Abstract
In this study, we propose a new approach to increase the success rate of single-oocyte dispensing and investigate the subsequent viability of the dispensed oocytes. We used a pair of capacitance sensors placed in a microfluidic chip to detect the oocyte, and custom-designed a special buffer zone in the microchannel to decelerate the flow velocity and reduce the hydraulic pressure acting on the oocyte. In the buffer zone, a semicircular bay, formed by equally spaced micro-pillars, is used to stop the oocyte at the dispensing nozzle hole. Finally, the oocyte is ejected by airflow to the culture array. The novel feature of the developed microfluidic system is that the extraordinary improvement in success rate is accompanied by a lack of change in oocyte survival rate (as assessed by a comparison of survival rates before and after the dispensing procedure). By using this device, we achieved a highly accurate single-oocyte dispensing process with a success rate of 100%. The oocyte survival rate is approximately 70%, regardless of whether or not the oocyte is dispensed. The newly proposed system has the advantages of high operation speed and potential usage for two-dimensional micropatterning.
INTRODUCTION
As a subset of microelectromechanical systems (MEMSs), lab-on-a-chip (LOC) systems have contributed significantly to biomedical applications. For example, microfluidic chips may provide advantages that are specific to their applications. In investigations on the functions of single cells, especially oocytes, microfluidic chips have achieved many functions such as single-cell manipulations and oocyte enucleation.1, 2 Therefore, many issues are resolved by using LOC techniques for single-cell analysis. However, since the birth of cloning, the enucleation process has been carried out by manual manipulator operation—a technique that has been used for more than 20 yr with very limited improvement. Recently, researchers have devised numerous techniques that do not require manual operation. Previously, we studied magnetically driven microtools (MMTs) with sufficient power for a wide range of cell manipulations.3, 4, 5, 6 Currently, we are developing a microgripper that will be applied to the oocyte enucleation process. By using such a microrobot, the enucleation process could be rapidly performed with far greater accuracy. However, following the enucleation process, the enucleated oocytes are manually collected by using an injection pipette7 or by centrifugation.8 Such time-consuming retrieval methods greatly affect oocyte viability and the subsequent analysis of single oocytes.
Conventionally, to isolate and sort different cell types, cell sorters based on flow cytometry, can sort cells in a continuous cell-laden flow, and sorted cells can be retrieved in cell suspensions.9 Cell sorting is performed electrically, after a shot of laser light to sense the cell. Thus, such kind of system tends to be large and expensive. In the meantime, several works have succeeded in cell ejection using inkjet technology.10, 11, 12, 13, 14, 15 Yusof et al.16 have outlined a non-contact method for the controlled separation of single cells confined in a droplet that can be printed inkjet-like onto predefined locations. This method is a suitable platform for printing single cells of various types. Mainly, the inkjet mechanism is composed of the solution tank and the nozzle part and the drive unit to generate droplets. However, such systems cannot have a module structure in order to be easily washed and are not disposable. Therefore, in cell manipulation the system encounters the risk of contamination problems. Tornay et al.17 have proposed the cell dispensing system with the disposable microchip, which consisted with an ejection mechanism by air and a capacitance sensor. This system succeeded in the dispensing a droplet with a single latex bead. However, since this system does not have a cell loading mechanism for supplying a single cell one by one to the microchannel, the success rate of dispensing is unsatisfactory. In addition, a bunch of non-inkjet technologies like dispensing valves18 or laser microdissection19 are available for single cell separation and manipulation. However, transporting continuously manipulated oocytes from the microfluidic chip to the incubation atmosphere is critical for single oocyte ejection. Kawahara et al.20 fabricated two pairs of capacitance sensors in a microfluidic chip to detect artificial beads with diameters of ∼100 μm. With this method, they were able to detect polystyrene beads very well. However, their success rate reached only 50%—an unsatisfactory level for polystyrene bead ejection. And the permittivity of oocyte and culture solution was quite close; therefore, detection of oocyte became quite difficult.
Here, our target are to (1) develop a system by improving the sensitivity and (2) custom-design a microchannel for single oocyte ejection. We report that the success rate of dispensing a single bovine oocyte is increased to 100% and that the dispensed oocytes are viable. The rest of the paper is divided into five main sections: (1) concept of the proposed dispensing system, (2) design and fabrication of the microchip, (3) experiments on dispensing bovine oocytes by using the proposed system, (4) viability evaluation, and (5) concluding remarks. In the final section, we also discuss the application of this dispensing system and future directions for research.
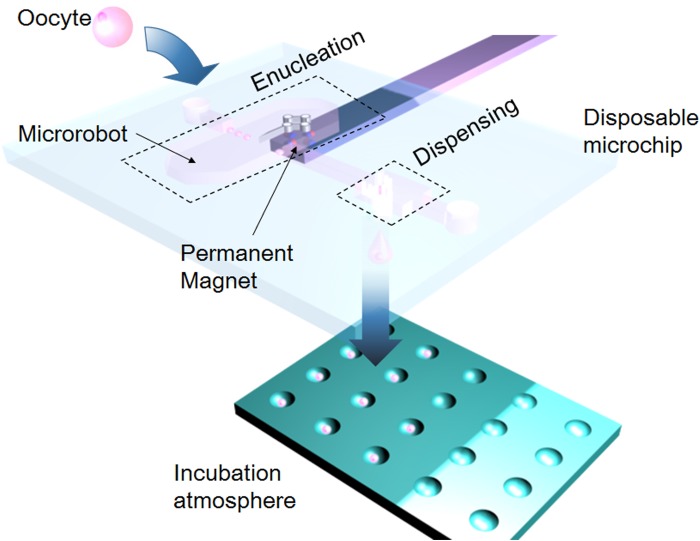
Figure 1 shows a conceptual diagram of the enucleation and dispensing system. The oocyte is injected into the microchip from the inlet, and the medium containing the oocytes from the inlet flows to the enucleation operation area. Subsequently, after the microrobots perform the enucleation process, the oocyte is delivered to the dispensing module. Finally, the oocytes are retrieved by a custom-designed culture vessel.
