Abstract
Human DNA polymerases mu (Polµ) and lambda (Polλ) are X family members involved in the repair of double-strand breaks in DNA during non-homologous end joining. Crucial abilities of these enzymes include bridging of the two 3′ single-stranded overhangs and trans-polymerization using one 3′ end as primer and the other as template, to minimize sequence loss. In this context, we have studied the importance of a previously uncharacterised sequence (‘brooch’), located at the N-terminal boundary of the Polß-like polymerase core, and formed by Tyr141, Ala142, Cys143, Gln144 and Arg145 in Polµ, and by Trp239, Val240, Cys241, Ala242 and Gln243 in Polλ. The brooch is potentially implicated in the maintenance of a closed conformation throughout the catalytic cycle, and our studies indicate that it could be a target of Cdk phosphorylation in Polµ. The brooch is irrelevant for 1 nt gap filling, but of specific importance during end joining: single mutations in the conserved residues reduced the formation of two ended synapses and strongly diminished the ability of Polµ and polymerase lambda to perform non-homologous end joining reactions in vitro.
INTRODUCTION
Most nucleic acid-synthesizing enzymes exhibit large structural changes on binding to the DNA substrate: some of the substrate binding energy is used to organize the active site and orient this substrate for catalysis. Similarly, the binding of a nucleotide to a high-fidelity DNA polymerase/DNA complex induces a subtle change in the structure of the enzyme from an ‘open’ to a ‘closed’ state [see Supplementary Figure S1A for an example with Polß; reviewed in (1)]. One role of this conformational change is to allow the rapid binding of substrates (and release of products) in the open state, while inducing optimal alignment of catalytic residues surrounding the substrates in the closed state. The shape of the base pair formed determines the fate of the weakly bound nucleotide during the conformational change step: only a correct base pair induces closing of the enzyme to form a tight catalytic complex; conversely, if a mismatched nucleotide is bound, the enzyme does not close, but rather proceeds to release the nucleotide while reducing the rate of catalysis.
Structural characterization of several DNA polymerases from families A, B and X suggests that deoxynucleotide (dNTP) binding induces large conformational changes affecting the relative positioning of both fingers and thumb subdomains (2–4). In the case of the human X family of polymerases, Polß has been the paradigm for structural examination of mechanistic details. The structure of free Polß showed that the polymerization domain (31 kDa), composed of subdomains fingers, palm and thumb, adopts a structure in space similar to a partially left hand, like other DNA polymerases evolutionarily unrelated (5,6); conversely its N-terminal domain (8 kDa) is shown to be located away from the polymerization domain in this structure (Supplementary Figure S1A, Polß – Apo). Crystallization of Polß complexed with different DNA molecules demonstrated the structural basis for its preference for small DNA gaps. Polß complexed with a template-primer substrate shows an arrangement in which the 8 kDa domain is not interacting with the DNA, being positioned at a distance from the polymerization domain, similar to the apoenzyme structure (7,8). In contrast, the crystal structure of Polß complexed with a 1 nt gapped substrate shows that the 8 kDa domain binds to the 5′-phosphate of the gap while also interacting with the DNA substrate through the thumb subdomain [(2,8); see Supplementary Figure S1A, Polß – Binary], thus promoting a more stable binding of the polymerase to this substrate. The resolution of the structure of Polß with a 1 nt gap and incoming nucleotide allowed further study of the polymerization mechanism and conformational changes that occur during catalysis. It was determined that the incoming nucleotide causes the transition from an open to a closed complex by induction of a conformational change of the thumb subdomain (2), which has a decisive influence on the fidelity of the reaction [(9,10), see Supplementary Figure S1A, Polß – Ternary].
Unexpectedly, structural characterization of polymerase lambda (Polλ) indicated that its catalytic cycle does not involve major subdomain motions [see comparison of a binary complex with gapped DNA (PDB ID: 1XSL) versus a pre-catalytic ternary complex with gapped DNA and incoming nucleotide (PDB ID: 1XSN), at Supplementary Figure S1B]. Thus, unlike Polß, Polλ is in a closed conformation before dNTP binding, and a shift of the DNA template is observed when comparing both crystal complexes (11) and predicted by molecular dynamic simulations (12). Informative crystal structures leading to this conclusion do not exist for polymerase mu (Polµ): the only one solved is a ternary complex with gapped DNA and incoming nucleotide [(13); PDB ID: 2IHM; Supplementary Figure S1C]. However, like for Polλ, an ab initio closed conformation of the Polµ apoenzyme is predicted by molecular dynamics simulations (14).
In this work, we describe the functional importance of a newly recognized sequence located in the N-terminal boundary of the polymerase ß-like core, formed by five residues highly conserved in Polµs (YACQR), Terminal deoxynucleotydil transferases (TdTs) (YACQR) and Polλs (WVCAQ) of different species, but absent in Polβ. This sequence, which we called ‘brooch’, seems to be involved in the maintenance of a closed conformation throughout the catalytic cycle, mediating interactions between the 8 kDa domain and the thumb subdomain. Our site-directed mutagenesis studies indicate that the lack of the conserved residues of the brooch affects specific functions of human Polµ and Polλ, such as end-bridging and trans-polymerization, or the filling-in of long gaps in DNA. These activities are of special importance during DNA repair processes such as non-homologous end joining (NHEJ), a pathway completely indispensable for the maintenance of genomic stability and cell viability.
MATERIALS AND METHODS
DNA and proteins
Synthetic DNA oligonucleotides were obtained from Isogen (Ijsselstein, Holland). PAGE-purified oligonucleotides were labelled at their 5′ ends with [γ-32P]ATP. The oligonucleotides used to generate the DNA substrates were the following: for 1 nt gapped substrates, Sp1C (5′GATCACAGTGAGTAC), T13C (5′AGAAGTGTATCTCGTACTCACTGTGATC) and DG1-P (5′AGATACACTTCT); for 5 nt gapped substrates, Sp1C, T18 (5′ACTGGCCGTCGTTCTATTGTACTCACTGTGATC) and DG5 (5′AACGACGGCCAGT). For NHEJ assays, four sets of oligonucleotides were used. A first set of primers sharing a common part (5′CCCTCCCTCCC …) and bearing different 3′ protrusions (1AC […CA3′], 1TG […GT3′], 1A […A3′], 1C […C3′], 1G […G3′], 1T […T3′]), were hybridized to 1D-NHEJ (5′GGGAGGGAGGG). A second set of primers sharing the common part (5′GCACTCACGTCCC…), and bearing different 3′ overhangs (2AC […CA3′], 2TG […GT3′], 2A […A3′], 2C […C3′], 2G […G3′], 2T […T3′]), were hybridized to oligonucleotide 2D-NHEJ (5′-GGGACGTGAGTGC). A third set of primers sharing the common part (5′CCCTCCCTCCG…), and bearing different 3′ overhangs (D3 […CGGC], D3+C […GCGGCC]), were hybridized to D1 (5′CGGAGGGAGGG). A fourth set of primers sharing the common part (5′ CCCTCCCTCCG…), and bearing different 3′ overhangs (D4 […CGCC], D4GC […CGGCC]), were hybridized to D2 (5′GGGACGTGAGTGCGCG). Oligonucleotides DG1-P, DG5, 2D-NHEJ and D2-P contain a 5′-P group when indicated. Ultrapure dNTPs, dideoxynucleotides (ddNTPs) and [γ-32P]ATP (3000 Ci/mmol) were purchased from GE Healthcare (USA). T4 polynucleotide kinase was obtained from New England Biolabs (Beverly, MA, USA). Pfu DNA polymerase was purchased from Promega Corporation (Madison, WI, USA).
Construction and purification of human Polµ and mutant versions
Site-directed mutagenesis by single PCR with oligonucleotides containing the desired mutation was performed on the over-expression plasmid pRSETA-hPolµ (15). The oligonucleotides used were the following:
Y141F (5′TGGATGCCTGCCTTTGCCTGCCAGCGC),
Y141S (5′TGGATGCCTGCCTCTGCCTGCCAGCGC),
C143G (5′CCTGCCTATGCCGGCCAGCGCCCTACG),
R145A (5′TATGCCTGCCAGGCCCCTACGCCCCTC),
R145K (5′TATGCCTGCCAGAAACCTACGCCCCTC),
N153G (5′CTCACACACCACGGCACTGGCCTCTCC),
R446G (5′CAGCGGGAGCTGCGCGGCTTCAGCCGGAAGGAG),
T147A (5′GCCTGCCAGCGCCCTGCGCCCCTCACACAC),
T147E (5′GCCTGCCAGCGCCCTGAGCCCCTCACACAC).
