Abstract
BACKGROUND AND PURPOSE
Topiramate improves insulin sensitivity, in addition to its antiepileptic action. However, the underlying mechanism is unknown. Therefore, the present study was aimed at investigating the mechanism of the insulin-sensitizing effect of topiramate both in vivo and in vitro.
EXPERIMENTAL APPROACH
Male C57Bl/6J mice were fed a run-in high-fat diet for 6 weeks, before receiving topiramate or vehicle mixed in high-fat diet for an additional 6 weeks. Insulin sensitivity was assessed by hyperinsulinaemic-euglycaemic clamp. The extent to which the insulin sensitizing effects of topiramate were mediated through the CNS were determined by concomitant i.c.v. infusion of vehicle or tolbutamide, an inhibitor of ATP-sensitive potassium channels in neurons. The direct effects of topiramate on insulin signalling and glucose uptake were assessed in vivo and in cultured muscle cells.
KEY RESULTS
In hyperinsulinaemic-euglycaemic clamp conditions, therapeutic plasma concentrations of topiramate (∼4 μg·mL−1) improved insulin sensitivity (glucose infusion rate + 58%). Using 2-deoxy-D-[3H]glucose, we established that topiramate improved the insulin-mediated glucose uptake by heart (+92%), muscle (+116%) and adipose tissue (+586%). Upon i.c.v. tolbutamide, the insulin-sensitizing effect of topiramate was completely abrogated. Topiramate did not directly affect glucose uptake or insulin signalling neither in vivo nor in cultured muscle cells.
CONCLUSION AND IMPLICATIONS
In conclusion, topiramate stimulates insulin-mediated glucose uptake in vivo through the CNS. These observations illustrate the possibility of pharmacological modulation of peripheral insulin resistance through a target in the CNS.
Keywords: type 2 diabetes mellitus, brain, KATP-channels, tolbutamide, insulin
Introduction
Type 2 diabetes mellitus (T2DM) is a syndrome characterized by impaired insulin secretion as well as reduced insulin sensitivity. Many drugs have been developed that act on these pathophysiological mechanisms. In recent years, evidence has accumulated that the CNS is also involved in the pathophysiology of T2DM. Experimental models have indicated that insulin-mediated effects on different organs are mediated in part through the CNS (Obici et al., 2002; Koch et al., 2008; Coomans et al., 2011a,b). Importantly, in insulin-resistant conditions, these effects of circulating insulin through the CNS are lost (Coomans et al., 2011a,b). The question arises whether the loss of these effects of insulin, mediated by the CNS, are amendable to pharmacological intervention.
Topiramate, a sulfamate-substituted derivative of the monosaccharide d-fructose (Shank et al., 2000), is used as an antiepileptic drug (McIntyre et al., 2005; Ferrari et al., 2011). The antiepileptic effects of topiramate are mediated by at least six mechanisms of action within the CNS (Zona et al., 1997; Dodgson et al., 2000; Gibbs, III et al. 2000; White et al., 1997; 2000; Zhang et al., 2000; Herrero et al., 2002). Studies in obese, diabetic rats demonstrated that topiramate treatment reduced plasma glucose levels and improved insulin sensitivity independently of weight loss (Picard et al., 2000; Wilkes et al., 2005a). However, the mechanism underlying this pharmacological, insulin-sensitizing effect of topiramate is unknown.
We hypothesized that topiramate improves insulin sensitivity not by a direct effect on peripheral organs, but, rather, through effects within the CNS. Therefore, we studied in high-fat fed mice the effects of i.c.v. administered vehicle versus tolbutamide on top of the effects of topiramate on tissue-specific insulin-mediated glucose uptake. Tolbutamide is an inhibitor of ATP-sensitive potassium (KATP) channels in neurons and i.c.v. administration of tolbutamide blocks the action of circulating insulin in the brain (Obici et al., 2002; Coomans et al., 2011a,b). In addition, we assessed the direct effects of topiramate on insulin signalling and glucose uptake in vivo and in cultured C2C12 muscle cells. In this study, we show that topiramate improves peripheral insulin sensitivity to a large extent by improving insulin sensitivity in the brain.
Methods
Animals
Eighty-six male C57Bl/6J mice obtained from Charles River Laboratories at an age of 8 weeks were housed in a temperature-controlled room on a 12 h light-dark cycle. From the age of 12 weeks, mice were fed ad libitum a run-in high-fat diet for 6 weeks (45 energy% of fat derived from lard; Research Diets Inc, New Brunswick, NJ, USA), which induces considerable insulin resistance, according to our previous experiments (van den Berg et al., 2010). Subsequently, the animals were randomized according to body weight and fasting plasma glucose levels and were fed ad libitum for 6 weeks a high-fat diet containing 3.33% anise (anise cubes, De Ruijter, Utrecht, The Netherlands) with (n = 42) or without (n = 44) 0.12% (w/w) topiramate (Abbott Products GmbH, Hannover, Germany). The mice had free access to water throughout the experiment. Food intake and body weight were measured regularly throughout the experiment. All animal experiments were performed in accordance with the regulations of Dutch law on animal welfare and the institutional ethics committee for animal procedures from the Leiden University Medical Center, Leiden, The Netherlands approved the protocol. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Krilkenny et al., 2010; McGrath et al., 2010).
I.c.v. cannula implantation
For i.c.v. cannula implantation, 15-week-old male mice were anaesthetized with 0.5 mg·kg−1 Medetomidine (Pfizer, Capelle a/d IJssel, The Netherlands), 5 mg·kg−1 Midazolam (Roche, Mijdrecht, The Netherlands) and 0.05 mg·kg−1 Fentanyl (Janssen-Cilag, Tilburg, The Netherlands) and placed in a stereotactic device (TSE systems, Homburg, Germany). A 25 gauge guide cannula was implanted into the left lateral ventricle using the following coordinates from Bregma: 1.0 mm lateral, 0.46 mm posterior and 2.2 mm ventral. The guide cannula was secured to the skull surface with dental cement (GC Europe N.V., Leuven, Belgium). Analgesia (0.03mg·kg−1 Buprenorphine (Schering-Plough, Houten, The Netherlands) was administered and anesthesia was antagonized using 2.5 mg·kg-1 Antipamezol (Pfizer, Capelle a/d IJssel, The Netherlands), 0.5 mg·kg−1 Flumazenil (Roche, Mijdrecht, The Netherlands) and 1.2 mg·kg−1 Naloxon (Orpha, Purkersdorf, Austria). After a recovery period of 1 week, cannula placement was verified. Mice that ate >0.3 g in 1 h in response to i.c.v. injection of 5 μg NPY (Bachem, St. Helens, UK) in 1 μl of artificial cerebrospinal fluid (aCSF, Harvard Apparatus, Natick, MA, USA) were considered to have the cannula correctly placed and were included in the study (van den Hoek et al., 2004; Coomans et al., 2011b).
