Abstract
RNA interference (RNAi) can be used to inhibit the expression of specific genes in vitro and in vivo, thereby providing an extremely useful tool for investigating gene function. Progress in the understanding of RNAi-based mechanisms has opened up new perspectives in therapeutics for the treatment of several diseases including ocular disorders. The eye is currently considered a good target for RNAi therapy mainly because it is a confined compartment and, therefore, enables local delivery of small-interfering RNAs (siRNAs) by topical instillation or direct injection. However, delivery strategies that protect the siRNAs from degradation and are suitable for long-term treatment would be help to improve the efficacy of RNAi-based therapies for ocular pathologies. siRNAs targeting critical molecules involved in the pathogenesis of glaucoma, retinitis pigmentosa and neovascular eye diseases (age-related macular degeneration, diabetic retinopathy and corneal neovascularization) have been tested in experimental animal models, and clinical trials have been conducted with some of them. This review provides an update on the progress of RNAi in ocular therapeutics, discussing the advantages and drawbacks of RNAi-based therapeutics compared to previous treatments.
Keywords: siRNAs, therapy, eye, glaucoma, retinitis pigmentosa, age-related macular degeneration, diabetic retinopathy, corneal neovascularization
Introduction
RNA interference (RNAi) technology has been used to elucidate gene function, to generate model systems and to identify new molecular targets. In addition, RNAi is currently progressing from basic research to potential therapeutic applications. Because any gene that causes or contributes to a disease is susceptible to suppression by RNAi, RNAi therapy represents a promising biomedical strategy for treating a diverse range of diseases including cancer, cardiovascular diseases, neurodegenerative diseases, inflammatory conditions, viral infections and ocular diseases (Guo et al., 2010). In particular, the accessibility of the eye facilitates small-interfering RNA (siRNA) delivery, and naked siRNA has been efficiently applied by topical administration to the anterior segment or by intravitreal injection to the posterior segment (de Fougerolles, 2008). As the localized delivery of siRNA to the eye is less challenging than for other tissues, there has been significant progress made towards its use as a therapeutic procedure for eye diseases. In fact, several siRNA-based therapeutic agents for ocular disorders have already reached clinical trials (http://www.allergan.com, http://www.opko.com, http://www.quarkpham.com; Table 1).
Table 1.
Clinical trials performed with siRNAs for ocular disease treatment
| Ocular diseases | siRNA | Target | Company | Status | References |
|---|---|---|---|---|---|
| Glaucoma | SYL040012 | β2-Adrenoceptor | Sylentis | Phase II (ongoing) | Pañeda et al., 2013 |
| AMD | Bevasiranib | VEGF | Opko Health, Inc. http://www.opko.com | Phase III (halted) | Brucker, 2006; ClinicalTrials, 2008 |
| AMD | siRNA-027 | VEGF receptor | Allergan http://www.allergan.com | Phase II (halted) | Kaiser et al., 2010 |
| AMD | PF-655 | RTP801 | Quark Pharmaceuticals http://www.quarkpharma.com | Phase II (completed) | Nguyen et al., 2012b; 2012c |
| DR | PF-655 | RTP801 | Quark Pharmaceuticals http://www.quarkpharma.com | Phase II (completed) | Nguyen et al., 2012a |
All siRNAs for AMD and DR treatment were administered by intravitreal injection. siRNA-027, also known as AGN211745; PF-655.
This review focuses on the potential therapeutic application of RNAi in ocular disorders, which could provide a valuable contribution to the prevention of blindness and other associated disabilities throughout the world. The advantages and challenges associated with the use of this technology are also discussed.
Molecular mechanism of RNAi
RNAi is an evolutionary conserved post-transcriptional gene-silencing pathway that was first demonstrated in the nematode worm Caenorhabditis elegans in 1998 (Fire et al., 1998). Soon after, RNAi was also shown to occur in mammalian cells (Elbashir et al., 2001a).
RNAi is a fundamental pathway by which sequence-specific siRNA is able to target and cleave complementary mRNA (Figure 1). RNAi is triggered by the presence of long pieces of double-stranded RNA (dsRNA), which are cleaved into the fragments known as siRNA [21–23 nucleotides (nt) long] by an RNase III enzyme called Dicer (Ketting et al., 2001). Alternatively, siRNA can be synthetically produced and then directly introduced into the cell, thus circumventing the Dicer mechanics (Elbashir et al., 2001a,b).
Figure 1.
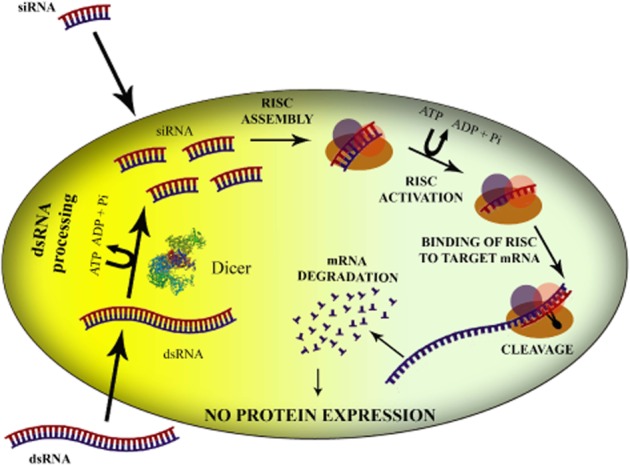
Schematic representation of mechanism of RNAi in mammalian systems. Long dsRNA molecules are processed by the Dicer complex, resulting in the formation of siRNAs. Alternatively, to induce RNAi, these small 21–23 base pair duplexes are directly delivered into the cell. The siRNAs are incorporated into a nuclease-containing multi-protein complex called RISC and single-stranded siRNA guides the RISC complex to its complementary target mRNA, which is then cleaved.
Once siRNA is present in the cytoplasm of the cell, it is incorporated into a protein complex called the RNA-induced silencing complex (RISC; Rand et al., 2004). RISC contains (i) an ATP helicase activity that unwinds the two strands of RNA molecules, allowing the antisense strand to bind to the targeted RNA molecule, and (ii) an endonuclease Argonaute 2, which hydrolyses the target mRNA. RISC activation is initially thought to involve Argonaute 2-mediated cleavage of the sense (or passenger strand) of the double-stranded siRNA (Rand et al., 2005), generating the single-stranded antisense strand that guides RISC to complementary sequences in target mRNAs. This guide strand is bound within the catalytic, RNase H-like PIWI domain of Argonaute 2 at the 5′-end (Parker et al., 2005) and a PIWI–Argonaute–Zwille domain that recognizes the siRNA 3′-end (Ma et al., 2004). The cleavage of targeted mRNA takes place between 10 and 11 relative to the 5′-end of the siRNA guide strand (Elbashir et al., 2001b), leading to subsequent degradation of the cleaved mRNA transcript by cellular exonucleases (Orban and Izaurralde, 2005). The activated RISC complex is capable of binding and destroying hundreds of complementary mRNA, which, in turn, abolishes the expression of the encoded protein, thus propagating gene silencing and making this a very potent process.
Despite the exceptional utility that RNAi technology appears to offer for the treatment of ocular pathologies, there are a number of critical issues that must be taken into account before siRNAs can be used clinically. These include minimizing off-target effects, increasing the stability of synthetic siRNA and optimizing siRNA delivery.
Off-target effects
Although specificity should be one of the main characteristics of RNAi, non-specific silencing resulting from binding of siRNA to sequences other than the specific target sequence can occur. Off-target effects have mostly been shown to be due to 3′-untranslated region (UTR) seed matches, but not to the overall homology between siRNA and its target (Birmingham et al., 2006). Avoiding siRNA seed matches with mRNA 3′-UTRs by using online 3′-UTRs search algorithms would, potentially, reduce these detrimental off-target effects. Moreover, it has been reported that a predominance of specific motifs such as -UGGC- and other -AU-rich pentamers, including -AUUUG, GUUUU, AUUUU, CUUUU, UUUUU, GUUUG-, can induce a toxic phenotype in cells (Fedorov et al., 2006); thus the use of such motifs should, if possible, be minimized.
Other non-specific effects
Exposure to long dsRNA (>30 nt) can trigger an innate immune response via activation of toll-like receptors (TLR; Alexander et al., 2011) (Alexopoulou et al., 2001). The 21 nt siRNAs can activate TLR3 (Kleinman et al., 2008; Cho et al., 2009) and TLR7/8 (Hornung et al., 2005; Sioud, 2005), which produce cytokines upon stimulation.
The presence of certain sequence motifs in the siRNAs might predispose them to initiate innate immune response. For example, siRNA sequence motifs such as 5′-GUCCUUCAA-3′ and 5′-UGUGU-3′ have been identified as having immunostimulating properties (Hornung et al., 2005; Judge et al., 2005). To prevent the activation of innate cellular immunity, these sequences should be avoided in siRNA design. Likewise, it has been shown that the introduction of some chemical modifications (2′-O-methyl, 2′-deoxy, etc.; Judge et al., 2006) or ensuring that siRNAs have a 2 nt 3′ overhang (Marques et al., 2006) can avert immune responses. Additionally, immune system recognition can be avoided by the use of siRNA duplexes shorter than 21 nt (Kleinman et al., 2012).
Stability
The gene-silencing effect of siRNA is transient with transfected siRNAs typically effective for about 3–7 days before they are naturally degraded. Therefore, there have been attempts to prolong the gene-silencing activity of siRNAs by the incorporation of chemical modifications to increase the stability of the siRNA molecules without compromising their gene-silencing potency. These modifications include the introduction of phosphorothioate backbone linkages at the 3′-end of the RNA strands (Gaglione and Messere, 2010), boranophosphate backbone modifications (Hall et al., 2004) or the incorporation of chemical modifications at the 2′ position of the ribose such as 2′-O-methyl or 2′-fluoro (Chiu and Rana, 2003). However, even chemically modified siRNAs can be susceptible to degradation as some endonucleases present in the extracellular environment, such as the vitreous humour, are able to avoid the terminal protection by bypassing them (Beverly et al., 2006).
Delivery
In the case of the eye, because of its accessibility, siRNA administration and delivery is clearly easier than in other organ/tissue targets. Important benefits of localized application of siRNAs include the potential for both higher bioavailability given the proximity to the target tissue and reduced adverse effects, which are typically associated with systemic administration.
Direct instillation of siRNA into the ocular surface has been used for the treatment of ocular surface and anterior segment disorders in vivo (Crooke et al., 2009; Martin-Gil et al., 2012). However, topical ocular administration of any compound, siRNAs included, is limited by ocular anatomic constraints and physiological protective mechanisms (Guzman-Aranguez et al., 2013). For this reason, new strategies such as iontophoresis (Myles et al., 2005) or the use of cell-penetrating peptides (Johnson et al., 2008) are being sought to improve the ocular delivery of siRNA.
Delivery of siRNAs by topical instillation to the posterior segment is truly challenging, because of the relatively large distance that the siRNAs have to go through the vitreous body before they reach the retina. The most common strategy used is direct intravitreal injection of siRNAs, which is less invasive than subretinal injection and bypasses the corneoscleral barriers (Tolentino et al., 2004). Successful knock-down of VEGF receptor-1 (VEGFR1) after injection of naked stability-modified VEGFR1 siRNA has been demonstrated in mouse models (Shen et al., 2006). This route of administration is also currently being used in clinical trials with siRNAs targeting VEGF (Brucker, 2006), VEGFR1 (Kaiser et al., 2010) and hypoxia-inducible RTP801 genes (Nguyen et al., 2012b). As chemically modified siRNAs can also be degraded by vitreous endonucleases (Beverly et al., 2006), siRNA-based treatments of posterior segment diseases could require multiple intravitreal injections, similar to conventional treatments. This need for multiple injections increases the potential for cataract, retinal detachment, vitreous haemorrhage and endophthalmitis (Kurz and Ciulla, 2002). Consequently, a sustained release of siRNAs for extended periods of time could be very helpful. To achieve this, encapsulation of siRNAs into non-viral nanocarriers might be a valuable option for intravitreal delivery. However, negatively charged glycosaminoglycans of the vitreous can interact with cationic carriers, thus limiting their diffusion and promoting their aggregation (Pitkanen et al., 2003; Peeters et al., 2005; 2007). The shielding of these polystyrene nanospheres and lipoplexes with polyethylene glycol (PEG) can prevent their aggregation (Peeters et al., 2005), but, unfortunately, pegylation can also prevent the cellular uptake of the complexes (Deshpande et al., 2004) as well as their endosomal transport and release (Shi et al., 2002; Song et al., 2002). This limitation can be overcome by using exchangeable PEG lipids or by coupling the PEG to carriers via a pH-sensitive linker (Peeters et al., 2007).
