Abstract
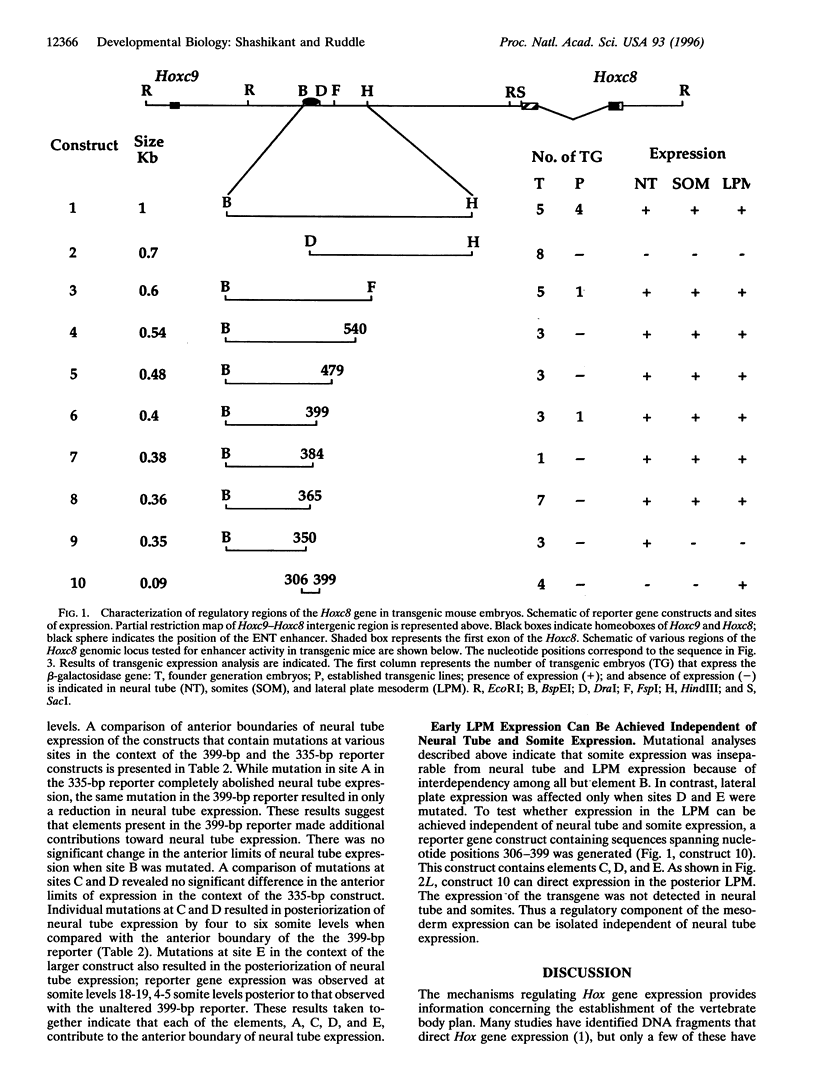
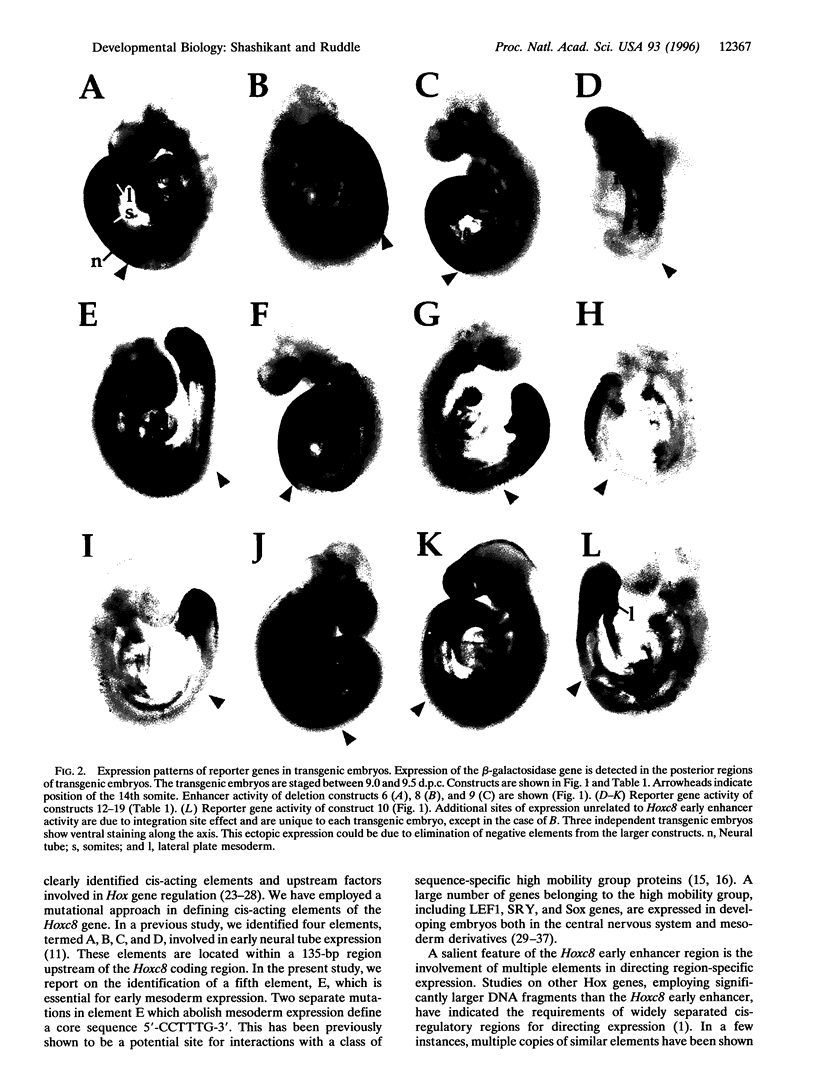
We have used a transgene mutation approach to study how expression domains of Hoxc8 are established during mouse embryogenesis. A cis-regulatory region located 3 kb upstream from the Hoxc8 translational start site directs the early phase of expression. Four elements, termed A, B, C, and D, were previously shown to direct expression to the neural tube. Here we report that a fifth element, E, located immediately downstream of D directs expression to mesoderm in combination with the other four elements. These elements are interdependent and partially redundant. Different combinations of elements determine expression in different posterior regions of the embryo. Neural tube expression is determined minimally by ABC, ABD, or ACD; somite expression by ACDE; and lateral plate mesoderm expression by DE. Neural tube and lateral plate mesoderm enhancers can be separated, but independent somite expression has not been achieved. Furthermore, mutations within these elements result in posteriorization of the reporter gene expression. Thus, the anterior extent of expression is determined by the combined action of these elements. We propose that the early phase of Hoxc8 expression is directed by two separate mechanisms: one that determines tissue specificity and another that determines anterior extent of expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aparicio S., Morrison A., Gould A., Gilthorpe J., Chaudhuri C., Rigby P., Krumlauf R., Brenner S. Detecting conserved regulatory elements with the model genome of the Japanese puffer fish, Fugu rubripes. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1684–1688. doi: 10.1073/pnas.92.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awgulewitsch A., Bieberich C., Bogarad L., Shashikant C., Ruddle F. H. Structural analysis of the Hox-3.1 transcription unit and the Hox-3.2--Hox-3.1 intergenic region. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6428–6432. doi: 10.1073/pnas.87.16.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awgulewitsch A., Jacobs D. Differential expression of Hox 3.1 protein in subregions of the embryonic and adult spinal cord. Development. 1990 Mar;108(3):411–420. doi: 10.1242/dev.108.3.411. [DOI] [PubMed] [Google Scholar]
- Awgulewitsch A., Utset M. F., Hart C. P., McGinnis W., Ruddle F. H. Spatial restriction in expression of a mouse homoeo box locus within the central nervous system. 1986 Mar 27-Apr 2Nature. 320(6060):328–335. doi: 10.1038/320328a0. [DOI] [PubMed] [Google Scholar]