Hyperinsulinaemic-euglycaemic clamp studies
Overnight fasted, body weight-matched male mice (vehicle-treated mice n = 10; topiramate-treated mice n = 7) were anaesthetized with 6.25 mg·kg−1 Acepromazine (Alfasan, Woerden, The Netherlands), 6.25 mg·kg−1 Midazolam (Roche), and 0.31 mg·kg−1 Fentanyl (Janssen-Cilag, Tilburg, The Netherlands). Anaesthesia, as well as body temperature, was maintained throughout the procedure. First, basal rates of glucose turnover were determined by administration of a primed continuous i.v. infusion of D-[1-14C]glucose (0.3 μCi·kg−1·min−1; Amersham, Little Chalfont, UK) for 60 min. Subsequently, insulin (Actrapid, Novo Nordisk, Denmark) was administered i.v. by primed (4.1 mU), continuous (6.8 mU·h−1) infusion to attain steady-state hyperinsulinemia together with D-[1-14C]glucose (0.3 μCi·kg−1·min−1; Amersham) for 90 min. A variable i.v. infusion of a 12.5% d-glucose solution was used to maintain euglycemia as determined at 10-min intervals via tail bleeding (<3 μL, Accu-chek, Sensor Comfort; Roche Diagnostics, Mannheim, Germany). To assess insulin-mediated glucose uptake in individual tissues, 2-deoxy-D-[3-3H]glucose (2-[3H]DG; Amersham) was administered as a bolus (1 μCi) 30 min before the end of both experiments. In the last 20 min of both experiments, blood samples were taken with intervals of 10 min. Subsequently, the mice were sacrificed by cervical dislocation and after perfusion with ice-cold PBS, organs were harvested and snap-frozen in liquid nitrogen.
I.c.v. tolbutamide treatment during clamp
Starting 30 min before the initiation of the hyperinsulinaemic-euglycaemic clamp, aCSF (vehicle-treated mice n = 8; topiramate-treated mice n = 10) or the KATP channel blocker tolbutamide (vehicle-treated mice n = 7; topiramate-treated mice n = 9), dissolved in 5% DMSO to a final concentration of 4.8 mM in aCSF, was continuously infused i.c.v. at a rate of 2.5 μL per hour (Obici et al., 2002; Plum et al., 2006). The concentration of i.c.v. tolbutamide was ascertained in a dose-finding study, to ensure that tolbutamide did not leak to the periphery.
In vivo insulin signalling in muscle
Overnight fasted mice were anaesthetized and received either an i.v. bolus of insulin (4.1 mU; vehicle-treated mice n = 10; topiramate-treated mice n = 8) or saline (vehicle-treated mice n = 9; topiramate-treated mice n = 8). After 10 min, the mice were sacrificed by cervical dislocation and skeletal muscles (upper limb) were harvested and snap-frozen in liquid nitrogen. Skeletal muscles were homogenized by Ultra-Turrax (22.000 rpm; 2 × 5 s) in a 10:1 (v/w) ratio of ice-cold buffer containing: 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, pH 7.6), 50 mM sodium fluoride, 50 mM potassium chloride (KCl), 5 mM sodium pyrophosphate, 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid, 5 mM β-glycerophosphate, 1 mM sodium vanadate, 1 mM dithiothreitol, 1% nonyl phenoxypolyethoxylethanol (Tergitol-type NP40) and protease inhibitors cocktail (Complete, Roche, Mijdrecht, The Netherlands). The effect of topiramate on the canonical insulin signalling pathway was subsequently determined by Western blot analysis.
Plasma analysis
Blood samples were taken from the tail tip into chilled paraoxon-coated capillaries to prevent ex vivo lipolysis. The tubes were immediately placed on ice and centrifuged at 4°C. Plasma levels of glucose and free fatty acids (FFA) were determined using commercially available kits and standards according to the instructions of the manufacturer (Instruchemie, Delfzijl, The Netherlands). Plasma insulin levels were measured using a mouse-specific insulin ELISA kit (Crystal Chem Inc., Downers Grove, IL, USA). Total plasma 14C-glucose and 3H-glucose were determined in supernatant of 7.5 μL plasma, after protein precipitation using 20% trichloroacetic acid and evaporation to eliminate tritiated water.
Tissue analysis
For determination of tissue 2-[3H]DG uptake, homogenates of heart, skeletal muscle and adipose tissue were boiled, and the supernatants were subjected to an ion-exchange column [described previously (Rossetti et al. 1990; van den Hoek et al., 2008; Parlevliet et al., 2009)] to isolate 2-[3H]DG-6-phosphate, a metabolic end-product of 2-[3H]DG that accumulates in muscle and fat cells.
C2C12 cells
C2C12 skeletal muscle cells were cultured in a humidified atmosphere containing 5% CO2 in DMEM containing 25 mM glucose, glutamine and pyruvate (Invitrogen, Bleiswijk, The Netherlands) supplemented with 10% FBS and 100 units per mL penicillin and 100 μg·mL−1 streptomycin (Invitrogen). For the deoxyglucose uptake assay ∼15 000 cells per well were seeded and cultured on 12-well plates. For Western blot analysis ∼25 000 cells per well were seeded and cultured on 6-well plates. After reaching confluence, the cells were differentiated into myotubes by replacing the complete growth medium with differentiation medium (same DMEM medium containing antibiotics, but supplemented with 2% horse serum instead of 10% FBS). The differentiation medium was changed every 48 h. The myotubes were used for experiments at day 7 of differentiation.
Deoxyglucose uptake assay in C2C12 myotubes
C2C12 myotubes were serum-starved for 4 h before the experiment. After serum starvation, cells were washed once with PBS and once with buffer (50 mM HEPES, 138 mM sodium chloride, 1.85 mM calcium chloride, 1.3 mM magnesium sulfate and 4.8 mM KCl, pH 7.4) followed by incubation with the same buffer for 45 min at 37°C in the presence of topiramate (1 or 100 μM) or vehicle. At the end of incubation, cells were challenged with or without 1 μM insulin (Sigma-Aldrich, St. Louis, MO, USA) for 10 min in the presence of topiramate or vehicle. 2-Deoxy-D-[1-14C] glucose (2DG) was added to the cells for another 10 min (0.012 μCi per dish). The reaction was ended by washing three times with ice-cold PBS and addition of a lysis buffer containing 1% sodium dodecyl sulfate (SDS)/0.2 M sodium hydroxide. The lysates were transferred into plastic vials, 2 mL of scintillation liquid (Instagel Plus, PerkinElmer, Waltham, MA, USA) was added and radioactivity was measured in a scintillation counter.