Figure 1.
On-chip cell enucleation and dispensing system.
METHODS AND CONCEPTS
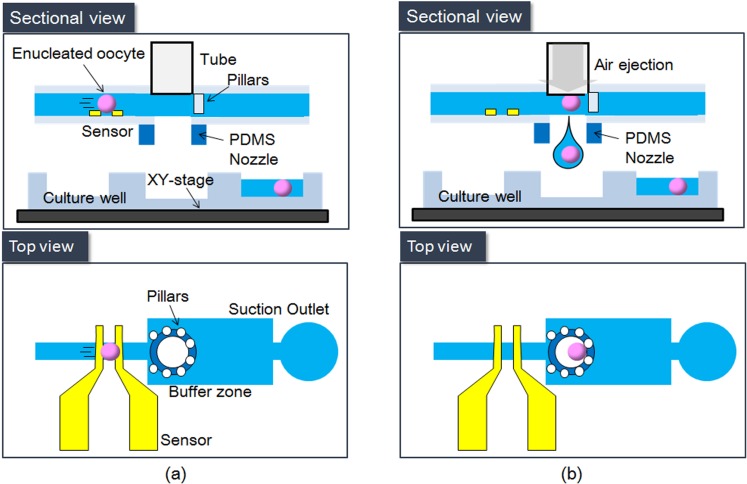
Figure 2 shows the proposed system, which comprises a coplanar capacitive sensor and a deceleration area with a circular array of micropillars. This oocyte ejection system is fabricated from polydimethylsiloxane (PDMS); hence, any risk of contamination is avoided because such a microfluidic chip is disposable.
Figure 2.
Basic concept of the on-chip oocyte dispensing system. (a) Oocyte detection. (b) Oocyte obstruction and dispensing.
Capacitance sensors
To eject a single oocyte into each hole of the culture well, a sensor is required for accurate detection. To achieve accurate single-oocyte ejection, we previously fabricated a pair of capacitance sensors on a microfluidic chip and placed a pair of electrodes in the microchannel so that a microsensor could be used to measure the flow velocity.20, 21 The great advantage of the capacitance sensor is that it can rapidly sense the oocyte without a visual system. In those studies, an oocyte delivered by a culture medium was detected by measuring the impedance changes between two electrodes. The signal was then demodulated by a lock-in amplifier to yield the impedance change due to passage of the oocyte. However, the noise signal of the capacitance sensors greatly interfered with the normal signal; at the same time, the sensitivity of the capacitance sensor decreased accordingly once the flow velocity increased. Therefore, the success rate of single-particle dispensing could only reach 50%. In the newly developed system, we improved the sensitivity of the microsensor by reducing the size of the microchannel; the width and height were reduced from 800 μm and 300 μm, respectively, to 200 μm for both dimensions. Whereas the occupied oocyte volume ratio at the sensing area was merely 2.45% in the previous system, it increased to 14.7% in the new system. Therefore, the change in permittivity at the sensing area becomes more distinct. Although the sensitivity may be increased further by reducing the channel size, there is a limit because the size of an oocyte is normally around 150 to 180 μm. The maximum amplitude of oocyte detection increases by 16.7 times from 15 mV in the previous system to 250 mV in the current system. Figure 2a shows an oocyte passing through the capacitance sensor.
Deceleration and obstruction mechanism
The success rate of our previous system in dispensing microbeads was low not only because of the low sensitivity of the sensor. Kawahara et al.20 used two pairs of sensors to estimate the flow velocity in the previous system. Owing to the large variation in the estimated velocity for calculating the timing of ejection; however, their method only achieved a success rate of 50%. In the newly developed system, a deceleration chamber is established in the microchannel and 50-μm micropillars are arranged into a circular shape to block the oocyte at the dispensing nozzle. An oocyte with the zona pellucida (ZP) is normally 150 μm in diameter; therefore, the distance between each pair of micropillars is set to 50 μm to block the oocyte successfully. Figure 2b is a sketch of an oocyte blocked by the micropillars. This mechanism works extremely well with the deceleration module in the developed system.
Air-flow-based inkjet mechanism
We use an air-flow-based inkjet system involving a high-speed solenoid valve with a maximum drive frequency of 500 Hz. When the solenoid valve is briefly opened, air force is applied to the microchannel (Fig. 2b). The oocyte is then ejected to the culture well.20
In Sec. 3, we describe the design and fabrication method for a high-accuracy single-oocyte dispensing mechanism by considering the characteristics of the system components.