DNA constructs were sequenced and transformed in Escherichia coli BL21(DE3)pLysS (Stratagene). Wild-type and mutant Polµ variants were over-expressed and purified in an Äkta Purifier FPLC system (GE Healthcare) with the following protocol: the cleared bacterial lysate was loaded on a heparin column followed by an SP Sepharose column. The selected fractions were then loaded on a HiPrep Sephacryl 26/60 to eliminate contaminant nucleases. The eluted fractions containing highly purified protein were concentrated and stored at −80°C.
Construction and purification of human Polλ and mutant versions
Site-directed mutagenesis by a single PCR with oligonucleotides containing the desired mutation was performed on the over-expression plasmid pET22b-Polλ (16). The oligonucleotides used were the following:
W239Y (5′CCTGCTGTCCTGGATAAGTATGTCTGTGCACAGCCC),
W239G (5′CCTGCTGTCCTGGATAAGGGGGTCTGTGCACAGCCC),
C241S (5′GTCCTGGATAAGTGGGTCAGTGCACAGCCCTCAAGC),
C241G (5′GTCCTGGATAAGTGGGTCGGTGCACAGCCCTCAAGC),
Q243R (5′GTGGGTCTGTGCACGGCCCTCAAGCCAGAAGGCG),
Q243G (5′GTGGGTCTGTGCAGGGCCCTCAAGCCAGAAGGCG).
Over-expression of wild-type and the desired mutants of Polλ was carried out in E. coli BL21-pRIL (Stratagene) as described (16). DNA in the cleared bacterial lysate was precipitated with 0.3% polyethyleneimine and sedimented by centrifugation. The supernatant was precipitated with ammonium sulphate to 35% saturation to obtain a polyethyleneimine-free protein pellet. Further purification was conducted on a Äkta Purifier FPLC system (GE Healthcare). The pellet was resuspended in Buffer A and diluted to 0.2 M NaCl. Then it was filtered and applied to a 5 ml of HiTrap Heparin column. The eluted fractions containing the protein of interest were loaded in a 1.7 ml of Mono S Sepharose column. The resulting fractions containing the protein of interest were diluted in Buffer A to 0.2 M NaCl and loaded again in a 5 ml of HiTrap Heparin column. The final pooled fractions were stored at −80°C in 50% glycerol.
DNA polymerization and NHEJ assays
Several DNA substrates, containing 5 nM 5′P-labelled primers, were incubated at 30°C with the indicated polymerase in the presence of dNTPs and activating metal ions. The concentrations of each of these components, as well as the incubation times, are indicated in the figure legends in each case. The reaction mixture, in 20 µl, contained 50 mM Tris–HCl (pH 7.5), 1 mM dithiothreitol (DTT), 4% glycerol and 0.1 mg/ml bovine serum albumin (with the addition of 10% PEG 8000 in the case of Polλ in NHEJ assays). After incubation, reactions were stopped by adding gel-loading buffer [95% (v/v) formamide, 10 mM EDTA, 0.1% (w/v) xylene cyanol and 0.1% (w/v) bromophenol blue] and analysed by 8 M urea/20% PAGE and autoradiography. When indicated, we used ddNTPs instead of dNTPs to limit incorporation to a single nucleotide on the 3′-end of the labelled oligonucleotide.
Electrophoretic mobility shift assays
Electrophoretic mobility shift assays (EMSAs), used to analyse the interaction with gapped-substrates and NHEJ intermediates, were performed in a final volume of 12.5 µl, containing 50 mM Tris–HCl (pH 7.5), 0.1 mg/ml bovine serum albumin, 1 mM DTT, 4% glycerol, 5 nM labelled DNA and different concentrations of the indicated proteins. After incubation for 10 min at 30°C, samples were mixed with 3 µl of 30% glycerol and resolved by native gel electrophoresis on a 4% polyacrylamide gel (80:1 [w/w] acrylamide/bisacrylamide). Gels were dried, and labelled DNA fragments were detected by autoradiography.
In vitro kinase assays
Phosphorylation was carried out in a final volume of 20 µl of containing kinase buffer [50 mM Tris (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 2 mM DTT, 0.01% Brij 35, 100 µM ATP and 0.3 µCi of [γ-32P]ATP], in the presence of substrate (either Histone H1 or 700 ng of Polµ) and five units of the indicated Cdk/cyclin complexes (New England Biolabs). Reactions were carried out for 30 min at 30°C, and the proteins were separated on a 10% SDS–PAGE. Reactions were stopped by addition of loading buffer and boiling for 2 min at 95°C. The gel was stained with Coomassie Brilliant Blue, dried and exposed on an X-ray film.
Amino acid sequence comparisons and 3D modelling
Multiple alignments of different DNA polymerases were done using the program MULTALIN (http://prodes.toulouse.inra.fr/multalin/). The different conformations of the studied residues, motifs and domains in the X family polymerases were analysed with the software MacPymol (http://delsci.com/macpymol/).
RESULTS
The brooch: a closed conformation of the Polµ polymerase core is optimal for NHEJ
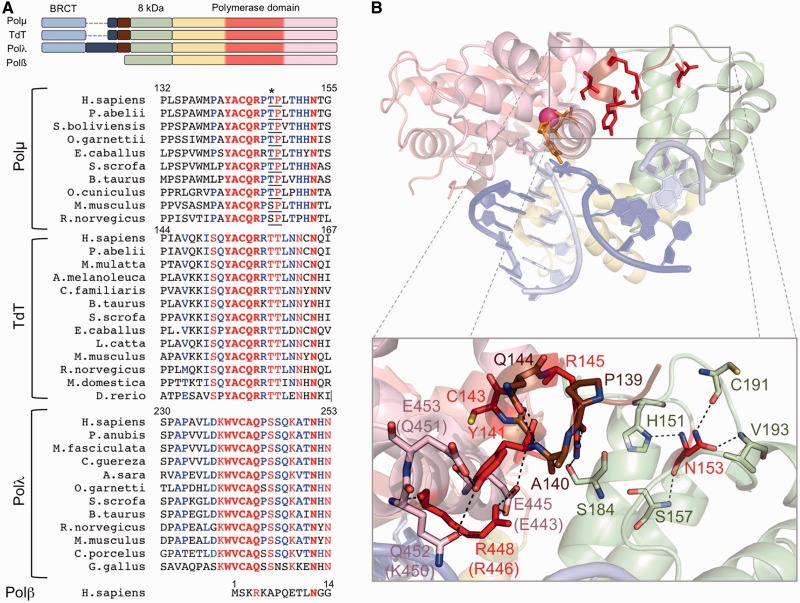
Despite the lack of enough structural information regarding Polµ catalytic mechanism, comparison of the existing structures and multiple amino acid sequence alignments guided us in the identification of an amino acid sequence that is present in the catalytic core of Polµ, TdT and Polλ, but absent in Polß. The brooch is formed by the five amino acids Tyr141, Ala142, Cys143, Gln144 and Arg145 (YACQR) in Polµs and TdTs, and it is extraordinarily conserved among different species (Figure 1A). In Polλs, the conserved sequence is slightly different but includes again five similar amino acids: Trp239, Val240, Cys241, Ala242 and Gln243 (WVCAQ, see Figure 1A). This sequence, located at the very N-terminal part of the core, preceding the 8 kDa domain, was not included in any of the Polλ structures that have been crystallized (17). Fortunately, the crystallized murine Polµ core did include the brooch, and therefore we could delineate its specific interactions with residues located in the thumb subdomain (Figure 1B): Tyr141 interacts directly with thumb residues Gln452 and Glu453 (Lys450 and Glu451 in human Polµ) through their side chain, and with the brooch residue Gln144 (in this case through the backbone of both residues). Arg448 (Arg446 in human Polµ) is also part of this network of interactions and directly stacks against Tyr141. Moreover, the brooch residue Arg145 is interacting with two other residues (Pro139, Ala140) and with Ser184 located at the 8 kDa subdomain, thus helping to maintain the general architecture of the brooch. We speculate that these interactions would be involved in the maintenance of the closed conformation of the core and consequently in the correct positioning of the DNA, specifically during the first step of a NHEJ reaction in which the protein has to interact with two separate DNA substrates, as modelled in Figure 1B. A constitutively closed core could be critical to correctly position the downstream DNA end, which would be in any case bound by its 5′-phosphate group to the 8 kDa domain of the polymerase, but also has to interact with protein residues for fine-tuned orientation during end-bridging. Therefore, if the protein architecture were not properly maintained, these interactions with the downstream side of the break (and also with the upstream side) would be less stable due to the constant ‘breathing’ of the polymerase core: the correct positioning of the two 3′-protrusions in the active site of the polymerase would not be achieved—lowering the efficiency of the NHEJ reaction.
Figure 1.