- Bieberich C. J., Utset M. F., Awgulewitsch A., Ruddle F. H. Evidence for positive and negative regulation of the Hox-3.1 gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8462–8466. doi: 10.1073/pnas.87.21.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw M. S., Shashikant C. S., Belting H. G., Bollekens J. A., Ruddle F. H. A long-range regulatory element of Hoxc8 identified by using the pClasper vector. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2426–2430. doi: 10.1073/pnas.93.6.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier G., Dressler G. R., Gruss P. Primary structure and developmental expression pattern of Hox 3.1, a member of the murine Hox 3 homeobox gene cluster. EMBO J. 1988 May;7(5):1329–1336. doi: 10.1002/j.1460-2075.1988.tb02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charité J., de Graaff W., Vogels R., Meijlink F., Deschamps J. Regulation of the Hoxb-8 gene: synergism between multimerized cis-acting elements increases responsiveness to positional information. Dev Biol. 1995 Oct;171(2):294–305. doi: 10.1006/dbio.1995.1282. [DOI] [PubMed] [Google Scholar]
- Collignon J., Sockanathan S., Hacker A., Cohen-Tannoudji M., Norris D., Rastan S., Stevanovic M., Goodfellow P. N., Lovell-Badge R. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development. 1996 Feb;122(2):509–520. doi: 10.1242/dev.122.2.509. [DOI] [PubMed] [Google Scholar]
- Connor F., Wright E., Denny P., Koopman P., Ashworth A. The Sry-related HMG box-containing gene Sox6 is expressed in the adult testis and developing nervous system of the mouse. Nucleic Acids Res. 1995 Sep 11;23(17):3365–3372. doi: 10.1093/nar/23.17.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn T. L., Mynett-Johnson L., Wright E. M., Hosking B. M., Koopman P. A., Muscat G. E. Sequence and expression of Sox-18 encoding a new HMG-box transcription factor. Gene. 1995 Aug 19;161(2):223–225. doi: 10.1016/0378-1119(95)00280-j. [DOI] [PubMed] [Google Scholar]
- Frasch M., Chen X., Lufkin T. Evolutionary-conserved enhancers direct region-specific expression of the murine Hoxa-1 and Hoxa-2 loci in both mice and Drosophila. Development. 1995 Apr;121(4):957–974. doi: 10.1242/dev.121.4.957. [DOI] [PubMed] [Google Scholar]
- Gaunt S. J. Mouse homeobox gene transcripts occupy different but overlapping domains in embryonic germ layers and organs: a comparison of Hox-3.1 and Hox-1.5. Development. 1988 May;103(1):135–144. doi: 10.1242/dev.103.1.135. [DOI] [PubMed] [Google Scholar]