However, more recently promising results have been reported using TransIT-TKO transfection reagent as a carrier for intraocular siRNA delivery into the mouse retina after intravitreal injection (Turchinovich et al., 2010).
Therapeutic applications of RNAi in ocular disorders
Recent findings have highlighted the effectiveness of RNAi in therapeutically appropriate conditions, such as the treatment of eye diseases. Indeed, the first clinical application of RNAi was directed at the treatment of the wet age-related macular degeneration (AMD). Since then, therapies based on RNAi are also being developed for other ocular diseases such as diabetic retinopathy (DR), retinitis pigmentosa (RP), corneal neovascularization, fibrotic eye diseases or glaucoma (Table 2).
Table 2.
Summary of therapeutic targets for siRNA-based treatment of several ocular diseases tested in vivo
| Ocular diseases | Target | References |
|---|---|---|
| Glaucoma | RhoA | Liu et al., 2012 |
| Cochlin | Goel et al., 2012 | |
| β2-Adrenoceptor | Mediero et al., 2009; Pintor, 2011 | |
| Na-K-ATPase | Mediero et al., 2009 | |
| Purine receptor P2Y2 | Martin-Gil et al., 2012 | |
| c-Jun | Lingor et al., 2005 | |
| Apoptosis regulator Bax | Lingor et al., 2005 | |
| Apaf-1 | Lingor et al., 2005 | |
| Caspase-2 | Ahmed et al., 2011 | |
| Fibrotic eye by glaucoma filtration surgery | TGF-β2 | Nakamura et al., 2004 |
| IκB kinase β | Ye et al., 2010 | |
| RP | Rhodopsin | Tessitore et al., 2006; O'Reilly et al., 2007; Mao et al., 2012 |
| Retinal degeneration slow-peripherin | Petrs-Silva et al., 2012 | |
| Guanylate cyclase 2 | Tosi et al., 2011 | |
| Cyclic nucleotide-gated ion channels | Tosi et al., 2011 | |
| AMD | VEGF | Reich et al., 2003 |
| VEGFR1 | Shen et al., 2006 | |
| RTP801 | Nozaki et al., 2006 | |
| DR | VEGF | Reich et al., 2003 |
| VEGFR1 | Shen et al., 2006 | |
| RTP801 | Brafman et al., 2004 | |
| HIF-1α | Jiang et al., 2009 | |
| Fibronectin | Roy et al., 2003 | |
| Connective tissue growth factor | Yang et al., 2010a; 2010b; Winkler et al., 2012 | |
| TXNIP | Perrone et al., 2010; Sbai et al., 2010; Devi et al., 2012 | |
| Corneal neovascularization | VEGF | Kim et al., 2004; Singh et al., 2007; Zuo et al., 2010; Qazi et al., 2012 |
| VEGFR1 | Kim et al., 2004 | |
| HIF-1α | Chen et al., 2012 | |
| Cytochome P450 enzyme CYP4B1 | Seta et al., 2007 | |
| Cannabinoid CB1 receptor | Pisanti et al., 2011 |
In this section, we review the main progress in RNAi-based therapies for ocular disorders and discuss the advances as well as the challenges in this field.
Glaucoma
Glaucoma consists of a group of progressive optic neuropathies that are characterized by progressive and permanent blindness resulting from the slow death of retinal ganglion cells (RGCs) and their axons, which form the optic nerve (Quigley, 2011).
The pathophysiology of glaucomatous neurodegeneration is not fully understood, and the factors contributing to its progression are not yet well characterized. The level of intraocular pressure (IOP) is related to the death of RGCs and optic nerve fibres; high IOP can cause blockade at the lamina cribrosa of axonal protein transport, causing neuronal RGC death by trophic insufficiency (Johnson et al., 2009). Independently or in addition to IOP, other factors contributing to neurodegeneration include local ischaemia-hypoxia, excessive stimulation of the glutamatergic system, alterations in glial cells and aberrant immunity (Weinreb and Khaw, 2004).
IOP is the only proven treatable risk factor (The AGIS Investigators, 2000; Heijl et al., 2002; Kass et al., 2002), and lowering IOP is the main approach for reducing disease progression. IOP is determined by the balance between the aqueous humour secreted into the eye by the ciliary body (Macknight et al., 2000) and the amount that is drained from the eye via the two major outflow pathways: the trabecular meshwork (TM; Tamm, 2009) and the uveoscleral pathway (consisting of the iris root, longitudinal ciliary muscle, anterior choroid and sclera; Alm and Nilsson, 2009). IOP is in a steady state when the rate of aqueous inflow is the same as the rate of aqueous outflow (Moses, 1981; Kiel et al., 2011). IOP reduction therapy is achieved through pharmacological treatment, laser therapy or surgical operation (trabeculectomy; Quigley, 2011).
Pharmacological treatment of glaucoma lowers IOP by decreasing the rate of aqueous inflow and/or increasing the rate of aqueous outflow (Figure 2). Drugs reducing the production of the aqueous humour include β-adrenoceptor blockers (timolol), carbonic anhydrase inhibitors (dorzolamide, brinzolamide) and α-adrenoceptor agonists (brimonidine, apraclonidine). β-Adrenoceptor blockers reduce aqueous humour secretion by inhibiting the activity of the predominant β-adrenoceptor in the ciliary epithelium (Nathanson, 1981). IOP has been noted to follow a 24 h rhythm with a nocturnal increase, at least in part, due to the supine position effected while sleeping (Liu et al., 1998; 1999; Fan et al., 2011). Unfortunately, β-adrenoceptor blockers are only effective during the day and not during the night. In addition, although they can reduce ocular perfusion pressure (Liu et al., 2004), they can also have substantial cardiovascular and respiratory side effects, especially in the elderly (Weinreb and Khaw, 2004).
Figure 2.
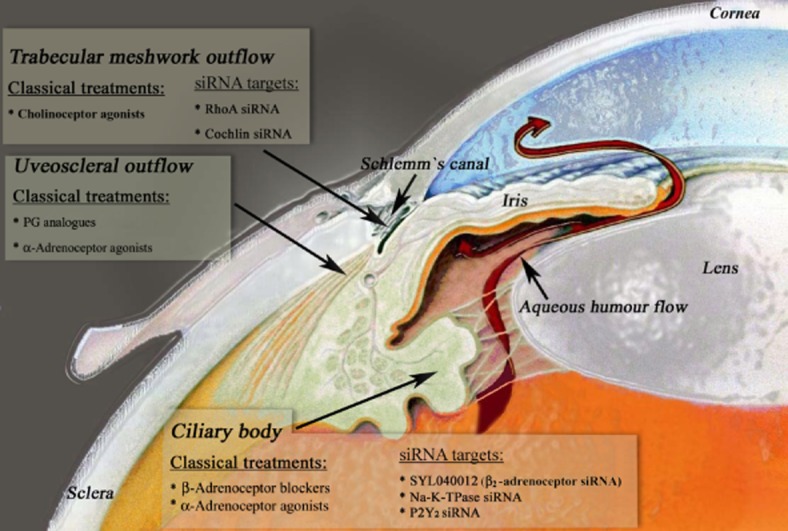
Treatments for lowering IOP. IOP is generated and maintained by the aqueous humour circulation system in the anterior eye. Aqueous humour is actively secreted by the ciliary body and is drained from the eye through the TM and the uveoscleral pathways. Conventional drugs as well as siRNA-based treatments for lowering IOP, by decreasing aqueous humour inflow and/or increasing aqueous humour outflow are indicated on the figure (modified from http://www.mdeyedocs.com/edcacuteglaucoma.htm).
Carbonic anhydrase inhibitors inhibit the activity of carbonic anhydrase 2 in the non-pigmented ciliary epithelium, slowing local bicarbonate production and so decreasing sodium and fluid transport, thus reducing aqueous humour secretion (Silver, 1998). Carbonic anhydrase inhibitors are sulphonamide-based drugs; therefore, they should not be used in individuals allergic to sulphonamides. Likewise, their use should be restricted in subjects with severe renal disease and patients with compromised endothelium (Mincione et al., 2008). Another approach to reduce aqueous humour formation is the use of α2-adrenoceptor agonists. These decrease aqueous humour inflow and also appear to increase uveoscleral outflow (Toris et al., 1999). Topical α2-adrenoceptor agonists are associated with allergic conjunctivitis, can cause sedation and have the potential to induce systemic sympathomimetic activity (Weinreb and Khaw, 2004).
Pharmacological agents that improve aqueous humour outflow include cholinoceptor agonists and PGs (Weinreb and Khaw, 2004). Cholinoceptor agonists act on muscarinic receptors on the ciliary muscle to increase outflow through the TM, but they have substantial ocular side effects such as miosis, myopia, brow ache and dimming of vision (Zhang et al., 2012). PGF2α analogues are the most effective outflow drugs and have been approved for clinical use. These drugs reduce IOP by stimulation of aqueous humour drainage, primarily through the uveoscleral outflow. These PG analogues activate matrix metalloproteinases, which then remodel the extracellular matrix (ECM) and reduce outflow resistance, allowing the aqueous humour to flow out via this route (Weinreb et al., 2002). The most common adverse reactions are conjunctival hyperaemia, lengthening and thickening of eyelashes, and changes in eye colour (Wistrand et al., 1997).
Therefore, all the drugs indicated can have various undesirable effects and their efficacy is also likely to be reduced after prolonged use (Quigley, 2011). Thus, there is the need to discover novel agents from the presently available pharmacological classes as well as potential new targets that should be validated and, subsequently, lead to new therapeutic alternatives. In this context, siRNA-based therapy for glaucoma is being explored. Initially, the main focus was to use it to lower IOP either by decreasing the production of aqueous humour or by improving its outflow. Hence, the effects of SYL040012 siRNA, which targets β2-adrenoceptors in the ciliary epithelium, on aqueous humour production were investigated. Topical instillation of the SYL040012 siRNA was found to decrease IOP in rabbits by around 30% as compared with the control (Mediero et al., 2009; Pintor, 2011). This reduction in IOP obtained using siRNA was similar to that found with drugs currently prescribed for glaucoma treatment such as dorzolamid, latanoprost or timolol (Mediero et al., 2009). However, the effect of the β2-adrenoceptor siRNA treatment was significantly longer in terms of time effect as compared with conventional pharmacological agents. While the commercial drugs reduced IOP for a few hours (around 8 h), the siRNA effect lasted for 2 days (Pintor, 2011). Therefore, the use of siRNA against β2-adrenoceptors produces a significant reduction in IOP that is long-lasting, whereas a continuous application of commercial drugs is required to induce a sustained effect on IOP. Moreover, this long-lasting effect was achieved by simple topical administration of the siRNA and the possible use of a non-viral vector carrier, which prevents siRNA degradation, may even prolong this hypotensive action of the siRNA.
This long-lasting reduction of IOP by siRNA may be one of the most attractive features of this new approach for glaucoma treatment as frequent application of some commercial anti-glaucoma drugs, currently used at high concentrations to achieve the desired therapeutic effects, can lead to adverse side effects due to systemic absorption (e.g. timolol can induce a deleterious effect on the heart). Additionally, a requirement for frequent instillation can lead to poor compliance of the patient, which represents another problem in glaucoma management.