The brooch: amino acid conservation and implications for the closing of the polymerase core. (A) Top: schemes showing the domain organization of the human PolXs. The subdomains in the polymerase domain are coloured as follows: fingers in yellow, palm in red and thumb in pink. Bottom: multiple sequence alignment of Polλs, Polµs and TdTs of different species and human Polβ. Invariant residues inside each group are shown in red, conserved residues in blue. The brooch and the invariant asparagine equivalent to Polµ Asn153 are highlighted in bold. Asterisk indicates a Cdk phosphorylation residue at the minimal consensus sequence (TP/SP; underlined). (B) Cartoon representation of the murine Polµ ternary complex with the domains coloured as in (A). Labels of some residues are given for both mouse and human (in parentheses) counterparts, as they differ. The DNA substrate is shown in sticks, dark blue for the template strand and light blue for the primer and downstream strands. One nucleotide of the template strand has been eliminated to mimic a NHEJ intermediate formed by two 3′-protruding substrates. Residues from the brooch are shown in brown, unless those subjected to mutagenesis, which are shown in red sticks; residues contacting them directly are shown in the colour of the subdomain they belong to.
To test this idea, we mutated each of the three most conserved residues in the brooch of human Polµ. The point mutations were designed as follows: Tyr141 was mutated either to phenylalanine (Y141F) to maintain the overall shape of the amino acid while eliminating the reactive group, or serine (Y141S) to get the opposite result; Cys143 was mutated to glycine (C143G), and Arg145 was mutated to either lysine (R145K), to maintain the positive charge, or alanine (R145A), to eliminate any function of this residue. We also targeted another residue, Asn153, which might be indirectly involved in maintenance of brooch conformation. Asn153 is located in the 8 kDa domain, and although it is not establishing direct interactions with any residue of the brooch or the thumb subdomain, it is part of a network of interactions [His151, Ser157, Cys191 (Ser191 in human Polµ), Val193] that maintains the general conformation of this part of the 8 kDa domain (Figure 1B). This structural function seems to be crucial, given the invariant conservation of this residue among Polµs, TdTs and Polλs of different species, and also in human Polβ (Figure 1A). We mutated Asn153 to glycine (N153G) to test whether this destabilization affects the function of the brooch.
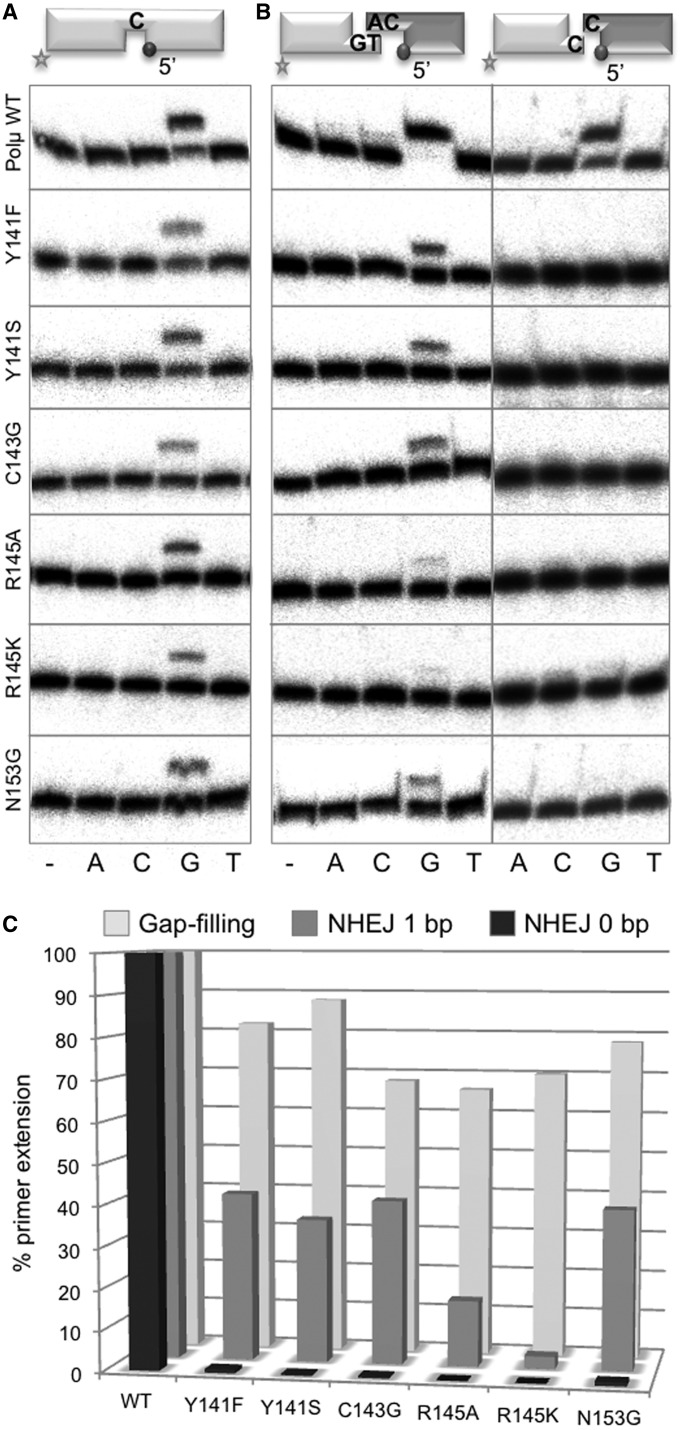
The purified mutants were firstly analysed on template-dependent polymerization activity assays. Gap-filling activity by these mutants was mildly affected (both in efficiency and nucleotide insertion fidelity) when compared with wild-type Polµ (Figure 2A and C). We next tested the activity of these mutants on either complementary or non-complementary NHEJ substrates (18). NHEJ activity on complementary ends was strongly reduced in all cases: mutants in Tyr141, Cys143 and Asn153 barely reached 40% of wild-type activity, whereas mutants R145A (16%) and R145K (3%) were much strongly affected (Figure 2B, left panels, and C). In the case of the non-complementary substrates, the activity of all the mutants was null when performing the assays in the conditions used for wild-type Polµ (Figure 2B, right panels, and C). When assayed with a 100-fold higher ddNTP concentration (Supplementary Figure S1E), we could detect some activity in the case of mutants Y141F (conservative mutation) and N153G, much less with mutants Y141S and C143G, and still none with mutants R145A and R145K. Therefore, our results demonstrate the relevance of the brooch, and by extrapolation, of a closed conformation of Polµ, during NHEJ. Moreover, brooch residue Arg145 is highly specific for NHEJ reactions: although the two mutants at this residue reached 70% of wild-type gap-filling activity, polymerization on NHEJ substrates, either complementary or non complementary, was very low or undetectable (Figure 2B and C).
Figure 2.
Mutagenesis of the brooch specifically affects the end-joining activity of Polµ. (A) Gap-filling reactions were performed for 30 min at 30°C with wild-type Polµ and the indicated mutants (25 nM) using a gapped substrate containing the oligonucleotides SP1C, T13C and DG1-P. dNTPs were added separately at 10 nM in the presence of 2.5 mM MgCl2. (B) NHEJ reactions were performed with 200 nM Polµ and using two sets of substrates: the labelled substrates contain 1TG or 1C and 1D-NHEJ, and the cold substrates, either 2AC or 2C and 2D-NHEJ. The dark spheres indicate the presence of a 5′-P group, which forces binding of this substrate to the 8 kDa domain of Polµ and thus its usage as the downstream part of the break. ddNTPs were added separately at 10 µM in the presence of 2.5 mM MgCl2. (C) Quantification of primer extension by wild-type Polµ and the indicated mutants in gap-filling and NHEJ reactions.