MICROCHIP DESIGN AND FABRICATION
Microchannel design
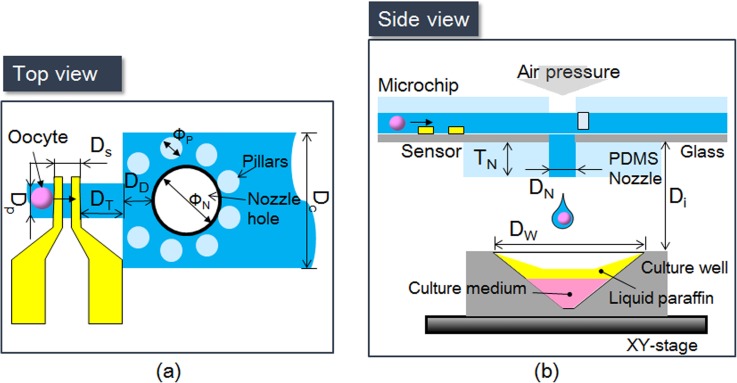
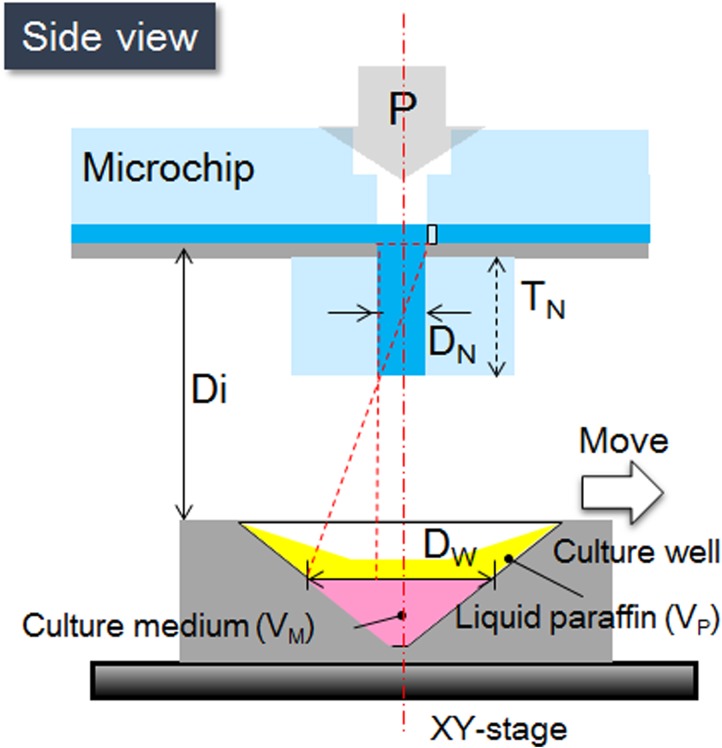
Figure 3 shows the entire single-oocyte dispensing system. Figure 3a shows the top view of the dispensing area, where Ds, DD, Dp, and DC are the width of the microsensor, width of the microchannel, distance from the microsensor to the center of the nozzle, and width of the deceleration microchannel, respectively. Figure 3b shows the side view of the dispensing system, where DN, Di, DW, DT, and TN are the diameter of the nozzle, distance between the microchip and the culture well, diameter of a single vessel, distance between the sensor and the deceleration microchamber, and thickness of the nozzle, respectively.
Figure 3.
Design parameters of the dispensing system. (a) Schematic diagram of microchannel viewed from above. (b) Side view of the dispensing system.
The width of a branch of the microsensor is set to 100 μm with a distance of 100 μm between each pair of branches.21 In current system, the time required for enucleation is at least 10 s for a single oocyte. Hence, our current goal is to sense and dispense one oocyte in 10 s. To this end, the length of the dispensing microchannel is set to 1 cm and the flow velocity to 1 mm/s. and distance between the microsensor and the deceleration microchamber is set to 0.5 mm. Thus, in 1 min we can dispense 6 oocytes. However, from the sensor to the dispensing nozzle, theoretically it only takes 0.63 s, therefore, for the other applications this system can achieve almost 95 oocytes sensing and dispensing per minute. Because an oocyte is softer without ZP than with it, the flow velocity should differ between these two cases. As noted above, the width and height of the microchannel are set equally to 200 μm. The diameters of the nozzle and pillar are 500 and 100 μm, respectively. To block the oocyte successfully, the distance between each pillar is set to 50 μm. The oocytes, which are delivered by flow, are detected when they pass through the sensor. After an oocyte is stopped by the pillars and the air dispenser triggered, the XY-stage with the culture well is moved to the position corresponding to the nozzle position to retrieve the oocyte.
One innovation in this study is the deceleration module. Because the parameters of the microchannel are fixed, we analyzed the flow distribution in the microchannel by using COMSOL Multiphysics 4.3. An oocyte is elastoplastic; therefore, in the case of high flow velocity, it could be deformed and flushed through a gap between the pillars.22 Two approaches are used for the current oocyte enucleation process: one with ZP, and the other without it. An oocyte with ZP has a much higher Young's modulus (22.8 ± 10.4 kPa) than that without ZP.23 The Young's modulus is difficult to measure in the latter case because the oocyte becomes soft and sticky on the surface. Here, we conducted preliminary experiments to find the optimum flow velocity.
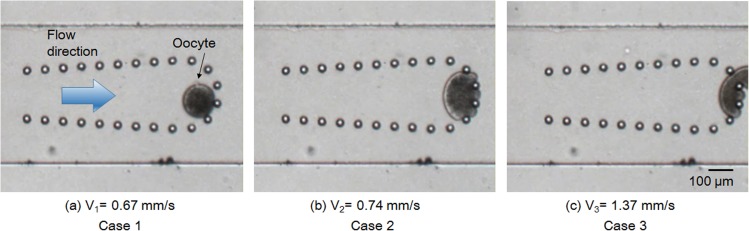
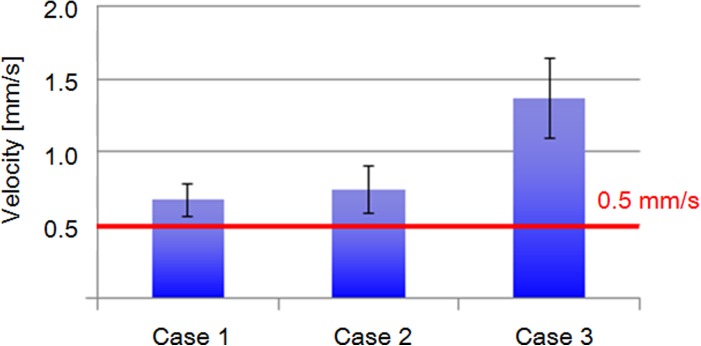
First, we investigated the minimum flow velocity that safely stops an oocyte by micropillars without obviously deforming it. Excessive deformation of an oocyte may harm it and affect its ensuing proliferation and division. Figure 4 shows three typical examples in a preliminary test at velocities of 0.67, 0.74, and 1.37 mm/s. In Case 1 (low flow velocity, 0.67 mm/s), an oocyte could be blocked without any obvious changes in its circular shape. When the flow velocity increased to 0.74 mm/s (Case 2), the oocyte was distinctly deformed. When the flow velocity further increased to 1.37 mm/s (Case 3), the oocyte was deformed and flushed away by the flow.
