Abstract
BACKGROUND AND PURPOSE
The beat-by-beat fluctuation (dynamics) of heart rate (HR) depends on centrally mediated control of the autonomic nervous system (ANS) reflecting the physiological state of an organism. 5-HT1A receptors are implicated in affective disorders,associated with ANS dysregulation which increases cardiac risk but their role in autonomic HR regulation under physiological conditions is insufficiently characterized.
EXPERIMENTAL APPROACH
The effects of subcutaneously administered 5-HT1A receptor ligands on HR dynamics were investigated in C57BL/6 mice during stress-free conditions and emotional challenge (recall of fear conditioned to an auditory stimulus and novelty exposure) using time domain and non-linear HR analyses.
KEY RESULTS
Pre-training treatment with of 8-OH-DPAT (0.5 mg·kg−1, s.c.) prevented conditioned tachycardia in the retention test indicating impaired fear memory. Pretest 5-HT1A receptor activation by 8-OH-DPAT (0.5 but not 0.1 and 0.02 mg·kg−1) caused bradycardia and increased HR variability. 8-OH-DPAT (0.5 mg·kg−1) lowered the unconditioned and conditioned tachycardia from ∼750 to ∼550 bpm, without changing the conditioned HR response to the sound. 8-OH-DPAT induced profound QT prolongation and bradyarrhythmic episodes. Non-linear analysis indicated a pathological state of HR dynamics after 8-OH-DPAT (0.5 mg·kg−1) with ANS hyperactivation impairing HR adaptability. The 5-HT1A receptor antagonist WAY-100635 (0.03 mg·kg−1) blocked these effects of 8-OH-DPAT.
CONCLUSIONS AND IMPLICATIONS
Pre-training 5-HT1A receptor activation by 8-OH-DPAT (0.5 mg·kg−1) impaired memory of conditioned auditory fear based on an attenuated HR increase, whereas pretest administration did not prevent the fear-conditioned HR increase but induced pathological HR dynamics through central ANS dysregulation with cardiac effects similar to acute SSRI overdose.
Keywords: fear conditioning, non-linear dynamics, novelty, radio-telemetry, 5-Hydroxytryptamine, autonomic nervous system
Introduction
Serotonin (5-hydroxytryptamine; 5-HT) signalling via its 14 receptor subtypes (Hoyer et al., 2002; receptor nomenclature follows Alexander et al., 2011) is implicated in a wide range of physiological functions (Barnes and Sharp, 1999) from cardiovascular regulation (see Ramage, 2001; Côte et al., 2004; Villalón and Centurión, 2007; Ramage and Villalón, 2008) to cognition (see Buhot, 1997). Affective disorders including depression and anxiety (Ressler and Nemeroff, 2000) have been linked to disturbances in 5-HT transmission. The 5-HT1A receptor, for which 5-HT has a high affinity, is of particular interest in affective disorders because the mechanisms of current antidepressants, for example the selective serotonin reuptake inhibitors (SSRIs), involves actions at both pre- and postsynaptic 5-HT1A receptors (Artigas, 2013). Moreover, PET studies have shown reductions in pre- and postsynaptic 5-HT1A receptor binding in depressed patients (see Savitz et al., 2009).
Patients with anxiety or anxiety co-morbid to depression show an increased risk for cardiovascular disease (Vogelzangs et al., 2010). Substantially increased cardiac risk exists especially during severe emotional challenge as indicated by epidemiological studies (Bhattacharyya and Steptoe, 2007). Emotional challenges alter autonomic nervous system (ANS) responses, generally by increasing sympathetic tone while decreasing parasympathetic tone (Nalivaiko and Sgoifo, 2009), and enhance thrombogenesis through increased platelet formation (Côte et al., 2004). These two conditions can contribute to elevated risk of cardiac mortality ranging from congestive heart failure to sudden cardiac death.
Previous investigations using pharmacological interventions (see Ramage, 2001; Côte et al., 2004; Ramage and Villalón, 2008) have shown that several 5-HT receptor subtypes are involved in central ANS regulation. These studies were performed under different experimental conditions mainly in anaesthetized rats and cats with externally placed ECG electrodes. The effects of the 5-HT1A/5-HT7 receptor agonist 8-OH-DPAT (100 μg·kg−1) on autonomic responses were investigated in rats subjected to severe behavioural stress (see Nalivaiko and Sgoifo, 2009). Injection of 8-OH-DPAT inhibited the stressor-induced increase of sympathetic tone in rats without changing baseline values (Ngampramuan et al., 2008). Based on these findings, 5-HT1A receptor activation was suggested to exert beneficial effects on ANS responses by attenuating sympathetic drive during aversive challenges (Nalivaiko, 2006; Ngampramuan et al., 2008; Nalivaiko and Sgoifo, 2009). However, 5-HT1A receptor activation is also associated with enhanced parasympathetic drive in rats and cats (see Ramage, 2001; Ramage and Villalón, 2008). As information on brain area-selective involvement of 5-HT1A receptors in central ANS regulation is limited, it is notable that overexpression of 5-HT1A receptors in the raphe complex of mice and increased autoinhibition leads to spontaneous death, which has been linked to sudden infant death syndrome (Audero et al., 2008).
Initially, the effects of subcutaneous pre-training injection of 8-OH-DPAT (0.5 mg·kg−1) were determined by two learning procedures, auditory delay and trace conditioning, as the latter but not the former procedure involves dorsal hippocampal function (Misane et al., 2005). Subcutaneous 8-OH-DPAT given at higher doses (≥0.3 mg·kg−1) before training impaired fear learning as show by the reduction in freezing response to both the context and, to a lesser degree, a tone previously paired to a shock (Stiedl et al., 2000a; Youn et al., 2009) and reducing transfer latencies in passive avoidance retention tests (Madjid et al., 2006). However, the consequences for aversively conditioned heart rate (HR) responses have not been investigated.
Experiments in mice based on the 0.5 mg·kg−1 dose of subcutaneously administered 8-OH-DPAT (Stiedl et al., 2009) indicate adverse effects of 5-HT1A receptor activation on ANS function. This dose also impairs emotional learning and memory (Stiedl et al., 2000a; Ögren et al., 2008; Youn et al., 2009). The aim of the present study was to investigate the contribution of 5-HT1A receptors to HR dynamics under physiological conditions in freely moving mice using radiotelemetry with a spectrum of tests, autonomic measures and 5-HT1A receptor ligands. Low and higher doses 8-OH-DPAT were used to address the involvement of pre- versus postsynaptic 5-HT1A receptors respectively (reviewed by Ögren et al., 2008). Autonomic effects were determined with respect to baseline HR and its adjustment in response to exposure to an auditory cue serving as conditioned fear stimulus (see Tovote et al., 2004; 2005). Therefore, 8-OH-DPAT was injected before the memory test to examine the effects on the expression of cued fear. In addition, HR effects were analysed during novelty exposure serving as unconditioned emotional challenge (Stiedl et al., 2004; Tovote et al., 2005) and under low stress conditions in the home cage. Effects of drug treatment on subintervals of the ECG were quantified because changes of electric propagation across the heart through altered ANS control may indicate cardiac risk states. Furthermore, 5-HT1A receptor-mediated effects on HR dynamics were analysed by non-linear methods because of their superior sensitivity of functional assessment with regard to physiological or pathological state of HR dynamics (Ivanov et al., 1996; Meyer and Stiedl, 2003; Stiedl et al., 2009).