A closed conformation of the Polµ core promotes binding to NHEJ substrates
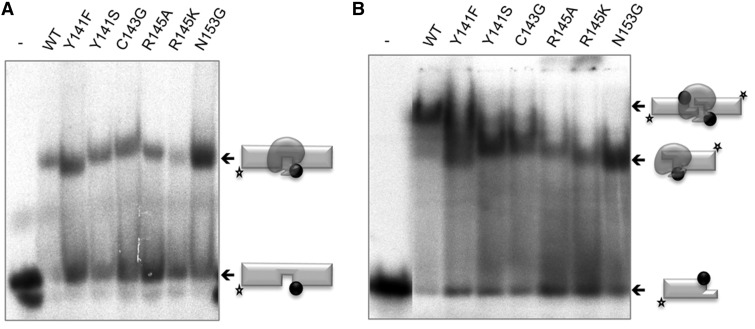
As a control, we first tested the binding of the brooch mutants to the same gapped DNA substrate used in the activity assays, and all displayed similar levels of DNA binding as the wild-type, as established by EMSA analysis of the enzyme/DNA binary complex formed (Figure 3A). These results are in agreement with the wild-type-like gap-filling activity shown by these mutants (Figure 2A). To test whether the inability of the brooch mutants to perform NHEJ of incompatible ends was primarily due to some defect in binding/bridging DNA ends with short, non-complementary, 3′-protrusions, we carried out EMSA experiments with the same 3′-protruding ends used in the NHEJ assays. Figure 3B shows that all the mutants displayed a similar binding to a single DNA end as the wild-type Polµ (lower shifted band); this can be expected, as binding to one end is largely dependent on its 5′-P pocket. Interestingly, wild-type (WT) Polµ produces a super-shifted band that must be a 2:1 (DNA:enzyme) synaptic complex for the following reasons: EMSA on gapped substrate showed that Polµ binds the substrate in a unique way (only one sharp retarded band), with the enzyme nested at the gap position flanked by the 5′-P [as confirmed by footprinting assays (18)], but not at the blunt ends of the DNA molecule. Extrapolation of this knowledge to the case of the NHEJ substrate implies that Polµ must be binding the 3′-protruding side of the substrate (through its 5′-P group), and not the blunt end. This rules out the possibility that more than one polymerase is stably bound to a single DNA molecule, producing the super-shift. Then, the super-shifted band must contain two DNA ends. As any of these two ends can provide one binding site to the polymerase, the super-shifted band could correspond to synapses involving two different complexes (either 2:1 or 2:2; DNA:prot). It could be envisioned that two polymerase molecules, each bound to one DNA end, could bring them together to form a 2:2 complex as the one observed in the bacterial NHEJ machinery (19). Nevertheless, the footprinting size (9 nt) produced by a single Polµ molecule (18), observed also in the structure of Polµ bound to a gapped substrate (PDBID: 2IHM), makes a single Polµ monomer competent to bridge the two sides of the break at the same time; conversely, the formation of a 2:2 complex would be hindered owing to sterical reasons. Thus, we infer that the super-shifted band does correspond to a 2:1 (DNA:pol) synapsis. Strikingly, none of the mutants were able to fully reproduce the second super-shifted band that corresponds to the synapsis. Mutant Y141F had a moderate defect in DNA binding in agreement with its residual activity. Mutation of Asn153 is clearly not affecting the general structure of the 8 kDa domain, as binding to the 5′-P group is not compromised; thus, its structural role must have to do with maintaining the framework needed for a correct functioning of the brooch. All these results reinforce the idea that the brooch is not essential to bind the template/downstream (T/D) DNA end, a task that relies on the recognition of the 5′-P by the 8 kDa domain (18). However, it is likely that the brooch is important for the correct orientation of the 3′-protrusion acting as a template. If that end is not correctly positioned, synapsis of the second end would be unstable, and catalysis would be impaired. This is especially true for non-complementary DNA ends, where the ability of Polµ to align the two 3′ overhangs, one as primer and the other as template, is crucial to achieve a correct synapsis.
Figure 3.
A closed conformation of the polymerase core promotes binding to NHEJ substrates. (A) EMSA was performed for the wild-type Polµ and the indicated mutants (200 nM) using a gapped substrate containing the oligonucleotides SP1C, T13C and DG1-P. (B) EMSA was performed for the wild-type Polµ and the indicated mutants (600 nM) using a 3′-protruding substrate formed by hybridization of 1TG with 1D-NHEJ.
We propose that the brooch is required to keep the closed conformation necessary for correct binding of NHEJ substrates and further catalysis. Although visual proof such as crystallization of the wild-type protein and comparison with selected mutants is unavailable, we took advantage of the fact that the ‘brooched’ domains are connected by linker regions that are possibly exposed to the solvent, therefore accessible to proteases. We performed partial proteolysis analyses on both Polµ and Polλ and a selection of strongly affected mutants to determine whether these mutations produce a defective close conformation. As shown in Supplementary Figure S2, both wild-type Polµ and Polλ showed a pattern of degradation that includes the appearance of a band corresponding to the polymerase core (red asterisks), lacking the Breast cancer gene 1 C-Terminal domain (BRCT domain) and the flexible Ser/Pro linker. Mutations on specific residues of the brooch changed this pattern: this band was less obvious, and a new band was formed (arrowheads). By using mass spectrometry, we have demonstrated that this new proteolytic fragment, running in a position close to the polymerase core, has lost the N-terminus of the protein (Supplementary Figure S2B). The appearance of this new band can only be interpreted as a change in the protease sensitivity of the linker, due to a defective brooch function that leads to a change of conformation of the polymerase in this region.
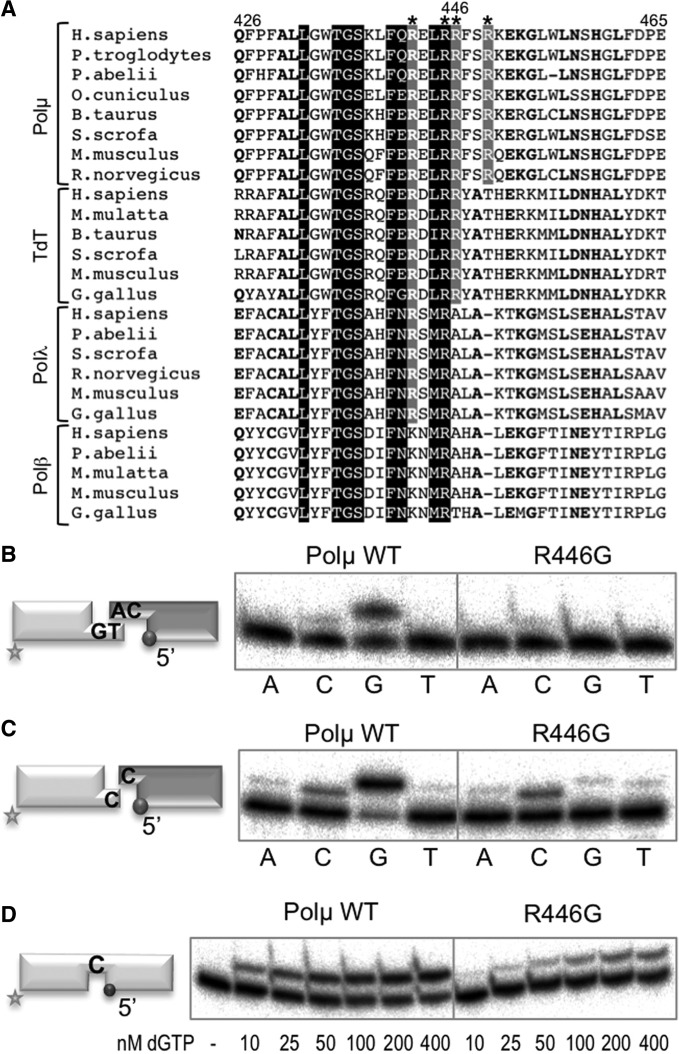
Mutation of Arg446 in human Polµ mimics disruption of the brooch
A specific arginine residue, located in the thumb subdomain, was predicted as important for the brooch function: in the murine structure, Arg448 interacts with Gln452 and Glu453, also in the thumb, and stacks against Tyr141, part of the brooch (see Figure 1B). Arg448 forms part of a positively charged α-helix that contains four arginine residues (Arg444, Arg447, Arg448 and Arg451) oriented towards the negatively charged phosphate backbone of the template strand (data not shown). Arg448 (Arg446 in human Polµ) is the only residue of these four that is exclusively conserved among Polµs and TdTs of different species (Figure 4A). Human Polµ mutant R446G was obtained to determine how Arg446 might be contributing to the function of the brooch in Polµ. When tested in NHEJ substrates, the only incorporation observed with mutant R446G corresponds to error-prone insertions, a result of the terminal transferase activity of Polµ, whereas the error-free templated insertion is only observed with the wild-type enzyme in either complementary (Figure 4B) or non-complementary DNA ends (Figure 4C). As a control, we tested R446G in polymerization on a 1 nt gap, and the mutant displayed a level of activity that was 60% wild-type (Figure 4D), a minor defect compared with the one shown in NHEJ substrates. These results clearly emphasize the importance of a perfectly orchestrated end synapsis during NHEJ, in which all the elements (two DNA ends and incoming nucleotide) must be stabilized by the polymerase in proper register for catalysis.
Figure 4.
Mutation of the interacting thumb residue Arg446 mimics disruption of the brooch. (A) Multiple sequence alignment showing the region corresponding to helix N in the four X family polymerases from different species. Numbers indicate the residues in the human Polµ sequence. The four conserved arginines (Arg444, Arg447, Arg448 and Arg451 in mouse Polµ) oriented towards the negatively charged phosphate backbone of the template strand are boxed in grey or black and indicated with an asterisk. Arg446 in human Polµ is numbered. (B) NHEJ reactions were performed for 30 min at 30°C with 400 nM of the indicated proteins and using two sets of substrates: the labelled substrate contained 1TG and 1D-NHEJ and the cold one 2AG and 2D-NHEJ. Each of the four ddNTPs (100 µM) was added in the presence of 2.5 mM MgCl2. (C) NHEJ reactions were performed as in (B) but using non-complementary DNA substrates, the labelled one containing 1C and 1D-NHEJ, and the cold one containing 1C and 2D-NHEJ. (D) Gap-filling reactions were performed for 30 min at 30°C with the indicated proteins (100 nM) using a gapped substrate containing SP1C, T13C and DG1-P. dGTP was added separately at the indicated concentrations in the presence of 2.5 mM MgCl2.