Insulin signalling in C2C12 myotubes
C2C12 myotubes were serum-starved for 4 h then washed once with PBS and once with 2DG buffer followed by incubation with the same buffer for 45 min at 37°C in the presence of topiramate (1 or 100 μM) or vehicle. At the end of incubation, cells were stimulated with 1 μM insulin. After 20 min, cells were rapidly washed one time with ice-cold PBS and lysed by addition of a buffer containing 12.5% glycerol, 3% SDS and 100 mM TrisPO4, pH 6.8. The cell lysates were then immediately boiled for 5 min and stored at −20°C until use.
Palmitate-induced insulin resistance in C2C12 myotubes
Palmitate-containing medium was prepared to induce insulin resistance in C2C12 myotubes. DMEM basic medium was supplemented with antibiotics and 2% FFA-free BSA. Palmitate was first dissolved in ethanol and then added to medium containing BSA at a final concentration of 0.75 mM. The final medium was sonicated for 5 min and warmed at 55°C for 10 min to allow complex formation between BSA and palmitate. Differentiated myotubes (day 6) were then treated with palmitate for 16 h before performing deoxyglucose uptake assay.
Western blot analysis
Protein content in cell or tissue homogenates was determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). Proteins (10 μg) were separated by 10% SDS-PAGE followed by transfer to a PVDF transfer membrane. Membranes were blocked for 1 h at room temperature in Tris Buffer Saline Tween20 (TBST) buffer with 5% non-fat dry milk followed by an overnight incubation with antibodies (all from Cell Signaling Technology, Beverly, MA, USA). Blots were then incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Bands were visualized by enhanced chemiluminescence and quantified using Image J (NIH, US).
Calculations
Turnover rates of glucose (μmol·min−1·kg−1) were calculated in basal and hyperinsulinaemic conditions as the rate of tracer infusion (dpm·min−1) divided by plasma specific activity of 14C-glucose (dpm·μmol−1). The ratio was corrected for body weight. Endogenous glucose production was identical to the glucose appearance rate under basal conditions and calculated as the difference between the tracer-derived rate of glucose appearance and the glucose infusion rate under hyperinsulinaemic-euglycaemic clamp conditions.
Tissue-specific glucose uptake in heart, skeletal muscle and adipose tissue was calculated from tissue 2-[3H]DG content, corrected for plasma specific activity and expressed as micromoles per gram of tissue.
Statistical analysis
Differences between groups were determined by Mann–Whitney non-parametric tests for two independent samples. The criterion for significance was set at P < 0.05. All values shown represent means ± SEM.
Results
In vivo studies
After 6 weeks of run-in high-fat diet, mice were randomized on body weight (25.5 ± 0.4 vs. 26.1 ± 0.8 g) and fasting plasma glucose levels (4.2 ± 0.2 vs. 4.1 ± 0.2 mmol·L−1) and received high-fat diet containing topiramate or vehicle during 6 subsequent weeks. After 5 weeks of topiramate treatment, the topiramate concentration in plasma, determined by liquid chromatography tandem mass spectrometry assay as previously described (Christensen et al., 2002), was 3.7 ± 0.2 μg·mL−1 (n = 7). After 6 weeks of topiramate treatment, the topiramate concentration in the brains of overnight fasted mice was 115 ± 18 ng per g brain tissue (n = 23).
Effect of topiramate on insulin sensitivity in mice
After 6 weeks of topiramate treatment, insulin sensitivity was assessed using hyperinsulinaemic-euglycaemic clamp studies. Body weight did not differ between topiramate- (n = 7) and vehicle-treated (n = 10) mice (Table 1). The reduction in body weight was also not statistically different between topiramate- (−2.9%) and vehicle-treated mice (−4.7%). In the basal period of the hyperinsulinaemic-euglycaemic clamp, endogenous glucose production (EGP), which equals glucose disposal (Rd), was not different between topiramate- and vehicle-treated animals.
Table 1.
Results obtained during the hyperinsulinaemic-euglycaemic clamp study in vehicle- (n = 10) and topiramate-treated (n = 7) animals. Data are represented as means ± SEM. There were no significant differences between vehicle- and topiramate-treated animals. *P < 0.05 basal versus hyperinsulinaemic-euglycaemic clamp period
| Vehicle | Topiramate | |
|---|---|---|
| Body weight (g) | 25.8 ± 0.4 | 24.9 ± 0.8 |
| Basal haematocrit (%) | 40.3 ± 0.2 | 38.5 ± 0.2 |
| Clamp haematocrit (%) | 36.8 ± 0.2 | 34.9 ± 0.5 |
| Basal insulin (ng·mL−1) | 0.5 ± 0.1 | 0.5 ± 0.1 |
| Clamp insulin (ng·mL−1) | 5.1 ± 0.4* | 4.3 ± 0.5* |
| Basal glucose (mmol·L−1) | 4.1 ± 0.1 | 4.2 ± 0.1 |
| Clamp glucose (mmol·L−1) | 4.5 ± 0.3 | 4.0 ± 0.2 |
The specific activity of 14C-glucose measured at 10-min intervals indicated the presence of steady-state conditions in both groups (Supporting Information Table S1). The glucose infusion rate (GIR) necessary to maintain euglycemia was 50% higher in topiramate-treated animals compared to vehicle-treated animals (average GIR 84 ± 8 vs. 56 ± 4 μmol·min−1·kg−1 for the last 20 min of the experiment, P < 0.05, Figure 1A), in the presence of similar glucose levels (Table 1, Supporting Information Fig. S1), indicating that topiramate improved insulin sensitivity.
Figure 1.