Methods
Animals
All animal care and experimental procedures were approved by local animal research committees and performed in accordance with the European Council Directive (86/609/EEC). All studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010). A total of 105 male C57BL/6NCrlBR (6N) and C57BL/6JIco (6J) mice aged 11–15 weeks were used in this study. These two C57BL/6 substrains show similar behavioural and autonomic responses (Stiedl et al., 1999). Mice (Charles River, Germany and Netherlands) were obtained at 8 weeks of age and individually housed in standard cages (Macrolon type II, Tecnilab BMI, Someren, The Netherlands) with free access to food and water. They were kept at constant ambient temperature (21 ± 1°C) and relative humidity (55 ± 10%) under a 12-h dark-light cycle with lights turned on at 0700 h. The experiments were performed during the light phase (low activity of nocturnal mice) to minimize the contribution of elevated physical activity to autonomic effects during the retention test.
ECG radiotelemetry
ECG recordings were obtained from intraperitoneally implanted ECG radio transmitters (TA10EA-F20, Data Sciences, St. Paul, MN, USA) with the two ECG electrodes placed subcutaneously in lead II position as previously described (Stiedl and Spiess, 1997). Surgery was performed in 8-week-old male mice, followed by analgesia with buprenorphine (0.1 mg·kg−1) and 2–3 weeks of recovery. ECG measurements by radiotelemetry allowed remote determination of HR dynamics in the home cage of mice (see Supporting Information).
Drugs and drug administration
The sources of the drugs used were as follows: 8-OH-DPAT (8-hydroxy-2-(di-N-propylamino)tetralin), WAY-100635 (N-(2-(1-(4-(2-methoxyphenyl)-1piperazinyl)ethyl)-N-2-pyridinylcyclohexanecarboamide; both from Sigma-Aldrich, Taufkirchen, Germany) and NAD-299 ((R)-3-N,N-dicyclobutylamino-8-fluoro-3,4-dihydro-2H-1-benzopyran-5-carboxamide hydrogen (2R,3R)-tartrate monohydrate; robalzotan; AZD-7371; AstraZeneca R&D, Södertälje, Sweden).. All compounds were freshly dissolved in sterile saline. Solutions were injected subcutaneously into the scruff of the neck in a volume of 8 mL·kg−1. For single injections, the drugs were given 15 min before the retention test or training and, for double injections, 30 and 15 min before the retention test. Injections occurred during a brief isoflurane anaesthesia period lasting maximally for 90 s as used before (e.g. Stiedl et al., 2000a; b; see Supporting Information). Drugs were given subcutaneously for comparison with previous studies in mice (e.g. Madjid et al., 2006; Youn et al., 2009; Eriksson et al., 2012) and because subcutaneous injection of 8-OH-DPAT results in improved pharmacokinetic properties due to reduced first-pass metabolism compared to intraperitoneal administration (see Stiedl et al., 2000a).
Fear conditioning
Fear conditioning experiments were carried out as previously described (e.g. Stiedl et al., 2000a, b) using a computer-controlled fear conditioning system (TSE-Systems, Bad Homburg, Germany). During training (acquisition) mice were subjected to a single paired (delay) presentation of a 30 s auditory cue (conditioned stimulus: CS; 10 kHz, pulsed 5 Hz, 75 dB sound pressure level) and, at sound offset, a shock (unconditioned stimulus: US; 2 s, 0.7 mA, constant current) delivered through a stainless steel floor grid in the fear conditioning box. Mice were returned to their home cage from the fear conditioning box 30 s after US termination. Additionally, training was performed by 30 s trace conditioning, that is separation of CS and US by a 30 s interval, repeated twice with a 60 s interval. This training sequence requires dorsohippocampal function for cued associative learning (Misane et al., 2005). Delay and 30 s trace conditioning were used to assess the effect of pre-training drug administration on conditioned HR responses 24 h after training.
Training occurred in a Plexiglas cage (36 cm × 21 cm × 20 cm; length × width × height) inside a constantly illuminated (120–500 lx) conditioning box made of dark grey acrylic plastic. A loudspeaker provided constant auditory background noise (white noise; 68 dB sound pressure level). The Plexiglas cage was thoroughly cleaned with 70% ethanol before each experiment and continuously ventilated by a fan during the experiments. Conditioned HR responses were measured in the home cage 24 h after training by recording ECG for 180 s without sound presentation (pre-CS phase) followed by 180 s sound presentation (CS phase) and a 30 s post-CS phase as previously described (Stiedl and Spiess, 1997; Stiedl et al., 1999; 2009).
Novelty exposure
Unconditioned fear responses were assessed 15 min after drug injection by the exposure of mice to a novel Plexiglas box (36 cm × 21 cm × 20 cm, length × width × height). This box had a plain floor and was cleaned with 1% acetic acid before the placement of mice. ECG of mice was recorded continuously for 34 min as previously described and used to determine beat-by-beat HR in 2-min intervals (e.g, Tovote et al., 2005).
Behavioural observations
Behaviour of mice was observed in their home cages to evaluate the effects of serotonergic drug treatment, such as the 5-HT syndrome (Blanchard et al., 1997; Stiedl et al., 2000a; Youn et al., 2009). The 5-HT syndrome in mice is characterized by unstable, ataxic gate and impaired coordination, hind limb abduction, flattening of the back, eye narrowing, Straub tail, reduced locomotion and rearing. Additionally, changes in physical activity, that is generally low in the home cage, were recorded. The 5-HT syndrome, which substantially reduces locomotor activity (Stiedl et al., 2000; Youn et al., 2009), prevented the evaluation of the acute effects of 8-OH-DPAT (0.5 mg·kg−1) on the expression of conditioned freezing responses.
HR analyses
HR was calculated from RR intervals of the ECG signal. Instantaneous HR (beat-by-beat HR) analyses were performed offline (LabChart 7.1, ADInstruments, Spechbach, Germany) with manual editing, that is removal of artifacts and addition of unrecognized beats using a software extension (HRV 1.4 for LabChart, ADInstruments) as described before (e.g. Stiedl et al., 2005). Ectopic beats were treated as described by Meyer and Stiedl (2006). HR variability was determined on the basis of the RMSSD (root-mean square of successive RR interval differences) value.
First-order variability analysis was performed to quantify HR variability based on changes of three consecutive heartbeat intervals (Stiedl and Meyer, 2003). Non-linear analysis of HR dynamics was included because non-stationarity and interdependence of HR intervals in their temporal sequence formally prohibit the use of linear analyses, unless the magnitude of difference is such that no statistical analysis is necessary to assess their (statistical) difference (see Meyer and Stiedl, 2003; Stiedl et al., 2009). Non-linear fractal dimensionless measures were determined to assess the complex dynamics of the cardiac time series because the irregular sequence of instantaneous HR intervals displays an intrinsic structure that cannot be captured by linear methods. These non-linear methods yield important novel information on complex regulatory processes such as autonomic control determining HR dynamics (Meyer and Stiedl, 2003). The dynamic properties provide for a highly sensitive qualitative assessment of physiological versus pathological states of autonomic control in man (Meyer and Stiedl, 2003; Stiedl et al., 2009) with the ability to predict cardiovascular risks in the absence of apparent cardiovascular disease (Meyer, 2002). An in-depth discussion of the significance of the used non-linear measures is provided elsewhere (Meyer and Stiedl, 2003; 2006).
Higher-order variability analysis determines the regularity as a function of increasing consecutive RR intervals (order). The average strength of cardiac acceleration and deceleration, defined as the mean incremental difference per beat (in ms) during acceleration and deceleration, is obtained from the first order variability data. Multiscale embedding-space decomposition determines the dynamic ‘complexity’ of the cardiac time series, where the number of available system states, ΩA, is calculated for aggregated samples (aggregation level, τ) of the original time series, hence accounting for multiple time scales inherent in the dynamics. The singularity spectrum of the multi-exponent multifractal analysis presents a statistical description of the set of singularities present in the time series and is characterized by the spectrum of the Hölder singularity exponents α, and probabilities of occurrence, f (α). It quantifies the dynamic complexity to identify pathological changes on a multifractal level (Ivanov et al., 1999). The singularity spectrum is ‘broad’ and implies multifractality, and when ‘narrowed’ and displaced to lower α-values, indicates reduced multifractality. The mode of the distribution is the global Hurst exponent h. Further explanations are provided in the Supporting Information. The mathematical bases for all non-linear analyses have been described elsewhere (see Meyer and Stiedl, 2006).