The brooch in Polµ may be a Cdk phosphorylation target
Inspection of the sequence of Polµ revealed the existence of a possible Cdk phosphorylation site very close to the brooch, at residue Thr147 (indicated with an asterisk in Figure 1A). This site contains the minimal consensus (TP, Thr-Pro) and is highly conserved among Polµs from different species. In murine Polµ, Ser147 occupies this position, and in the crystal structure, Ser147 was shown to be indirectly contacting residue Arg145 of the brooch and acting itself as a bridge between the 8 kDa domain and the thumb subdomain, through direct contacts with Gln493 and Asn495 (Figure 5A). In vitro kinase assays with the wild-type human Polµ and mutant T147A were carried out with two different complexes: Cdk2/cyclin A (associated with S phase) and Cdc2/cyclin B (important for G2/M transition and progression through M phase). Histone HI was used as a positive control of phosphorylation. As shown in Figure 5B, a clear phosphorylation signal could be observed with the two cyclins (although the phosphorylation signal is more intense in the case of Cdk2/cyclin A), meaning that Polµ is a substrate of Cdk complexes and thus could be cell-cycle regulated. The second conclusion is that the Cdk site at Thr147 does not seem to be the main Cdk phosphorylation site in the sequence of Polµ, in agreement with our recent data (Esteban V., Martin MJ. and Blanco L., manuscript under revision). It was not possible to detect this putative phosphorylation site by mass-spectrometry after in vitro phosphorylation, as none of the peptides detected contained this site. However, and to elucidate the possible impact of phosphorylation-mediated regulation in a region so closely related with the brooch, the phospho-mimicking mutation T147E was obtained and tested it on a range of in vitro assays. As expected, mutants T147E and T147A displayed a wild-type phenotype in 1 nt gap-filling reactions (Figure 5C). On the other hand, when tested for NHEJ on non-complementary ends, the activity of mutant T147E was completely abolished, whereas the control mutation to alanine did not produce any detectable effect (Figure 5D). These results lead us to propose that the maintenance of a closed conformation of the polymerase core, which impacts highly specialized functions of Polµ, might be regulated in a cell-cycle-dependent manner. This hypothesis needs to be further tested in vivo, as our data cannot yet confirm the existence of a functional Cdk site at this position and its implications for Polµ activity during the cell cycle.
Figure 5.
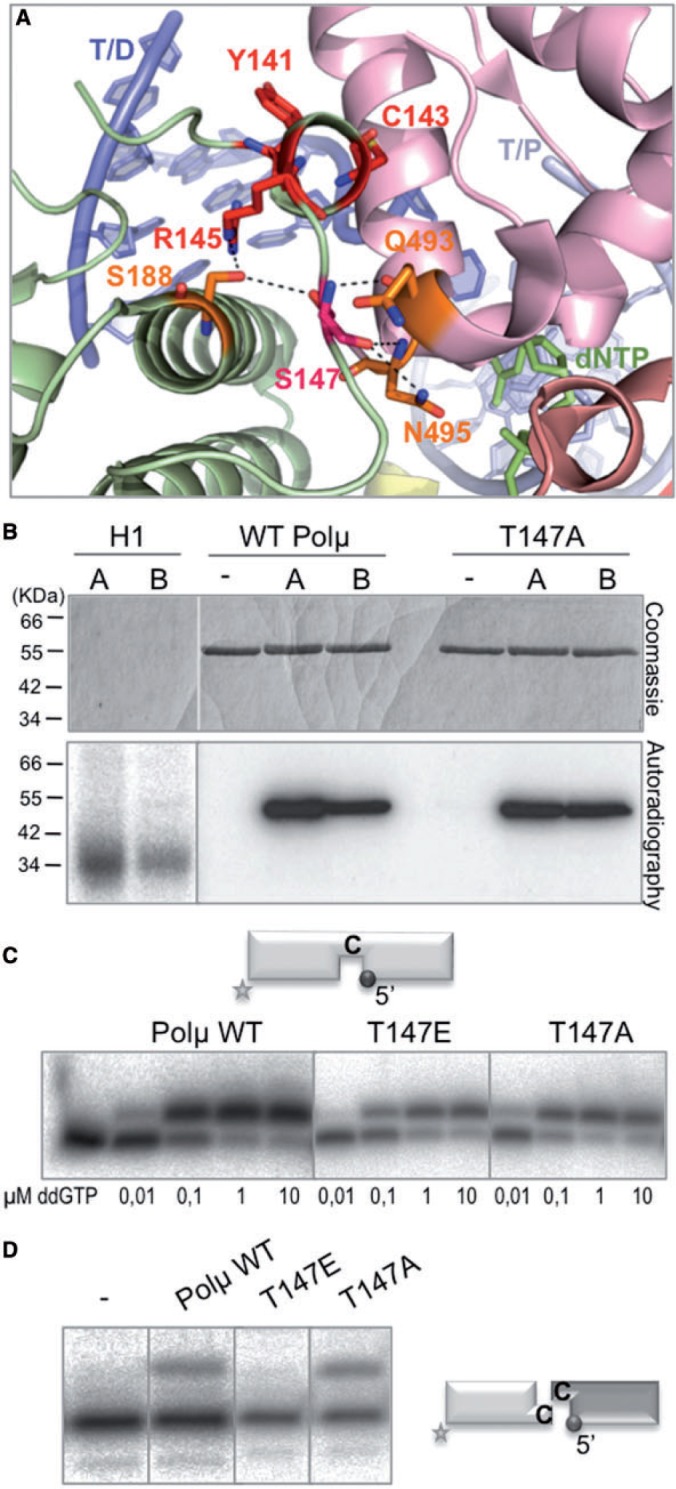
The brooch in Polµ may be regulated via Cdk phosphorylation. (A) Cartoon representation of the murine Polµ ternary complex with the domains coloured as in Figure 1A. Residues of the brooch are shown in red sticks, Ser147 (equivalent to Thr147 in human Polµ) in hot pink and residues contacting it directly in orange. (B) In vitro Cdk phosphorylation assay of histone H1 (100 ng), wild-type Polµ and T147A mutant (700 ng) was carried out as described in ‘Materials and Methods’ section. Two different Cdk complexes were used in this assay, Cdk2/cyclin A (A) and Cdc2/cyclin B (B). The amount of the different proteins was visualized by Coomassie staining, the labelled portion was detected by autoradiography. (C) Gap-filling reactions were performed for 30 min at 30°C with the indicated proteins (25 nM) using a gapped substrate containing SP1C, T13C and DG1-P. As indicated, a range of ddGTP concentrations was used in the presence of 2.5 mM MgCl2. (D) NHEJ reactions were performed for 30 min at 30°C with 200 nM of the indicated proteins and using two substrates: the labelled substrate contained 1C and 1D-NHEJ, and the cold one contained 2C and 2D-NHEJ. ddGTP was added at 10 µM in the presence of 2.5 mM MgCl2.