- German M. S., Wang J., Chadwick R. B., Rutter W. J. Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: building a functional insulin minienhancer complex. Genes Dev. 1992 Nov;6(11):2165–2176. doi: 10.1101/gad.6.11.2165. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Giese K., Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994 Mar;10(3):94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Rowader K. E., Stevens K., Jiang C., Milos P., Zaret K. S. Modulation of liver-specific transcription by interactions between hepatocyte nuclear factor 3 and nuclear factor 1 binding DNA in close apposition. Mol Cell Biol. 1993 Apr;13(4):2401–2410. doi: 10.1128/mcb.13.4.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Laudet V., Stehelin D., Clevers H. Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res. 1993 May 25;21(10):2493–2501. doi: 10.1093/nar/21.10.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mouellic H., Condamine H., Brûlet P. Pattern of transcription of the homeo gene Hox-3.1 in the mouse embryo. Genes Dev. 1988 Jan;2(1):125–135. doi: 10.1101/gad.2.1.125. [DOI] [PubMed] [Google Scholar]
- Le Mouellic H., Lallemand Y., Brûlet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992 Apr 17;69(2):251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Liu J. K., DiPersio C. M., Zaret K. S. Extracellular signals that regulate liver transcription factors during hepatic differentiation in vitro. Mol Cell Biol. 1991 Feb;11(2):773–784. doi: 10.1128/mcb.11.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H., Studer M., Pöpperl H., Aparicio S., Kuroiwa A., Brenner S., Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994 Aug 18;370(6490):567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- Nonchev S., Vesque C., Maconochie M., Seitanidou T., Ariza-McNaughton L., Frain M., Marshall H., Sham M. H., Krumlauf R., Charnay P. Segmental expression of Hoxa-2 in the hindbrain is directly regulated by Krox-20. Development. 1996 Feb;122(2):543–554. doi: 10.1242/dev.122.2.543. [DOI] [PubMed] [Google Scholar]
- Overdier D. G., Porcella A., Costa R. H. The DNA-binding specificity of the hepatocyte nuclear factor 3/forkhead domain is influenced by amino-acid residues adjacent to the recognition helix. Mol Cell Biol. 1994 Apr;14(4):2755–2766. doi: 10.1128/mcb.14.4.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock R. A., Jay G., Bieberich C. J. Altering the boundaries of Hox3.1 expression: evidence for antipodal gene regulation. Cell. 1992 Dec 11;71(6):911–923. doi: 10.1016/0092-8674(92)90388-s. [DOI] [PubMed] [Google Scholar]