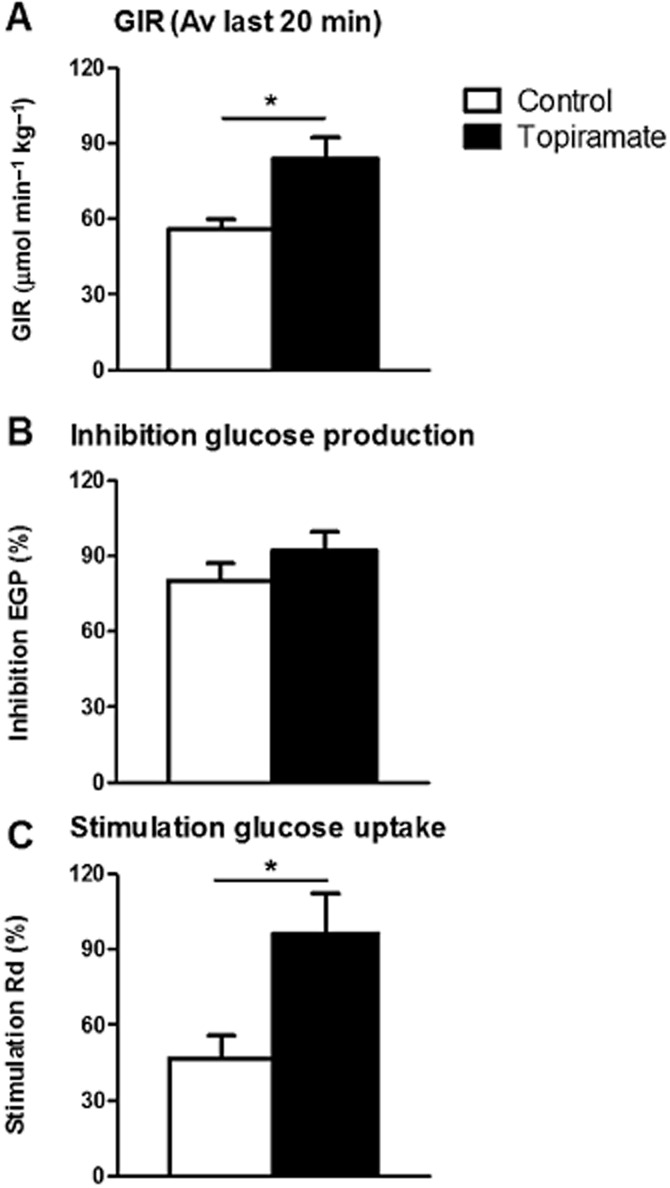
Glucose infusion rate (GIR, A), endogenous glucose production (EGP, B) and glucose disposal (Rd, C) in vehicle-treated (white) and topiramate-treated (black) animals as measured in hyperinsulinaemic-euglycaemic clamp studies. Values represent means ± SEM for n = 7–10. *P < 0.05 topiramate versus vehicle. Av, average.
In the hyperinsulinaemic-euglycaemic period, insulin inhibited EGP to the same extent in both groups of mice (92 ± 8 vs. 80 ± 7%, n.s., Figure 1B). Insulin-mediated Rd, however, was increased by 104% in the topiramate-treated group compared to the vehicle-treated group (96 ± 16 vs. 47 ± 9%, respectively, P < 0.05, Figure 1C), indicating that topiramate treatment improved peripheral insulin sensitivity.
Effect of i.c.v. administration of tolbutamide on the effects of topiramate on insulin sensitivity in mice
To determine whether topiramate improved peripheral insulin sensitivity by affecting insulin signalling in the brain, tolbutamide (vehicle-treated mice n = 7; topiramate-treated mice n = 9), an inhibitor of KATP channels in neurons, or vehicle (vehicle-treated mice n = 8; topiramate-treated mice n = 10) was infused into the lateral ventricle (i.c.v.) during hyperinsulinaemic-euglycaemic clamp experiments. The specific activity in plasma of 14C-glucose measured at 10-min intervals indicated the presence of steady-state conditions in all groups (Supporting Information Table S2). In agreement with the first experiment, topiramate-treated animals receiving vehicle (aCSF) had higher GIR compared to vehicle-treated animals receiving aCSF (average GIR 85 ± 9 vs. 55 ± 11 μmol·min−1·kg−1 for the last 20 min of the experiment, P < 0.05, Figure 2A), in the presence of similar glucose levels (Table 2, Supporting Information Figure S2). I.c.v. tolbutamide administration in vehicle-treated animals did not affect any of the parameters tested compared to i.c.v. aCSF infused vehicle-treated animals (Figure 2A–D), in line with our previous study in high-fat fed mice (Coomans et al., 2011a). In topiramate-treated animals, i.c.v. administration of tolbutamide decreased GIR compared to i.c.v. aCSF (average GIR 75 ± 7 vs. 85 ± 9 μmol·min−1·kg−1 for the last 20 min of the experiment, P < 0.05, Figure 2A), indicating that i.c.v. tolbutamide counteracted, at least in part, the improvement in insulin sensitivity induced by topiramate. In the basal period as well as in the hyperinsulinaemic period, EGP was not different between all groups of mice (Figure 2B). Insulin-stimulated Rd was again significantly higher in topiramate-treated animals compared to vehicle-treated animals receiving aCSF (101 ± 15 vs. 71 ± 12%, respectively, P < 0.05, Figure 2C). However, i.c.v. administration of tolbutamide in topiramate animals diminished the improvement of Rd induced by the drug (81 ± 12 vs. 101 ± 15%, respectively, P < 0.05, Figure 2C). Assessment of tissue-specific 2-[3H]DG uptake revealed that topiramate treatment increased insulin-stimulated glucose uptake in heart, skeletal muscle and adipose tissue (gonadal fat pad) (Figure 2D). Interestingly, i.c.v. tolbutamide in topiramate-treated animals abrogated the improvement in insulin-stimulated 2-[3H]DG uptake in heart, skeletal muscle and adipose tissue.
Figure 2.
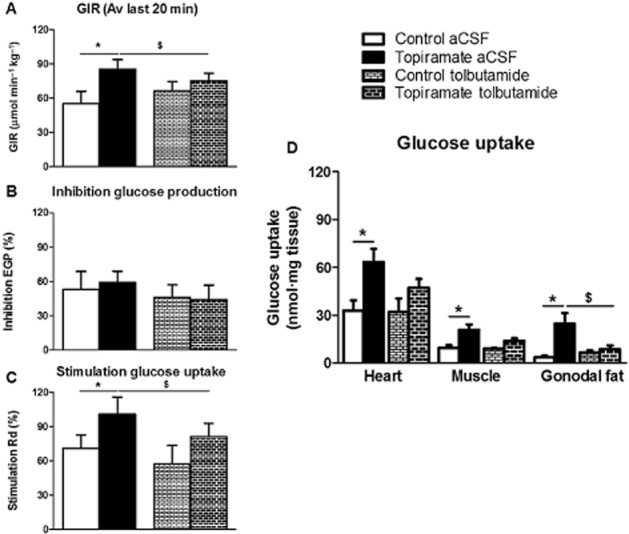
Glucose infusion rate (GIR, A), endogenous glucose production (EGP, B), glucose disposal (Rd, C) and organ-specific glucose uptake (D) in vehicle-treated (white) and topiramate-treated animals (black) receiving i.c.v. vehicle (artificial cerebrospinal fluid, aCSF) or tolbutamide. Values represent means ± SEM for n = 7–10. *P < 0.05 topiramate versus vehicle. $P < 0.05 i.c.v. tolbutamide versus vehicle. Av, average.