Because of these promising results, phase I clinical trials have been carried out (Sylentis Company, Madrid, Spain). SLY040012 was topically administered to healthy volunteers and individuals with increased IOP. All of the subjects analysed showed excellent local and systemic tolerance, and SLY040012 was able to lower IOP in individuals with elevated IOP (Pañeda et al., 2013). Phase II clinical trials are currently ongoing with a dose–range finding study in which the efficacy of different doses of SYL040012 will be compared with placebo (Table 1).
This long-lasting effect in IOP reduction, compared to commercial drugs, was also apparent in studies where other siRNAs against different targets were used to reduce aqueous humour production. In this regard, the potential hypotensive effect of siRNAs targeting Na-K-ATPase subunits was tested in rabbits (Mediero et al., 2009). Na-K-ATPase was selected as a target as it establishes ion gradients in the ciliary epithelium that contribute to aqueous humour production (Riley and Kishida, 1986). The silencing of Na-K-ATPase induced a 15% IOP reduction in the treated rabbits and this hypotensive effect was maintained for around 100 h (Mediero et al., 2009).
Finally, it is worth mentioning another different target recently identified to reduce IOP, the purine receptor P2Y2. Elevated levels of agonists of this receptor (ATP and dinucleotides) have been found in the aqueous humour from glaucomatous patients (Zhang et al., 2007; Castany et al., 2011), suggesting the involvement of these compounds in glaucoma pathology. In fact, it has been reported that activation of P2Y2 receptors present in rabbit ciliary processes by different nucleotides induces an increase in IOP (Markovskaya et al., 2008; Peral et al., 2009). On consideration of these findings, a siRNA targeting P2Y2 receptor has been developed (Martin-Gil et al., 2012). Topical application of the P2Y2 siRNA produced a robust decrease in IOP of 48 ± 5% in normotensive rabbits compared with control animals, with this maximal reduction in IOP remaining for 2 days. Once more, in terms of time of hypotensive effect, RNAi was more effective than commercial drugs.
Potential new targets aimed at improving aqueous humour outflow have also been proposed, although the biological data are preliminary and they have not been pharmacologically validated yet. These potential new targets are localized in the TM. To reach this location, siRNAs used in animal models have been solely administered by anterior chamber injection through the corneal limbus. However, it has been demonstrated that naked siRNAs can penetrate human TM when perfused at physiological pressure (Comes and Borras, 2007). This finding, together with the results obtained in the clinical trials (mentioned earlier), in which siRNAs were administered topically, suggests that this target has potential as an effective route of administration. Further research is needed to confirm this.
Many of these new targets are related to the ECM of TM. There is an important relationship between the structure of the ECM of TM and aqueous humour outflow. Excessive amounts of cross-linked ECM proteins in the TM have been associated with increased aqueous humour outflow resistance in glaucoma (Buller and Johnson, 1994). Consequently, various components of the ECM have become an important target for siRNA delivery to regulate aqueous humour outflow and lower IOP. In this sense, the TGF-β2/signalling mothers against decapentaplegic (Smad) pathway is known to regulate the ECM deposits in TM (Verrecchia and Mauviel, 2002). Down-regulation of Smad7 by RNAi interrupted the effects of TGF-β2 on the expression of several ECM components (fibronectin and laminin) in human TM cells in in vitro assays (Su et al., 2012). Likewise, silencing of other member of the Smad signalling pathway, Smad3, suppressed TGF-β2 induction of tissue transglutaminase, which is involved in cross-linking of ECM proteins (Tovar-Vidales et al., 2011).
Another critical mediator of the effects of TGF-β2 on the synthesis of ECM protein in TM cells appears to be the connective TGF (CTGF). Attenuation of CTGF expression by RNAi in cultured human TM cells prevented the TGF-β2-induced accumulation of fibronectin (Junglas et al., 2009). Interestingly, it has also recently been reported that depletion of CTGF by RNAi in cultured human TM cells causes a marked reduction in the actin cytoskeleton (Junglas et al., 2012). Disruption of TM actin stress fibres (Tian et al., 2000) evokes a reduction in outflow resistance; accordingly, siRNA against CTFG could provide a dual beneficial effect in the improvement of aqueous humour outflow in TM by both preventing ECM protein deposits and modifying the actin cytoskeleton.
However, because the contraction of actin stress fibres is predominantly regulated by the activity of RhoA/Rho kinase, the use of RhoA siRNA as a potential pharmaceutical intervention for reducing IOP has also been described (Liu et al., 2012). A liposome-RhoA siRNA complex, administered to mice by injection into the anterior chamber, caused a large decrease in the both the mRNA and protein expression of RhoA. Furthermore, siRNA silencing of RhoA in TM effectively decreased IOP in a mouse model with high IOP (Liu et al., 2012).
Cochlin is another potential target for siRNA glaucoma therapy localized in TM. Although its function has not been fully elucidated, it has been suggested that it has a role as mechanosensor detecting fluid shear changes in ECM (Goel et al., 2012). TM cells in glaucomatous eyes are subjected to a significant level of fluid shear change due to IOP fluctuation. Cochlin multimerization is induced in response to fluid shear changes (Goel et al., 2012). This multimerized cochlin is resistant to proteolysis and accumulates in the TM ECM in glaucomatous eyes (Bhattacharya et al., 2005). These cochlin deposits observed in glaucomatous TM could obstruct aqueous outflow across a wide region and increase IOP. Silencing of cochlin by RNAi in the glaucoma mouse model DBA/2J reduced IOP and maintained low IOP levels for the next 2 months as compared with control injected eyes (Goel et al., 2012).
In a few glaucoma patients, deficiencies in the ability of the TM to manage aqueous humour outflow have been attributed to the deleterious effects of mutated myocilin (Fingert et al., 1999). Misfolded mutant myocilin accumulates in the endoplasmic reticulum, inducing endoplasmic reticulum stress and apoptosis of TM cells with subsequent obstruction of TM and increased resistance of aqueous humour outflow (Sohn et al., 2002). Yuan and co-workers (2007) designed different self-looped short hairpin RNAs as siRNA precursors to suppress mutant myocilin in TM5 cells. The expression of mutant, but also wild-type myocilin was reduced to around 60% of the control level in TM5 cells. Because these results were detected in vitro, myocilin siRNAs need to be evaluated in animal models before definitive conclusions about their potential clinical application.
Despite successful lowering of IOP, many glaucoma patients continue to have progressive retina and optic nerve damage, and others acquire damage at normal or low IOP, indicating that other factors contribute to the pathophysiology of glaucoma. This has led to the need to develop new therapeutic approaches focused on offering effective neuroprotection to preserve vision. Consequently, siRNA-based treatments to prevent apoptosis and provide ocular neuroprotection have also been proposed. Several siRNAs have been designed to target proteins related to the initiation or execution of the programmed cell death cascade in RGC such as c-Jun-amino-terminal kinase-interacting protein 1 (c-Jun), the pro-apoptotic Bcl-2 family member Bax and the mediator of caspase-induced cell death apoptotic protease-activating factor-1 (Apaf-1; Lingor et al., 2005). SiRNAs, injected into the optic nerve stump, were shown to inhibit axotomy-induced apoptosis in rats. Rat retinas that were injected with Apaf-1 siRNA and c-Jun siRNA showed significantly more surviving RGC than non-injected or eGFP siRNA-injected controls (approximately two- to threefold respectively). Bax siRNA-injected retinas tended to have increased RGC numbers, but the levels reached were not significant (Lingor et al., 2005).
The siRNA targeting caspase-2 has also been demonstrated to have a neuroprotective action. A single intravitreal injection of this siRNA protected RGC during the first week after optic nerve crush (ONC), maintaining enhanced survival for 30 days after ONC (Ahmed et al., 2011). Likewise, caspase-2 siRNA significantly improved RGC survival in the more severe and prolonged optic nerve transection (axotomy) rat model (Ahmed et al., 2011).
Despite these promising results, as yet no clinical evidence exists about the neuroprotective action of these siRNAs preventing disease progression in patients with glaucoma.
Fibrotic eye
Filtration surgery to enhance the drainage of the eye is the most effective method for reducing IOP in patients with glaucoma that is refractory to topical ocular hypotensive treatments (The AGIS Investigators, 2000). However, the post-operative healing response can render the outcome of such surgery unsuccessful if scar formation increases outflow resistance of the artificially generated drainage pathway. The wound healing response at the operated site is characterized by several molecular events that include cell proliferation (particularly subconjunctival Tenon's capsule fibroblasts proliferation; Jampel et al., 1988), enhanced expression of ECM proteins (Saika et al., 2001) and inflammation (Chang et al., 2001b). Different approaches to modulate these processes during the wound healing response after filtering surgery by RNAi have been proposed. Nakamura et al. (2004) using RNAi targeting TGF-β2 reported a significant reduction in the inflammatory response and matrix deposition in a mouse model of subconjunctival scarring. Recently, the siRNA silencing of secreted protein acidic and rich in cysteine (SPARC) has also been shown to reduce the pro-fibrotic TGF-β2 in in vitro experiments with human Tenon's fibroblasts (Seet et al., 2012). Likewise, SPARC down-regulation induced a cell migration delay, reduced collagen contractility as well as the expression of other pro-fibrotic and pro-inflammatory genes, which indicates that SPARC silencing has potential as an anti-fibrotic strategy. Furthermore, the effects of siRNA silencing SPARC were compared with those of mitomycin C, which is commonly used to attenuate the post-operative scarring response. Mitomycin C-treated human Tenon's fibroblasts maintained their migratory ability and continued to express TGF-β2 as well as pro-fibrotic and pro-inflammatory genes. These observations explain why sometimes filtration surgery is not effective despite the use of mitomycin C and support the targeting of SPARC expression as an improved anti-fibrotic strategy rather than the use of mitomycin C for treating post-operative scarring.
In another attempt to improve surgical outcome, a subconjunctival injection of cationic nano-copolymers mediated IκB kinase β targeting siRNA was administered to a monkey model of trabeculectomy (Ye et al., 2010), and found to markedly reduce the subconjunctival scar tissue in the treated eyes.
Retinitis pigmentosa
RP is a disease of the retina caused by degenerating photoreceptors. It is characterized by night blindness and loss of peripheral vision in the early stages, followed later by loss of central vision (Kalloniatis and Fletcher, 2004). Currently there is no cure for RP, but some treatments have been suggested to slow down the progression of the pathology. In a recent clinical trial, patients receiving vitamin A palmitate, 15 000 IU·day−1, with an omega-3 fatty acid intake of at least 0.20 g·day−1, showed an almost 50% slower rate of decline in their central visual field sensitivity as compared with control subjects (Berson et al., 2012). A lutein supplement (12 mg·day−1) combined with vitamin A uptake has also been shown to have a beneficial effect on preserving mid-peripheral field sensitivity (Berson et al., 2010). However, these clinical trials have been severely criticized (Massof and Fishman, 2010; Seigel and Richoz, 2013), and further investigations are required to provide evidence of the beneficial effects of nutritional supplements on RP rate progression. In contrast, a retinal prosthesis system (The Argus II Retinal Prosthesis System), which electrically stimulates the retina to induce visual perception, has received market approval in the United States and the European Economic Area. However, the effectiveness of this device in blind patients with severe to profound RP is still being tested in clinical trials (Humayun et al., 2012; da Cruz et al., 2013).