The brooch of Polλ improves end-bridging efficiency and binding to long DNA gaps
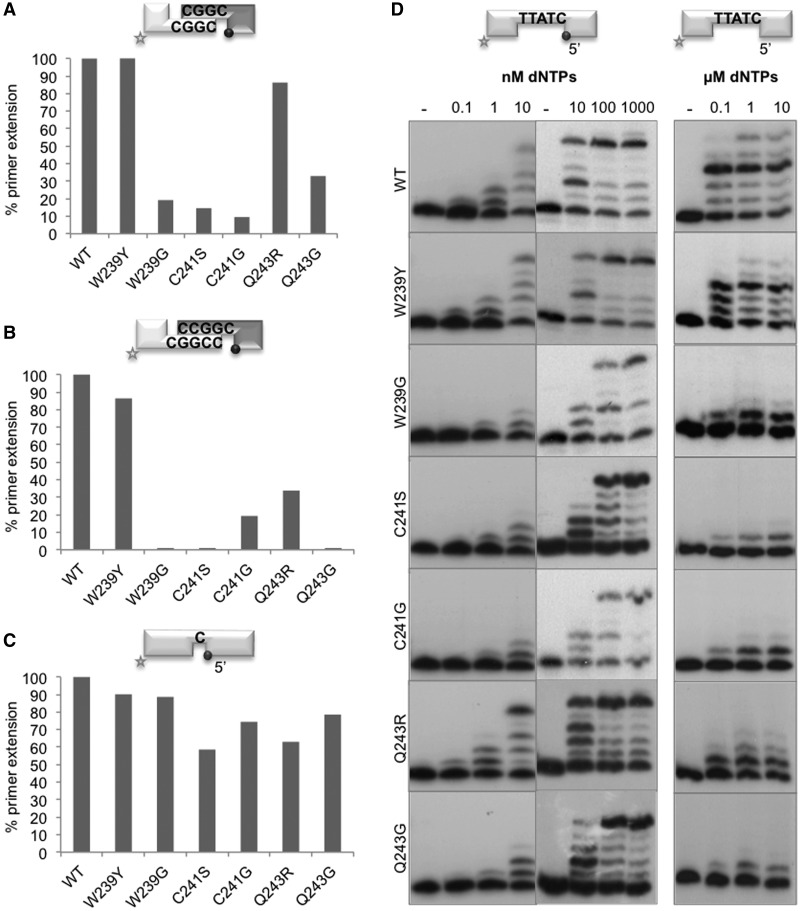
As previously indicated, the brooch is also conserved in Polλ, but its function during the catalytic cycle and its structural and mechanistic implications have not been studied to date. Thus, we mutated the WVCAQ brooch as follows: Trp239 was changed either to tyrosine (mimicking the Polµ sequence) or glycine; Cys241 was mutated to either serine or glycine (having a similar size); Gln243 was changed either to arginine (to modify the charge of the side chain while maintaining its size and structure) or glycine. As Polλ needs complementarity to achieve end bridging (20), we used substrates with a limited (3 bp) microhomology. These reactions were performed in the presence of manganese, a metal cofactor known to increase the rate of catalysis by stabilizing the nucleotide in the active site (21). Under these conditions, the mutants showed <30% of wild-type activity, with the exception of W239Y and Q243R (Figure 6A and Supplementary Figure S3C).
Figure 6.
The brooch of Polλ improves end-bridging efficiency and binding to long DNA gaps. (A) NHEJ reactions were performed with the wild-type Polλ and the indicated mutants (500 nM) in the presence of 1 µM ddGTP and 0.1 mM MnCl2 at 30°C for 60 min. Two separate sets of substrates were used: the labelled substrates contain D3 and D1, and the cold substrates, D4 and D2-P. (B) NHEJ reactions were performed with Polλ and the indicated mutants (500 nM) in the presence of 1 µM ddGTP and 2.5 mM MgCl2 at 30°C for 60 min. Two separate sets of substrates were used: the labelled substrates contain D3+C and D1, and the cold substrates contain D4GC and D2-P. (C) Gap-filling reactions were performed with the wild-type Polλ and the indicated mutants (50 nM) in the presence of 10 nM ddGTP and 2.5 mM MgCl2, using a gapped substrate containing the oligonucleotides SP1C, T13C and DG1-P at 30°C, 20 min. (D) Polymerization reactions were performed with the wild-type Polλ and the indicated mutants (50 nM) in the presence of 2.5 mM MgCl2 at 30°C for 20 min, using two different DNA substrates: GAP5-P (left panels) containing SP1C, T18 and DG5-P and GAP5-OH (right panel) containing SP1C, T18 and DG5. dNTPs concentration is indicated. After denaturing electrophoresis, the labelled fragments were detected by autoradiography.
Interestingly, Polλ was much more efficient (4–5-fold) during NHEJ of DNA ends with 4 bp of complementarity, thus allowing the efficient use of magnesium as cofactor (compare Supplementary Figure S3C and D, WT panels). Even using this more favourable connection, and perhaps a more physiological metal cofactor, the mutants (with the exception of W239Y) displayed very low (C241G and Q243R) or even undetectable (W239G, C241S, Q243G) levels of NHEJ activity compared with the wild-type enzyme (Figure 6B and Supplementary Figure S3D). However, when the mutants were tested on a 1 nt gap as the most favourable substrate, their activity was only slightly affected (to a much lesser extent than in NHEJ reactions; Figure 6C and Supplementary Figure S3A and B). As longer gaps can eventually arise as NHEJ intermediates to be handled by Polλ, the brooch mutants were also tested for gap filling on a 5 nt gapped substrate bearing a 5′-P group. As shown in Figure 6D (left panels), the activity of mutants W239G, C241S, C241G and Q243G was significantly affected. Moreover, the activity of the brooch mutants was further reduced when tested on DNA substrates with a lower binding affinity, such as a 5 nt gap lacking the 5′P group (Figure 6D, right panel). This defective activity of some brooch mutants was primarily due to an impaired binding to long gaps as demonstrated by EMSA: all the mutants were perfectly able to stably bind a 1 nt gapped substrate (Supplementary Figure S4A), whereas their binding to the 5 nt gap was affected (Supplementary Figure S4B), except for mutants W239Y and Q243R, in agreement with their high-activity levels. Formation of a more stable ternary complex in this 5 nt gap, which requires providing the first complementary nucleotide in the EMSA assays, but also the positioning of four template bases in the ‘scrunching’ pocket (22), was even more difficult for the mutants (Supplementary Figure S4C), Interestingly, the ‘scrunching’ pocket is located between the 8 kDa domain and the thumb subdomain, very close to the brooch. Thus, according to our mutational data, the Polλ brooch could be involved in the architecture of the scrunching pocket, contributing to Polλ ability to bind the ‘yet-to-be-copied’ downstream nucleotides.
In summary, these results show that the Polλ brooch is important for its NHEJ function, as described for Polµ, but it is also particularly important to bind substrates containing gaps longer than 1 nt, which require a functional scrunching site.
DISCUSSION
Compelling evidence indicates that the NHEJ pathway minimizes loss of genetic material by using any template available: the overhangs formed during alignment of DSBs are usually filled in, allowing retention of the maximum sequence possible (23–25). The NHEJ pathway takes advantage of highly specialized polymerases, capable of dealing with two DNA ends at once: one providing a primer strand with a protruding 3′-OH, and another that provides a recessive 5′-P and a template strand, also 3′-protruding. Thus, for this task, the polymerase must extend the primer using a templating base provided in trans: ‘alignment based gap fill-in’. In the case of family X polymerases, the ability to act during NHEJ is dictated by their template dependence (26), which relies on structural differences among the four polymerases.
Some of the DNA polymerases belonging to the X-family contain non-catalytic regulatory domains, such as an N-terminal BRCT domain implicated in protein–protein (27) and protein–DNA interactions (28,29), followed by a flexible linker known as the Ser-Pro domain. In the past few years, data from different laboratories have indicated the possible implication of the non-catalytic domains, and specifically the Ser-Pro domain, in the regulation of the activity of the polymerase domain: first, a deletion mutant of the first 244 amino acids in Polλ (BRCT and Ser-Pro domains) was shown to display stronger strand-displacement that the wild-type Polλ (30); second, pre-steady-state kinetics comparing full-length Polλ with a truncated form that again lacks the first 244 amino acids indicated that this Ser-Pro domain confers an increase in fidelity by lowering the incorporation rate constants of incorrect nucleotides, thus allowing Polλ to reach the fidelity levels of Polß (31). However, all the biochemical studies leading to the conclusion that the Ser-Pro domain is implicated in the regulation of the catalytic activity of the polymerase have been performed on deletion mutants, which not only lacked the Ser-Pro (and BRCT) domain but also a previously unnoticed region of the protein that has been studied in depth in the work presented here: the brooch, present in Polλ, Polµ and also TdT, but absent in Polβ.
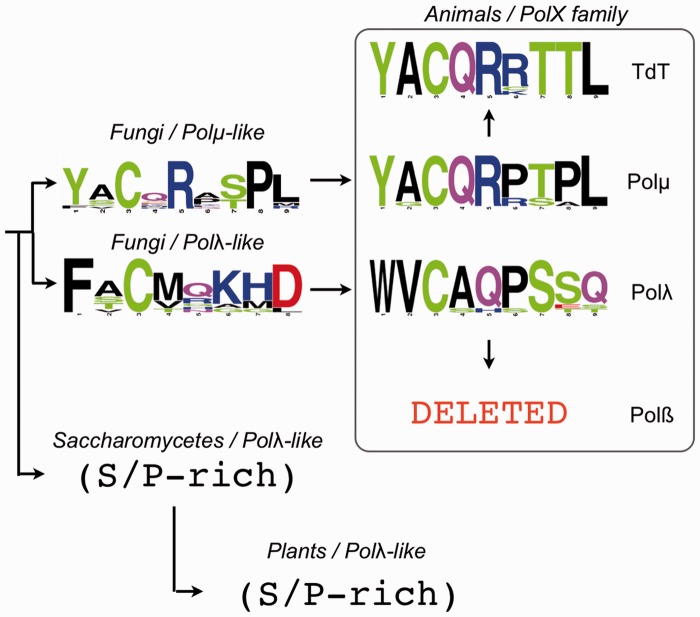
The brooch is a conserved N-terminal extension of the 8 kDa domain of eukaryotic Polµ, TdT and Polλ, but it is also highly conserved in other PolXs from different species, even from different kingdoms of life. Figure 7 shows the sequence consensus of different types of brooch, indicating the conserved residues in each case (full sequence alignments from which these logos were generated are shown in Supplementary Figure S5). According to the sequence conservation of the brooch, the PolXs found in Ascomycota can be divided into three groups: Polµ-like, Polλ-like and PolXs from Saccharomycetales. Strikingly, PolXs from Saccharomycetales (as Pol4 from Saccharomyces cerevisiae) do not contain a brooch, and the Ser-Pro domain is followed immediately by the 8 kDa domain. Polλ sequences from plants also lack this sequence, possibly indicating an evolutionary relationship amongst these two groups. In the case of Polµs from animals, the sequence is almost invariant (YACQR), as in the case of TdTs (Figure 7 and Supplementary Figure S5). In the case of Polλs from animals, the sequence is different (WVCAQ), but it is equally conserved, whereas in Polßs, the brooch has been apparently lost, together with the BRCT and Ser-Pro domains, in agreement with a more specific role of Polß in base excision repair, not dealing with DSBs as its family siblings.