Table 2.
Results of i.c.v. administration of aCSF (vehicle-treated mice n = 8; topiramate-treated mice n = 10) and tolbutamide (vehicle-treated mice n = 7; topiramate-treated mice n = 9) obtained from the hyperinsulinaemic-euglycaemic clamp study in vehicle- and topiramate-treated animals. Data are represented as means ± SEM. There were no significant differences between vehicle- and topiramate-treated animals. *P < 0.05 basal versus hyperinsulinaemic-euglycaemic clamp period. aCSF, artificial cerebrospinal fluid; FFA, free fatty acids
| Vehicle | Topiramate | |||
|---|---|---|---|---|
| aCSF | Tolbutamide | aCSF | Tolbutamide | |
| Body weight (g) | 28.6 ± 0.7 | 28.7 ± 0.7 | 27.7 ± 0.5 | 27.8 ± 0.3 |
| Basal haematocrit (%) | 40.3 ± 0.2 | 39.0 ± 0.8 | 38.5 ± 0.2 | 37.1 ± 0.3 |
| Clamp haematocrit (%) | 36.8 ± 0.2 | 36.3 ± 0.2 | 34.9 ± 0.5 | 34.4 ± 0.4 |
| Basal insulin (ng·mL−1) | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| Clamp insulin (ng·mL−1) | 4.1 ± 0.4* | 3.3 ± 0.1* | 3.4 ± 0.2* | 3.8 ± 0.4* |
| Basal glucose (mmol·L−1) | 5.0 ± 0.2 | 5.4 ± 0.3 | 5.5 ± 0.2 | 4.9 ± 0.2 |
| Clamp glucose (mmol·L−1) | 5.6 ± 0.3 | 6.0 ± 0.5 | 5.8 ± 0.3 | 5.2 ± 0.3 |
| Basal FFA (mmol·L−1) | 0.7 ± 0.0 | 0.6 ± 0.1 | 0.6 ± 0.0 | 0.7 ± 0.0 |
| Clamp FFA (mmol·L−1) | 0.3 ± 0.0* | 0.3 ± 0.0* | 0.3 ± 0.0* | 0.3 ± 0.0* |
Effect of topiramate on muscle insulin signalling in mice
To determine the direct effect of topiramate on muscle, we determined insulin signalling in skeletal muscle (upper hindlimb) 10 min after insulin (vehicle-treated mice n = 10; topiramate-treated mice n = 8) or saline i.v. injection (vehicle-treated mice n = 9; topiramate-treated mice n = 8). As expected, insulin strongly increased the phosphorylation state of PKB and proline-rich Akt substrate of 40 kDa in muscle (Figure 3A-C). The expression of insulin receptor β (IRβ) and its downstream signalling pathway were not differently affected between vehicle and topiramate-treated mice. Insulin decreased the phosphorylation state of AMP-activated protein kinase (AMPK) in vehicle-treated mice as expected (Figure 3D). In topiramate-treated mice, AMPK phosphorylation was increased under insulin-stimulated conditions compared to vehicle-treated mice.
Figure 3.
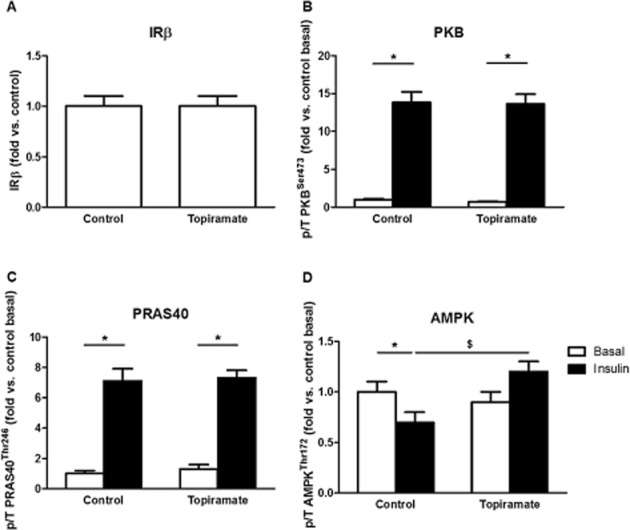
Expression of IRβ (A) and phosphorylation state of PKB (B), PRAS40 (C) and AMPK (D) in basal state (black bars) or hyperinsulinaemic state (white bars) in muscle of mice-treated with vehicle or topiramate. The Western blot data were expressed as fold change compared to vehicle at basal condition. Data are represented as means ± SEM for n = 8–10, *P < 0.05 insulin versus basal. $P < 0.05 topiramate versus vehicle.
In vitro studies
Effect of topiramate on glucose uptake in differentiated myotubes
To exclude that topiramate directly increased glucose uptake at the tissue level, we investigated the direct effects of topiramate on glucose uptake in differentiated C2C12 myotubes (Figure 4A). Cells were treated with increasing concentrations of topiramate (1 or 100 μM) or vehicle for 45 min and glucose uptake was then measured during the last 20 min, after addition, or not, of 1 μM insulin. Insulin stimulated glucose uptake by about +25% in control condition, that is without topiramate. Topiramate did not increase basal or insulin-stimulated glucose uptake (Figure 4A).
Figure 4.
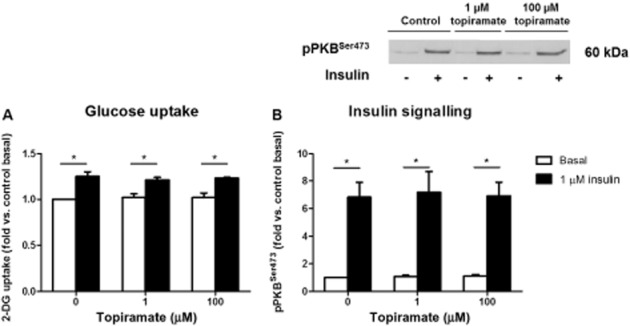
Glucose uptake (A) and insulin signalling (B,C) in C2C12 myotubes at basal (white) or insulin-stimulated (black) condition with topiramate (1 or 100 μM) or vehicle. The quantification was normalized for total protein and expressed as fold change compared to vehicle at basal condition. Data are represented as means ± SEM for n = 3–4, *P < 0.05 insulin versus basal.
Effect of topiramate on insulin signalling in differentiated myotubes
Western blot analyses were performed to determine whether topiramate affects the insulin signalling pathway in differentiated myotubes by assessing the phosphorylation state of PKB (Figure 4B). As expected, insulin strongly increased the phosphorylation state of PKB on Ser473 (+583%) in control condition. Topiramate did not affect basal or insulin-stimulated phosphorylation of PKB. (Figure 4B).