The lack of a clear treatment for RP means there is demand for new therapeutic approaches. In this context, RNAi-based gene therapy could be a particularly attractive therapeutic strategy. There are almost 1.5 million patients with autosomal-dominant RP who have mutations in 18 different genes (Phelan and Bok, 2000; Hartong et al., 2006). Among these genes, mutations in the rod photoreceptor-specific gene encoding rhodopsin, the pigment responsible for phototransduction, cause up to 25% of the cases of autosomal-dominant RP (Morris et al., 2009). Over 100 mutations in rhodopsin have been characterized (http://www.retinainternational.org/sci-news/rhomut.htm), most of which are single-point missense mutations. To prevent the pathological progress of autosomal-dominant RP, rhodopsin mRNA silencing by siRNAs, using two different approaches, has been proposed. The first approach includes the use of allele-specific siRNAs, which block the expression of mutant transcripts while allowing normal expression of wild-type alleles (Hernan et al., 2011). However, the preferential silencing of a common dominant rhodopsin mutation was not sufficient to inhibit retinal degeneration in a transgenic rat model (Tessitore et al., 2006). Moreover, the considerable heterogeneity among rhodopsin gene mutations would require the development of individual therapeutics for each mutation, which would represent an expensive and time-consuming process. To circumvent this limitation an alternative strategy consisting of mutation-independent RNAi suppression and gene replacement has been proposed. In essence, the approach involves suppression of both mutant and wild-type alleles of the rhodopsin gene by siRNAs (Cashman et al., 2005; Kiang et al., 2005; Gorbatyuk et al., 2007) and concomitant provision of the rhodopsin gene, which has been sequence-modified to evade suppression. Using this approach, O'Reilly and colleagues (2007) demonstrated a substantial RNAi-based suppression of rhodopsin mRNA and the effective expression of RNAi-resistant replacement genes in the presence of siRNAs in mice subretinally injected with adeno-associated virus expressing both a siRNA and a replacement rhodopsin. Likewise, intravitreal injection of this adeno-associated virus induced a short-term (10 day) beneficial effect in the outer nuclear layer of the mouse simulating human rhodopsin-linked RP with a mutation consisting of proline-23 substituted by histidine (P23H). More recently, a long-term restoration of retinal structure and function by mutant-independent rhodopsin siRNA combined with rhodopsin RNA replacement was observed in these P23H rhodopsin transgenic RP mice (Mao et al., 2012). At 9 months post-injection, the outer nuclear layer and rod outer segment thickness were significantly increased in the treated compared to control eyes. The improved survival of photoreceptors was concomitant with the preservation of an electroretinogram response.
Other genes associated with autosomal-dominant RP such as retinal degeneration slow-peripherin have similarly been a target for RNAi-based suppression coupled with gene replacement technology for the development of RP therapies (Palfi et al., 2006; Petrs-Silva et al., 2012). Alternatively, a combined therapy introducing wild-type photoreceptor PDE-6 expression and knocking-down guanylate cyclase or cyclic nucleotide-gated ion channels by RNAi has been proposed to prevent an excessive influx of calcium leading to photoreceptor cell death in RP (Tosi et al., 2011).
Despite all these promising results, the possibility that the combined use of RNAi and gene therapy could also have potential adverse effects must not be overlooked. For instance, more data from animal models are required to fully assess the potential risk of insertional mutagenesis from integrating vectors, even the mostly episomal adeno-associated virus vectors.
Age-related macular degeneration
AMD is the leading cause of irreversible blindness in the Western world (Chopdar et al., 2003), with an estimated prevalence of 30–50 million persons.
AMD is a progressive disease that affects the central portion of the retina (the macula). In the earliest stage, deposits called drusen form in the area between the retinal pigment epithelium and the underlying choroid. Advanced AMD, responsible for profound vision loss, has two forms: dry and wet. The dry form of advanced AMD results from atrophy of the retinal pigment epithelial layer below the retina. In wet AMD (neovascular AMD), the principal cause of severe vision loss is the invasion of aberrant blood vessels into the retina from the choroid, a pathological process known as choroidal neovascularization (CNV).
In the past few years, therapeutic intervention for AMD has shifted from a predominantly laser-based treatment approach to a more targeted pharmacotherapeutic strategy (Emerson and Lauer, 2008). As VEGF and its receptor VEGFR1 are critical molecules in the pathogenesis of CNV, several anti-VEGF drugs have been developed (Campa and Harding, 2011). Two agents have been approved by the Food and Drug Administration (FDA) for the treatment of neovascular AMD: pegaptanib (Macugen®, a pegylated aptamer that targets VEGF165 isoform) and ranibizumab (Lucentis®, a humanized monoclonal antibody fragment against VEGF). Also, bevacizumab (Avastin®), a full-length recombinant humanized anti-VEGF antibody, originally approved by the FDA for cancer therapy, is widely used to treat patients with AMD. These last two agents, ranibizumab and bevacizumab, appear to be more efficacious than pegaptanib (Emerson and Lauer, 2008).
Similarly, the siRNA strategy for AMD has been mainly based on the blocking the expression of VEGF and VEGFR1 mRNA. The potency of siRNA against VEGF is thought to be 100–1000 times greater than that of VEGF protein antagonists due to the mechanism of action of RNAi (Gu et al., 2010). Moreover, siRNA-induced suppression of VEGF production may allow the time intervals between the administrations by intravitreal injections to be increased, so decreasing the risk of toxicity or infection.
Reich et al. (2003) tested the ability of siRNA targeting VEGF to reduce growth and vascular permeability in a murine laser-induced model of CNV. The area of CNV was significantly less in eyes that had received a subretinal injection of VEGF siRNA as compared with those injected with GFP siRNA. Similarly, in another study, both intravitreous and periocular injections of siRNA directed against VEGFR1 (siRNA-027) significantly reduced VEGFR1 mRNA levels and suppressed the development of CNV in mice with oxygen-induced ischaemic retinopathy (Shen et al., 2006).
In view of these hopeful results in animals, two pioneering human trials with siRNAS were conducted in the eye. Naked siRNAs targeting VEGFA (bevasiranib) or one of its receptors VEGFR1 (siRNA-027/AGN 211745), administered intravitreously, were tested as drug candidates in clinical trials with CNV due to AMD (Table 1). The randomized, double-blind, phase II study with bevasiranib (Opko Health Inc., Miami, FL, USA) compared three doses (0.2, 1.5 or 3.0 mg) injected intravitreally 6 weeks apart in 129 patients with CNV secondary to AMD (Brucker, 2006). The safety data were encouraging; however, bevasiranib-treated patients lost visual acuity over 18 weeks and lesion size also increased, as measured by fluorescein angiography at 12 weeks. Compared with the improvement in vision seen with other therapies, including ranibizumab and bevacizumab, the vision loss in these patients does not bode well for the use of bevasiranib as a monotherapy. Because bevasiranib only inhibits new VEGF synthesis, but does not eliminate the VEGF already present in the eye at the time of administration, the effect of the compound on CNV can appear to be delayed. For this reason, a phase III trial evaluating the combination of bevasiranib and ranibizumab in 330 patients with neovascular AMD was performed (ClinicalTrials, 2008). More than 30% of the subjects who received ranibizumab followed by bevasiranib showed an improvement of at least three lines of visual acuity. The greatest difference compared with the group treated with ranibizumab only was at 6 weeks. These preliminary results suggested a possible benefit of using ranibizumab–bevasiranib combination therapy to treat AMD. However, Opko Health decided to terminate its phase III trial prematurely, after the Independent Data Monitoring Committee found that, although bevasiranib showed activity when used in conjunction with ranibizumab and safety was acceptable, the trial was unlikely to meet its primary end point. Similarly, a clinical trial of the siRNA AGN211745 targeting VEGFR1 (formerly siRNA-027; Allergan Inc., Irvine, CA, USA) was also halted, despite initial positive reports of efficacy from an earlier study enrolling 26 patients with CNV resulting from AMD (Kaiser et al., 2010). No safety issues were associated with AGN211745 targeting VEGFR1, but it failed to meet a key efficacy in a phase II study.
Importantly, while these clinical studies were being conducted, a shocking discovery was reported by Kleinman et al. (2008). These authors demonstrated that the suppression of neovascularization is a generic property of siRNAs independent of sequence and target. siRNAs of 21 nt (but not shorter siRNAs), with different sequences and targets, showed an anti-angiogenic effect. This effect is mediated by the binding of any 21 nt siRNA to TLR3. This results in receptor dimerization and NF-κB activation of genes encoding IFN-γ and IL-12, which, it seems, are the effectors of the anti-angiogenesis. In this scheme of events, therefore, inhibition of angiogenesis does not seem to occur through the specific action of siRNAs in suppressing the messenger RNA of VEGF or its receptor.
Further studies also confirmed that 21 nt siRNAs, regardless of their targeting sequences, are able to inhibit experimental CNV in mice (Ashikari et al., 2010; Gu et al., 2010). Moreover, a recent study has determined that 21 nt siRNAs can induce caspase-3-mediated apoptotic death of retinal pigmented epithelial cells in mice by activating surface TLR3 and subsequent nuclear translocation of IFN regulatory factor-3 (Kleinman et al., 2012). Retinal pigment epithelium degeneration was not observed after treatment with siRNAs shorter than 21 nt. Accordingly, in order to enhance therapeutic specificity and avoid dsRNA-induced retinal pigment epithelium toxicity, the use of siRNAs (small interference RNA or RNAi) with lengths below 21 nt should be considered. In this context, PF-04523655 (also known as PF-655), an siRNA (19 nt in length) with 2′-O-methyl nucleosides in every pair of oligonucleotide sequences, is in development for the treatment of wet AMD. PF-655 inhibits the expression of the hypoxia-inducible RTP801 gene, which plays a critical role in the pathological process of acute or chronic stress-induced retinal disease and potentiates VEGF expression during hypoxic stress (Brafman et al., 2004). The methylation and short length of this siRNA are considered to prevent TLR3 activation and immune stimulation (Kariko et al., 2005), and, certainly experimental evidence confirmed that PF-655 produced gene silencing into the retinal cells while avoiding TLR3 activation (Feinstein et al., 2009). Moreover, methylation could prevent its degradation (Chiu and Rana, 2003).
In animal models of laser-induced CNV, intravitreal injection of PF-655 reduced CNV formation and vessel linkage (Nozaki et al., 2006). In addition, this siRNA targeting RTP801 gene is currently in clinical trials for the treatment of wet AMD. A phase I trial testing the efficacy and safety of stable, modified PF-655 administered alone by intravitreal injection (Nguyen et al., 2012b), and a phase II study (MONET) comparing PF-655 efficacy with that of ranibizumab as well as a ranibizumab/PF-655 combination therapy versus ranibizumab monotherapy (Nguyen et al., 2012c) have recently been performed (Table 1). The best-corrected visual acuity in AMD patients treated with PF-655 alone was less than that in the ranibizumab group. In contrast, the ranibizumab/PF-655 combination group achieved a numerically greater improvement in mean best-corrected visual acuity from baseline than the ranibizumab group at week 16, although the difference was not statistically significant. Regarding the results of anatomic measurements, the combination (ranibizumab/PF-655) and ranibizumab monotherapy groups had similar mean reductions in central subfield retinal thickness and total CNV area, which were greater than those of the PF-655 monotherapy group.
Thus, despite its favourable structural and chemical properties suggesting that PF-655 should be more effective than other siRNAs for the treatment of neovascular AMD, the clinical trial results indicate that PF-655 monotherapy did not improve AMD as compared with standard of care monotherapy (ranibizumab). However, the combination therapy (ranibizumab/PF-655) showed promising results with an improvement in best-corrected visual acuity in AMD patients as compared with patients treated with only ranibizumab.
However, although anti-VEGF agents such as ranibizumab or bevacizumab are clearly important therapeutic breakthroughs in the treatment of AMD, there are increasing concerns regarding their long-term safety profile because systemic and local side effects can occur. Firstly, although the drugs are administered by intravitreous injection, systemic side effects caused by VEGF blockade can still occur. Thus, systemic exposure to anti-VEGF agents is associated with heightened risks of systemic thromboembolic complications, such as systemic hypertension, stroke, myocardial infarction as well as non-ocular (e.g. gastric and renal) haemorrhage (Costagliola et al., 2012). Secondly, it is also important to remember that VEGF is an important factor in retinal physiology. In fact, VEGF protects RGCs from insults such as H2O2-mediated oxidative stress (Brar et al., 2010). Furthermore, it has recently been reported that VEGF has an essential trophic role in maintaining a healthy choroid vasculature and cone photoreceptors (Kurihara et al., 2012). VEGF deletion in adult mouse retinal pigmented epithelial cells causes rapid retinal dysfunction, especially in cone receptors, whereas deletion of the upstream regulatory transcriptional factors, hypoxia-inducible transcription factors (HIFs), does not produce vision loss. Yet, elimination of these HIFs prevented pathological angiogenesis in a laser photocoagulation model of CNV (Kurihara et al., 2012).