Figure 4.
Initial investigation of oocyte deformation by hydraulic pressure in microchannel.
These results reveal that the maximum flow velocity must not exceed 0.6 mm/s. After analyzing 15 oocytes for each case and removing deviations in the data, a velocity of under 0.5 mm/s was set as the maximum velocity for blocking an oocyte without ZP (Fig. 5). To determine the microchannel design for decelerating the flow velocity from 1 to 0.5 mm/s, a two-dimensional structure was modeled using COMSOL Multiphysics 4.3 to analyze the flow condition in the microchannel. The flow in the microchannel changed dramatically from the narrow channel (width: 200 μm) to the wide channel (width: 800 μm). At the intersection of the channels (x = 0.8 mm), the flow velocity decreased sharply (Fig. 6).
Figure 5.
Experimental results under three different cases.
Figure 6.
FEM results of velocity distribution in the microchannel. (a) Velocity distribution. (b) Flow velocity curve (A-A′).
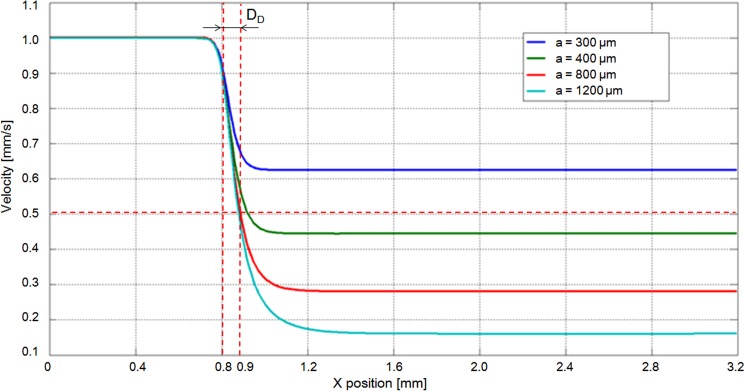
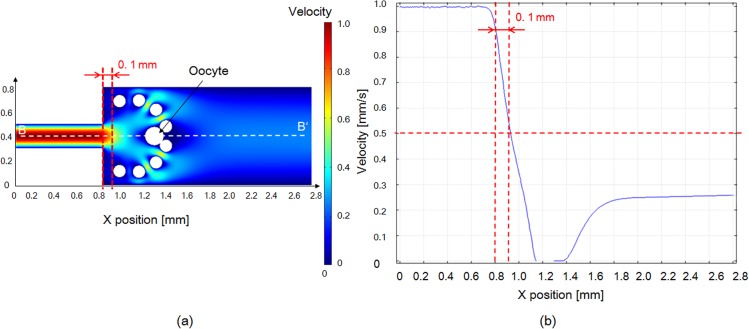
The finite element method (FEM) simulation results suggest that to achieve a flow velocity of 0.5 mm/s, the width of the microchannel should be increased to at least 400 μm. Although the flow velocity significantly decelerated when the width was increased to 800 μm, the rate at which the velocity decreased was not markedly different even when the width of the microchannel was increased to 1200 μm (Fig. 7). Meanwhile, the nozzle hole should be placed with 0.1 mm distance from the intersection. Therefore, in our experiments, we selected an 800 μm-wide microchannel for oocyte deceleration. Moreover, the velocity was noticeably reduced in the simulation results with the oocyte and pillars shown in Fig. 8. The velocity decreased under 0.5 mm/s from the distance of more than 0.1 mm. Therefore, oocyte under the velocity of 0.5 mm/s makes it feasible that the pillars could block the oocyte successfully, and the pressure acting on the oocyte was released. As mentioned earlier, the stiffness of an oocyte with ZP is greater than that without ZP. Even when the flow velocity was increased to 60 mm/s, oocytes with an intact ZP were still blocked by pillars without deformation.
Figure 7.
FEM results of velocity changes with microchannels of different widths (a: width of deceleration channel).
Figure 8.
FEM simulation results of velocity distribution in the microchannel with micropillars and an oocyte.
Design of dispensing nozzle
In our system, we use a high-speed solenoid valve. Air pressure is applied to the microchannel when the solenoid valve is briefly opened, and a minimum pressure of 1 kPa is achieved. The surface tension of the liquid is the main force that prevents leakage, because the dispensing hole is always open. Although this suggests that a higher surface tension can generate a higher force to maintain the air-liquid interface in balance, a higher surface tension also implies that a higher air pressure is required for dispensing, which may increase the risk of unintended damages to the oocyte. For this reason, we explain how to determine the size of the dispensing hole and thickness of the nozzle. The surface tension at the nozzle hole is
| (1) |
where Δp is the maximum surface tension of the air-fluid interface, γ is the surface tension, and R1 and R2 are radii of curvature (R1 = R2 = ½ DN). Ideally, increasing the diameter of the dispensing hole should make it easier to dispense the oocyte. However, the surface tension should also be considered to prevent leakage. A minimum nozzle hole of 0.5 mm could be achieved with the current fabrication technique. The values of surface tension Δp are 0.58 and 0.29 kPa in cases where the diameters of the dispensing hole are 0.5 and 1 mm, respectively. The dispensing hole with a diameter of 0.5 mm is more appropriate because the dispensing air pressure is 1 kPa.