Figure 7.
Brooch ancestry. Sequence logo representations and evolution of the brooch sequence in groups of species belonging to different eukaryotic kingdoms: animals, fungi and plants. The sequences used for generating the logos are indicated in Supplementary Figure S5.
Taking into account the structural data available for Polµ, we could initially postulate the implication of the brooch in maintaining the constitutively closed conformation of Polµ, TdT and Polλ through the catalytic cycle, whereas Polß is not constrained by this brooch and allows free motion of the 8 kDa domain with respect to the thumb (Supplementary Figure S1). Unfortunately, in the case of all the structures solved for Polλ, this small region is missing (a model of the predicted position of the brooch in this polymerase can be seen in Supplementary Figure S1D). This fact could well explain the 5-fold difference in Km observed between the full-length Polλ and the crystallized form of the enzyme (16) and also the conformation adopted by the 8 kDa domain in this Polλ structure, which is intermediate between the open and closed conformation of Polß (PDB IDs: 1BPZ, 1BPY). This lack of the brooch could also affect the results obtained by Fiala et al. (31) and Fan et al. (30) using truncated mutants, thus not allowing assignment of a specific role for the Ser-Pro domain from their data.
The 8 kDa domain is one of the structural features that allow X family polymerases to bind gapped and NHEJ substrates, and it is located either at the N-terminus (Polß), or at the N-terminal portion of the polymerase domain (Polλ, Polµ, TdT and yeast PolXs), preceded by the Ser/Pro-rich region and/or the BRCT domain. The 8 kDa domain is involved in contacting the downstream 5′-P group (13), and in some of the members of the X family, it bears a dRP-lyase activity, highly related to the BER pathway (32,33) but also needed to process the DNA ends of a ‘dirty’ DSB. The Polλ binary structure with a 2 nt gap (16) shows that the location of the polymerase domain in a gap is dictated by the binding of the 8 kDa domain to the downstream part of the substrate, and not by interactions with the primer terminus. This observation has implications of great interest for the binding of the polymerase to NHEJ substrates, as 8 kDa-mediated binding would occur, irrespective of the conformation of the 3′ end. An extreme example of a situation like this would be a recent structure of the NHEJ polymerase from Mycobacterium tuberculosis in which the polymerase is forming a ‘pre-ternary’ complex (34): the enzyme is binding the downstream side of the break via the 5′-P group and also the incoming nucleotide dictated by the templating base. The active site is poised for catalysis, with both metals A and B present and the residues in their active conformations—all in the absence of a primer strand. In the case of the eukaryotic polymerases, the order of events could be similar during end bridging, as hinted by the Polλ structure. Therefore, a tightly controlled conformation of the body of the polymerase would help to correctly position all the components needed for catalysis: primer terminus, templating base, incoming nucleotide, metal cofactors and protein side chains building the active site. Accordingly, our mutagenesis results indicate that the most affected reaction when the brooch is disturbed in both Polµ or Polλ was end bridging/trans-polymerization. In Polλ, it is also affecting its ‘scrunching’ capacities used to deal with long gaps. This brooch is thus ‘scrunching’-compatible, probably allowing a certain opening of the 8 kDa domain with respect to the thumb, whereas Polµ’s might be much more static. On the other hand, the support provided by the brooch was not necessary during 1 nt gap filling, an expected result as Polß, a polymerase specialized in this reaction, does not contain a brooch.
In addition to large-scale domain motions (in Polß), several side chains in the thumb domain rearrange to assemble the active site. Specifically, the side chains of the Tyr and Phe residues forming the YFTGS motif in Polß and Polλ move on binding of the incoming nucleotide, resulting in the correct positioning of the primer terminus for catalysis. This motif is different in Polµ and TdT (GWTGS), where the equivalent residues are a glycine and a tryptophan, responsible for a decreased discrimination between deoxyribo- versus ribonucleotides (35–37) and a more spacious active site that allows Polµ to bridge non-complementary ends (38), contrary to Polß and Polλ, which largely favour the use of deoxyribonucleotides (8,39). The thumb subdomain is also interacting with the downstream part of the template strand through the already mentioned α-helix N, which is strongly positively charged in Polµ. An interaction common to Polµ and Polλ is the stacking of the templating base with one of the residues included in this positive path, Arg442 in Polµ and Arg514 in Polλ (11,13). The equivalent residue in Polß is a lysine residue (Lys280) with a similar function. Polµ has a few minor groove interactions through residue Arg445, some of which are mediated by bridging water molecules (13). Arg446 of this helix in Polµ is involved in interactions with the brooch; accordingly, mutation of this residue to alanine had the same effects as mutations in the brooch.
In conclusion, this work describes a small protein sequence (named brooch) that modulates the catalytic cycle of polymerases from the human X family, of main importance for DNA repair reactions such as those occurring during the NHEJ pathway. Polµ and Polλ are highly specialized polymerases, singularly suited for their functions: engagement of two different DNA chains and trans-directed polymerization (even in the case of very low or inexistent complementarity) with the only objective of fulfilling the most efficient reaction possible with the minimal loss of sequence. The physiological importance of the brooch as a controller of the most specific activities of these enzymes remains an open question of special interest, taking into account the existence of several human single nucleotide polymorphisms affecting residues studied here, such as R145C and N153S in Polµ, and also of regulatory sites such as the possible Cdk phosphorylation site at Polµ’s Thr147, the possible Chk target site at the brooch in TdT (YACQRRTT) and Polλ’s Ser246, an ATM/ATR target (40). Future studies will focus on understanding the mechanistic and physiological effects of these allelic variants and also the in vivo regulation of the brooch.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Funding for open access charge: Ministerio de Ciencia y Tecnología Grant [BFU2009-10085] and CONSOLIDER [CSD2007-00015]; and Centro de Biología Molecular ‘Severo Ochoa’ from Fundación Ramón Areces; the recipients of fellowships from the Comunidad Autónoma de Madrid (to M.J.M. and A.G.B.).
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The proteomic analysis ‘Protein Identification by MS-MALDI-TOF’ was carried out in the Cbmso Protein Chemistry Facility, a member of ProteoRed network.
