Abstract
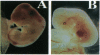
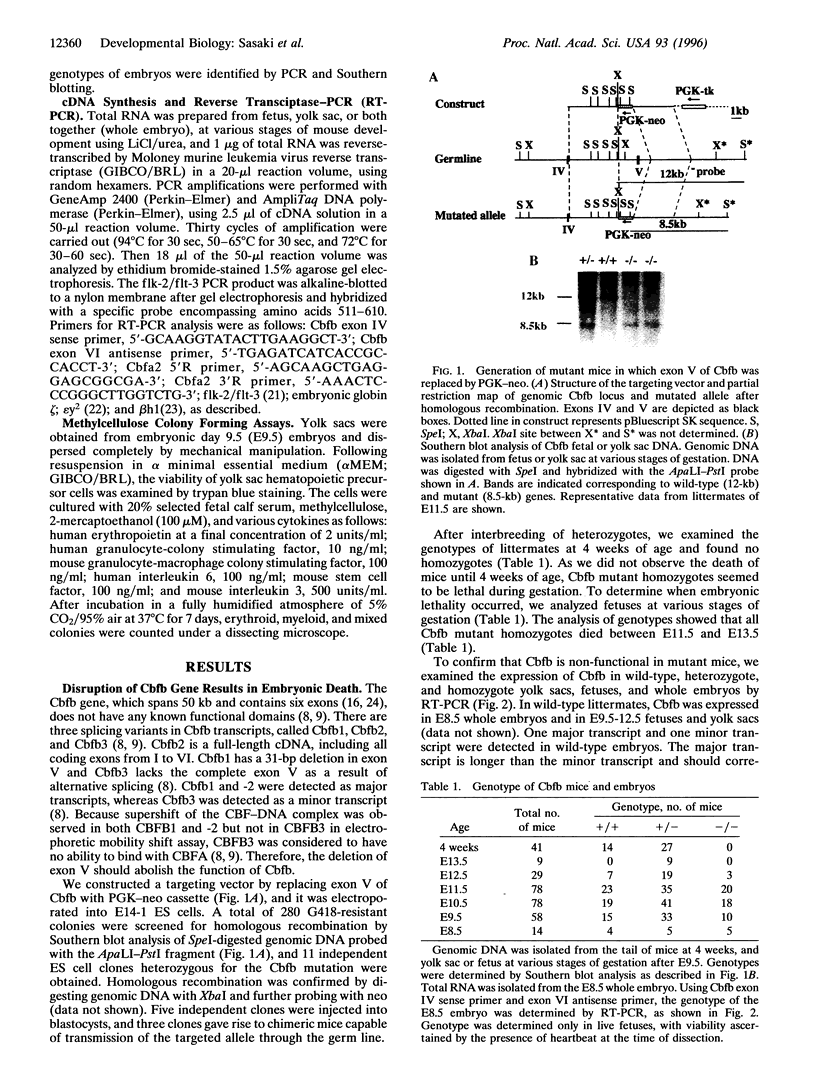
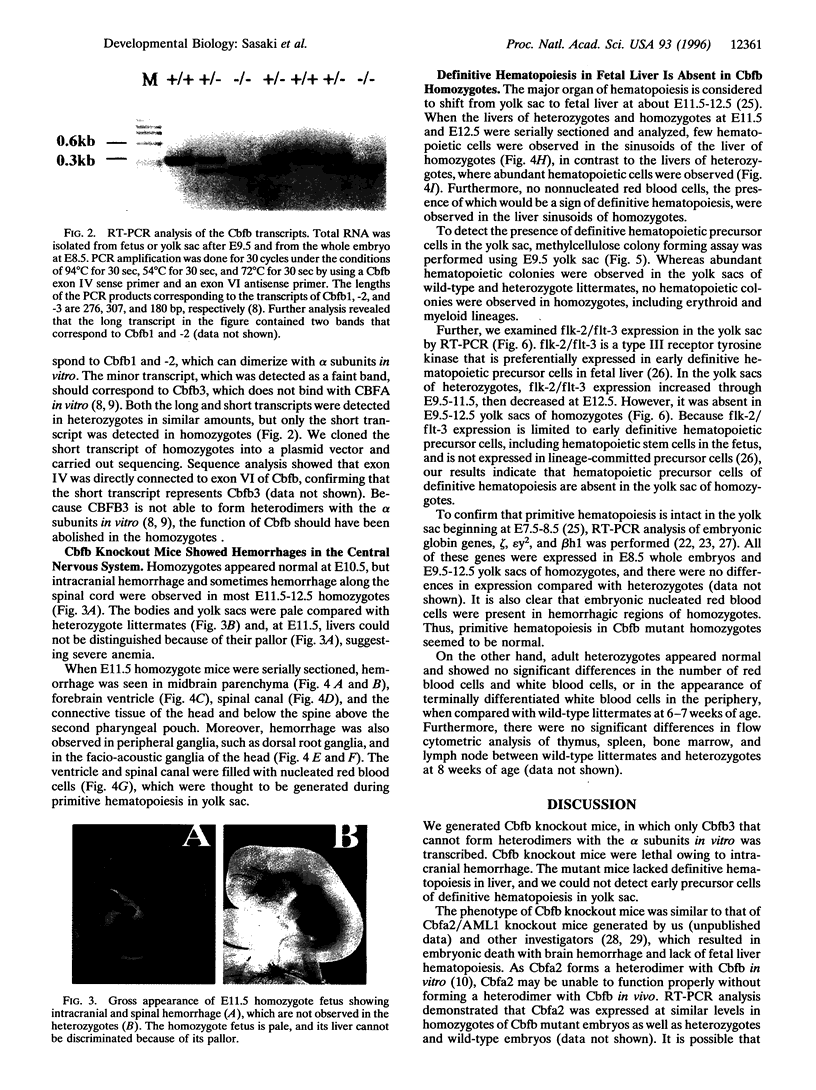
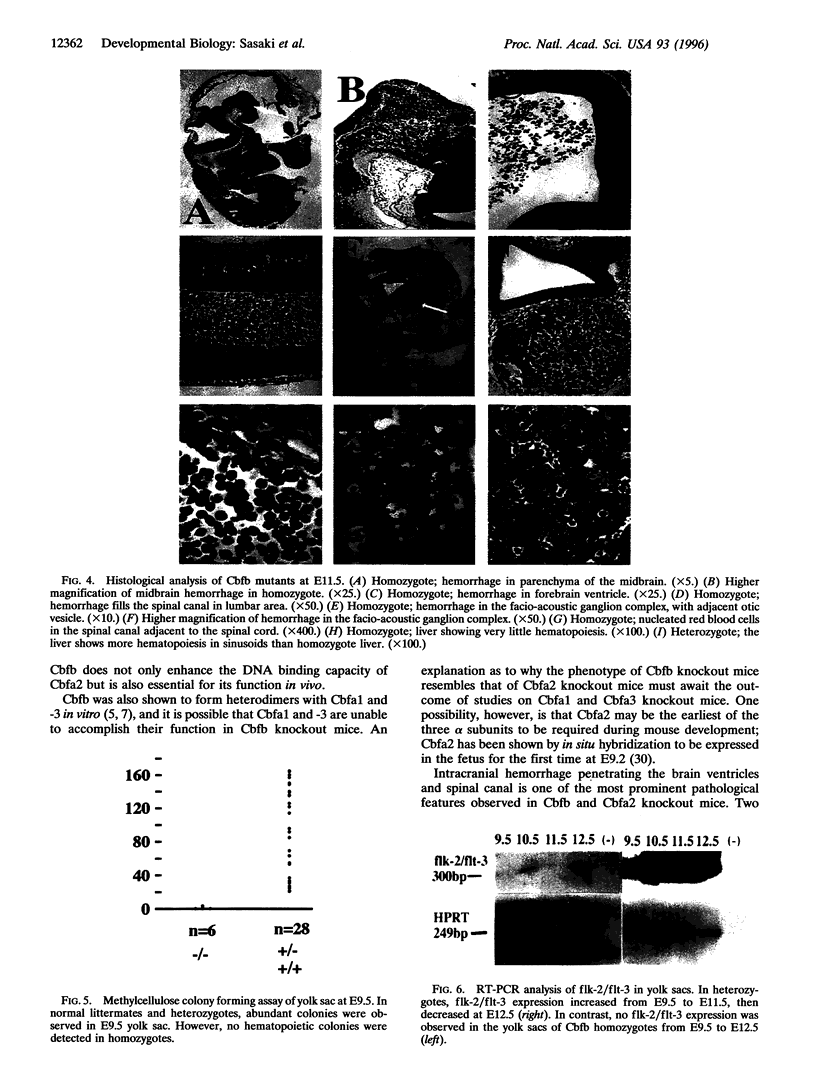
Core binding factor beta (CBF beta) is considered to be a transcriptional coactivator that dimerizes with transcription factors core binding factor alpha 1 (CBFA1), -2, and -3, and enhances DNA binding capacity of these transcription factors. CBF beta and CBFA2, which is also called acute myeloid leukemia 1 gene, are frequently involved in chromosomal translocations in human leukemia. To elucidate the function of CBF beta, mice carrying a mutation in the Cbfb locus were generated. Homozygous mutant embryos died between embryonic days 11.5-13.5 due to hemorrhage in the central nervous system. Mutant embryos had primitive erythropoiesis in yolk sac but lacked definitive hematopoiesis in fetal liver. In the yolk sac of mutant embryos, no erythroid or myeloid progenitors of definitive hematopoietic origin were detected, and the expression of flk-2/flt-3, the marker gene for early precursor cells of definitive hematopoiesis, was absent. These data suggest that Cbfb is essential for definitive hematopoiesis in liver, especially for the commitment to early hematopoietic precursor cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bae S. C., Ogawa E., Maruyama M., Oka H., Satake M., Shigesada K., Jenkins N. A., Gilbert D. J., Copeland N. G., Ito Y. PEBP2 alpha B/mouse AML1 consists of multiple isoforms that possess differential transactivation potentials. Mol Cell Biol. 1994 May;14(5):3242–3252. doi: 10.1128/mcb.14.5.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S. C., Takahashi E., Zhang Y. W., Ogawa E., Shigesada K., Namba Y., Satake M., Ito Y. Cloning, mapping and expression of PEBP2 alpha C, a third gene encoding the mammalian Runt domain. Gene. 1995 Jul 4;159(2):245–248. doi: 10.1016/0378-1119(95)00060-j. [DOI] [PubMed] [Google Scholar]
- Bae S. C., Yamaguchi-Iwai Y., Ogawa E., Maruyama M., Inuzuka M., Kagoshima H., Shigesada K., Satake M., Ito Y. Isolation of PEBP2 alpha B cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993 Mar;8(3):809–814. [PubMed] [Google Scholar]