The diameter of each retrieving vessel, DW, is 2 mm. To prevent the oocyte from being sprayed out of the vessel, the thickness of the nozzle is set to
| (2) |
According to calculations, the minimum thickness of the nozzle should be 667 μm. In the experiment, we set the nozzle thickness to 0.7 mm (Fig. 9). All parameters are shown in Table TABLE I.. The liquid paraffin can prevent evaporation of the culture medium and is non-toxic.
Figure 9.
Design of nozzle and parameters of current structure.
TABLE I.
Parameters of dispensing system.
| Parameter | Value |
|---|---|
| DN | 0.5 mm |
| TN | 0.7 mm |
| Di | 3 mm |
| DW | 2 mm |
| VP | 10 μl |
| VM | 3 μl |
| P | 1 kPa |
Fabrication of microfluidic chip
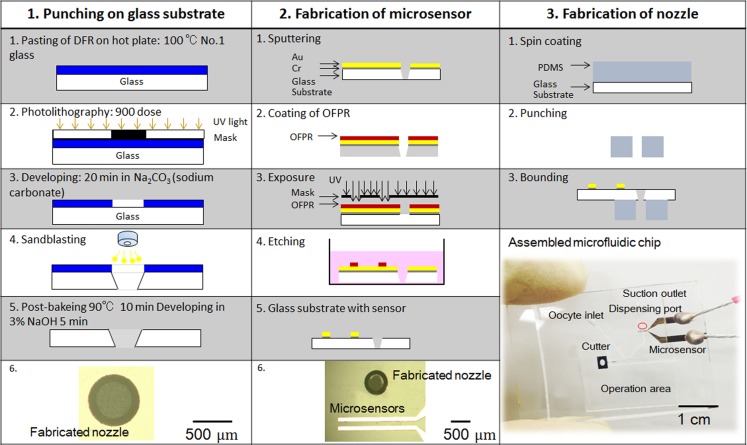
The fabrication process for the microchip, which involves integrating a nozzle hole and two pairs of capacitive sensors, can be summarized as follows (Fig. 10):
Figure 10.
Fabrication process of microchip.
Step 1: To fabricate the dispensing hole, a positive photoresist—a 50 μm-thick dry film resist (DFR; Asahi Kasei Co., Tokyo, JAPAN)—was pasted onto the prepared 0.12-mm-thick glass substrate. After h-line (λ = 405 nm) exposure of the DFR, a hollow circle was developed by using a sodium carbonate solution for 20 min at 26 °C. By using the sandblast technique, we fabricated a hole on the glass substrate. Finally, a NaOH solution was used to remove the remaining photoresist. After Step 3, a 0.5 × 1 × 0.7 mm PDMS nozzle was attached to the glass substrate.
Step 2: To fabricate the capacitive sensors, layers of 30-nm-thick Cr and 300-nm-thick Au were sputtered onto the prepared glass substrate. Next, OAP (Tokyo Ohka Kogyo) was spin-coated for 30 s at 2000 rpm to bond the posi-resist onto the glass substrate and baked for 2 min at 90 °C. Then, the positive photoresist, OFPR (Tokyo Ohka Kogyo), was spin-coated for 30 s at 2000 rpm and baked for 30 min at 90 °C. After h-line (λ = 405 nm) exposure of the OFPR, the sensor pattern was developed by NMD-3 (Tokyo Ohka Kogyo). Subsequently, the Au and Cr layers were wet etched, and OFPR was removed by acetone. Finally, the electrode on the glass was wired with Ag paste.
Step 3: A microfluidic chip consisting of a PDMS microchannel was bonded to a glass substrate. A PDMS microchannel was produced by replica molding with a master mold fabricated by photolithography. Ultraviolet light was irradiated through a photomask to produce a microchannel pattern with a mask aligner (LA410, Nanometrich Technology Inc., Tokyo, Japan). The substrate was then developed and rinsed. We employed SU-8 film (DuPont Co., Wilmington, DE, USA) and exposure to produce the microchannel in the microfluidic chip.
Disposable microfluidic chip was used to prevent contamination after experiments that lasted 2–3 h.
EXPERIMENTS
Experimental setup
For the setup, we used a Teflon tube to connect the microchip and silicone tube (Fig. 11b). This prevents air leakage between the microchip (PDMS) and the silicone tube, because the Teflon tube is reasonably stiff and the input pressure is not excessively high (∼10 kPa). The diameter of the air inlet (air hole) is 0.5 mm and the outer diameter of the Teflon tube is 1.0 mm.
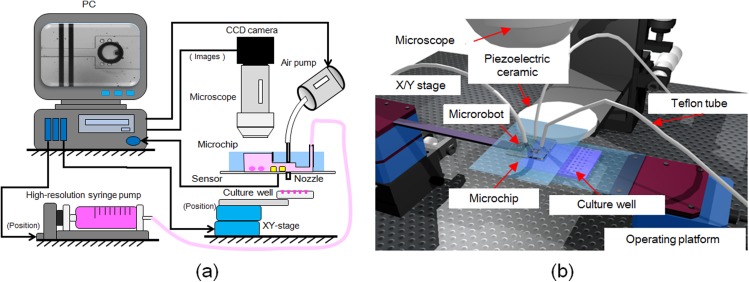
Figure 11.
Components of the experimental system. (a) Experimental setup for dispensing oocytes, which includes a solenoid valve, chip holder, culture well, and camera. (b) Architecture of the three-dimensional rendering system.