Effect of topiramate on glucose uptake in insulin-resistant myotubes
Next, we investigated whether topiramate was able to reverse palmitate-induced insulin resistance in C2C12 myotubes (Figure 5). After incubation with palmitate for 16 h, C2C12 myotubes were incubated with increasing concentrations of topiramate (1 or 100 μM) for 45 min and challenged with or without 1 μM of insulin for 20 min. At maximal concentration of insulin, glucose uptake was increased by +25% in control condition, that is without palmitate and topiramate (Figure 4A). Preincubation with palmitate resulted in a decrease of insulin-stimulated glucose uptake. There was no effect of topiramate on basal or insulin-stimulated glucose uptake (Figure 5B,C).
Figure 5.
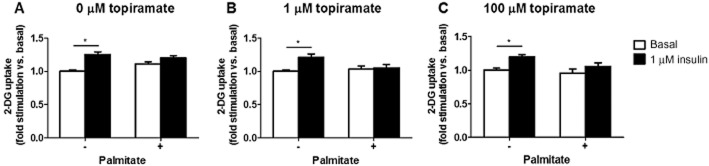
Glucose uptake (A-C) by healthy or palmitate-induced insulin resistant C2C12 myotubes at basal (white) or insulin-stimulated (black) condition with topiramate (1 or 100 μM) or vehicle. The quantification was normalized for total protein and expressed as fold change compared to vehicle at basal condition. Data are represented as means ± SEM for n = 3–4, *P < 0.05 insulin versus basal.
Discussion and conclusions
In the present study, topiramate improved insulin sensitivity by increasing glucose uptake by skeletal and cardiac muscle and by adipose tissue in high-fat fed mice. In addition, inhibition of the central action of circulating insulin by i.c.v. administration of tolbutamide, a KATP channel blocker in neurons, prevented this insulin-sensitizing effect of topiramate. Topiramate had no direct effect on muscle insulin signalling and glucose uptake in vivo and in vitro. Collectively, these data indicate that topiramate improves peripheral insulin sensitivity indirectly via the brain, rather than by directly targeting peripheral organs.
The mice were fed a high-fat diet for 6 weeks to reduce insulin sensitivity before topiramate treatment was started, as topiramate exerts its greatest effects in obese, insulin resistant subjects (Astrup and Toubro, 2004). The half-life of topiramate in humans is 21 h, whereas the half-life in rodents is only 1–2 h (Bialer et al., 2004). To obtain stable concentrations of topiramate in plasma of our mice, topiramate was mixed through the diet with addition of 3.33% anise to cover the bitter taste. The dose of topiramate used in the present study resulted in plasma concentrations within the therapeutic range (∼4 μg·mL−1). Hyperinsulinaemic-euglycaemic clamp analyses revealed that topiramate improved insulin sensitivity independent of weight loss, in agreement with previous studies (Wilkes et al., 2005a,b; Stenlof et al., 2007). In the present study, we extend these observations by showing that this insulin-sensitizing effect of topiramate was present in cardiac and skeletal muscle, as well as in adipose tissue. In contrast, topiramate did not improve hepatic insulin sensitivity. This improved insulin sensitivity in muscle and adipose tissue, but not in liver, has previously been associated with enhanced AMPK phosphorylation in muscle, but not in liver (Wilkes et al., 2005b). Also in the current study, we show enhanced AMPK phosphorylation in skeletal muscle upon topiramate treatment. As α-adrenergic stimulation enhances AMPK phosphorylation, the increased glucose uptake by peripheral organs might be related to increased sympathetic nervous system activation (Minokoshi et al., 2002). The topiramate concentration in plasma correlates with that in CSF (Christensen et al., 2001). Moreover, the effects of topiramate on body weight, body composition and energy metabolism have been associated with altered neuropeptide expression in the hypothalamus (York et al., 2000). Combined with the absence of direct effects of topiramate on insulin sensitivity in muscle cells, we therefore hypothesized that the brain mediated the effects of topiramate on insulin sensitivity.
Topiramate has been shown to act at the level of the central nervous system by modifying and inhibiting neurotransmission: (i) enhancement of GABA-ergic activity (White et al., 1997; 2000), (ii) inhibition of kainite/AMPA receptors (Gibbs et al., 2000), (iii) inhibition of voltage-dependent sodium channels (Zona et al., 1997), (iv) inhibition of high-voltage-activated calcium channels (Zhang et al., 2000) and (v) inhibition of carbonic anhydrase (Dodgson et al., 2000). As a result of carbonic anhydrase inhibition, potassium currents are activated (Herrero et al., 2002). Potassium (K+) channels are membrane-spanning proteins that selectively conduct K+ ions across the cell membrane. There are several classes of K+ channels, including the voltage-gated potassium channels and the inwardly rectifying potassium channels. Although topiramate has effects on voltage-gated potassium channels (Danielsson, et al., 2005), topiramate has no direct effect on the inwardly rectifying G-protein coupled or the KATP channels (Kobayashi et al., 2009).
KATP channels are widely expressed in the cytoplasmic membranes of neurons and play an important role in the regulation of cell excitability (Ashcroft and Gribble, 1998). These channels open when the intracellular ATP to ADP ratio decreases, resulting in membrane hyperpolarization and close when this ratio increases, resulting in membrane depolarization. The KATP channel is composed of the sulfonylurea receptor type-1 (SUR1) and the inwardly rectifying K+ channel (Kir6.2) subunits (Seino and Miki, 2003). Tolbutamide binds to the SUR1 subunit of the KATP channel, thereby preventing opening of this channel (Babenko et al., 2000; Moreau et al., 2005). We and others have shown that in lean animals, tolbutamide blocks activation of KATP channels by insulin (Spanswick et al., 2000; Obici et al., 2002; Coomans et al., 2011b). In obese animals, however, hypothalamic KATP channels are insensitive to insulin and blockage of the KATP channels by tolbutamide has no effect (Coomans et al., 2011a,b). To test our hypothesis that the CNS is involved in the anti-diabetic effects of topiramate by improving insulin signalling in the brain, we administered tolbutamide i.c.v. in topiramate-treated animals on a high-fat diet during hyperinsulinaemic-euglycaemic clamp conditions. In the present study, high-fat feeding resulted in similar central insulin resistance as these previous studies, since i.c.v. tolbutamide in vehicle-treated animals had no effect on insulin-inhibited glucose production or insulin-stimulated glucose uptake. Interestingly, we show that topiramate is able to restore hypothalamic insulin sensitivity, thereby restoring hypothalamic sensitivity to KATP channels blockage by tolbutamide. This is in line with a recent study showing that short-term topiramate treatment improved hypothalamic insulin signalling (Caricilli et al., 2012). As topiramate has no direct effects on KATP channels (Kobayashi et al., 2009), the exact mechanism by which topiramate restores hypothalamic insulin sensitivity remains to be identified.