Subintervals of the ECG (PQ, QRS and QT duration) were analysed to determine potential drug-induced changes of autonomic control affecting electric propagation across the heart and ectopic beats/arrhythmias characterized according to Bennett (2006).
Statistical analyses
Overall effects of treatment were examined by one-way or two-way anova and anova for repeated measures (StatView 5.0.1, SAS Institute, Cary, NC, USA) and the Greenhouse-Geisser correction was used whenever appropriate (SPSS 20, IBM, Armonk, NY, USA). HR differences over the time course of pre-CS and CS phase were analysed with linear contrast (General Linear Model: Within Subjects Contrast). Post hoc comparisons were performed by Tukey HSD or Dunnett's T3 when the Levene's test was significant. An error probability of P < 0.05 was accepted as statistically significant in all tests.
Results
Behavioural effects of 8-OH-DPAT
All mice injected subcutaneously with 8-OH-DPAT at 0.5 mg·kg−1 showed the typical signs of the 5-HT syndrome (see Behavioural observations above). Pre-injection of WAY-100635 (0.03 mg·kg−1) or NAD-299 (0.3 mg·kg−1) prevented the 5-HT syndrome caused by 8-OH-DPAT.
Effects of pre-training 8-OH-DPAT on HR responses during expression of cued fear
Because the effect of pre-training 8-OH-DPAT (0.5 mg·kg−1) on conditioned HR responses had not been investigated, experiments were performed with injections 15 min before training and testing 24 h later. Two-way anova with factors of training sequence and drug treatment indicated a significant effect of training sequence (F1,20 = 4.74; P < 0.05), drug treatment (F1,20 = 38.32; P < 0.0001) and a significant training sequence × drug treatment interaction (F1,20 = 5.34; P < 0.05) for the sound-induced HR increase in the first 60 s of CS presentation indicative of conditioned fear. The same effect was observed for the whole (180 s) CS period training sequence (F1,20 = 6.91; P < 0.05), drug treatment (F1,20 = 42.09; P < 0.0001) and training sequence × drug treatment interaction (F1,20 = 6.50; P < 0.05). Under both experimental conditions, pre-training injection of 8-OH-DPAT (0.5 mg·kg−1) prevented the conditioned tachycardia to the auditory CS that was observed in saline-injected controls (Figure 1). The HR increase elicited by the auditory CS reached maximum physiological levels [∼800 beats per min (bpm)] in saline-injected mice starting from pre-CS (baseline) HR (583 ± 15 bpm) that did not differ significantly between drug-treated groups (data not shown). In addition, 24 h after 8-OH-DPAT administration, none of the HR effects that are described below, were observed after pretest injection of 8-OH-DPAT.
Figure 1.
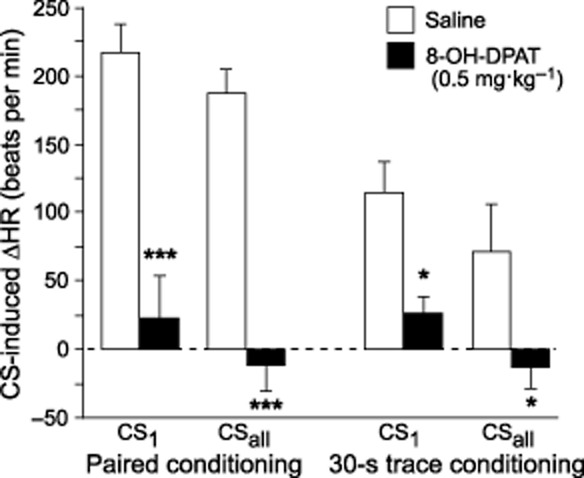
Effects of 8-OH-DPAT (0.5 mg·kg−1) injected subcutaneously 15 min before training on fear-conditioned heart rate changes (ΔHR). Mice were subjected to either paired CS/US or CS separated from US by a 30 s trace interval during training. Heart rate was determined during the retention test in the home cage 24 h after training. ΔHR denotes changes from baseline values [180 s pre-CS phase; mean HR (0 bpm) = 583 ± 15 bpm]. CS1: the first 60 s of CS presentation; CSall: throughout the 180 s CS presentation; values represent means ± SEM; n = 6 per group; *P < 0.05; ***P < 0.001 versus saline control.
Effects of 5-HT1A receptor ligands on HR responses during expression of cued fear
Baseline HR and its fear-induced adjustment was measured 24 h after training in the home cage during exposure to the sound serving as the conditioned fear stimulus. Typical HR responses of a saline- and an 8-OH-DPAT-injected mouse show substantial differences (Figure 2A). anova for repeated measures indicated significant HR differences as a function of drug treatment (F4,29 = 17.95, P < 0.0001; Figure 2B). In addition, there was a significant effect of time on HR (F4,12 = 34.75, P < 0.0001). There was no significant interaction between time and drug treatment (F4,48 = 1.17, P = 0.325). The post hoc test showed a significant difference between 0.5 mg·kg−1 8-OH-DPT and the saline control group (P < 0.002).
Figure 2.
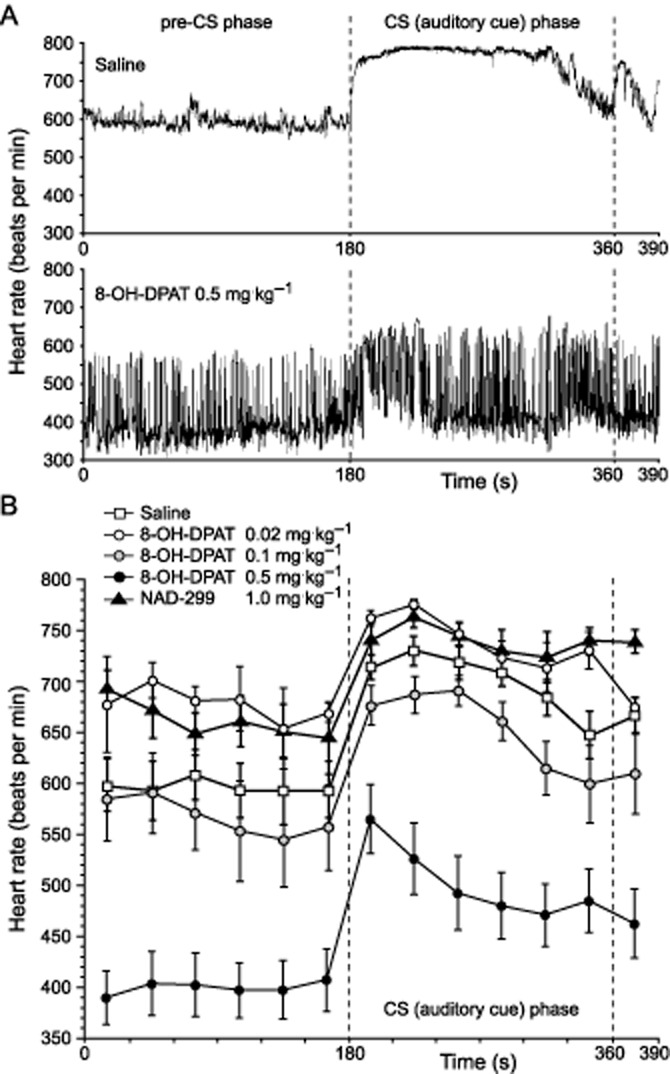
Effects of 5-HT1A receptor ligands on heart rate responses during expression of conditioned fear to the auditory cue. Typical instantaneous heart rate responses of mice injected subcutaneously with saline and 8-OH-DPAT (A), and mean heart rate responses in 30 s bins (B) after injection of mice with either saline, 8-OH-DPAT or NAD-299 at the given dose 15 min before the cue-dependent memory test. CS: conditioned stimulus (sound); values represent means ± SEM; n = 12 (saline), n = 7 (8-OH-DPAT 0.5 mg·kg−1; NAD-299 1 mg·kg−1), n = 5 (8-OH-DPAT 0.1 mg·kg−1) and n = 3 (8-OH-DPAT 0.02 mg·kg−1).