Targeting factors upstream of VEGF may be therapeutically advantageous compared with direct VEGF antagonists, which may have more off-target inhibitory trophic effects. From this perspective, PF-655 siRNA treatment could have the benefit of avoiding the detrimental long-term side effects associated with current anti-VEGF drugs. Alternatively, bearing in mind the clinical trial results with PF-655 siRNA, a sequential combination therapy, which would include an initial treatment with an anti-VEGF drug like ranibizumab followed by maintenance with PF-655 siRNA as a long-term therapy, could be another strategy.
Diabetic retinopathy
DR is a common complication of diabetes, and because the worldwide prevalence of diabetes mellitus continues to increase (it is expected to affect an estimated 500 million by 2030), DR remains a leading cause of vision loss in many developed countries (Congdon et al., 2003; Cheung et al., 2010).
DR is a microvascular disease characterized by vessel basement membrane thickening, blood–retinal barrier breakdown, capillary cell death, acellular capillary, neovascularization and retinal detachment (Cheung et al., 2010). In addition to vascular abnormalities, glial dysfunction and death of retinal neurons also occur in DR.
Pathophysiological mechanisms responsible for the development of this slow-progressing disease have not been fully elucidated yet. Chronic exposure to hyperglycaemia is believed to initiate several biochemical pathways (accumulation of sorbitol and advanced glycation end products, increased oxidative stress, up-regulation of the renin–angiotensin system and VEGF), inducing pathophysiological changes that finally produce microvascular damage and retinal dysfunction (Robinson et al., 2012). Among the pathological changes that occur early and are linked causally to the development of retinopathy in diabetes are inflammation, altered ECM gene expression, and premature death of retinal capillary cells, photoreceptors and ganglion cells (Antonetti et al., 2006; 2012; Gastinger et al., 2006; Kern, 2007).
Laser retinal photocoagulation is the mainstay of ophthalmic therapy for vision-threatening DR.
However, laser treatment reduces the risk of moderate visual loss by approximately 50%, with no significant recovery of vision (Iacono et al., 2010). Moreover, the destructive nature of laser therapy is associated with significant ocular side effects. Thus, new approaches for the treatment of DR have emerged, such as intraocular administration of the anti-VEGF agent ranibizumab (Cheung et al., 2010; Iacono et al., 2010).
Similarly, siRNAs targeting VEGF (bevasiranib) or its receptor VEGFR1 (siRNA-027) designed to inhibit the choroidal neovascularization that occurs in AMD have also been tested, and the results were obtained in mouse models of retinal neovascularization, the process associated with DR, have so far been promising (Reich et al., 2003; Shen et al., 2006). Despite these results, clinical trials using these siRNAs have not been conducted in patients with DR, presumably due to previously failed clinical trials with AMD patients. In contrast, recently, a phase II clinical trial (DEGAS) of the siRNA PF-655, which blocks the RTP801 hypoxia/stress pathway, has been completed in DR patients (Nguyen et al., 2012a; Table 1). This clinical trial was designed to evaluate the effects of three dose levels (0.4, 1.0 and 3.0 mg) of PF-655 compared with laser photocoagulation therapy.
All three dose levels of PF-655 improved visual acuity from baseline by month 12 in the patients. The improvement in best-corrected visual acuity from baseline occurred within the first month after the intravitreal injection of PF-655, and by 6 months, a dose–dependent effect was evident that was maintained until month 12. Both the 1 and 3 mg doses produced greater mean gains in visual acuity than laser therapy. Because there were no dose-limiting toxicities, studies of higher doses (>3 mg) are planned to determine the optimal efficacious dose of PF-655. Moreover, in the future trial the efficacy of higher doses of PF-655 will be compared with those of ranibizumab. Considering the adverse events associated with long-term treatment with anti-VEGF drugs like ranibizumab, as already discussed, intravitreous administration of PF-655 may be a more desirable approach for DR treatment.
Likewise, siRNA targeting of other factors upstream of VEGF, such as the hypoxia-inducible factor-1α (HIF-1α), could be a safer option than using the current anti-VEGF drugs. It has been reported that the silencing of HIF-1α is effective at inhibiting retinal neovascularization in experimental mice models (Jiang et al., 2009).
However, the elucidation of VEGF-independent pathways involved in the pathological progress of DR has provided new potential targets for the design of siRNA-based therapies that could offer future treatment strategies. Thus, as indicated earlier, hyperglycaemia modifies the expression of the ECM gene inducing an excess synthesis of retinal capillary basement membrane components such as fibronectin, collagen type IV and laminin (Evans et al., 2000; Oshitari et al., 2006). The consequent retinal capillary basement membrane thickening is a long-lasting lesion of DR and promotes other characteristic lesions, including acellular capillaries and pericyte loss, vascular leakage and a disturbance in the overall vascular homeostasis (Roy et al., 2003; Oshitari et al., 2006). Bearing this in mind, a siRNA strategy targeting ECM components has been shown to prevent basement membrane thickening (Roy et al., 2011). In streptozotocin-induced diabetic rats, intravitreal injection of a siRNA targeting fibronectin over a period of 4.5 months (at 6 week intervals) significantly reduced basement membrane thickening. Fibronectin siRNA treatment also prevented the loss of pericytes and formation of acellular capillaries, suggesting that fibronectin siRNA could be an effective preventative strategy in patients with DR. Other possible approaches also based on the prevention of ECM protein increase are the use of a siRNA against CTGF. This is a profibrotic factor that induces ECM production, angiogenesis and apoptosis (Brigstock, 2003; van Setten et al., 2005; Liu et al., 2008), and its levels are significantly increased in the retinas of diabetic rats (Winkler et al., 2012). Treatment of diabetic rats with CTGF siRNA decreased laminin β1 and collagen IV mRNA levels whereas fibronectin mRNA levels remain unchanged (Winkler et al., 2012).
Remarkably, intravitreal injection of CTGF siRNA in streptozotocin-induced diabetic rats not only reduced laminin β1 and collagen IV mRNA levels, but also inhibited VEGF and TGF-β2 expression (Yang et al., 2010a), and ameliorated retinal cell apoptosis (Yang et al., 2010b). Collectively, these findings indicate the key role that CTGF plays in DR development and support the potential ability of a CTGF siRNA-based treatment as a therapy for DR.
Thioredoxin-interacting protein (TXNIP) has recently been described as another potential gene target for preventing neovascular injury/cell death and the pathogenesis of DR (Devi et al., 2012). TXNIP is significantly increased in the diabetic rat retina (Perrone et al., 2009) and causes pro-inflammatory gene expression of VEGFA, COX-2, Intercellular Adhesion Molecule 1 and sclerotic fibronectin (Perrone et al., 2009; 2010; Sbai et al., 2010). Knock-down of TXNIP by siRNA was found to prevent the early abnormalities associated with DR in streptozocin-induced diabetic rats, such as retinal inflammation, fibrosis, gliosis and neuronal injury/apoptosis (Perrone et al., 2010; Sbai et al., 2010; Devi et al., 2012).
Corneal neovascularization
Corneal neovascularization is an important cause of blindness that affects up to 4.14% of patients presenting for eye care (Bachmann et al., 2010). Ischaemia, trauma, inflammation and rejection of corneal transplants can induce corneal neovascularization (Tshionyi et al., 2012). Infectious keratitis, caused by a virus, bacteria or fungi, can also lead to corneal neovascularization (Chang et al., 2001a). Interestingly, the use of a TLR2 siRNA to reduce the inflammation induced by fungal infection, was recently found to also prevent the subsequent severe damage (i.e. corneal neovascularization) to the cornea (Guo et al., 2012).
Other causes of corneal neovascularization include the overuse of contact lenses, chemical burns, limbal stem cell deficiency or degenerative diseases (Chang et al., 2012).
Current treatments for corneal neovascularization include topical corticosteroid and non-steroid anti-inflammatory medications, photodynamic therapy, laser photocoagulation, fine needle diathermy and conjunctival, limbal and amniotic membrane transplantation (Gupta and Illingworth, 2011). Unfortunately, these all have limited clinical efficacy and also cause undesirable side effects, such as the elevated IOP and posterior subcapsular cataracts subsequent to corticosteroid use.
There is an overlap in the molecular signals involved in the development of corneal neovascularization with those involved in retinal and CNV, although there are also differences as there are among most vascular beds either within or outside the eye (Oshima et al., 2005). One similarity for all these neovascularization processes is the involvement of VEGF, and therapeutic approaches based on anti-VEGF agents such as bevacizumab are being investigated (Chang et al., 2012). Likewise, siRNAs targeting VEGF or its receptors have been tested in several animal models of corneal neovascularization. Kim et al. (2004) reported that i.v. administration (administered via the polymer vehicle TargeTran) or subconjunctival administration of siRNAs targeting VEGFA, VEGFR1 and VEGFR2 into mice led to a significant reduction in the corneal neovascularization induced by herpes simplex virus infection or CpG oligodeoxynucleotides. The combination of all three siRNAs provided the maximal effectiveness (∼60% reduction in neovascularization). Consistent with these results, subconjunctival injection of siRNA targeting VEGFA in mice eyes in which corneal neovascularization was induced by an alkali burn, reduced VEGF expression, the neovascularized area, as well as the number of new vessels (Zuo et al., 2010).
In another example, intrastromal delivery of a plasmid expressing siRNA against VEGF suppressed injury-induced VEGF production, leukocyte infiltration and angiogenesis, and was able to reverse the corneal neovascularization (Singh et al., 2007). In particular, the suppression of neovascularization exceeded 73% as compared with the 30–50% reduction observed with the anti-VEGF antibodies bevacizumab and ranibizumab (Dursun et al., 2012).
Further, an intrastromal injection of poly(lactic co-glycolic acid) nanoparticles loaded with the plasmid expressing siRNA against VEGF has been shown to reverse the corneal neovascularization in a more sustained and robust manner than the naked plasmid alone, suggesting its usefulness as a therapeutic tool for sustainable and effective treatment of corneal neovascularization (Qazi et al., 2012).
Research on the development of new anti-VEGF siRNA therapies for corneal neovascularization has also been focused on the inhibition of upstream regulators of VEGF expression and action. Hence, the silencing of HIF-1α significantly reduced VEGF expression and corneal neovascularization (about 69–72% at day 10) in a mouse model of closed eye contact lens wear (Chen et al., 2012). Another regulator of VEGF expression in the cornea is the enzyme CYP4B1, a cytochrome P450 enzyme that metabolizes arachidonic acid to the potent inflammatory and angiogenic 12-hydroxyeicosatrienoic acid (Mezentsev et al., 2005). siRNA targeting CYP4B1 greatly diminished VEGF mRNA levels (75% reduction) and attenuated the corneal neovascular response in a rabbit model of inflammatory neovascularization (Seta et al., 2007). The cannabinoid CB1 receptor has also been considered as a novel target. Down-regulation of CB1 receptors by RNAi inhibited both basic fibroblast growth factor- and VEGF-induced angiogenesis in in vitro experiments with human umbilical vein endothelial cells and in rabbit cornea assays in vivo (Pisanti et al., 2011).
These targets upstream of VEGF could be interesting alternative when taking into account the detrimental side effects coupled to long-term direct anti-VEGF therapy.
Concluding remarks and futures perspectives
siRNA technology holds great promise for the treatment of several ocular disorders that affect both the anterior and the posterior segment of the eye. However, implementation of safe siRNA-based ocular therapies might be more challenging than originally thought as there are still a number of concerns about the safety and true specificity of the current generation of siRNAs. On a positive note, the range of non-specific effects observed in siRNA studies is becoming better defined, as well as the approaches to reduce them. In terms of safety, further long-term studies are required, as long-term data in animals are still limited and it is entirely feasible that toxic effects will not show up for months, or perhaps even years.