- Pöpperl H., Bienz M., Studer M., Chan S. K., Aparicio S., Brenner S., Mann R. S., Krumlauf R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell. 1995 Jun 30;81(7):1031–1042. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- Sham M. H., Vesque C., Nonchev S., Marshall H., Frain M., Gupta R. D., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993 Jan 29;72(2):183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Shashikant C. S., Bieberich C. J., Belting H. G., Wang J. C., Borbély M. A., Ruddle F. H. Regulation of Hoxc-8 during mouse embryonic development: identification and characterization of critical elements involved in early neural tube expression. Development. 1995 Dec;121(12):4339–4347. doi: 10.1242/dev.121.12.4339. [DOI] [PubMed] [Google Scholar]
- Studer M., Pöpperl H., Marshall H., Kuroiwa A., Krumlauf R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science. 1994 Sep 16;265(5179):1728–1732. doi: 10.1126/science.7916164. [DOI] [PubMed] [Google Scholar]
- Suh E., Chen L., Taylor J., Traber P. G. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol. 1994 Nov;14(11):7340–7351. doi: 10.1128/mcb.14.11.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman J., Harris E., Wilson D., Desplan C. The homeodomain: a new face for the helix-turn-helix? Bioessays. 1992 Mar;14(3):145–150. doi: 10.1002/bies.950140302. [DOI] [PubMed] [Google Scholar]
- Utset M. F., Awgulewitsch A., Ruddle F. H., McGinnis W. Region-specific expression of two mouse homeo box genes. Science. 1987 Mar 13;235(4794):1379–1382. doi: 10.1126/science.2881353. [DOI] [PubMed] [Google Scholar]
- Uwanogho D., Rex M., Cartwright E. J., Pearl G., Healy C., Scotting P. J., Sharpe P. T. Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev. 1995 Jan;49(1-2):23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Snopek B., Koopman P. Seven new members of the Sox gene family expressed during mouse development. Nucleic Acids Res. 1993 Feb 11;21(3):744–744. doi: 10.1093/nar/21.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Corbi N., Basilico C., Dailey L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 1995 Nov 1;9(21):2635–2645. doi: 10.1101/gad.9.21.2635. [DOI] [PubMed] [Google Scholar]
- van Genderen C., Okamura R. M., Fariñas I., Quo R. G., Parslow T. G., Bruhn L., Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994 Nov 15;8(22):2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]