soil, Cameron Highlands | 1829 | 2500 | 15.2 | 21.9 |
| C12 | Genting Highlands | 2000 | 2150 | 16.0 | 23.0 |
| C13 | Fraser’s Hill | 1200 | 2350 | 16.2 | 21.3 |
| C14 | Penang Hill | 1805 | 2250 | 19.0 | 25.9 |
| C15 | Gunung Jerai | 1217 | 2500 | 21.0 | 27.0 |
| C16 | Kundasang, Sabah | 2000 | 2500 | 19.0 | 32.0 |
| C17 | Kinabalu Park, Sabah | 1866 | 305 | 13.5 | 20.0 |
| C18 | Begunan’s Hill, Sarawak | 400 | 2100 | 18.5 | 24.5 |
| C19 | Kubah National Park, Sarawak | 800 | 3000 | 12.0 | 32.0 |
Note:
Rainfall and temperature data were provided by the Malaysian Meteorological Department
Isolation of Fusarium Isolates
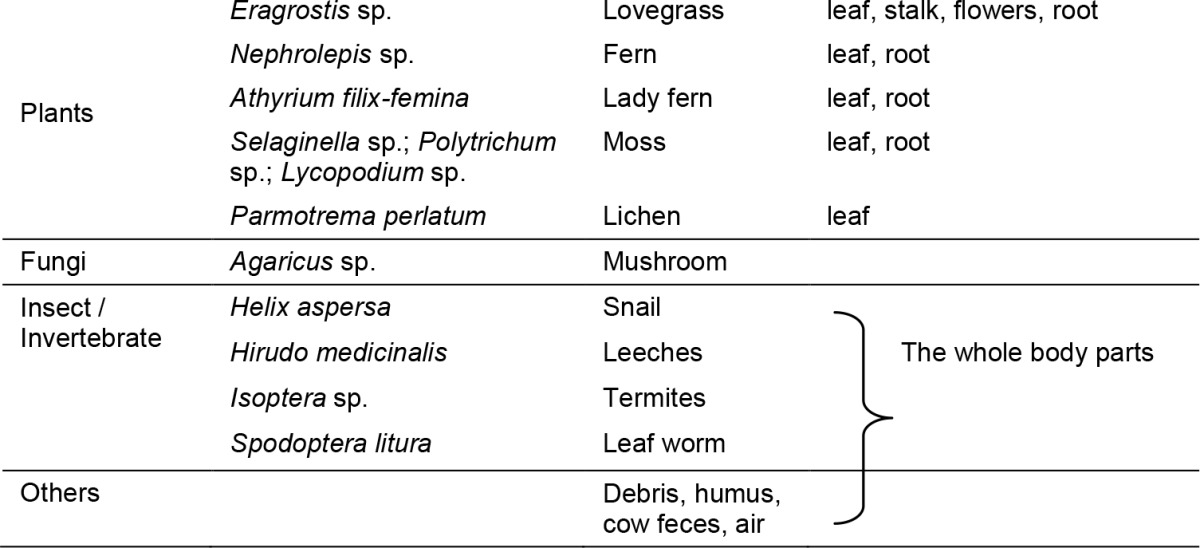
Isolates of Fusarium were isolated from various hosts and substrate as shown in Table 2. Isolation of Fusarium was done by directly plating the plant parts, debris and other substrates onto a semi-selective media, pentachloronitrobenzene (PCNB) agar (Nash & Snyder 1962). For identification, four media were used, namely potato dextrose agar (PDA), potato sucrose agar (PSA), carnation leaf agar (CLA) and water agar (WA) as described in The Fusarium Laboratory Manual (Leslie & Summerell 2006). Microscopic and macroscopic characteristics as described in the manual were used for species identification. Species descriptions were based on Wollenweber and Reinking (1935), Booth (1971), Joffe (1974), Gerlach and Nirenberg (1982), Nelson et al. (1983), Burgess et al. (1994) and, Leslie and Summerell (2006).
Table 2:
Various hosts and substrates for isolation of Fusarium isolates.