The system comprised a PC, solenoid valve, chip holder, culture well, and camera. By using the camera, we could observe delivery of the oocyte to the dispensing area. The position of the culture well was controlled by a motorized XY-stage. Measurements of the sensor outputs, timing of ejection, and signals sent to the solenoid valve, in addition to position control of the culture well, were performed with a computer. For the initial detection step, we used a digital lock-in amplifier (LI 5640, NF Corporation) with the following settings for the sinusoidal waveform to the microsensor: output voltage (Vp-p), 0.5 V; frequency, 10 kHz; and time constant, 30 μs. We obtained the voltage value under flow with an oocyte in the microchannel. To dispense the oocytes stably, we typically used 1 kPa air pressure and a 1 ms delay, on-time signal processing to the solenoid valve. A high-resolution syringe pump connected to the microchip was used to control the flow velocity in the microchannel.
Oocyte-dispensing experiments
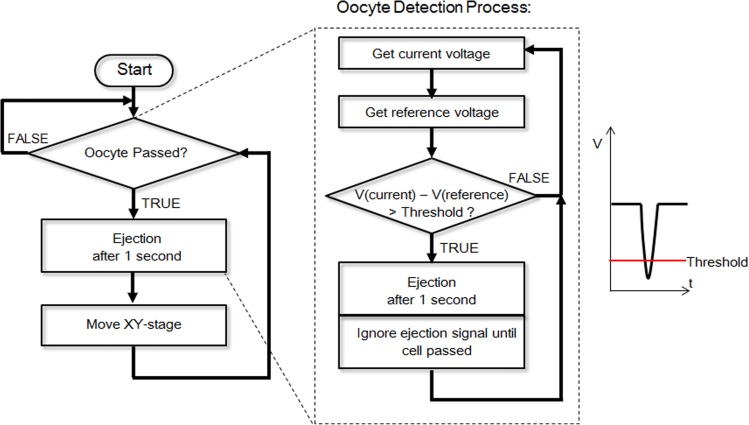
Figure 12 shows the detection, ejection, and retrieving processes. In the beginning, a signal is sent to the computer if an oocyte passes the sensor, and after a 1 s delay, the PC opens a solenoid valve and dispenses the oocyte. To determine the arrival of an oocyte, the voltage signal is continuously recorded and compared with the reference voltage. Once a wave trough is detected, the PC sends a command to open the solenoid valve, and the oocyte is dispensed to the culture well.
Figure 12.
Flowchart for oocyte detection.
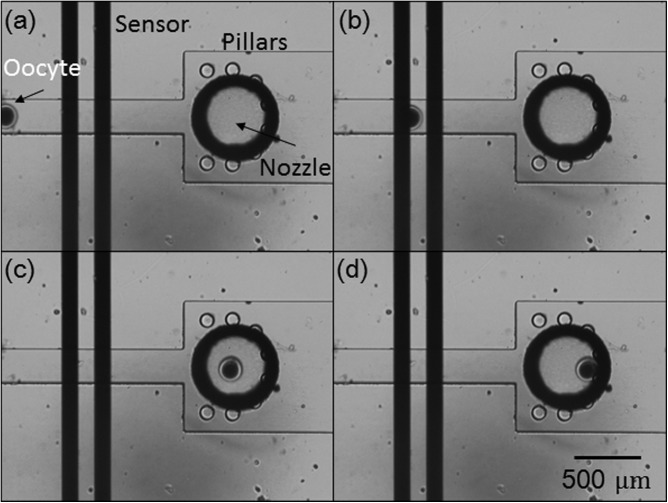
The oocytes were obtained from Livestock Improvement Association of Japan, Inc., Tokyo, Japan. Before the experiments, bovine oocytes were prepared in advance with hyaluronidase (0.1% of TCM 199) for 10 min to remove the cumulus cells surrounding the oocytes. After this manipulation, each oocyte was delivered to the dispensing area by flow suction from the outlet (Fig. 13a). The oocyte was then passed through the microsensor (Fig. 13b). After flowing into the deceleration microchamber, the oocyte suddenly slowed down and was blocked by the micropillars at the nozzle hole. Finally, the air pump ejected the oocyte to the culture environment. To investigate the success rate, the experiments were conducted during three different days with three different PDMS chips. Totally we tried 60 oocytes for dispensing, and all of the dispensed oocytes were retrieved. Therefore, we claim that the success rate after repeating 60 dispensing experiments was 100%.
Figure 13.
Experiment showing detection, deceleration, and obstruction (by micropillars) of an oocyte.
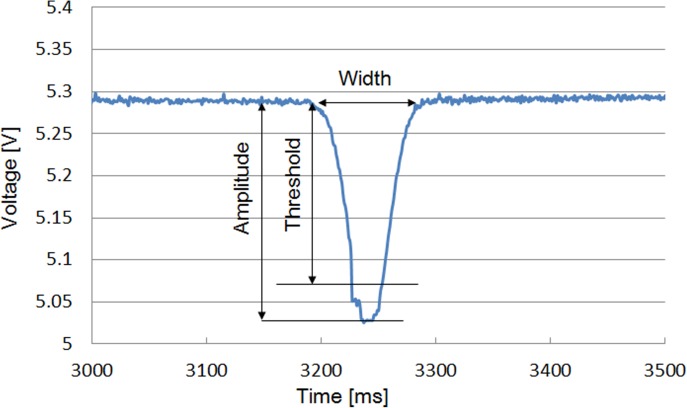
Figure 14 shows a typical experimental result of oocyte detection. The threshold was determined experimentally to 0.25 V. From the voltage changes in the sensor outputs, the passage of the oocyte could be detected.
Figure 14.
Detection of oocyte passing through the microsensor.