Moreover, although the eye is a good target for this type of molecule mainly because it is a confined compartment and their delivery is close to the target site, the use of controlled and/or targeted delivery systems is recommended to improve the efficiency of siRNA therapy. These systems can provide protection against degradation, increase the intracellular penetration and permit long-term delivery, avoiding repeated administrations. Sustained siRNA levels could be particularly valuable for minimizing the number of intraocular injections needed to treat posterior segment diseases, as repetitive injections increase the risk of deleterious side effects.
Despite the therapeutic potential expected for RNAi, the reality is that the results obtained with siRNAs in the first clinical trials, which focused on AMD treatment, were not better than those obtained with other pharmacological treatments, at least, when siRNAs were applied alone. However, combining existing drugs with RNAi treatment improved the results, indicating that this combination approach could provide an interesting therapeutic strategy. Although siRNAs did not completely succeed in clinical trials for AMD treatment, their potential merits continue to inspire research. In this way, several siRNAs have been tested for glaucoma treatment and clinical trials are ongoing. Their use offers a significant advantage as compared with conventional drugs as they were found to produce a more sustained reduction in IOP, circumventing the drawbacks associated with a requirement of frequent injections. Moreover, the RNAi strategy is particularly attractive for the treatment of ocular pathologies that do not have a specific cure such as RP.
Future advances in improving the safety, efficiency and duration of action of siRNAs will make it possible to maximize the clinical significance of RNAi technology for the treatment of ocular diseases.
Acknowledgments
This work was supported by Universidad Complutense de Madrid (Project GR35/10-A-920777), the Ministry of Economy (SAF 2010/16024) and the Institute Carlos III (Redes temáticas de investigación cooperativa en salud RD12/0034/0003).
Glossary
- AMD
age-related macular degeneration
- Apaf-1
apoptotic protease-activating factor-1
- CNV
choroidal neovascularization
- CTGF
connective tissue growth factor
- DR
diabetic retinopathy
- dsRNA
double-stranded RNA
- ECM
extracellular matrix
- HIF-1α
hypoxia-inducible factor-1α
- IOP
intraocular pressure
- ONC
optic nerve crush
- POAG
open-angle glaucoma
- RGC
retinal ganglion cell
- RISC
RNA-induced silencing complex
- RNAi
RNA interference
- RP
retinitis pigmentosa
- Smad
signalling mathers against decapentaplegic
- SPARC
secreted protein acidic and rich in cysteine
- TLR3
toll-like receptor 3
- TM
trabecular meshwork
- TXNIP
thioredoxin interacting protein
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- Ahmed Z, Kalinski H, Berry M, Almasieh M, Ashush H, Slager N, et al. Ocular neuroprotection by siRNA targeting caspase-2. Cell Death Dis. 2011;2:e173. doi: 10.1038/cddis.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) 5th edition. Br J Pharmacol. 2011;164:S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Alm A, Nilsson SF. Uveoscleral outflow – a review. Exp Eye Res. 2009;88:760–768. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- Ashikari M, Tokoro M, Itaya M, Nozaki M, Ogura Y. Suppression of laser-induced choroidal neovascularization by nontargeted siRNA. Invest Ophthalmol Vis Sci. 2010;51:3820–3824. doi: 10.1167/iovs.09-5121. [DOI] [PubMed] [Google Scholar]
- Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. 2010;117:1300–1305. doi: 10.1016/j.ophtha.2010.01.039. e7. [DOI] [PubMed] [Google Scholar]
- Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Brockhurst RJ, Hayes KC, et al. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol. 2010;128:403–411. doi: 10.1001/archophthalmol.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Willett WC. Omega-3 intake and visual acuity in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol. 2012;130:707–711. doi: 10.1001/archophthalmol.2011.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly M, Hartsough K, Machemer L, Pavco P, Lockridge J. Liquid chromatography electrospray ionization mass spectrometry analysis of the ocular metabolites from a short interfering RNA duplex. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;835:62–70. doi: 10.1016/j.jchromb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Rockwood EJ, Smith SD, Bonilha VL, Crabb JS, Kuchtey RW, et al. Proteomics reveal Cochlin deposits associated with glaucomatous trabecular meshwork. J Biol Chem. 2005;280:6080–6084. doi: 10.1074/jbc.M411233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham A, Anderson EM, Reynolds A, Ilsley-Tyree D, Leake D, Fedorov Y, et al. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods. 2006;3:199–204. doi: 10.1038/nmeth854. [DOI] [PubMed] [Google Scholar]
- Brafman A, Mett I, Shafir M, Gottlieb H, Damari G, Gozlan-Kelner S, et al. Inhibition of oxygen-induced retinopathy in RTP801-deficient mice. Invest Ophthalmol Vis Sci. 2004;45:3796–3805. doi: 10.1167/iovs.04-0052. [DOI] [PubMed] [Google Scholar]
- Brar VS, Sharma RK, Murthy RK, Chalam KV. Bevacizumab neutralizes the protective effect of vascular endothelial growth factor on retinal ganglion cells. Mol Vis. 2010;16:1848–1853. [PMC free article] [PubMed] [Google Scholar]
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Brucker A. Small interfering RNA (CAND5) for the treatment of subfoveal choroidal neovascularization due to age-related macular degeneration. 2006. Retina society. Combined Retina Society/Gonin Society Meeting.
- Buller C, Johnson D. Segmental variability of the trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1994;35:3841–3851. [PubMed] [Google Scholar]
- Campa C, Harding SP. Anti-VEGF compounds in the treatment of neovascular age related macular degeneration. Curr Drug Targets. 2011;12:173–181. doi: 10.2174/138945011794182674. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Binkley EA, Kumar-Singh R. Towards mutation-independent silencing of genes involved in retinal degeneration by RNA interference. Gene Ther. 2005;12:1223–1228. doi: 10.1038/sj.gt.3302512. [DOI] [PubMed] [Google Scholar]
- Castany M, Jordi I, Catala J, Gual A, Morales M, Gasull X, et al. Glaucoma patients present increased levels of diadenosine tetraphosphate, Ap(4)A, in the aqueous humour. Exp Eye Res. 2011;92:221–226. doi: 10.1016/j.exer.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001a;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol. 2012;57:415–429. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Crowston JG, Sabin CA, Khaw PT, Akbar AN. Human tenon's fibroblast-produced ifnbeta and the prevention of t-cell apoptosis. Invest Ophthalmol Vis Sci. 2001b;42:1531–1538. [PubMed] [Google Scholar]
- Chen P, Yin H, Wang Y, Xie L. Inhibition of VEGF expression and corneal neovascularization by shRNA targeting HIF-1alpha in a mouse model of closed eye contact lens wear. Mol Vis. 2012;18:864–873. [PMC free article] [PubMed] [Google Scholar]
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- Chiu YL, Rana TM. siRNA function in RNAi: a chemical modification analysis. RNA. 2003;9:1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WG, Albuquerque RJ, Kleinman ME, Tarallo V, Greco A, Nozaki M, et al. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc Natl Acad Sci U S A. 2009;106:7137–7142. doi: 10.1073/pnas.0812317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopdar A, Chakravarthy U, Verma D. Age related macular degeneration. BMJ. 2003;326:485–488. doi: 10.1136/bmj.326.7387.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials. 2008. Safety and efficacy study evaluating the combination of bevasiranib and Lucentis therapy in wet AMD (COBALT)
- Comes N, Borras T. Functional delivery of synthetic naked siRNA to the human trabecular meshwork in perfused organ cultures. Mol Vis. 2007;13:1363–1374. [PubMed] [Google Scholar]
- Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–2060. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- Costagliola C, Agnifili L, Arcidiacono B, Duse S, Fasanella V, Mastropasqua R, et al. Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration. Expert Opin Biol Ther. 2012;12:1299–1313. doi: 10.1517/14712598.2012.707176. [DOI] [PubMed] [Google Scholar]
- Crooke A, Mediero A, Guzman-Aranguez A, Pintor J. Silencing of P2Y2 receptor delays Ap4A-corneal re-epithelialization process. Mol Vis. 2009;15:1169–1178. [PMC free article] [PubMed] [Google Scholar]
- da Cruz L, Coley BF, Dorn J, Merlini F, Filley E, Christopher P, et al. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br J Ophthalmol. 2013;97:632–636. doi: 10.1136/bjophthalmol-2012-301525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande MC, Davies MC, Garnett MC, Williams PM, Armitage D, Bailey L, et al. The effect of poly(ethylene glycol) molecular architecture on cellular interaction and uptake of DNA complexes. J Control Release. 2004;97:143–156. doi: 10.1016/j.jconrel.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Devi TS, Lee I, Huttemann M, Kumar A, Nantwi KD, Singh LP. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: implications for diabetic retinopathy. Exp Diabetes Res. 2012;2012:438238. doi: 10.1155/2012/438238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun A, Arici MK, Dursun F, Ozec AV, Toker MI, Erdogan H, et al. Comparison of the effects of bevacizumab and ranibizumab injection on corneal angiogenesis in an alkali burn induced model. Int J Ophthalmol. 2012;5:448–451. doi: 10.3980/j.issn.2222-3959.2012.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001a;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001b;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson MV, Lauer AK. Current and emerging therapies for the treatment of age-related macular degeneration. Clin Ophthalmol. 2008;2:377–388. doi: 10.2147/opth.s1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T, Deng DX, Chen S, Chakrabarti S. Endothelin receptor blockade prevents augmented extracellular matrix component mRNA expression and capillary basement membrane thickening in the retina of diabetic and galactose-fed rats. Diabetes. 2000;49:662–666. doi: 10.2337/diabetes.49.4.662. [DOI] [PubMed] [Google Scholar]
- Fan S, Hejkal JJ, Gulati V, Galata S, Camras CB, Toris CB. Aqueous humor dynamics during the day and night in volunteers with ocular hypertension. Arch Ophthalmol. 2011;129:1162–1166. doi: 10.1001/archophthalmol.2011.226. [DOI] [PubMed] [Google Scholar]
- Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K, et al. Off-target effects by siRNA can induce toxic phenotype. RNA. 2006;12:1188–1196. doi: 10.1261/rna.28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein HA, Kleinman ME, Nozaki M, Kalinski H, Mett I, Ambati J, et al. PF-04523655 (REDD14), an siRNA compound targeting RTP801, penetrates retinal cells producing target gene knockdown and avoiding TLR3 activation. Invest Ophthalmol Vis Sci (ARVO Meeting Abstracts) 2009;50:5693. [Google Scholar]
- Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther. 2008;19:125–132. doi: 10.1089/hum.2008.928. [DOI] [PubMed] [Google Scholar]