RESULTS AND DISCUSSION
A total of 1449 isolates of Fusarium were successfully isolated from 19 highland areas in Malaysia. Twenty species from seven sections were identified based on morphological characteristics. The species were from section Arthrosporiella (F. camptoceras, F. decemcellulare and F. semitectum), Discolor (F. crookwellense, F. culmorum and F. graminearum), Elegans (F. oxysporum), Gibbosum (F. compactum and F. equiseti), Liseola (F. subglutinans, F. nygamai, F. proliferatum, F. sacchari, F. verticillioides and F. sterilihyphosum), Martiella (F. solani), Roseum (F. avenaceum) and Sporotrichiella (F. chlamydosporum and F. sporotrichioides). Table 3 shows the number of Fusarium species and the location from where the species were isolated. From Table 4, the most common species of Fusarium isolated from the highland areas was F. solani (66.1%) followed by F. graminearum (8.5%), F. oxysporum (7.8%), F. semitectum (5.7%), F. subglutinans (3.5%), F. proliferatum (3.4%) and other Fusarium species which comprised 1% recoveries.
Table 3:
Number of Fusarium isolates from various locations.
| Fusarium species |
*Location
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | C13 | C14 | C15 | C16 | C17 | C18 | C19 | |
| F. avenaceum | **– | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F. camptoceras | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F. chlamydosporum | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F. compactum | 1 | 3 | 8 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – |
| F. crookwellense | – | 1 | – | – | – | – | – | – | – | – | – | – | 6 | – | – | – | – | – | – |
| F. culmorum | 8 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F. decemcellulare | 1 | – | 2 | – | 1 | – | – | – | – | – | – | – | 3 | – | – | – | 1 | – | – |
| F. equiseti | – | 2 | – | 1 | 5 | – | – | – | – | – | – | – | – | – | – | 3 | – | – | – |
| F. graminearum | 11 | 5 | 17 | 11 | 73 | 1 | – | – | – | – | – | – | 4 | – | – | – | 1 | – | – |
| F. nygamai | – | – | – | – | – | – | – | 3 | 5 | – | – | – | 2 | – | – | – | – | – | – |
| F. oxysporum | 7 | 5 | 30 | 1 | 7 | 1 | – | 4 | 7 | – | 4 | 16 | 21 | 5 | 2 | – | 3 | – | – |
| F. poae | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F. proliferatum | 4 | 6 | 3 | 1 | 4 | – | – | – | 3 | 1 | – | 3 | 7 | 3 | – | 12 | 1 | – | 1 |
| F. sacchari | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F. semitectum | 21 | 10 | 14 | 5 | 4 | 1 | – | 1 | – | – | – | 1 | 2 | 5 | 1 | 3 | 12 | 2 | – |
| F. solani | 74 | 79 | 167 | 2 | 13 | 9 | 17 | 14 | 1 | 1 | 18 | 41 | 251 | 10 | 74 | 55 | 79 | 8 | 45 |
| F. sporotrichioides | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F. sterilihyphosum | – | – | – | – | 1 | – | – | – | – | – | – | 1 | 1 | – | – | – | – | – | – |
| F. subglutinans | 9 | 4 | 7 | 3 | 2 | 1 | – | – | – | 2 | 3 | 3 | 8 | 1 | 6 | – | 1 | – | – |
| F. verticillioides | – | – | 1 | – | 1 | – | – | – | – | – | – | 1 | 5 | – | – | – | 1 | – | – |
Table 4:
Number of Fusarium isolates recovered from different substrates.