- Godin I., Dieterlen-Lièvre F., Cumano A. Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):773–777. doi: 10.1073/pnas.92.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A., Collins F. S. Structure of the leukemia-associated human CBFB gene. Genomics. 1995 Apr 10;26(3):571–579. doi: 10.1016/0888-7543(95)80177-n. [DOI] [PubMed] [Google Scholar]
- Hajra A., Liu P. P., Speck N. A., Collins F. S. Overexpression of core-binding factor alpha (CBF alpha) reverses cellular transformation by the CBF beta-smooth muscle myosin heavy chain chimeric oncoprotein. Mol Cell Biol. 1995 Sep;15(9):4980–4989. doi: 10.1128/mcb.15.9.4980. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hajra A., Liu P. P., Wang Q., Kelley C. A., Stacy T., Adelstein R. S., Speck N. A., Collins F. S. The leukemic core binding factor beta-smooth muscle myosin heavy chain (CBF beta-SMMHC) chimeric protein requires both CBF beta and myosin heavy chain domains for transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):1926–1930. doi: 10.1073/pnas.92.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kamachi Y., Ogawa E., Asano M., Ishida S., Murakami Y., Satake M., Ito Y., Shigesada K. Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J Virol. 1990 Oct;64(10):4808–4819. doi: 10.1128/jvi.64.10.4808-4819.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Kennedy M., Papayannopoulou T., Wiles M. V. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993 Jan;13(1):473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Okada A., Stewart V., Alt F. W. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993 Aug 27;261(5125):1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- Liu P. P., Hajra A., Wijmenga C., Collins F. S. Molecular pathogenesis of the chromosome 16 inversion in the M4Eo subtype of acute myeloid leukemia. Blood. 1995 May 1;85(9):2289–2302. [PubMed] [Google Scholar]
- Liu P., Tarlé S. A., Hajra A., Claxton D. F., Marlton P., Freedman M., Siciliano M. J., Collins F. S. Fusion between transcription factor CBF beta/PEBP2 beta and a myosin heavy chain in acute myeloid leukemia. Science. 1993 Aug 20;261(5124):1041–1044. doi: 10.1126/science.8351518. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Matthews W., Jordan C. T., Wiegand G. W., Pardoll D., Lemischka I. R. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991 Jun 28;65(7):1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- McClanahan T., Dalrymple S., Barkett M., Lee F. Hematopoietic growth factor receptor genes as markers of lineage commitment during in vitro development of hematopoietic cells. Blood. 1993 Jun 1;81(11):2903–2915. [PubMed] [Google Scholar]