Significant HR differences as a function of drug treatment were observed in the pre-CS phase (baseline HR) before sound presentation (F4,29 = 11.90, P < 0.0001). Post hoc analysis indicated that 8-OH-DPAT at 0.5 mg·kg−1 significantly decreased baseline HR (P < 0.0001). Both, NAD-299 at 1 mg·kg−1 and 8-OH-DPAT at 0.02 mg·kg−1 did not alter the baseline HR (P > 0.45; Figure 2B), compared with that of saline-injected controls. There was no significant interaction between drug treatment and time interval.
Difference contrast analysis was used following the repeated measures anova to determine whether the relative HR changes were different between pre-CS and different 30 s CS phases in the different drug treatment group (see Supporting Information). Since there were no significant differences, this indicated a parallel HR shift from baseline irrespective of drug treatment. However, repeated contrasts indicated a significant difference from the first 30 s CS phase to the second 30 s CS phase that was reconfirmed by comparison of HR responses of mice treated with saline versus 8-OH-DPAT (0.5 mg·kg−1) due to the rise of HR in saline but fall in HR in mice in the second versus the first CS phase.
Effects of 5-HT1A receptor ligands on HR responses during novelty exposure
To determine whether the observed HR effects also occur in response to unconditioned emotional challenge, experiments were performed with novelty exposure that normally elevates HR to maximum physiological levels of close to 800 bpm (Stiedl et al., 2004; Tovote et al., 2005).
Drug treatment (8-OH-DPAT and NAD-299 compared with saline control) had a significant effect on HR values (F2,15 = 121.38, P < 0.0001; Figure 3A,B). Saline-injected control mice exhibited a maximum HR of ∼750 bpm in the first 2 min of novelty exposure and HR remained elevated in the two additional 2-min intervals analysed at 16–18 and 32–34 min (Figure 3B). The 5-HT1A receptor antagonist NAD-299 (1 mg·kg−1) did not alter the HR response when compared to saline-injected controls. 8-OH-DPAT (0.5 mg·kg−1) significantly lowered HR (P < 0.0001 versus saline and NAD-299) to maximum values of ∼600 bpm (Figure 3A) and mean values of ∼500 bpm throughout the 34-min test period (Figure 3B). HR variability based on the RMSSD measure showed an inverse pattern (F2,15 = 36.57, P < 0.0001) with significantly higher RMSSD values in 8-OH-DPAT-injected mice than in NAD-299 and saline-injected controls (P < 0.0001) and no difference between mice injected with saline and NAD-299 (Figure 3C). The 8-OH-DPAT-mediated effects exceeded the 34 min novelty test.
Figure 3.
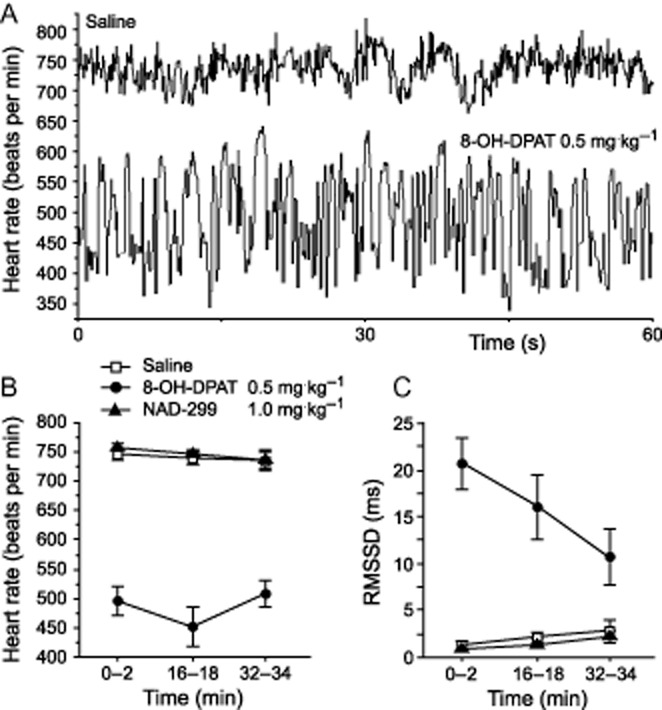
Heart rate responses during novelty exposure. Instantaneous heart rate patterns of a 1 min interval are shown for two mice injected subcutaneously with saline or 8-OH-DPAT, respectively (A). Mean heart rate responses (B) and heart rate variability based on the RMSSD measure (C) were determined during three 2 min intervals (0–2, 16–18 and 32–24 min) of the total 34 min novelty exposure as a function of drug treatment. Values represent means ± SEM; n = 6 per group.
Effects of 5-HT1A receptor ligands on HR dynamics in the home cage
Additional experiments were performed in the home cage with 18-min ECG recording which started 15 min after subcutaneous injection of either saline, 8-OH-DPAT (0.5 mg·kg−1) or WAY-100635 (0.03 mg·kg−1). Injections of WAY-100635 addressed the specificity of 5-HT1A receptor involvement. Under these conditions, similar results were obtained as for baseline HR (pre-CS HR) in fear conditioning experiments. This 18-min recording period yielded a substantially increased number of RR intervals (nmax ∼104) that were used for higher-order variability analysis and are essential to perform non-linear analysis.
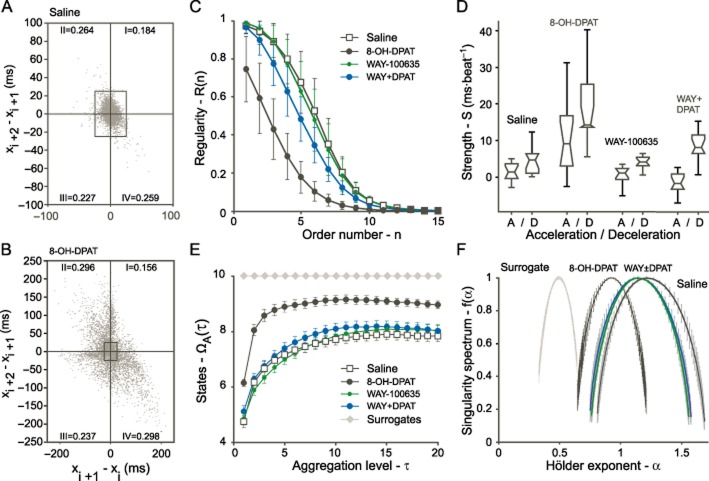
The first-order variability diagram based on the interval change of three successive heartbeat intervals indicated a substantially increased variability in mice injected with 8-OH-DPAT (0.5 mg·kg−1) in comparison to saline-injected controls (Figure 4A,B). The regularity R(n) decreased in saline-injected controls in a sigmoid fashion with increasing order, the increasing number of successive RR intervals (Figure 4C). In contrast, 0.5 mg·kg−1 8-OH-DPAT resulted in an exponentially declining regularity versus order reflecting weakened intrinsic correlations in the time series. Higher-order variability analysis revealed a reduced regularity of the HR pattern (Figure 4C), an increased strength of acceleration and deceleration (Figure 4D), a decreased complexity (Figure 4E) and a shift towards that of a random HR pattern (Figure 4F). For mathematical details on these measures see Meyer and Stiedl (2006). All these effects were observed only in 8-OH-DPAT-treated but not saline-injected mice, and were blocked by pre-injection of the 5-HT1A receptor antagonist WAY-100635 (0.03 mg·kg−1), while WAY-100635 alone at this dose had no significant effect on any of these measures.