Peripheral and hypothalamic insulin effects are both required for physiological regulation of glucose homeostasis. Activation of KATP channels in hypothalamic neurons by insulin provides a signal inhibiting gluconeogenesis, that is conveyed to the liver by parasympathetic vagal fibres. KATP channels, however, are not only expressed in neurons of different brain regions, but also in microglial cells (McLarnon et al., 2001). In activated microglia, the KATP channel components SUR-1 and SUR-2 are present together with glucokinase, suggesting that microglia also have glucosensing properties (Ramonet et al., 2004). This energy-sensing capacity might be a key element for controlling microglial activation and secretion of proinflammatory cytokines following CNS injury. However, until now, the function of these KATP channels in microglial cells has not been studied in relation to peripheral glucose homeostasis. We cannot exclude the possibility that such mechanisms in microglial cells might be involved in the results presented in the current study.
Topiramate treatment did not exert any direct effects on insulin signalling in muscle in vivo. Furthermore, both basal or insulin-stimulated glucose uptake and insulin signalling in insulin-sensitive or insulin-resistant cultured muscle cells (differentiated C2C12 myotubes) were not affected by topiramate. Our data are in contrast to a previous study that reported that topiramate increases glucose uptake in cultured insulin-sensitive L6 cells, a rat skeletal muscle cell line, via an AMPK-mediated pathway (Ha et al., 2006). The phosphorylation level of AMPK (Thr172) was not increased when we treated C2C12 myotubes with topiramate (data not shown). The apparent contradiction between the previous study performed in L6 cells and our study could be due to difference in origin of cell-type, the differentiation status of the muscle cells or other differences in experimental conditions.
In conclusion, topiramate improves insulin sensitivity in high-fat fed mice by stimulating insulin-mediated glucose uptake by skeletal muscle, heart and adipose tissue through effects within the CNS. Inhibition of KATP channel activation in the brain abrogates the insulin-sensitizing effect of topiramate in these mice. These observations indicate that the anti-diabetic effects of topiramate are the result of actions in the brain, rather than of direct effects of topiramate on peripheral organs. These observations demonstrate the possibility of pharmacological treatment of peripheral insulin resistance through targets in the CNS.
Acknowledgments
This work was supported by grants from TI Pharma (TIP project T2-105, to J. A. R., L. M. H. and D. M. O.), the Netherlands Heart Foundation (NHS project 2007B81, to P. C. N. R. and J. A. R.) and the Dutch Diabetes Research Foundation (DFN project 2007.00.010, to P. C. N. R. and J. A. R.). PCN is an Established Investigator of the Netherlands Heart Foundation (NHS 2009T038). We thank Delphine Chevillon (Abbott Products GmbH, Germany) and Dr. Edwin Parlevliet (Department of Endocrinology, LUMC, The Netherlands) for excellent technical support, and Dr. Stephan Michel for discussion.
Glossary
- aCSF
artificial cerebrospinal fluid
- EGP
endogenous glucose production
- GIR
glucose infusion rate
- KATP channel
ATP-sensitive potassium channel
- Rd
rate of disposal
- T2DM
type 2 diabetes mellitus
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Hyperinsulinaemic-euglycaemic clamp: glucose concentrations (A) and glucose infusion rates (GIR, B). Values represent means ± SEM for 7–10 mice per group. *P < 0.05, topiramate versus vehicle.
Figure S2 Hyperinsulinaemic-euglycaemic clamp: glucose concentrations (A) and glucose infusion rates (GIR, B). Values represent means ± SEM for 7–10 mice per group. *P < 0.05, topiramate versus other groups.
Table S1 Specific activity of 14C-glucose (dpm·mmol−1) in the basal period or in the hyperinsulinaemic-euglycaemic period. Values are means ± SEM for n = 7–10.
Table S2 Specific activity of 14C-glucose (dpm·mmol−1) in the basal period or in the hyperinsulinaemic-euglycaemic period. Values are means ± SEM for n = 7–10.
References
- Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- Astrup A, Toubro S. Topiramate: a new potential pharmacological treatment for obesity. Obes Res. 2004;12(Suppl):167S–173S. doi: 10.1038/oby.2004.284. [DOI] [PubMed] [Google Scholar]
- van den Berg SA, Guigas B, Bijland S, Ouwens M, Voshol PJ, Frants RR, et al. High levels of dietary stearate promote adiposity and deteriorate hepatic insulin sensitivity. Nutr Metab (Lond) 2010;7:24. doi: 10.1186/1743-7075-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko AP, Gonzalez G, Bryan J. Pharmaco-topology of sulfonylurea receptors. Separate domains of the regulatory subunits of K(ATP) channel isoforms are required for selective interaction with K(+) channel openers. J Biol Chem. 2000;275:717–720. doi: 10.1074/jbc.275.2.717. [DOI] [PubMed] [Google Scholar]
- Bialer M, Doose DR, Murthy B, Curtin C, Wang SS, Twyman RE, et al. Pharmacokinetic interactions of topiramate. Clin Pharmacokinet. 2004;43:763–780. doi: 10.2165/00003088-200443120-00001. [DOI] [PubMed] [Google Scholar]
- Caricilli AM, Penteado E, de Abreu LL, Quaresma PG, Santos AC, Guadagnini D, et al. Topiramate treatment improves hypothalamic insulin and leptin signaling and action and reduces obesity in mice. Endocrinology. 2012;153:4401–4411. doi: 10.1210/en.2012-1272. [DOI] [PubMed] [Google Scholar]