- Mitani K., Ogawa S., Tanaka T., Miyoshi H., Kurokawa M., Mano H., Yazaki Y., Ohki M., Hirai H. Generation of the AML1-EVI-1 fusion gene in the t(3;21)(q26;q22) causes blastic crisis in chronic myelocytic leukemia. EMBO J. 1994 Feb 1;13(3):504–510. doi: 10.1002/j.1460-2075.1994.tb06288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Kozu T., Shimizu K., Enomoto K., Maseki N., Kaneko Y., Kamada N., Ohki M. The t(8;21) translocation in acute myeloid leukemia results in production of an AML1-MTG8 fusion transcript. EMBO J. 1993 Jul;12(7):2715–2721. doi: 10.1002/j.1460-2075.1993.tb05933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., McLain K., Kier A. B., Swerdlow S. H., Schreiner C. M., Miller T. A., Pietryga D. W., Scott W. J., Jr, Potter S. S. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991 May 17;65(4):677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- Nucifora G., Begy C. R., Erickson P., Drabkin H. A., Rowley J. D. The 3;21 translocation in myelodysplasia results in a fusion transcript between the AML1 gene and the gene for EAP, a highly conserved protein associated with the Epstein-Barr virus small RNA EBER 1. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7784–7788. doi: 10.1073/pnas.90.16.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa E., Inuzuka M., Maruyama M., Satake M., Naito-Fujimoto M., Ito Y., Shigesada K. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993 May;194(1):314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- Ogawa E., Maruyama M., Kagoshima H., Inuzuka M., Lu J., Satake M., Shigesada K., Ito Y. PEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T., van Deursen J., Hiebert S. W., Grosveld G., Downing J. R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996 Jan 26;84(2):321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Redondo J. M., Pfohl J. L., Hernandez-Munain C., Wang S., Speck N. A., Krangel M. S. Indistinguishable nuclear factor binding to functional core sites of the T-cell receptor delta and murine leukemia virus enhancers. Mol Cell Biol. 1992 Nov;12(11):4817–4823. doi: 10.1128/mcb.12.11.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romana S. P., Mauchauffé M., Le Coniat M., Chumakov I., Le Paslier D., Berger R., Bernard O. A. The t(12;21) of acute lymphoblastic leukemia results in a tel-AML1 gene fusion. Blood. 1995 Jun 15;85(12):3662–3670. [PubMed] [Google Scholar]