Figure 4.
Heart rate dynamics based on successive interval changes and nonlinear analyses. First-order variability diagram based on the interval change of three successive heartbeat intervals (X-axis) in mice injected with saline (A) and 8-OH-DPAT (0.5 mg·kg−1; (B). Increasing intervals, reflecting cardiac deceleration, appear in quadrant I. Gradual shortening of intervals, reflecting cardiac acceleration, yields points appearing in quadrant III. Short-long-short values of x fall into quadrant II and long-short-long values of x appear in quadrant IV respectively. The residual values of 0.068 (ΣI-IV = 0.932 for saline) and 0.013 (ΣI-IV = 0.987 for 8-OH-DPAT) denote the fraction of three successive heartbeat intervals without difference, that is located on 0. The experimental cardiac time series shows a patchy clustering of points, and all four quadrants exhibit different fractional (related to the total number of threesomes in the times series) densities of the populations of points of which the dissymmetry between I and III is the most salient feature. Black squares of a width of 50 ms (± 25 ms centred around 0) indicate the substantially increased variability of the cardiac rhythm over three beats by 8-OH-DPAT. Higher-order variability analysis indicated a loss of complexity when the curve is shifted to the left (C). The strength of acceleration and deceleration in ms·beat−1 as a function of drug treatment is shown in the box plots (D). Multiscale embedding-space decomposition, an entropy-based measure of system complexity to reveal structure on multiple time scales, indicated a loss of complexity by 8-OH-DPAT when the curve is shifted up towards random dynamics as shown for the surrogate (E). The singularity spectrum of the multi-exponent multifractal analysis, an estimation of the multifractal properties of a given time series, is characterized by the range (the width of the inverted-shaped curve) and the mode (the maximum of the curve). This analysis again indicates a shift of the spectrum of 8-OH-DPAT-treated mice toward that of the surrogate with reduced complexity (F). The sequence-randomized surrogates, representing random dynamics (white noise) for each experimental group, all superimpose at ΩA(τ) = 10 (E) and are shifted downwards (F). Note that the singularity spectra of mice treated with WAY-100635 (WAY; 0.03 mg·kg−1) alone or followed by 8-OH-DPAT (0.5 mg·kg−1) largely overlap (F); WAY±DPAT). Drugs were injected subcutaneously. Values represent means ± SE; n = 8 per group.
Comparison of HR variability changes by 8-OH-DPAT versus sleep
To compare the effect of 0.5 mg·kg−1 8-OH-DPAT on HR variability with that of the physiological state at similar HR, ECG was analysed from the same mice under undisturbed conditions during sleep in their home cage. At similarly low HR (∼430 bpm), 8-OH-DPAT resulted in a significantly (F1,126 = 44.82, P < 0.0001) increased RMSSD value (∼2-fold), compared with untreated mice (Table 1), providing further evidence for its deviation from the physiological state.
Table 1.
Comparison of heart rate variability (RMSSD) at similar low heart rates
| Treatment | Mice [n] | Count [n]a | HR [bpm] | RMSSD [ms] |
|---|---|---|---|---|
| Untreated (sleep) | 8 | 64 | 434.8 ± 3.1 | 10.5 ± 0.9 |
| 8-OH-DPAT (0.5 mg·kg−1) | 8 | 64 | 427.0 ± 6.1n.s. | 23.4 ± 1.7*** |
RMSSD, root-mean square of successive RR interval differences.
not significant.
P < 0.0001 versus untreated (sleep).
8 × 2 min bins per mouse.
Effects of 5-HT1A receptor ligands on ECG subintervals and arrhythmias
Analysis of ECG subintervals indicated a significant effect of drug treatment on PQ (F3,48 = 10.45, P < 0.0001), QRS (F3,48 = 5.17, P < 0.005) and QT intervals (F3,48 = 25.92, P < 0.0001; Figure 5A–D). The duration of all intervals was significantly prolonged (P < 0.0005) in mice injected with 8-OH-DPAT (0.5 mg·kg−1), compared with saline-injected controls. The prolongation of all subintervals, except the QRS duration, was significantly lowered by subcutaneous pre-injection of WAY-100635 at 0.03 mg·kg−1, a dose that was ineffective when injected alone. Furthermore, 5-HT1A receptor activation by 8-OH-DPAT resulted in the occurrence of third-degree atrioventricular conduction blocks with complete interruption of transmission of atrial impulses and emergence of escape beats generated in the subsidiary bundle of His. These effects are the consequence of parasympathetic overactivation as indicated by the loss of the normally observed stable temporal relation between subsequent PP and RR intervals (Figure 5E,F). Consequently, the number of ectopic beats (sinus arrhythmias and third-degree atrioventricular conduction blocks without escape beats) was quantified on a total of ∼384 beats. While the number of ectopic beats was low (∼1 per 104 beats) in saline-injected and naïve mice, it was markedly increased to ∼34 per 104 beats by 8-OH-DPAT at 0.5 mg·kg−1. This increase was completely prevented (∼1 per 104 beats) by pre-injection of the 5-HT1A receptor antagonists WAY-100635 (0.03 mg·kg−1) and NAD-299 (0.3 mg·kg−1), which did not induce ectopic beats on their own (data not shown).
Figure 5.
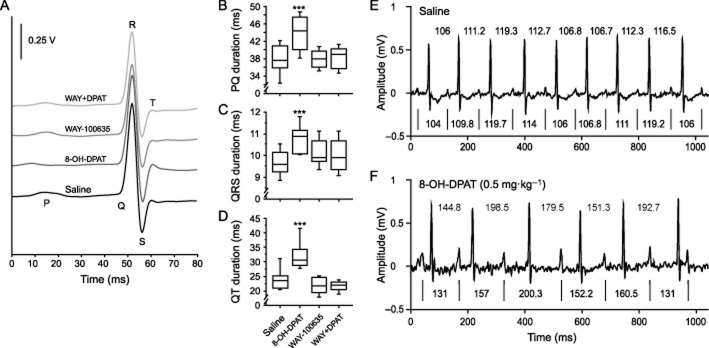
ECG subinterval analyses and RR versus PP intervals of successive ECG signals. ECG waveforms were synchronized on the R peak (A). Durations of PQ (B), QRS (C) and QT (D) intervals as a function of drug treatment are presented as box plots. Examples of the temporal relation of RR versus PP intervals of successive ECG signals in two mice after administration of saline (E) or 8-OH-DPAT (DPAT; F). RR intervals are indicated above the two ECG traces, whereas PP intervals are provided below the two ECG traces with the vertical lines (|) indicating P peaks. All intervals are given in ms. Drugs were injected subcutaneously at the following doses: 8-OH-DPAT (0.5 mg·kg−1), WAY-100635 (WAY: 0.03 mg·kg−1); n = 13 per group; ***P < 0.001 versus saline control.
Discussion
This study investigated the effects of 5-HT1A receptor activation on fear learning and expression, as assessed by the HR in mice. Pre-training injection of 8-OH-DPAT (0.5 mg·kg−1) resulted in impaired fear memory as shown by the absence of sound-induced tachycardia, irrespective of hippocampal involvement (Misane et al., 2005; Chowdhury et al., 2005). This finding extends previous behavioural results (Stiedl et al., 2000a; Youn et al., 2009) to autonomic function. Pretest injections indicated that 5-HT1A receptor activation by 8-OH-DPAT (0.5 mg·kg−1) elicited the 5-HT syndrome and lowered HR with preserved auditory CS-induced HR increase but reduced maximum HR during retention of conditioned auditory fear in mice. Additionally, 8-OH-DPAT (0.5 mg·kg−1) reduced the unconditioned tachycardia during novelty exposure. The reduced maximum HR values were the consequence of a pathological state of HR dynamics caused by 8-OH-DPAT (0.5 mg·kg−1) that confounded the expression of physiological HR responses with maximum HR normally close to 800 bpm. Analyses of the dynamics of the heartbeat interval fluctuations by non-linear methods revealed a pathological state of HR dynamics by 5-HT1A receptor activation. This was prevented by the selective 5-HT1A receptor antagonist WAY-100635. The 0.5 mg·kg−1 dose of 8-OH-DPAT suggests the involvement of postsynaptic 5-HT1A receptors (Ögren et al., 2008; Youn et al., 2009). Taken together, the results indicated that the effect of 5-HT1A receptor activation on fear learning differed from that on fear expression, based on HR responses.