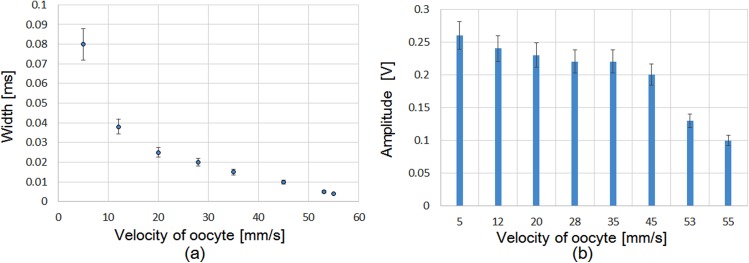
In accord with Fig. 14, the graphs in Fig. 15 show the evaluated signal changes in both the width and the aptitude due to the change in flow velocity. Figure 15a shows that the width of the detected signal decreased by increasing the flow velocity. At the same time, the amplitude of the detected signal also decreased by increasing the flow velocity. The system could detect oocytes up to a maximum velocity of 55 mm/s.
Figure 15.
Detection of oocyte according to its velocity.
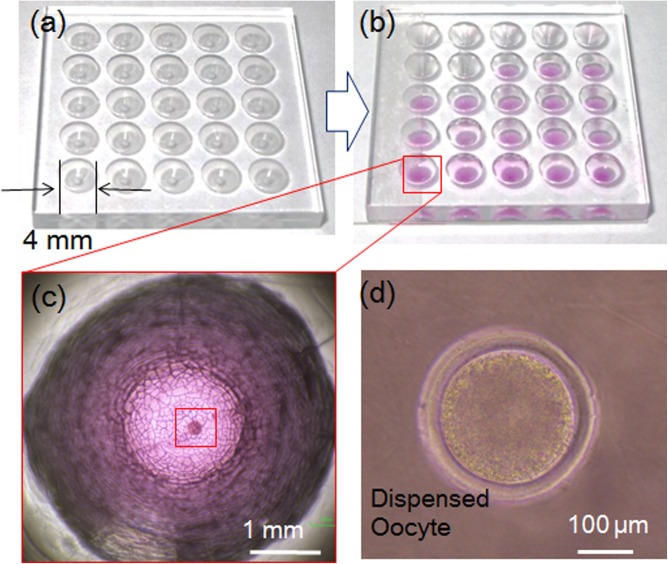
Figure 16 shows a representative dispensing result. In this experiment, we used a 3 cm × 3 cm culture well with vessels 4 mm in diameter (Fig. 16a). After the dispensing process, each vessel with the pink medium contained a single oocyte (Fig. 16b). The oocyte could be observed clearly in magnified views of the vessel (Figs. 16c, 16d).
Figure 16.
Experimental result of dispensing.
Viability evaluation
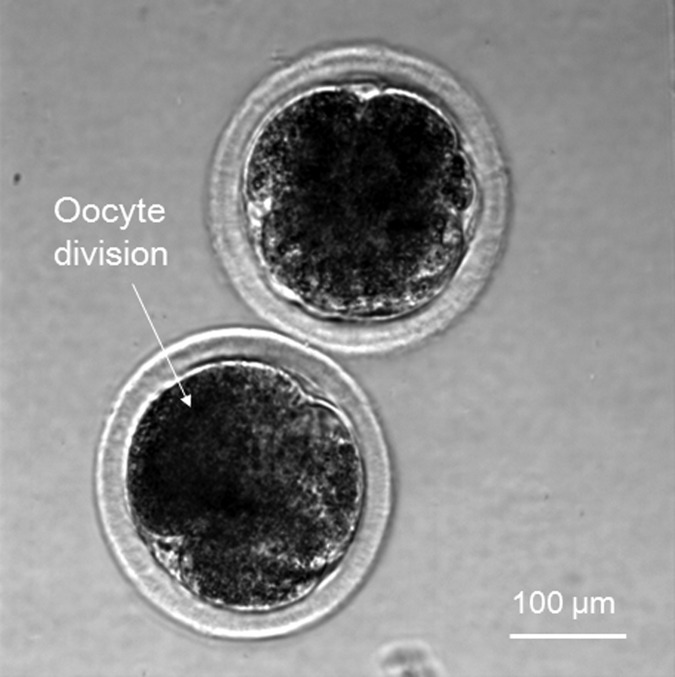
Dispensed oocytes were washed with Medium 199 without FBS (Fetal bovine serum) (Gibco Co., Tokyo, Japan) twice to remove any paraffin residue; they were then washed with three droplets of 10 μM Ca ionophore (Calbiochem Co., Darmstadt, Germany), which included mPBS (marine phosphate-buffered saline) (without FBS) prepared in a dish, by transferring them to three separate droplets each time. The latter process was carried out within 5 min to prevent degradation due to light. The oocytes were then washed with three droplets of 6-Dimethylaminopurine (6-DMAP) (Sigma Co., Tokyo, Japan), which included Medium 199 (with FBS), and incubated in 5 ml of the same reagent for 4 h. Subsequently, the oocytes were transferred to IVD-101 (IFP Co., Yamagata, Japan) and incubated for over 20 h.24 Finally, we observed oocyte division (Fig. 17). To investigate the viability rates before and after the dispensing procedure, we separated the oocytes into two groups, one was experimental group, and the other one was control group. We randomly picked 31 oocytes for the control group. These oocytes have been treated in the same way as the dispensed oocytes for the viability test. Among these 31 oocytes, we observed 22 oocytes division by using microscope. Meanwhile, 32 oocytes were selected from experimental group, 22 oocytes division have been observed by microscope. Therefore, the viability ratios of the oocytes before and after dispensing were 71% and 69%, respectively. This suggests that the dispensing process is non-harmful to oocytes.
Figure 17.
Division of dispensed oocyte in viability test.