| Species | Substrate | Number of isolates |
|---|---|---|
| F. solani (958) | Soils | 239 |
| Pines | 155 | |
| Flowers | 127 | |
| Mosses | 120 | |
| Trees | 117 | |
| Grasses | 72 | |
| Fruits | 27 | |
| Ferns | 23 | |
| Lichens | 21 | |
| Algae | 19 | |
| Mushrooms | 15 | |
| Insect/Invertebrate | 9 | |
| Faeces | 3 | |
| Weeds | 3 | |
| Debris | 3 | |
| Air sampling | 3 | |
| Humus | 2 | |
|
| ||
| F. graminearum (123) | Grasses | 73 |
| Flowers | 31 | |
| Pines | 14 | |
| Ferns | 3 | |
| Mosses | 2 | |
|
| ||
| F. oxysporum (113) | Soils | 31 |
| Flowers | 33 | |
| Grasses | 22 | |
| Mosses | 8 | |
| Asparagus | 7 | |
| Pines | 7 | |
| Lichens | 4 | |
| Air sampling | 1 | |
|
| ||
| F. semitectum (82) | Flowers | 22 |
| Pines | 20 | |
| Mosses | 14 | |
| Grasses | 13 | |
| Soils | 9 | |
| Air sampling | 1 | |
| Sugarcane | 1 | |
| Asparagus | 1 | |
| Mushroom | 1 | |
|
| ||
| F. subglutinans (50) | Flowers | 16 |
| Pines | 10 | |
| Mosses | 9 | |
| Soils | 6 | |
| Grasses | 5 | |
| Air sampling | 2 | |
| Sugarcane | 2 | |
|
| ||
| F. proliferatum (49) | Asparagus | 14 |
| Pines | 15 | |
| Grasses | 9 | |
| Flowers | 5 | |
| Air sampling | 2 | |
| Mushrooms | 2 | |
| Soil | 1 | |
| Sugarcane | 1 | |
|
| ||
| F. compactum (13) | Pine | 6 |
| Soils | 4 | |
| Mosses | 3 | |
|
| ||
| F. equiseti (11) | Grasses | 5 |
| Asparagus | 4 | |
| Mosses | 2 | |
|
| ||
| F. nygamai (8) | Asparagus | 5 |
| Flowers | 3 | |
|
| ||
| F. culmorum (9) | Grasses | 5 |
| Pines | 4 | |
|
| ||
| F. crookwellense (7) | Grasses | 7 |
|
| ||
| F. verticillioides (9) | Mosses | 5 |
| Pines | 2 | |
| Grasses | 2 | |
|
| ||
| F. decemcellulare (8) | Mosses | 3 |
| Pines | 2 | |
| Soil | 1 | |
| Weed | 1 | |
| Flower | 1 | |
|
| ||
| F. sterilihyphosum (3) | Fern | 1 |
| Flower | 1 | |
| Pine | 1 | |
|
| ||
| F. avenaceum (1) | Moss | 1 |
| F. camptoceras (1) | Pine | 1 |
| F. chlamydosporum (1) | Pine | 1 |
| F. poae (1) | Pine | 1 |
| F. sacchari (1) | Grass | 1 |
F. solani was recovered in all sampling sites, and was the most prevalent in Fraser’s Hill (C13) with 251 isolates. F. solani was recovered from various hosts and substrates which included different types of plants such as pine, flower and grasses as well as from the insect (termites, snail and leeches) (Tables 3 and 4). F. solani was mostly isolated from the soils (239 isolates) followed by leaves from different species of plants. F. solani is a cosmopolitan species, widely distributed in tropical region especially at lowland areas and in the soils in different environments (Leslie & Summerell 2006). F. solani can easily be isolated from different types of soils and had been isolated in subtropical, semiarid and grassland soils (Burgess & Summerell 1992), cultivated soils (Latiffah et al. 2007), forested area (Latiffah et al. 2009), sandy soils (Sarquis & Borba 1997) and from arid and saline environments (Sangalang et al. 1995b; Mandeel 2006).
Although F. graminearum is a well-known pathogen of cereal grains, causing scab or head blight, in the present study, 73 isolates of F. graminearum were isolated from grass (Family: Gramineae) especially from xElyhordeum montanense, which is a wild grass growing at the hillside of Cameron Highlands. F. graminearum has been isolated from wild grass such as Agrostis stolonifera L. (creeping bent grass), Echinochloa crusgalli (L.) Beauv. (barnyard grass), Agropyron trachycaulum (Link) Malte (slender wheat grass) and Bromus ciliates L. (fringed brome) as reported by Inch and Gilbert (2003) and, Goswami and Kistler (2004). Besides grasses, 50 isolates of F. graminearum was also recovered from flowers, stalk pine, leaves and mosses. Burgess et al. (1988) reported that F. graminearum has been isolated from non-agricultural host such as grasses in temperate region.