Specificity of 5-HT1A receptor-mediated HR effects
The 8-OH-DPAT-mediated HR effects were prevented by pre-injection of the selective 5-HT1A receptor antagonist WAY-100635 (Fletcher et al., 1997; Martel et al., 2007) ruling out a potential contribution of 5-HT7 receptor activation by 8-OH-DPAT (Bickmeyer et al., 2002). No evidence was provided for the contribution of 5-HT7 receptors in the expression of conditioned fear elicited by the auditory CS on the basis of HR adjustments (Eriksson et al., 2012).
Several studies reported bradycardic effects of 5-HT1A receptor agonists in anaesthetized rats and cats (see Ramage, 2001; Ramage and Villalón, 2008). However, intraperitoneal injection of 0.25 mg·kg−1 8-OH-DPAT in rats did not affect baseline HR and blood pressure effects but significantly reduced HR and blood pressure increases to emotional (but not cold) stress (Vianna and Carrive, 2009). This is attributed to sympathoinhibition via limbic and/or downstream autonomic sites such as the rostral ventrolateral medulla, the nucleus ambiguus and the dorsal motor nucleus of the nervus vagus. The absence of baseline HR effect in rats contrasts with the baseline bradycardia reported before and here. Besides the use of a lower dose, species-specific differences related to 5-HT1A receptor expression levels cannot be ruled out. For a discussion on potential effects of respiration and blood pressure see the Supporting Information.
Effects of 5-HT1A receptor modulation on conditioned and unconditioned HR responses
While the relative initial increase of HR (∼150 bpm) elicited by the auditory fear stimulus was preserved in mice injected with 8-OH-DPAT, maximum HR did not exceed 600 bpm. However, HR increases to maximum physiological level of ∼800 bpm even from resting HR values of 400–450 bpm (Stiedl et al., 2007). The preserved CS-induced HR increase does not support an anxiolytic action as claimed for the partial 5-HT1A receptor agonist buspirone (see Ögren et al., 2008; see Supporting Information).
The tachycardia elicited by novelty exposure as unconditioned stressor with a mean HR in the range of ∼750 bpm was prevented by 8-OH-DPAT (0.5 mg·kg−1) implying an anxiolytic-like action. However, the lack of novelty-induced tachycardia was attributed to an altered autonomic state. Novelty exposure and injection stress increase HR and body temperature in 5-HT1A receptor-deficient mice compared to wild-type controls (Pattij et al., 2002). The 5-HT1A receptor agonist flesinoxan lowers the tachycardia elicited by the injection procedure, as an aversive stimulus, in wild-type mice without affecting baseline HR (Bouwknecht et al., 2000). This effect of flesinoxan is absent in 5-HT1A receptor-deficient mice (Pattij et al., 2002) indicating a 5-HT1A receptor-mediated reduction of HR as observed here. Similarly, injection of 0.25 mg·kg−1 8-OH-DPAT caused a faster return to baseline HR and higher HR variability in wild-type than in 5-HT1A receptor-deficient mice (Carnevali et al., 2012).
The attenuated tachycardia in response to an adverse challenge after 5-HT1A receptor activation in rats is reported to be beneficial (Nalivaiko and Sgoifo, 2009) because of attenuated stress responsiveness and reduced stress-induced arrhythmias. This conclusion is in contrast to the adverse effects reported previously (Stiedl et al., 2009) and extended by the present study in mice. The reduction of the magnitude of HR adjustments is considered insufficient (maladaptive) with respect to physiological needs (see Koolhaas et al., 2011).
ECG alterations, arrhythmias and similarity with SSRI-mediated cardiovascular effects
ANS dysregulation affects the duration of ECG subintervals through altered electric conduction in the heart. The increased duration of ECG subintervals is used to identify potential cardiovascular risks. The QT prolongation predisposes to the development of ventricular tachyarrhythmias such as torsades des pointes and ventricular fibrillation that are implicated in syncope, cardiac arrest and sudden cardiac death (Zareba, 2007). Adverse effects of serotonergic antidepressants such as SSRIs include PQ and QT interval prolongation and arrhythmias, especially after overdose (Pacher and Kecskeméti, 2004). Notably, serum levels of the SSRI citalopram correlate with QT interval prolongation after intoxication (Unterecker et al., 2012). These effects are mimicked by 5-HT1A receptor activation in mice suggesting the involvement of this receptor in cardiac risk through ANS dysregulation caused by SSRIs. Impairment of electric propagation across the heart by 8-OH-DPAT is attributed to parasympathetic overstimulation causing the increased sinus arrhythmias and third-degree atrioventricular conduction blocks (without escape beats) while bradyarrhythmias are low (∼1 per 104 beats in control mice) under physiological conditions in C57BL/6 mice, as reported before (Meyer and Stiedl, 2003). These findings provide further evidence for negative consequences of central 5-HT1A receptor overactivation for cardiovascular function mediated through the ANS. It remains to be clarified whether these effects are also observed during SSRI treatment which elevates endogenous 5-HT levels and thereby activates multiple 5-HT receptor subtypes, in particular 5-HT1A receptors for which 5-HT has a high-affinity. The involvement of other 5-HT receptor subtypes in HR regulation is discussed in the Supporting Information.
Central 5-HT1A receptor involvement and the brain areas implicated
5-HT1A receptors are found predominantly in the CNS (brain and spinal cord) and parasympathetic control is exerted via direct outputs from the brain stem. Peripheral 5-HT1A receptors are unlikely to contribute to the HR effects reported here (see Supporting Information). The baseline bradycardia is probably mediated by postsynaptic 5-HT1A receptor activation tonically inhibiting GABA-ergic interneurons which exert inhibitory control of preganglionic vagal cardiac neurons of the parasympathetic system. These originate in the nucleus ambiguus and the dorsal motor nucleus of the nervus vagus (Jordan, 2005; Ramage and Villalón, 2008). Disinhibition of these neurons is expected to result in an increased parasympathetic activity of the vagal outputs leading to a HR decrease. In contrast, 5-HT1A receptor antagonists should attenuate parasympathetic tone and increase HR. However, NAD-299 at 0.3 mg·kg−1 did not affect HR in mice (Madjid et al., 2006) consistent with the lack of effects of WAY-100635 at the dose of 0.03 mg·kg−1 reported here (see Supporting Information). NAD-299, which has a higher selectivity than WAY-100635 for the 5-HT1A receptor (Johansson et al., 1997), failed to significantly increase baseline HR (∼660 bpm) even at 1 mg·kg−1, presumably, because parasympathetic inhibition by atropine, a muscarinic acetylcholine receptor antagonist that blocks the vagal control of the heart, will increase HR in unstressed mice to ∼670 bpm (Stiedl et al., 2009).
The importance of the 5-HT1A receptor for HR changes was recently demonstrated in 5-HT1A receptor-deficient mice. Deletion of the 5-HT1A receptor increased the tachycardia to an acute stressor and caused cardiac arrest during psychosocial stress in 27% of tested mice (Carnevali et al., 2012). These and the present results, in combination with the adverse outcome of 5-HT1A autoreceptor overexpression shunting postsynaptic 5-HT1A release (Audero et al., 2008), suggest an adverse role of both postsynaptic 5-HT1A receptor hypo- and hyperactivation for cardiovascular function and increased risk for cardiac morbidity. However, here we could not provide evidence for an involvement of the 5-HT1A receptor in HR regulation under these experimental conditions.