- Gaglione M, Messere A. Recent progress in chemically modified siRNAs. Mini Rev Med Chem. 2010;10:578–595. doi: 10.2174/138955710791384036. [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006;47:3143–3150. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- Goel M, Sienkiewicz AE, Picciani R, Wang J, Lee RK, Bhattacharya SK. Cochlin, intraocular pressure regulation and mechanosensing. PLoS One. 2012;7:e34309. doi: 10.1371/journal.pone.0034309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk M, Justilien V, Liu J, Hauswirth WW, Lewin AS. Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery. Vision Res. 2007;47:1202–1208. doi: 10.1016/j.visres.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Chen H, Tuo J, Gao X, Chen L. Inhibition of experimental choroidal neovascularization in mice by anti-VEGFA/VEGFR2 or non-specific siRNA. Exp Eye Res. 2010;91:433–439. doi: 10.1016/j.exer.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Guo H, Gao J, Wu X. Toll-like receptor 2 siRNA suppresses corneal inflammation and attenuates Aspergillus fumigatus keratitis in rats. Immunol Cell Biol. 2012;90:352–357. doi: 10.1038/icb.2011.49. [DOI] [PubMed] [Google Scholar]
- Guo J, Fisher KA, Darcy R, Cryan JF, O'Driscoll C. Therapeutic targeting in the silent era: advances in non-viral siRNA delivery. Mol Biosyst. 2010;6:1143–1161. doi: 10.1039/c001050m. [DOI] [PubMed] [Google Scholar]
- Gupta D, Illingworth C. Treatments for corneal neovascularization: a review. Cornea. 2011;30:927–938. doi: 10.1097/ICO.0b013e318201405a. [DOI] [PubMed] [Google Scholar]
- Guzman-Aranguez A, Colligris B, Pintor J. Contact lenses: promising devices for ocular drug delivery. J Ocul Pharmacol Ther. 2013;29:189–199. doi: 10.1089/jop.2012.0212. [DOI] [PubMed] [Google Scholar]
- Hall AH, Wan J, Shaughnessy EE, Ramsay Shaw B, Alexander KA. RNA interference using boranophosphate siRNAs: structure-activity relationships. Nucleic Acids Res. 2004;32:5991–6000. doi: 10.1093/nar/gkh936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- Hernan I, Gamundi MJ, Planas E, Borras E, Maseras M, Carballo M. Cellular expression and siRNA-mediated interference of rhodopsin cis-acting splicing mutants associated with autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52:3723–3729. doi: 10.1167/iovs.10-6933. [DOI] [PubMed] [Google Scholar]
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- Humayun MS, Dorn JD, da Cruz L, Dagnelie G, Sahel JA, Stanga PE, et al. Interim results from the international trial of Second Sight's visual prosthesis. Ophthalmology. 2012;119:779–788. doi: 10.1016/j.ophtha.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono P, Battaglia Parodi M, Bandello F. Antivascular endothelial growth factor in diabetic retinopathy. Dev Ophthalmol. 2010;46:39–53. doi: 10.1159/000320008. [DOI] [PubMed] [Google Scholar]
- Jampel HD, McGuigan LJ, Dunkelberger GR, L'Hernault NL, Quigley HA. Cellular proliferation after experimental glaucoma filtration surgery. Arch Ophthalmol. 1988;106:89–94. doi: 10.1001/archopht.1988.01060130095036. [DOI] [PubMed] [Google Scholar]
- Jiang J, Xia XB, Xu HZ, Xiong Y, Song WT, Xiong SQ, et al. Inhibition of retinal neovascularization by gene transfer of small interfering RNA targeting HIF-1alpha and VEGF. J Cell Physiol. 2009;218:66–74. doi: 10.1002/jcp.21566. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Guo Y, Cepurna WO, Morrison JC. Neurotrophin roles in retinal ganglion cell survival: lessons from rat glaucoma models. Exp Eye Res. 2009;88:808–815. doi: 10.1016/j.exer.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol Ther. 2008;16:107–114. doi: 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Judge AD, Bola G, Lee AC, MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Junglas B, Yu AH, Welge-Lussen U, Tamm ER, Fuchshofer R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp Eye Res. 2009;88:1065–1075. doi: 10.1016/j.exer.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bosl M, et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012;180:2386–2403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Kaiser PK, Symons RC, Shah SM, Quinlan EJ, Tabandeh H, Do DV, et al. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol. 2010;150:33–39. doi: 10.1016/j.ajo.2010.02.006. e2. [DOI] [PubMed] [Google Scholar]
- Kalloniatis M, Fletcher EL. Retinitis pigmentosa: understanding the clinical presentation, mechanisms and treatment options. Clin Exp Optom. 2004;87:65–80. doi: 10.1111/j.1444-0938.2004.tb03152.x. [DOI] [PubMed] [Google Scholar]
- Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007(95103):1–14. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang AS, Palfi A, Ader M, Kenna PF, Millington-Ward S, Clark G, et al. Toward a gene therapy for dominant disease: validation of an RNA interference-based mutation-independent approach. Mol Ther. 2005;12:555–561. doi: 10.1016/j.ymthe.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Kiel JW, Hollingsworth M, Rao R, Chen M, Reitsamer HA. Ciliary blood flow and aqueous humor production. Prog Retin Eye Res. 2011;30:1–17. doi: 10.1016/j.preteyeres.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165:2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman ME, Kaneko H, Cho WG, Dridi S, Fowler BJ, Blandford AD, et al. Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3. Mol Ther. 2012;20:101–108. doi: 10.1038/mt.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest. 2012;122:4213–4217. doi: 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz D, Ciulla TA. Novel approaches for retinal drug delivery. Ophthalmol Clin North Am. 2002;15:405–410. doi: 10.1016/s0896-1549(02)00034-2. [DOI] [PubMed] [Google Scholar]
- Lingor P, Koeberle P, Kugler S, Bahr M. Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain. 2005;128(Pt 3):550–558. doi: 10.1093/brain/awh382. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang R, Tinner B, Choudhry A, Schutze N, Chaqour B. Cysteine-rich protein 61 and connective tissue growth factor induce deadhesion and anoikis of retinal pericytes. Endocrinology. 2008;149:1666–1677. doi: 10.1210/en.2007-1415. [DOI] [PubMed] [Google Scholar]
- Liu JH, Kripke DF, Hoffman RE, Twa MD, Loving RT, Rex KM, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39:2707–2712. [PubMed] [Google Scholar]
- Liu JH, Kripke DF, Twa MD, Hoffman RE, Mansberger SL, Rex KM, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40:2912–2917. [PubMed] [Google Scholar]
- Liu JH, Kripke DF, Weinreb RN. Comparison of the nocturnal effects of once-daily timolol and latanoprost on intraocular pressure. Am J Ophthalmol. 2004;138:389–395. doi: 10.1016/j.ajo.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wu K, Qiu X, Yang Y, Lin X, Yu M. siRNA silencing of gene expression in trabecular meshwork: RhoA siRNA reduces IOP in mice. Curr Mol Med. 2012;12:1015–1027. doi: 10.2174/156652412802480907. [DOI] [PubMed] [Google Scholar]
- Ma JB, Ye K, Patel DJ. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature. 2004;429:318–322. doi: 10.1038/nature02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight AD, McLaughlin CW, Peart D, Purves RD, Carre DA, Civan MM. Formation of the aqueous humor. Clin Exp Pharmacol Physiol. 2000;27:100–106. doi: 10.1046/j.1440-1681.2000.03208.x. [DOI] [PubMed] [Google Scholar]
- Mao H, Gorbatyuk MS, Rossmiller B, Hauswirth WW, Lewin AS. Long-term rescue of retinal structure and function by rhodopsin RNA replacement with a single adeno-associated viral vector in P23H RHO transgenic mice. Hum Gene Ther. 2012;23:356–366. doi: 10.1089/hum.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovskaya A, Crooke A, Guzman-Aranguez AI, Peral A, Ziganshin AU, Pintor J. Hypotensive effect of UDP on intraocular pressure in rabbits. Eur J Pharmacol. 2008;579:93–97. doi: 10.1016/j.ejphar.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Marques JT, Devosse T, Wang D, Zamanian-Daryoush M, Serbinowski P, Hartmann R, et al. A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat Biotechnol. 2006;24:559–565. doi: 10.1038/nbt1205. [DOI] [PubMed] [Google Scholar]
- Martin-Gil A, de Lara MJ, Crooke A, Santano C, Peral A, Pintor J. Silencing of P2Y(2) receptors reduces intraocular pressure in New Zealand rabbits. Br J Pharmacol. 2012;165:1163–1172. doi: 10.1111/j.1476-5381.2011.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massof RW, Fishman GA. How strong is the evidence that nutritional supplements slow the progression of retinitis pigmentosa? Arch Ophthalmol. 2010;128:493–495. doi: 10.1001/archophthalmol.2010.46. [DOI] [PubMed] [Google Scholar]
- Mediero A, Alarma-Estrany P, Pintor J. New treatments for ocular hypertension. Auton Neurosci. 2009;147:14–19. doi: 10.1016/j.autneu.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Mezentsev A, Mastyugin V, Seta F, Ashkar S, Kemp R, Reddy DS, et al. Transfection of cytochrome P4504B1 into the cornea increases angiogenic activity of the limbal vessels. J Pharmacol Exp Ther. 2005;315:42–50. doi: 10.1124/jpet.105.088211. [DOI] [PubMed] [Google Scholar]
- Mincione F, Scozzafava A, Supuran CT. The development of topically acting carbonic anhydrase inhibitors as antiglaucoma agents. Curr Pharm Des. 2008;14:649–654. doi: 10.2174/138161208783877866. [DOI] [PubMed] [Google Scholar]
- Morris MB, Dastmalchi S, Church WB. Rhodopsin: structure, signal transduction and oligomerisation. Int J Biochem Cell Biol. 2009;41:721–724. doi: 10.1016/j.biocel.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Moses RA. Intraocular pressure. In: Moses RA, editor. Adler's Physiology of the Eye: Clinical Application. St Louis: C.V. Mosby Co; 1981. pp. 227–254. [Google Scholar]
- Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv Drug Deliv Rev. 2005;57:2063–2079. doi: 10.1016/j.addr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Siddiqui SS, Shen X, Malik AB, Pulido JS, Kumar NM, et al. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol Vis. 2004;10:703–711. [PubMed] [Google Scholar]
- Nathanson JA. Human ciliary process adrenergic receptor: pharmacological characterization. Invest Ophthalmol Vis Sci. 1981;21:798–804. [PubMed] [Google Scholar]
- Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ, et al. Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS Study) Invest Ophthalmol Vis Sci. 2012a;53:7666–7674. doi: 10.1167/iovs.12-9961. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ, et al. Phase 1 dose-escalation study of a siRNA targeting the RTP801 gene in age-related macular degeneration patients. Eye (Lond) 2012b;26:1099–1105. doi: 10.1038/eye.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Klamerus KJ, Chi-Burris K, et al. Evaluation of the siRNA PF-04523655 versus ranibizumab for the treatment of neovascular age-related macular degeneration (MONET Study) Ophthalmology. 2012c;119:1867–1873. doi: 10.1016/j.ophtha.2012.03.043. [DOI] [PubMed] [Google Scholar]
- Nozaki RB, Mett I, Notkin N, Papismadov I, Shalom L, Takeda A, et al. RTP801i: a novel anti-angiogenic strategy superior to and cooperative with VEGF-A blockade in suppressing CNV. Invest Ophthalmol Vis Sci (ARVO Meeting Abstracts) 2006;47:900. [Google Scholar]
- O'Reilly M, Palfi A, Chadderton N, Millington-Ward S, Ader M, Cronin T, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban TI, Izaurralde E. Decay of mRNAs targeted by RISC requires XRN1, the Ski complex, and the exosome. RNA. 2005;11:459–469. doi: 10.1261/rna.7231505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y, Oshima S, Nambu H, Kachi S, Takahashi K, Umeda N, et al. Different effects of angiopoietin-2 in different vascular beds: new vessels are most sensitive. FASEB J. 2005;19:963–965. doi: 10.1096/fj.04-2209fje. [DOI] [PubMed] [Google Scholar]
- Oshitari T, Polewski P, Chadda M, Li AF, Sato T, Roy S. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes. 2006;55:86–92. [PubMed] [Google Scholar]
- Palfi A, Ader M, Kiang AS, Millington-Ward S, Clark G, O'Reilly M, et al. RNAi-based suppression and replacement of rds-peripherin in retinal organotypic culture. Hum Mutat. 2006;27:260–268. doi: 10.1002/humu.20287. [DOI] [PubMed] [Google Scholar]