A total of 113 F. oxysporum isolates were recovered from 14 sampling sites (Table 1) and 31 isolates were isolated mainly from soils, and the other 82 isolates were isolated from asparagus, flowers, grasses, moss, pine, lichen and air sampling. Similar with F. solani, F. oxysporum is a cosmopolitan species and is widespread in different types of soil worldwide. F. oxysporum is also a well-known plant pathogen in tropical and temperate regions; causing wilt and root rot diseases in a variety of agricultural crops, and can be easily isolated from agricultural soils as well as non-agricultural soils (Ooi & Salleh 1999; Baayen et al. 2000; Flood 2006; Latiffah et al. 2010). Some isolates of F. oxysporum are saprophytes especially on plant debris (Moss & Smith 1984; Gordon & Martyn 1997). F. oxysporum also has been reported to be among the most frequently isolated fungus from arid and saline environments (Sangalang et al. 1995b; Mandeel 2006).
Five species of Fusarium, namely F. culmorum, F. crookwellense, F. sporotrichioides, F. poae and F. avenaceum were recovered from different substrates such as moss, grasses and pine (Table 4). The number of isolates recovered was between 1 to 13 isolates. The five species of Fusarium are commonly found in temperate region and are frequently associated with cereal crops or small grains such as barley and wheat. Friebe et al. (1998) reported that F. culmorum was isolated from grasses in temperate region causing root rot disease. Similar with F. culmorum, F. sporotrichioides, F. crookwellense and F. poae was also isolated from grasses and small grains (Perkowski et al. 2003; Inch & Gilbert 2003; Mielniczuk et al. 2004). F. sporotrichioides and F. culmorum have been isolated from pine seed (Douglas-fir) however these species are nonpathogenic towards conifer seedlings (Hoefnagels & Linderman 1999; James & Perez 1999). F. avenaceum was also reported to cause pre- and post-emergence damping-off diseases to conifer germinates (James 1993) and dry rot on potato tubers (Satyaprasad et al. 1997). It is not surprising that these common temperate species occur in highland areas in Malaysia as the highland areas have cooler temperatures ranging from 16°C to 23°C and wetter weathers compared with the lowland areas.
A total of 117 isolates of section Liseola comprising six species namely F. proliferatum (49), F. subglutinans (50), F. nygamai (8), F. verticillioides (9), F. sacchari (14) and F. sterilihyphosum (3) were recovered from different substrates such as asparagus, grasses, pine, soil, maize and others (Table 4). The six species are common plant pathogen, infecting various crops in tropical and temperate regions. F. proliferatum, F. verticillioides and F. subglutinans are common pathogen of ear-rot disease of maize (Zea mays) in both temperate and tropical regions (Magnoli et al.1999; Voss et al. 2007) F. nygamai, F. proliferatum and F. subglutinans has been recovered from soils in three different climate regions in Australia namely tropical, arid and Mediterranean (Sangalang et al. 1995b) and also from soils of tropical highlands. In addition, F. sterilihyphosum is commonly associated with malformation of inflorescence of mango (Mangifera indica) especially in Asia, Africa and the Americas (Britz et al. 2002; Iqbal et al. 2006; Marasas et al. 2006). However, from the present study, F. sterilihyphosum was isolated from flowers and fern. Further studies on F. sterilihyphosum from the two substrates need to be carried out as the morphological characteristics of F. sterilihyphosum are very similar with other species of Fusarium in the section Liseola.