HR dynamics
Under physiological conditions (saline) the balanced strength of HR acceleration and deceleration was generally low. However, the strength of both acceleration and deceleration was markedly enhanced in 8-OH-DPAT-treated mice, with pronounced predominance of negative chronotropic effects from vagal activation. The HR dynamics elicited by 8-OH-DPAT (0.5 mg·kg−1) is similar, but even more pronounced, to that following central activation of the corticotropin-releasing factor CRF1 receptors by intracerebroventricular ovine CRF (Stiedl et al., 2005; Meyer and Stiedl, 2006). Particularly, the increased acceleration/deceleration strength elicited by 8-OH-DPAT indicates central hyperactivation of both ANS branches. This is further supported by the twofold increased HR variability compared with physiological values during sleep. 5-HT1A receptor activation elicited dynamic properties with a significant loss of intrinsic structural complexity of cardiac control. Multifractal scaling of HR dynamics suggests that the control mechanisms regulating the heartbeat interact as part of a coupled cascade of feedback loops for neuroautonomic regulation that is also impaired in congestive heart failure (Ivanov et al., 1999). The loss of complexity leads to a significant impairment of HR adaptability in response to emotional challenge (Meyer and Stiedl, 2006) as indicated by the reduced maximum HR elicited by the unconditioned (novelty) and conditioned (auditory) stressors. However, the conclusion of pathological HR dynamics elicited by 8-OH-DPAT at 0.5 mg·kg−1 can only be drawn on the basis of the nonlinear results.
In conclusion, we provide evidence that 5-HT1A receptor activation impaired the acquisition but not the expression of the fear-conditioned HR response suggesting that the underlying neuronal circuits for fear learning differ from those for fear expression. 5-HT1A receptor activation before fear expression decreased HR, increased HR variability and blunted the HR adjustment in response emotional challenges because of compromised dynamic range. Together with impaired HR dynamics, prolongation of PQ intervals, atrioventricular conduction blocks and emergence of escape beats this identifies a pathological state of ANS control of the heart by 5-HT1A receptor overactivation in mice. This demonstrates the importance of a more extensive data analysis for the qualitative interpretation of observed cardiovascular effects, beyond mean HR. In view of the present findings, the elevation of the endogenous 5-HT level by SSRIs may increase cardiovascular risk via enhanced 5-HT1A receptor activation, at least during the onset of treatment, in view of reported side effects. Taken together, these results suggest the involvement of 5-HT1A receptor activation in emotional memory impairments and elevated cardiac risk, associated with SSRI-based pharmacotherapy in affective disorders.
Acknowledgments
We are grateful to Anne-Kathrin Streit and Anna Günther for support in initial ECG data analysis and to Dr. Sophie van der Sluis and one reviewer for statistical advice. NAD-299 was kindly provided by Dr. Johan Sandin, AstraZeneca R&D, Södertälje, Sweden. We mourn the loss of our dear colleague Dr. René F. Jansen. This work was supported by the CNCR, VU University Amsterdam (J. Y., A. W. P., R. F. J., O. S.), the Canadian Institute of Health Research (200509OPD to I. M., O. S.), the Max Planck Society (M. M.) and the Swedish Research Council (S. O. Ö.). The European Union Seventh Framework Program under grant agreement no. PEOPLE-ITN-2008–238055 (BrainTrain) provided funding for T. H. (O. S.).
Glossary
- ANS
autonomic nervous system
- CS
conditioned stimulus
- HR
heart rate
- US
unconditioned stimulus
Author contributions
JY, TH, AWP and OS performed the experiments. JY, IM, SOÖ and OS designed the experiments. TH, AWP and OS performed ECG surgery. JY, TH, IM, RFJ, MM and OS performed data analysis. AWP, MM and OS generated figures. OS, JY and SOÖ wrote the paper with support from all authors.
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12325
Table S1 P-values of linear contrasts of time × drug interaction from pre-CS to CS phases across the five drug treatment groups.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edn. Br J Pharmacol. 2011;164(Suppl 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas F. Developments in the field of antidepressants, where do we go now? Eur Neuropsychopharmacol. 2013 doi: 10.1016/j.euroneuro.2013.04.013. . doi: 10.1016/j.euroneuro.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Audero E, Coppi E, Mlinar B, Rossetti T, Caprioli A, Al Banchaabouchi M, et al. Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science. 2008;321:130–133. doi: 10.1126/science.1157871. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bennett DH. Cardiac Arrhythmias. 7th edn. London: Hodder-Arnold; 2006. [Google Scholar]
- Bhattacharyya MR, Steptoe A. Emotional triggers of acute coronary syndromes: strength of evidence, biological processes, and clinical implications. Prog Cardiovasc Dis. 2007;49:353–365. doi: 10.1016/j.pcad.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Bickmeyer U, Heine M, Manzke T, Richter DW. Differential modulation of Ih by 5-HT receptors in mouse CA1 hippocampal neurons. Eur J Neurosci. 2002;16:209–218. doi: 10.1046/j.1460-9568.2002.02072.x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Griebel G, Guardiola-Lemaitre B, Brush MM, Lee J, Blanchard DC. An ethopharmacological analysis of selective activation of 5-HT1A receptors: the mouse 5-HT1A syndrome. Pharmacol Biochem Behav. 1997;57:897–908. doi: 10.1016/s0091-3057(96)00472-8. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Hijzen TH, van der Gugten J, Maes RAA, Olivier B. Stress-induced hyperthermia in mice: effects of flesinoxan on heart rate and body temperature. Eur J Pharmacol. 2000;400:59–66. doi: 10.1016/s0014-2999(00)00387-3. [DOI] [PubMed] [Google Scholar]
- Buhot M-C. Serotonin receptors in cognitive behaviors. Curr Opin Neurobiol. 1997;7:243–254. doi: 10.1016/s0959-4388(97)80013-x. [DOI] [PubMed] [Google Scholar]
- Carnevali L, Mastorci F, Audero E, Graiani G, Rossi S, Macchi E, et al. Stress-induced susceptibility to sudden cardiac death in mice with altered serotonin homeostasis. PLoS ONE. 2012;7:e41184. doi: 10.1371/journal.pone.0041184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Côte F, Fligny C, Fromes Y, Malles J, Vodjdani G. Recent advances in understanding of serotonin regulation of cardiovascular function. Trends Mol Med. 2004;10:232–238. doi: 10.1016/j.molmed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Eriksson TM, Holst S, Stan TL, Hager T, Sjögren B, Ögren SO, et al. 5-HT1A and 5-HT7 receptors crosstalk in the regulation of emotional learning: implications for effects of selective serotonin reuptake inhibitors. Neuropharmacology. 2012;63:1150–1160. doi: 10.1016/j.neuropharm.2012.06.061. [DOI] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, et al. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1997;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Ivanov PC, Amaral LAN, Goldberger AL, Havlin S, Rosenblum MG, et al. Multifractality in human heartbeat dynamics. Nature. 1999;399:461–485. doi: 10.1038/20924. [DOI] [PubMed] [Google Scholar]
- Ivanov PC, Rosenblum MG, Peng C-K, Mietus J, Havlin S, Stanley HE, et al. Scaling behavior of heartbeat intervals obtained by wavelet-based time-series analysis. Nature. 1996;383:323–327. doi: 10.1038/383323a0. [DOI] [PubMed] [Google Scholar]
- Johansson L, Sohn D, Thorberg S-O, Jackson DM, Kelder D, Larsson L-G, et al. The pharmacological characterization of a novel selective 5-hydroxytryptamine1A receptor antagonist, NAD-299. J Pharmacol Exp Ther. 1997;283:216–225. [PubMed] [Google Scholar]