- Pañeda C, Martinez T, Gonzalez V, Ruz V, Jimenez AI. Development of SYL040012, a siRNA for treating increased intraocular pressure associated to glaucoma. AOPT 2013 Scientific Meeting. 2013;1:96. [Google Scholar]
- Parker JS, Roe SM, Barford D. Structural insights into mRNA recognition from a PIWI domain-siRNA guide complex. Nature. 2005;434:663–666. doi: 10.1038/nature03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters L, Sanders NN, Braeckmans K, Boussery K, Van de Voorde J, De Smedt SC, et al. Vitreous: a barrier to nonviral ocular gene therapy. Invest Ophthalmol Vis Sci. 2005;46:3553–3561. doi: 10.1167/iovs.05-0165. [DOI] [PubMed] [Google Scholar]
- Peeters L, Sanders NN, Demeester J, De Smedt SC. Challenges in non-viral ocular gene transfer. Biochem Soc Trans. 2007;35(Pt 1):47–49. doi: 10.1042/BST0350047. [DOI] [PubMed] [Google Scholar]
- Peral A, Gallar J, Pintor J. Adenine nucleotide effect on intraocular pressure: involvement of the parasympathetic nervous system. Exp Eye Res. 2009;89:63–70. doi: 10.1016/j.exer.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Perrone L, Devi TS, Hosoya K, Terasaki T, Singh LP. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009;221:262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- Perrone L, Devi TS, Hosoya KI, Terasaki T, Singh LP. Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis. 2010;1:e65. doi: 10.1038/cddis.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrs-Silva H, Yasumura D, Matthes MT, LaVail MM, Lewin AS, Hauswirth WW. Suppression of rds expression by siRNA and gene replacement strategies for gene therapy using rAAV vector. Adv Exp Med Biol. 2012;723:215–223. doi: 10.1007/978-1-4614-0631-0_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JK, Bok D. A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Mol Vis. 2000;6:116–124. [PubMed] [Google Scholar]
- Pintor J. Silencing Beta2-Adrenergic Receptors reduces intraocular pressure: a new approach for glaucoma therapy. An R Acad Nac Farm. 2011;78:230–240. [Google Scholar]
- Pisanti S, Picardi P, Prota L, Proto MC, Laezza C, McGuire PG, et al. Genetic and pharmacologic inactivation of cannabinoid CB1 receptor inhibits angiogenesis. Blood. 2011;117:5541–5550. doi: 10.1182/blood-2010-09-307355. [DOI] [PubMed] [Google Scholar]
- Pitkanen L, Ruponen M, Nieminen J, Urtti A. Vitreous is a barrier in nonviral gene transfer by cationic lipids and polymers. Pharm Res. 2003;20:576–583. doi: 10.1023/a:1023238530504. [DOI] [PubMed] [Google Scholar]
- Qazi Y, Stagg B, Singh N, Singh S, Zhang X, Luo L, et al. Nanoparticle-mediated delivery of shRNA. VEGF-a plasmids regresses corneal neovascularization. Invest Ophthalmol Vis Sci. 2012;53:2837–2844. doi: 10.1167/iovs.11-9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- Rand TA, Ginalski K, Grishin NV, Wang X. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc Natl Acad Sci U S A. 2004;101:14385–14389. doi: 10.1073/pnas.0405913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- Riley MV, Kishida K. ATPases of ciliary epithelium: cellular and subcellular distribution and probable role in secretion of aqueous humor. Exp Eye Res. 1986;42:559–568. doi: 10.1016/0014-4835(86)90046-1. [DOI] [PubMed] [Google Scholar]
- Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS. Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis Model Mech. 2012;5:444–456. doi: 10.1242/dmm.009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Sato T, Paryani G, Kao R. Downregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed rats. Diabetes. 2003;52:1229–1234. doi: 10.2337/diabetes.52.5.1229. [DOI] [PubMed] [Google Scholar]
- Roy S, Nasser S, Yee M, Graves DT. A long-term siRNA strategy regulates fibronectin overexpression and improves vascular lesions in retinas of diabetic rats. Mol Vis. 2011;17:3166–3174. [PMC free article] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Baba Y, Kawashima Y, Shirai K, Miyamoto T, et al. Accumulation of latent transforming growth factor-beta binding protein-1 and TGF beta 1 in extracellular matrix of filtering bleb and of cultured human subconjunctival fibroblasts. Graefes Arch Clin Exp Ophthalmol. 2001;239:234–241. doi: 10.1007/s004170100275. [DOI] [PubMed] [Google Scholar]
- Sbai O, Devi TS, Melone MA, Feron F, Khrestchatisky M, Singh LP, et al. RAGE-TXNIP axis is required for S100B-promoted Schwann cell migration, fibronectin expression and cytokine secretion. J Cell Sci. 2010;123(Pt 24):4332–4339. doi: 10.1242/jcs.074674. [DOI] [PubMed] [Google Scholar]
- Seet LF, Su R, Toh LZ, Wong TT. In vitro analyses of the anti-fibrotic effect of SPARC silencing in human Tenon's fibroblasts: comparisons with mitomycin C. J Cell Mol Med. 2012;16:1245–1259. doi: 10.1111/j.1582-4934.2011.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigel D, Richoz O. Omega-3 Intake in patients with retinitis pigmentosa receiving vitamin A. JAMA Ophthalmol. 2013;131:267–268. doi: 10.1001/jamaophthalmol.2013.590. [DOI] [PubMed] [Google Scholar]
- Seta F, Patil K, Bellner L, Mezentsev A, Kemp R, Dunn MW, et al. Inhibition of VEGF expression and corneal neovascularization by siRNA targeting cytochrome P450 4B1. Prostaglandins Other Lipid Mediat. 2007;84:116–127. doi: 10.1016/j.prostaglandins.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Setten G, Berglin L, Blalock TD, Schultz G. Detection of connective tissue growth factor in subretinal Fluid following retinal detachment: possible contribution to subretinal scar formation, preliminary results. Ophthalmic Res. 2005;37:289–292. doi: 10.1159/000087698. [DOI] [PubMed] [Google Scholar]
- Shen J, Samul R, Silva RL, Akiyama H, Liu H, Saishin Y, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13:225–234. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- Shi F, Wasungu L, Nomden A, Stuart MC, Polushkin E, Engberts JB, et al. Interference of poly(ethylene glycol)-lipid analogues with cationic-lipid-mediated delivery of oligonucleotides; role of lipid exchangeability and non-lamellar transitions. Biochem J. 2002;366(Pt 1):333–341. doi: 10.1042/BJ20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver LH. Clinical efficacy and safety of brinzolamide (Azopt), a new topical carbonic anhydrase inhibitor for primary open-angle glaucoma and ocular hypertension. Brinzolamide Primary Therapy Study Group. Am J Ophthalmol. 1998;126:400–408. doi: 10.1016/s0002-9394(98)00095-6. [DOI] [PubMed] [Google Scholar]
- Singh N, Higgins E, Amin S, Jani P, Richter E, Patel A, et al. Unique homologous siRNA blocks hypoxia-induced VEGF upregulation in human corneal cells and inhibits and regresses murine corneal neovascularization. Cornea. 2007;26:65–72. doi: 10.1097/ICO.0b013e31802b4201. [DOI] [PubMed] [Google Scholar]
- Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Sohn S, Hur W, Joe MK, Kim JH, Lee ZW, Ha KS, et al. Expression of wild-type and truncated myocilins in trabecular meshwork cells: their subcellular localizations and cytotoxicities. Invest Ophthalmol Vis Sci. 2002;43:3680–3685. [PubMed] [Google Scholar]
- Song LY, Ahkong QF, Rong Q, Wang Z, Ansell S, Hope MJ, et al. Characterization of the inhibitory effect of PEG-lipid conjugates on the intracellular delivery of plasmid and antisense DNA mediated by cationic lipid liposomes. Biochim Biophys Acta. 2002;1558:1–13. doi: 10.1016/s0005-2736(01)00399-6. [DOI] [PubMed] [Google Scholar]
- Su Y, Yang CY, Li Z, Xu F, Zhang L, Wang F, et al. Smad7 siRNA inhibit expression of extracellular matrix in trabecular meshwork cells treated with TGF-beta2. Mol Vis. 2012;18:1881–1884. [PMC free article] [PubMed] [Google Scholar]
- Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009;88:648–655. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Parisi F, Denti MA, Allocca M, Di Vicino U, Domenici L, et al. Preferential silencing of a common dominant rhodopsin mutation does not inhibit retinal degeneration in a transgenic model. Mol Ther. 2006;14:692–699. doi: 10.1016/j.ymthe.2006.07.008. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- Tolentino MJ, Brucker AJ, Fosnot J, Ying GS, Wu IH, Malik G, et al. Intravitreal injection of vascular endothelial growth factor small interfering RNA inhibits growth and leakage in a nonhuman primate, laser-induced model of choroidal neovascularization. Retina. 2004;24:660. doi: 10.1097/00006982-200408000-00039. [DOI] [PubMed] [Google Scholar]
- Toris CB, Camras CB, Yablonski ME. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am J Ophthalmol. 1999;128:8–14. doi: 10.1016/s0002-9394(99)00076-8. [DOI] [PubMed] [Google Scholar]
- Tosi J, Sancho-Pelluz J, Davis RJ, Hsu CW, Wolpert KV, Sengillo JD, et al. Lentivirus-mediated expression of cDNA and shRNA slows degeneration in retinitis pigmentosa. Exp Biol Med (Maywood) 2011;236:1211–1217. doi: 10.1258/ebm.2011.011053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Vidales T, Clark AF, Wordinger RJ. Transforming growth factor-beta2 utilizes the canonical Smad-signaling pathway to regulate tissue transglutaminase expression in human trabecular meshwork cells. Exp Eye Res. 2011;93:442–451. doi: 10.1016/j.exer.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tshionyi M, Shay E, Lunde E, Lin A, Han KY, Jain S, et al. Hemangiogenesis and lymphangiogenesis in corneal pathology. Cornea. 2012;31:74–80. doi: 10.1097/ICO.0b013e31821dd986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchinovich A, Zoidl G, Dermietzel R. Non-viral siRNA delivery into the mouse retina in vivo. BMC Ophthalmol. 2010;10:25. doi: 10.1186/1471-2415-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002;47(Suppl. 1):S53–S64. doi: 10.1016/s0039-6257(02)00306-5. [DOI] [PubMed] [Google Scholar]
- Winkler JL, Kedees MH, Guz Y, Teitelman G. Inhibition of connective tissue growth factor by small interfering ribonucleic acid prevents increase in extracellular matrix molecules in a rodent model of diabetic retinopathy. Mol Vis. 2012;18:874–886. [PMC free article] [PubMed] [Google Scholar]
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(Suppl. 2):S129–S138. doi: 10.1016/s0039-6257(97)80020-3. [DOI] [PubMed] [Google Scholar]
- Yang H, Huang Y, Chen X, Liu J, Lu Y, Bu L, et al. The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-beta(2), elucidated by treatment with CTGFsiRNA. Acta Ophthalmol. 2010a;88:652–659. doi: 10.1111/j.1755-3768.2009.01641.x. [DOI] [PubMed] [Google Scholar]
- Yang HW, Chen XL, Liu ZL, Liu J, Bu LM. CTGFsiRNA ameliorates retinal cells apoptosis in streptozotocin-induced diabetic rats. Int J Ophthalmol. 2010b;3:120–124. doi: 10.3980/j.issn.2222-3959.2010.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Qian Y, Lin M, Duan Y, Sun X, Zhuo Y, et al. Cationic nano-copolymers mediated IKKbeta targeting siRNA to modulate wound healing in a monkey model of glaucoma filtration surgery. Mol Vis. 2010;16:2502–2510. [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Zins EJ, Clark AF, Huang AJ. Suppression of keratoepithelin and myocilin by small interfering RNAs (siRNA) in vitro. Mol Vis. 2007;13:2083–2095. [PubMed] [Google Scholar]
- Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov. 2012;11:541–559. doi: 10.1038/nrd3745. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li A, Ge J, Reigada D, Laties AM, Mitchell CH. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res. 2007;85:637–643. doi: 10.1016/j.exer.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Zuo L, Fan Y, Wang F, Gu Q, Xu X. A siRNA targeting vascular endothelial growth factor-A inhibiting experimental corneal neovascularization. Curr Eye Res. 2010;35:375–384. doi: 10.3109/02713681003597230. [DOI] [PubMed] [Google Scholar]