Three species from section Arthrosporiella namely, F. camptoceras, F. decemcellulare and F. semitectum were isolated from pine, grasses, moss, weeds, mushroom, sugarcane, air, asparagus, and soils (Table 4). The three species especially F. semitectum are commonly isolated from various substrates such as soils and plant debris in the tropical region. F. semitectum in particular, has been isolated from different types of soil such as soil from arid regions (Sangalang et al. 1995b), tropical and temperate regions (Burgess et al. 1988). The species is probably found as soil inhabitants (Leslie et al. 1990). F. camptoceras was isolated from leaf and pine (Table 4) and this species is limited, found only in subtropical and tropical regions (Jimenez et al. 1997; Leslie & Summerell 2006). In the present study, F. decemcellulare was isolated from leaf, grass, pine, Sellaginella, seed, flower and weed (Table 4). F. decemcellulare is commonly found in tropical regions (Ploetz et al. 1996) and is often associated with canker of various tree species (Leslie & Summerell 2006).
Two species of Fusarium, F. equiseti and F. compactum from section Gibbosum were isolated from asparagus, grasses, soils and pine tree (Table 4). Both species are well-distributed in warm temperature and subtropical areas (Burgess et al. 1988; Burgess & Summerell 1992). The occurrence of F. equiseti was reported in many tropical, subtropical and temperate countries worldwide (Burgess 1981; Backhouse & Burgess 1995). Whereas, F. compactum is generally recovered in hot arid and semi-arid climates and commonly occurs as soil saprophyte and is rarely found in cooler areas (Backhouse & Burgess 2002). Sangalang et al. (1995b) reported that F. compactum is commonly recovered from a variety of soils.
F. chlamydosporum (section Sporotrichiella) was isolated from pine (Table 4). This species is a common saprophyte on a variety of substrates especially in soils of arid and semi-arid areas and has been reported to cause damping-off of rooibos tea plants (Engelbrecht et al. 1983) and stem canker of okra (Fugro 1999).
Among all the sampling sites, the majority (21.39%) of Fusarium isolates was recovered from Fraser’s Hill (C13) and the least number of isolates (0.28%) were from sugarcane field (C10) in Cameron Highlands. The results of the present study showed that a variety of Fusarium species occurs in tropical highland areas in Malaysia. Fusarium species which are commonly found in the temperate region were also found in tropical highland although in fewer numbers such as F. graminearum, F. culmorum, F. sporotrichioides and F. avenaceum, and several of these species are pathogenic to agricultural crops. Most of Fusarium species found in tropical region such as F. solani, F. oxysporum and F. semitectum can also be found in temperate region. Further studies on these species should be conducted to determine whether the species are climate or geographically dependent.
Acknowledgments
USM Fellowship was granted to the first author and Research University Postgraduate Research Grant Scheme (1001/PBIOLOGI/833011) from Universiti Sains Malaysia is duly acknowledged. The project was also supported by Research University Grant (1001/PBIOLOGI/811179).The authors would like to thank Mohd Kamarudin Mohd Maidin, Siti Norsyila Mahmud, Nurul Farizah Azzudin, Noor Fazila Mohamed Yahaya, Bintra Mailina and Darnetty for their cooperation and contribution in sample collection.
REFERENCES
- Andrews RL, Freestone CS. A geography of Indonesia, Malaysia, Singapore. 2nd ed. Australia: George Philip & O’Neil Pty Ltd; 1972. [Google Scholar]
- Arney KL, Tiernan R, Judson MA. Primary pulmonary involvement of Fusarium solani in lung transplant recipient. The American College of Chest Physicians. 1997;112(4):1128–1130. doi: 10.1378/chest.112.4.1128. [DOI] [PubMed] [Google Scholar]