REFERENCES
- 1.Johnson KA. The kinetic and chemical mechanism of high-fidelity DNA polymerases. Biochim. Biophys. Acta. 2010;1804:1041–1048. doi: 10.1016/j.bbapap.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawaya MR, Prasad R, Wilson SH, Kraut J, Pelletier H. Crystal structures of human DNA polymerase beta complexed with gapped and nicked DNA: evidence for an induced fit mechanism. Biochemistry. 1997;36:11205–11215. doi: 10.1021/bi9703812. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Kong Y, Korolev S, Waksman G. Crystal structures of the Klenow fragment of Thermus aquaticus DNA polymerase I complexed with deoxyribonucleoside triphosphates. Protein Sci. 1998;7:1116–1123. doi: 10.1002/pro.5560070505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doublie S, Sawaya MR, Ellenberger T. An open and closed case for all polymerases. Structure. 1999;7:R31–R35. doi: 10.1016/S0969-2126(99)80017-3. [DOI] [PubMed] [Google Scholar]
- 5.Holm L, Sander C. DNA polymerase beta belongs to an ancient nucleotidyltransferase superfamily. Trends Biochem. Sci. 1995;20:345–347. doi: 10.1016/s0968-0004(00)89071-4. [DOI] [PubMed] [Google Scholar]
- 6.Brautigam CA, Steitz TA. Structural and functional insights provided by crystal structures of DNA polymerases and their substrate complexes. Curr. Opin. Struct. Biol. 1998;8:54–63. doi: 10.1016/s0959-440x(98)80010-9. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994;264:1891–1903. [PubMed] [Google Scholar]
- 8.Beard WA, Wilson SH. Structural insights into DNA polymerase beta fidelity: hold tight if you want it right. Chem. Biol. 1998;5:R7–R13. doi: 10.1016/s1074-5521(98)90081-3. [DOI] [PubMed] [Google Scholar]
- 9.Kunkel TA, Bebenek K. DNA replication fidelity. Annu. Rev. Biochem. 2000;69:497–529. doi: 10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]
- 10.Beard WA, Shock DD, Wilson SH. Influence of DNA structure on DNA polymerase beta active site function: extension of mutagenic DNA intermediates. J. Biol. Chem. 2004;279:31921–31929. doi: 10.1074/jbc.M404016200. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Diaz M, Bebenek K, Krahn JM, Kunkel TA, Pedersen LC. A closed conformation for the Pol lambda catalytic cycle. Nat. Struct. Mol. Biol. 2005;12:97–98. doi: 10.1038/nsmb876. [DOI] [PubMed] [Google Scholar]
- 12.Foley MC, Arora K, Schlick T. Sequential side-chain residue motions transform the binary into the ternary state of DNA polymerase lambda. Biophys. J. 2006;91:3182–3195. doi: 10.1529/biophysj.106.092080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon AF, Garcia-Diaz M, Bebenek K, Davis BJ, Zhong X, Ramsden DA, Kunkel TA, Pedersen LC. Structural insight into the substrate specificity of DNA Polymerase mu. Nat. Struct. Mol. Biol. 2007;14:45–53. doi: 10.1038/nsmb1180. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Schlick T. Modeling DNA polymerase mu motions: subtle transitions before chemistry. Biophys. J. 2010;99:3463–3472. doi: 10.1016/j.bpj.2010.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz JF, Dominguez O, Lain de Lera T, Garcia-Diaz M, Bernad A, Blanco L. DNA polymerase mu, a candidate hypermutase? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:99–109. doi: 10.1098/rstb.2000.0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Diaz M, Bebenek K, Krahn JM, Blanco L, Kunkel TA, Pedersen LC. A structural solution for the DNA polymerase lambda-dependent repair of DNA gaps with minimal homology. Mol. Cell. 2004;13:561–572. doi: 10.1016/s1097-2765(04)00061-9. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Diaz M, Bebenek K, Gao G, Pedersen LC, London RE, Kunkel TA. Structure-function studies of DNA polymerase lambda. DNA Repair (Amst) 2005;4:1358–1367. doi: 10.1016/j.dnarep.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Martin MJ, Juarez R, Blanco L. DNA-binding determinants promoting NHEJ by human Polmu. Nucleic Acids Res. 2012;40:11389–11403. doi: 10.1093/nar/gks896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brissett NC, Pitcher RS, Juarez R, Picher AJ, Green AJ, Dafforn TR, Fox GC, Blanco L, Doherty AJ. Structure of a NHEJ polymerase-mediated DNA synaptic complex. Science. 2007;318:456–459. doi: 10.1126/science.1145112. [DOI] [PubMed] [Google Scholar]
- 20.Nick McElhinny SA, Havener JM, Garcia-Diaz M, Juarez R, Bebenek K, Kee BL, Blanco L, Kunkel TA, Ramsden DA. A gradient of template dependence defines distinct biological roles for family X polymerases in nonhomologous end joining. Mol. Cell. 2005;19:357–366. doi: 10.1016/j.molcel.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Diaz M, Bebenek K, Sabariegos R, Dominguez O, Rodriguez J, Kirchhoff T, Garcia-Palomero E, Picher AJ, Juarez R, Ruiz JF, et al. DNA polymerase lambda, a novel DNA repair enzyme in human cells. J. Biol. Chem. 2002;277:13184–13191. doi: 10.1074/jbc.M111601200. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Diaz M, Bebenek K, Larrea AA, Havener JM, Perera L, Krahn JM, Pedersen LC, Ramsden DA, Kunkel TA. Template strand scrunching during DNA gap repair synthesis by human polymerase lambda. Nat. Struct. Mol. Biol. 2009;16:967–972. doi: 10.1038/nsmb.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thode S, Schafer A, Pfeiffer P, Vielmetter W. A novel pathway of DNA end-to-end joining. Cell. 1990;60:921–928. doi: 10.1016/0092-8674(90)90340-k. [DOI] [PubMed] [Google Scholar]
- 24.Roth DB, Wilson JH. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol. Cell. Biol. 1986;6:4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer KM, Brock JA, Bloom K, Moore JK, Haber JE. Two different types of double-strand breaks in Saccharomyces cerevisiae are repaired by similar RAD52-independent, nonhomologous recombination events. Mol. Cell. Biol. 1994;14:1293–1301. doi: 10.1128/mcb.14.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsden DA. Polymerases in nonhomologous end joining: building a bridge over broken chromosomes. Antioxid. Redox Signal. 2011;14:2509–2519. doi: 10.1089/ars.2010.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller GA, Moon AF, Derose EF, Havener JM, Ramsden DA, Pedersen LC, London RE. A comparison of BRCT domains involved in nonhomologous end-joining: introducing the solution structure of the BRCT domain of polymerase lambda. DNA Repair (Amst) 2008;7:1340–1351. doi: 10.1016/j.dnarep.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto T, Go K, Hyodo M, Koiwai K, Maezawa S, Hayano T, Suzuki M, Koiwai O. BRCT domain of DNA polymerase mu has DNA-binding activity and promotes the DNA polymerization activity. Genes Cells. 2012;17:790–806. doi: 10.1111/j.1365-2443.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 29.Martin MJ, Juarez R, Blanco L. DNA-binding determinants promoting NHEJ by human Polµ. Nucleic Acids Res. 2012;40:11389–11403. doi: 10.1093/nar/gks896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan W, Wu X. DNA polymerase lambda can elongate on DNA substrates mimicking non-homologous end joining and interact with XRCC4-ligase IV complex. Biochem. Biophys. Res. Commun. 2004;323:1328–1333. doi: 10.1016/j.bbrc.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Fiala KA, Duym WW, Zhang J, Suo Z. Up-regulation of the fidelity of human DNA polymerase lambda by its non-enzymatic proline-rich domain. J. Biol. Chem. 2006;281:19038–19044. doi: 10.1074/jbc.M601178200. [DOI] [PubMed] [Google Scholar]
- 32.Prasad R, Beard WA, Strauss PR, Wilson SH. Human DNA polymerase beta deoxyribose phosphate lyase. Substrate specificity and catalytic mechanism. J. Biol. Chem. 1998;273:15263–15270. doi: 10.1074/jbc.273.24.15263. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Diaz M, Bebenek K, Kunkel TA, Blanco L. Identification of an intrinsic 5'-deoxyribose-5-phosphate lyase activity in human DNA polymerase lambda: a possible role in base excision repair. J. Biol. Chem. 2001;276:34659–34663. doi: 10.1074/jbc.M106336200. [DOI] [PubMed] [Google Scholar]
- 34.Brissett NC, Martin MJ, Pitcher RS, Bianchi J, Juarez R, Green AJ, Fox GC, Blanco L, Doherty AJ. Structure of a preternary complex involving a prokaryotic NHEJ DNA polymerase. Mol. Cell. 2011;41:221–231. doi: 10.1016/j.molcel.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Boule JB, Rougeon F, Papanicolaou C. Terminal deoxynucleotidyl transferase indiscriminately incorporates ribonucleotides and deoxyribonucleotides. J. Biol. Chem. 2001;276:31388–31393. doi: 10.1074/jbc.M105272200. [DOI] [PubMed] [Google Scholar]
- 36.Nick McElhinny SA, Ramsden DA. Polymerase mu is a DNA-directed DNA/RNA polymerase. Mol. Cell. Biol. 2003;23:2309–2315. doi: 10.1128/MCB.23.7.2309-2315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz JF, Juarez R, Garcia-Diaz M, Terrados G, Picher AJ, Gonzalez-Barrera S, Fernandez de Henestrosa AR, Blanco L. Lack of sugar discrimination by human Pol mu requires a single glycine residue. Nucleic Acids Res. 2003;31:4441–4449. doi: 10.1093/nar/gkg637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin MJ, Garcia-Ortiz MV, Esteban V, Blanco L. Ribonucleotides and manganese ions improve non-homologous end joining by human Polmu. Nucleic Acids Res. 2013;41:2428–2436. doi: 10.1093/nar/gks1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shevelev I, Blanca G, Villani G, Ramadan K, Spadari S, Hubscher U, Maga G. Mutagenesis of human DNA polymerase lambda: essential roles of Tyr505 and Phe506 for both DNA polymerase and terminal transferase activities. Nucleic Acids Res. 2003;31:6916–6925. doi: 10.1093/nar/gkg896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.