- Christensen J, Hojskov CS, Dam M, Poulsen JH. Plasma concentration of topiramate correlates with cerebrospinal fluid concentration. Ther Drug Monit. 2001;23:529–535. doi: 10.1097/00007691-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Christensen J, Hojskov CS, Poulsen JH. Liquid chromatography tandem mass spectrometry assay for topiramate analysis in plasma and cerebrospinal fluid: validation and comparison with fluorescence-polarization immunoassay. Ther Drug Monit. 2002;24:658–664. doi: 10.1097/00007691-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Coomans CP, Biermasz NR, Geerling JJ, Guigas B, Rensen PC, Havekes LM, et al. Stimulatory effect of insulin on glucose uptake by muscle involves the central nervous system in insulin-sensitive mice. Diabetes. 2011a;60:3132–3140. doi: 10.2337/db10-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomans CP, Geerling JJ, Guigas B, van den Hoek AM, Parlevliet ET, Ouwens DM, et al. Circulating insulin stimulates fatty acid retention in white adipose tissue via KATP channel activation in the central nervous system only in insulin-sensitive mice. J Lipid Res. 2011b;52:1712–1722. doi: 10.1194/jlr.M015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson BR, Lansdell K, Patmore L, Tomson T. Effects of the antiepileptic drugs lamotrigine, topiramate and gabapentin on hERG potassium currents. Epilepsy Res. 2005;63:17–25. doi: 10.1016/j.eplepsyres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41(Suppl 1):S35–S39. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Tiraferri I, Neri L, Sternieri E. Clinical pharmacology of topiramate in migraine prevention. Expert Opin Drug Metab Toxicol. 2011;7:1169–1181. doi: 10.1517/17425255.2011.602067. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, III, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41(Suppl 1):S10–S16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Ha E, Yim SV, Jung KH, Yoon SH, Zheng LT, Kim MJ, et al. Topiramate stimulates glucose transport through AMP-activated protein kinase-mediated pathway in L6 skeletal muscle cells. Pharmacogenomics J. 2006;6:327–332. doi: 10.1038/sj.tpj.6500366. [DOI] [PubMed] [Google Scholar]
- Herrero AI, Del ON, Gonzalez-Escalada JR, Solis JM. Two new actions of topiramate: inhibition of depolarizing GABA(A)-mediated responses and activation of a potassium conductance. Neuropharmacology. 2002;42:210–220. doi: 10.1016/s0028-3908(01)00171-x. [DOI] [PubMed] [Google Scholar]
- van den Hoek AM, Voshol PJ, Karnekamp BN, Buijs RM, Romijn JA, Havekes LM, et al. Intracerebroventricular neuropeptide Y infusion precludes inhibition of glucose and VLDL production by insulin. Diabetes. 2004;53:2529–2534. doi: 10.2337/diabetes.53.10.2529. [DOI] [PubMed] [Google Scholar]
- van den Hoek AM, Teusink B, Voshol PJ, Havekes LM, Romijn JA, Pijl H. Leptin deficiency per se dictates body composition and insulin action in ob/ob mice. J Neuroendocrinol. 2008;20:120–127. doi: 10.1111/j.1365-2826.2007.01626.x. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Hirai H, Iino M, Fuse I, Mitsumura K, Washiyama K, et al. Inhibitory effects of the antiepileptic drug ethosuximide on G protein-activated inwardly rectifying K+ channels. Neuropharmacol. 2009;56:499–506. doi: 10.1016/j.neuropharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, et al. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon JG, Franciosi S, Wang X, Bae JH, Choi HB, Kim SU. Acute actions of tumor necrosis factor-alpha on intracellular Ca(2+) and K(+) currents in human microglia. Neurosci. 2001;10:1175–1184. doi: 10.1016/s0306-4522(01)00119-1. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Riccardelli R, Binder C, Kusumakar V. Open-label adjunctive topiramate in the treatment of unstable bipolar disorder. Can J Psychiatry. 2005;50:415–422. doi: 10.1177/070674370505000705. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Moreau C, Prost AL, Dérand R, Vivaudou M. SUR, ABC proteins targeted by KATP channel openers. J Mol Cell Cardiol. 2005;38:951–963. doi: 10.1016/j.yjmcc.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Parlevliet ET, Schroder-van der Elst JP, Corssmit EP, Picha K, O'Neil K, Stojanovic-Susulic V, et al. CNTO736, a novel glucagon-like peptide-1 receptor agonist, ameliorates insulin resistance and inhibits very low-density lipoprotein production in high-fat-fed mice. J Pharmacol Exp Ther. 2009;328:240–248. doi: 10.1124/jpet.108.144154. [DOI] [PubMed] [Google Scholar]
- Picard F, Deshaies Y, Lalonde J, Samson P, Richard D. Topiramate reduces energy and fat gains in lean (Fa/?) and obese (fa/fa) Zucker rats. Obes Res. 2000;8:656–663. doi: 10.1038/oby.2000.84. [DOI] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonet D, Ríguez MJ, Pugliese M, Mahy N. Putative glucosensing property in rat and human activated microglia. Neurobiol Dis. 2004;17:1–9. doi: 10.1016/j.nbd.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Rossetti L, Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990;85:1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(Suppl 1):S3–S9. [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- Stenlof K, Rossner S, Vercruysse F, Kumar A, Fitchet M, Sjostrom L. Topiramate in the treatment of obese subjects with drug-naive type 2 diabetes. Diabetes Obes Metab. 2007;9:360–368. doi: 10.1111/j.1463-1326.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate enhances GABA-mediated chloride flux and GABA-evoked chloride currents in murine brain neurons and increases seizure threshold. Epilepsy Res. 1997;28:167–179. doi: 10.1016/s0920-1211(97)00045-4. [DOI] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41(Suppl 1):S17–S20. [PubMed] [Google Scholar]
- Wilkes JJ, Nelson E, Osborne M, Demarest KT, Olefsky JM. Topiramate is an insulin-sensitizing compound in vivo with direct effects on adipocytes in female ZDF rats. Am J Physiol Endocrinol Metab. 2005a;288:E617–E624. doi: 10.1152/ajpendo.00437.2004. [DOI] [PubMed] [Google Scholar]
- Wilkes JJ, Nguyen MT, Bandyopadhyay GK, Nelson E, Olefsky JM. Topiramate treatment causes skeletal muscle insulin sensitization and increased Acrp30 secretion in high-fat-fed male Wistar rats. Am J Physiol Endocrinol Metab. 2005b;289:E1015–E1022. doi: 10.1152/ajpendo.00169.2005. [DOI] [PubMed] [Google Scholar]
- York DA, Singer L, Thomas S, Bray GA. Effect of topiramate on body weight and body composition of osborne-mendel rats fed a high-fat diet: alterations in hormones, neuropeptide, and uncoupling-protein mRNAs. Nutrition. 2000;16:967–975. doi: 10.1016/s0899-9007(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Zhang X, Velumian AA, Jones OT, Carlen PL. Modulation of high-voltage-activated calcium channels in dentate granule cells by topiramate. Epilepsia. 2000;41(Suppl 1):S52–S60. doi: 10.1111/j.1528-1157.2000.tb02173.x. [DOI] [PubMed] [Google Scholar]
- Zona C, Ciotti MT, Avoli M. Topiramate attenuates voltage-gated sodium currents in rat cerebellar granule cells. Neurosci Lett. 1997;231:123–126. doi: 10.1016/s0304-3940(97)00543-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