- Rosnet O., Marchetto S., deLapeyriere O., Birnbaum D. Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene. 1991 Sep;6(9):1641–1650. [PubMed] [Google Scholar]
- Shivdasani R. A., Rosenblatt M. F., Zucker-Franklin D., Jackson C. W., Hunt P., Saris C. J., Orkin S. H. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995 Jun 2;81(5):695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- Simeone A., Daga A., Calabi F. Expression of runt in the mouse embryo. Dev Dyn. 1995 May;203(1):61–70. doi: 10.1002/aja.1002030107. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Wang Q., Stacy T., Binder M., Marin-Padilla M., Sharpe A. H., Speck N. A. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. W., Speck N. A. Purification of core-binding factor, a protein that binds the conserved core site in murine leukemia virus enhancers. Mol Cell Biol. 1992 Jan;12(1):89–102. doi: 10.1128/mcb.12.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wang Q., Crute B. E., Melnikova I. N., Keller S. R., Speck N. A. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol Cell Biol. 1993 Jun;13(6):3324–3339. doi: 10.1128/mcb.13.6.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Keller G., Orkin S. H. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 1994 May 15;8(10):1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- Whitelaw E., Tsai S. F., Hogben P., Orkin S. H. Regulated expression of globin chains and the erythroid transcription factor GATA-1 during erythropoiesis in the developing mouse. Mol Cell Biol. 1990 Dec;10(12):6596–6606. doi: 10.1128/mcb.10.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler F. C., Bennett B. D., Jordan C. T., Spencer S. D., Baumhueter S., Carroll K. J., Hooley J., Bauer K., Matthews W. Cellular and molecular characterization of the role of the flk-2/flt-3 receptor tyrosine kinase in hematopoietic stem cells. Blood. 1994 Oct 15;84(8):2422–2430. [PubMed] [Google Scholar]
- Zon L. I. Developmental biology of hematopoiesis. Blood. 1995 Oct 15;86(8):2876–2891. [PubMed] [Google Scholar]