- Baayen RP, O’Donnell K, Bonants PJM, Cigelnik E, Kroon LPNM, Roebroeck EJA, Waalwijk C. Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae specials causing wilt and rot disease. Phytopathology. 2000;90(8):891–900. doi: 10.1094/PHYTO.2000.90.8.891. [DOI] [PubMed] [Google Scholar]
- Backhouse D, Burgess LW. Climatic analysis of the distribution of Fusarium graminearum, F. pseudograminearum and F. culmorum on cereals in Australia. Australasian Plant Pathology. 2002;31(4):321–327. [Google Scholar]
- Backhouse D, Burgess LW. Mycogeography of Fusarium: Climatic analysis of the distribution within Australia of Fusarium species in section Gibbosum. Mycological Research. 1995;99(10):1218–1224. [Google Scholar]
- Booth C. The genus Fusarium. Kew, Surrey, England: Commonwealth Mycological Institute; 1971. [Google Scholar]
- Britz H, Steenkamp ET, Coutinho TA, Wingfield BD, Marasas WFO, Wingfield MJ. Two new species of Fusarium section Liseola associated with mango malformation. Mycologia. 2002;94(4):722–730. doi: 10.1080/15572536.2003.11833199. [DOI] [PubMed] [Google Scholar]
- Burgess LW. General ecology of Fusaria. In: Nelson PE, Toussoun TA, Cook RJ, editors. Fusarium: Diseases, biology, and taxonomy. Pennsylvania, USA: Pennsylvania State University Press; 1981. pp. 276–286. [Google Scholar]
- Burgess LW, Summerell BA. Mycogeography of Fusarium: Survey of Fusarium species from subtropical and semiarid grassland soils from Queensland Australia. Mycological Research. 1992;96(9):780–784. [Google Scholar]
- Burgess LW, Nelson PE, Toussoun TA, Forbes GA. Distribution of Fusarium species in section Roseum, Arthrosporiella, Gibbosum and Discolor recovered from grassland, pasture and pine nursery soils of Eastern Australia. Mycologia. 1988;80(6):815–824. [Google Scholar]
- Burgess LW, Summerell BA, Bullock S, Gott KP, Backhouse D. Laboratory manual for Fusarium research. Sydney: University of Sydney; 1994. pp. 116–117. [Google Scholar]
- Engelbrecht MC, Smit WA, Knox-Davies PS. Damping off of rooibos tea, Aspalathus linearis. Phytophylactica. 1983;15(3):121–124. [Google Scholar]
- Flood J. A review of fusarium wilt of oil palm caused by Fusarium oxysporum f. sp. elaeidis. Phytopathology. 2006;96(6):660–662. doi: 10.1094/PHYTO-96-0660. [DOI] [PubMed] [Google Scholar]
- Friebe A, Vilich V, Hennig I, Kluge M, Sicker D. Detoxification of benzoxazolinone allelochemicals from wheat by Gaeumannomyces graminis var. tritici, G. graminis var. graminis, G. graminis var. avenar, and Fusarium culmorum. Applied and Environmental Microbiology. 1998;64(7):2386–2391. doi: 10.1128/aem.64.7.2386-2391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugro PA. A new disease of okra (Abelmoschus esulentus L.) in India. Journal of Mycology and Plant Pathology. 1999;29(2):264. [Google Scholar]
- Gerlach W, Nirenberg HI. The genus Fusarium – A pictorial atlas Mitteilungen aus der Biologischen Bundesanstalt für Land-und Forstwirtschaft Berlin-Dahlem, 209. Germany: Kommissionsverlag Paul Parey; 1982. [Google Scholar]
- Gordon WL. The taxonomy and habitats of Fusarium species from tropical and temperate regions. Canadian Journal of Botany. 1960;38(4):643–658. [Google Scholar]
- Gordon TR, Martyn RD. The evolutionary biology of Fusarium oxysporum. Annual Review of Phytopathology. 1997;35:111–128. doi: 10.1146/annurev.phyto.35.1.111. [DOI] [PubMed] [Google Scholar]
- Goswami RS, Kistler HC. Heading for disaster: Fusarium graminearum on cereal crops. Molecular Plant Pathology. 2004;5(6):515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- Hoefnagels MH, Linderman RG. Biological suppression of seedborne Fusarium spp. during cold stratification of Douglas-fir seeds. Plant Disease. 1999;83(9):845–852. doi: 10.1094/PDIS.1999.83.9.845. [DOI] [PubMed] [Google Scholar]