- Jordan D. Vagal control of the heart: central serotonergic mechanisms. Exp Physiol. 2005;90:175–181. doi: 10.1113/expphysiol.2004.029058. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, et al. Stress revisited: A critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;34:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjid N, Elvander Tottie E, Lüttgen M, Meister B, Sandin J, Kuzmin A, et al. 5-HT1A receptor blockade facilitates aversive learning in mice: Interactions with cholinergic and glutamatergic mechanisms. J Pharmacol Exp Ther. 2006;316:581–591. doi: 10.1124/jpet.105.092262. [DOI] [PubMed] [Google Scholar]
- Martel JC, Leduc N, Ormière A-M, Faucillon V, Danty N, Culie C, et al. WAY-100635 has high selectivity for serotonin 5-HT1A versus dopamine D4 receptors. Eur J Pharmacol. 2007;574:15–19. doi: 10.1016/j.ejphar.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Meyer M. Fractal scaling of heart rate dynamics in health and disease. In: Losa A, Merlini D, Nonnenmacher TF, Weibel ER, editors. Fractals in Biology and Medicine. Vol. 3. Basel: Birkhäuser; 2002. pp. 181–193. [Google Scholar]
- Meyer M, Stiedl O. Self-affine fractal variability of human heartbeat interval dynamics in health and disease. Eur J Appl Physiol. 2003;90:305–316. doi: 10.1007/s00421-003-0915-2. [DOI] [PubMed] [Google Scholar]
- Meyer M, Stiedl O. Fractal rigidity by enhanced sympatho-vagal antagonism in heartbeat interval dynamics elicited by central application of corticotropin-releasing factor in mice. J Math Biol. 2006;52:830–874. doi: 10.1007/s00285-006-0375-5. [DOI] [PubMed] [Google Scholar]
- Misane I, Tovote M, Meyer M, Spiess J, Ögren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E. 5-HT1A receptors in stress-induced cardiac changes: a possible link between mental and cardiac disorders. Clin Exp Pharmacol Physiol. 2006;33:1259–1264. doi: 10.1111/j.1440-1681.2006.04521.x. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Sgoifo A. Central 5-HT receptors in cardiovascular control during stress. Neurosci Biobehav Rev. 2009;33:95–106. doi: 10.1016/j.neubiorev.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Ngampramuan S, Baumert M, Beig MI, Kotchabhakdi N, Nalivaiko E. Activation of 5-HT1A receptors attenuates tachycardia induced by restraint stress in rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R132–R141. doi: 10.1152/ajpregu.00464.2007. [DOI] [PubMed] [Google Scholar]
- Ögren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekström JC, Svenningsson P, et al. The role of the 5-HT1A receptor in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Pacher P, Kecskeméti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10:2463–2475. doi: 10.2174/1381612043383872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Groenink L, Hijzen TH, Oosting RS, Maes RAA, van der Gugten J, et al. Autonomic changes associated with enhanced anxiety in 5-HT1A receptor knockout mice. Neuropharmacology. 2002;27:380–390. doi: 10.1016/S0893-133X(02)00317-2. [DOI] [PubMed] [Google Scholar]
- Ramage A. Central cardiovascular regulation and 5-hydroxytryptamine. Brain Res Bull. 2001;56:425–439. doi: 10.1016/s0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- Ramage A, Villalón CM. 5-hydroxytryptamine and cardiovascular regulation. Trends Pharmacol Sci. 2008;29:472–481. doi: 10.1016/j.tips.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT1A receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl O, Meyer M. Cardiac dynamics in corticotropin-releasing factor receptor subtype 2-deficient mice. Neuropeptides. 2003;37:3–16. doi: 10.1016/s0143-4179(02)00135-x. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Spiess J. Effect of tone-dependent fear conditioning on heart rate and behavior of C57BL/6N mice. Behav Neurosci. 1997;111:703–711. doi: 10.1037//0735-7044.111.4.703. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Radulovic J, Lohmann R, Birkenfeld K, Palve M, Kammermeier J, et al. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav Brain Res. 1999;104:1–12. doi: 10.1016/s0166-4328(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Misane I, Spiess J, Ögren SO. Involvement of the 5-HT1A receptors in classical fear conditioning in C57BL/6J mice. J Neurosci. 2000a;20:8515–8527. doi: 10.1523/JNEUROSCI.20-22-08515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl O, Birkenfeld K, Palve M, Spiess J. Impairment of conditioned contextual fear of C57BL/6J mice by intracerebral injections of the NMDA receptor antagonist APV. Behav Brain Res. 2000b;116:157–168. doi: 10.1016/s0166-4328(00)00269-2. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Tovote P, Ögren SO, Meyer M. Behavioral and autonomic dynamics during contextual fear conditioning in mice. Auton Neurosci. 2004;115:15–27. doi: 10.1016/j.autneu.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Meyer M, Jahn O, Ögren SO, Spiess J. Corticotropin-releasing factor receptor 1 and central heart rate regulation in mice during expression of conditioned fear. J Pharmacol Exp Ther. 2005;312:905–916. doi: 10.1124/jpet.104.075820. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Misane I, Koch M, Pattij T, Meyer M, Ögren SO. Activation of the brain 5-HT2C receptors causes hypolocomotion without anxiogenic-like cardiovascular adjustments in mice. Neuropharmacology. 2007;52:949–957. doi: 10.1016/j.neuropharm.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Jansen RF, Pieneman AW, Ögren SO, Meyer M. Assessing aversive emotional states through the heart in mice: implications for cardiovascular dysregulation in affective disorders. Neurosci Biobehav Rev. 2009;33:181–190. doi: 10.1016/j.neubiorev.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Tovote P, Meyer M, Beck-Sickinger AG, von Hörsten S, Ögren SO, Spiess J, et al. Central NPY receptor-mediated alteration of heart rate dynamics in mice during expression of fear conditioned to an auditory cue. Regul Pept. 2004;120:205–214. doi: 10.1016/j.regpep.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Tovote P, Meyer M, Ronnenberg A, Ögren SO, Spiess J, Stiedl O. Heart rate dynamics and behavioral responses during acute emotional challenge in corticotropin-releasing factor receptor 1-deficient and corticotropin-releasing factor overexpressing mice. Neuroscience. 2005;134:1113–1122. doi: 10.1016/j.neuroscience.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Unterecker S, Warrings B, Deckert J, Pfuhlmann B. Correlation of QTc interval prolongation and serum level of citalopram after intoxication – a case report. Pharmacopsychiatry. 2012;45:30–34. doi: 10.1055/s-0031-1286346. [DOI] [PubMed] [Google Scholar]
- Vianna DLM, Carrive P. Inhibition of the cardiovascular response to stress by systemic 5-HT1A receptor activation: sympathoinhibition or anxiolysis? Am J Physiol Regul Integr Comp Physiol. 2009;297:R495–R501. doi: 10.1152/ajpregu.00232.2009. [DOI] [PubMed] [Google Scholar]
- Villalón CM, Centurión D. Cardiovascular responses induced by 5-hydroxytryptamine: a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:45–63. doi: 10.1007/s00210-007-0179-1. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Seldenrijk A, Beekman ATF, van Hout HPJ, de Jonge P, Penninx BWJH. Cardiovascular disease in persons with depressive and anxiety disorders. J Affect Disord. 2010;125:241–248. doi: 10.1016/j.jad.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J, Misane I, Eriksson TM, Millan MJ, Ögren SO, Verhage M, et al. Bidirectional modulation of classical fear conditioning in mice by 5-HT1A receptor ligands with contrasting intrinsic activities. Neuropharmacology. 2009;57:567–576. doi: 10.1016/j.neuropharm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Zareba W. Drug induced QT prolongation. Cardiol J. 2007;14:523–533. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.