- Inch SA, Gilbert J. Survival of Gibberella zeae in Fusarium-damaged wheat kernels. Plant Disease. 2003;87(3):282–287. doi: 10.1094/PDIS.2003.87.3.282. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Mehboob-ur-Rahman, Dasti AA, Saleem A, Zafar Y. RAPD analysis Fusarium isolates causing “Mango Malformation” disease in Pakistan. World Journal Microbiology and Biotechnology. 2006;22(11):1161–1167. [Google Scholar]
- James RL. Northern Region Nursery Disease Notes #129. USA: US Department of Agriculture; 1993. Fusarium species associated with post-emergence damping-off and root disease of young conifer-grown Douglas-fir seedlings in USDA Forest Service Nursery, Coeur d’Alene, Idaho. [Google Scholar]
- James RL, Perez R. Forest Health Protection Report 99-8. USA: US Department of Agriculture; 1999. Pathogenic characteristics of Fusarium sporotrichioides isolated from inland Pacific Northwest forest nurseries. [Google Scholar]
- Jeschke N, Nelson PE, Marasas WFO. Fusarium spp. isolated from soil samples collected at different altitudes in the Transkei, southern Africa. Mycologia. 1990;82(6):727–733. [Google Scholar]
- Jimenez M, Huerta T, Mateo R. Mycotoxin production by Fusarium species isolated from bananas. Applied and Environmental Microbiology. 1997;63(2):364–369. doi: 10.1128/aem.63.2.364-369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe AZ. A modern system of Fusarium taxonomy. Mycopathologia. 1974;53(1):201–228. doi: 10.1007/BF02127209. [DOI] [PubMed] [Google Scholar]
- Kommedahl T, Abbas HK, Burnes PM, Mirocha CJ. Prevalence and toxigenicity of Fusarium spp. from soils of Norway near the Arctic Circle. Mycologia. 1988;80(6):790–794. [Google Scholar]
- Latiffah Z, Mohd. Zariman M, Baharuddin S. Diversity of Fusarium species in cultivated soils in Penang. Malaysian Journal of Microbiology. 2007;3(1):27–30. [Google Scholar]
- Latiffah Z, Nurul Izzati H, Baharuddin S. Fusarium species isolated from peat soil of Pondok Tanjung and Sungai Beriah, Perak. Malaysian Journal of Microbiology. 2010;6(1):102–105. [Google Scholar]
- Latiffah Z, Padzilah MI, Baharuddin S, Maziah Z. Fusarium species in forest soil of Bird Valley. Malaysian Journal of Microbiology. 2009;5(2):132–133. [Google Scholar]
- Leslie JF, Summerell BA. The Fusarium laboratory manual. Oxford, UK: Blackwell Publishing Ltd; 2006. [Google Scholar]
- Leslie JF, Pearson CAS, Nelson PE, Toussoun TA. Fusarium species from corn, sorghum, and soybean fields in the central and eastern United States. Phytopathology. 1990;80(4):343–350. [Google Scholar]
- Magnoli CE, Saenz MA, Chiacchiera SM, Dalcero AM. Natural occurrence of Fusarium species and fumonisin-production toxigenic strains isolated from poultry feeds in Argentina. Mycopathologia. 1999;145(1):35–41. doi: 10.1023/a:1007053617961. [DOI] [PubMed] [Google Scholar]
- Mandeel QA. Biodiversity of the genus Fusarium in saline soil habitats. Journal of Basic Microbiology. 2006;46(6):480–494. doi: 10.1002/jobm.200510128. [DOI] [PubMed] [Google Scholar]
- Marasas WFO, Burgess LW, Anelich RY, Lamprecht SC, Van Shalkwyk DJ. Survey of Fusarium species associated with plant debris in South African soils. South African Journal of Botany. 1988;54(1):63–71. [Google Scholar]
- Marasas WFO, Ploetz RC, Wingfield MJ, Steenkamp ET. Mango malformation disease and the associated Fusarium species. Phytopathology. 2006;96(6):667–672. doi: 10.1094/PHYTO-96-0667. [DOI] [PubMed] [Google Scholar]
- Mielniczuk E, Kiecana I, Perkowski J. Susceptibility of oat genotypes to Fusarium crookwellense Burgess, Nelson and Toussoun infection and mycotoxins accumulation in kernels. Biologia Bratislava. 2004;59(6):809–816. [Google Scholar]
- Money DC. Climate, soils and vegetation. London: University Tutorial Press; 1972. [Google Scholar]
- Moss MO, Smith JE. The applied mycology of Fusarium. New York: Cambridge University Press; 1984. [Google Scholar]
- Nash SN, Snyder WC. Quantitative estimations by plate counts of propagules of the bean rot Fusarium in field soils. Phytopathology. 1962;52:567–572. [Google Scholar]
- Nelson PE, Toussoun TA, Marasas WFO. Fusarium species: An illustrated manual for identification. Pennsylvania, USA: Pennsylvania State University Press; 1983. [Google Scholar]
- Nelson PE, Dignani MC, Anaissie EJ. Taxonomy, biology and clinical aspect of Fusarium species. Clinical Microbiology Review. 1994;7(4):479–504. doi: 10.1128/cmr.7.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi JB. Peninsular Malaysia. London: Longman Group Limited; 1976. [Google Scholar]
- Ooi KH, Salleh B. Vegetative compatibility groups of Fusarium oxysporum, the causal organism of vascular wilt on Roselle in Malaysia. Biotropia. 1999;12:31–41. [Google Scholar]
- Perkowski J, Kiecana I, Stachowiak J, Basinski T. Natural occurrence of scirpentriol in cereals infected by Fusarium species. Food Additives and Contaminants. 2003;20(6):572–578. doi: 10.1080/0265203031000100773. [DOI] [PubMed] [Google Scholar]
- Ploetz R, Vazquez A, Benscher D. First report of Fusarium decemcellulare as a pathogen of mango in the United States. Plant Disease. 1996;80(10):1207. [Google Scholar]
- Sangalang AE, Backhouse D, Burgess LW. Survival and growth in culture of four Fusarium species in relation to occurrence in soils from hot climatic regions. Mycological Research. 1995a;99(5):529–533. [Google Scholar]
- Sangalang AE, Burgess LW, Backhouse D, Duff J, Wurst M. Mycogeography of Fusarium species in soils from tropical, arid and Mediterranean regions of Australia. Mycological Research. 1995b;99(5):523–528. [Google Scholar]
- Sarquis MIM, Borba CM. Fusarium species in sandy soils from Ipanema Beach, Rio de Janeiro, Brazil. Journal of Basic Microbiology. 1997;37(6):425–439. [Google Scholar]
- Satyaprasad K, Bateman GL, Read PJ. Variation in pathogenicity on potato tubers and sensitivity to thiabendazole of the dry rot fungus Fusarium avenaceum. Potato Research. 1997;40(4):357–366. [Google Scholar]
- Stover RH. Fusarium diseases in the tropics. In: Nelson PE, Toussoun TA, Cook RJ, editors. Fusarium: Diseases, biology and taxonomy. Pennsylvania USA: Pennsylvania State University Press; 1981. pp. 114–120. [Google Scholar]
- Summerell BA, Rugg CA, Burgess LW. Mycogeography of Fusarium: Survey of Fusarium species associated with forest and woodland communities in north Queensland, Australia. Mycological Research. 1993;97(8):1015–1019. [Google Scholar]
- Summerell BA, Salleh B, Leslie JF. A utilitarian approach to Fusarium identification. Plant Disease. 2003;87(2):117–128. doi: 10.1094/PDIS.2003.87.2.117. [DOI] [PubMed] [Google Scholar]
- Summerell BA, Laurence MH, Liew ECY, Leslie JF. Biogeography and phylogeography of Fusarium: A review. Fungal Diversity. 2010;44(1):3–13. [Google Scholar]
- Voss KA, Smith GW, Haschek WM. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Animal Feed Sciences and Technology. 2007;137(3–4):299–325. [Google Scholar]
- Wollenweber HW, Reinking OA. Die Fusarien, ihre Beschreibung, Schadwirkung und Bekampfung. Berlin: Paul Parey; 1935. [Google Scholar]
- Young NA, Kwon-Chung KJ, Kubota TT, Jennings AE, Fisher RI. Disseminated infection by Fusarium moniliforme during treatment for malignant lymphoma. Journal Clinical Microbiology. 1978;7(6):589–594. doi: 10.1128/jcm.7.6.589-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