CONCLUSIONS
A new scheme that involves the use of microsensors for detection and deceleration with micropillars for oocyte dispensing has been demonstrated. This system, which is specifically used to dispense single oocytes, is composed of a capacitance sensor, a deceleration module, and micropillars for stopping the oocytes. A design and fabrication strategy to increase the success rate of dispensing was also demonstrated. Through simulations and calculations, we characterized a prototype of this system. Furthermore, in preliminary experiments, we confirmed the characteristics of the system components and determined the parameters of microchip design. Subsequently, we demonstrated that by using this newly designed system, the success rate of dispensing oocytes greatly increased to 100%. Finally, through viability tests that compared the viability ratios before and after dispensing, we found that the dispensing process had no adverse effects on oocyte viability.
We believe that this system will be highly useful for cell dispensing and those similar systems, in combination with the on-chip oocyte enucleation process, could usher in a new era of newly improved cloning techniques.
ACKNOWLEDGMENTS
This work was partially supported by SENTAN, JST, the Nagoya University Global COE program for Education and Research of Micro-Nano Mechatronics, and the Japan Society for the Promotion of Science (JSPS).
References
- Hagiwara M., Kawahara T., Yamanishi Y., Masuda T., and Feng L., Lab Chip 11(12), 2049–2054 (2011). 10.1039/c1lc20164f [DOI] [PubMed] [Google Scholar]
- Feng L., Hagiwara M., Ichikawa A., and Arai F., “On-chip enucleation of bovine oocytes using microrobot-assisted flow-speed control,” Micromachines 4(2), 272–285 (2013). 10.3390/mi4020272 [DOI] [Google Scholar]
- Yamanishi Y., Feng L., and Arai F., Adv. Rob. 24(14), 2005–2018 (2010). 10.1163/016918610X529084 [DOI] [Google Scholar]
- Hagiwara M., Kawahara T., Yamanishi Y., and Arai F., Appl. Phys. Lett. 97, 013701 (2010). 10.1063/1.3459040 [DOI] [Google Scholar]
- Yamanishi Y., Sakuma S., Onda K., and Arai F., Biomed. Microdevices 12(4), 745–752 (2010). 10.1007/s10544-010-9428-z [DOI] [PubMed] [Google Scholar]
- Feng L., Hagiwara M., Uvet H., Yamanishi Y., Kawahara T., Kosuge K., and Arai F., in 16th IEEE International Solid-State Sensors, Actuators, and Microsystems Conference (TRANSDUCERS) (IEEE, 2011), pp. 1312–1315.
- Wakayama T., Perry A. C., Zuccotti M., Johnson K. R., and Yanagimachi R., Nature 394(6691), 369–374 (1998). 10.1038/28615 [DOI] [PubMed] [Google Scholar]
- Tatham B. G., Dowsing A. T., and Trounson A. O., Biol. Reprod. 53(5), 1088–1094 (1995). 10.1095/biolreprod53.5.1088 [DOI] [PubMed] [Google Scholar]
- Lindmo T., Peters D. C., and Sweet R. G., Flow Cytometry and Sorting, 2nd ed., edited by Melamed M. R., Lindmo T., and Mendelsohn (Wiley-Liss, New York, 1990). [Google Scholar]
- Dijksman J. F. and Pierik A., Biomicrofluidics 2, 044101 (2008). 10.1063/1.2994715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavana H. and Shuichi T., Biomicrofluidics 5, 013404 (2011). 10.1063/1.3516658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Doh l., and Cho Y., Biomicrofluidics 6, 034107 (2012). 10.1063/1.4739460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth E. A., Xu T., Das M., Gregory C., Hickman J. J., and Boland T., Biomaterials 25(17), 3707–3715 (2004). 10.1016/j.biomaterials.2003.10.052 [DOI] [PubMed] [Google Scholar]
- Calvert P., Science 318(5848), 208–209 (2007). 10.1126/science.1144212 [DOI] [PubMed] [Google Scholar]
- Uvet H., Feng L., Ohashi S., Hagiwara M., Kawahara T., Yamanishi Y., and Arai F., in IEEE International Conference on Robotics and Automation (ICRA) (IEEE, 2011), pp. 3151–3156.
- Yusof A., Keegan H., Spillane C. D., Sheils O. M., Martin C. M., O'Leary J. J., and Koltay P., Lab Chip 11(14), 2447–2454 (2011). 10.1039/c1lc20176j [DOI] [PubMed] [Google Scholar]
- Tornay R., Chapuis V., Haguet V., Chatelain F., and Renaud P., in 14th IEEE International Solid-State Sensors, Actuators, and Microsystems Conference (TRANSDUCERS) (IEEE, 2007), pp. 695–698. [Google Scholar]
- Drobish J. L. and Taske L. E., U.S. patent 4,728,006 (1988).
- Emmert-Buck M. R., Bonner R. F., Smith P. D., Chuaqui R. F., Zhuang Z., Goldstein S. R., Weiss R. A., and Liotta L. A., Science 274(5289), 998–1001 (1996). 10.1126/science.274.5289.998 [DOI] [PubMed] [Google Scholar]
- Kawahara T., Ohashi S., Hagiwara M., Yamanishi Y., and Arai F., Adv. Rob. 26(3–4), 291–306 (2012). 10.1163/156855311X614572 [DOI] [Google Scholar]
- Feng L., Kawahara T., Yamanishi Y., Hagiwara M., Kosuge K., and Arai F., J. Rob. Mechatron. 24(1), 133–140 (2012). [Google Scholar]
- Hochmuth R. M., J. Biomech. 33(1), 15–22 (2000). 10.1016/S0021-9290(99)00175-X [DOI] [PubMed] [Google Scholar]
- Murayama Y., Mizuno J., Kamakura H., Fueta Y., Nakamura H., Akaishi K., Anzai K., Watanabe A., Inui H., and Omata S., Hum. Cell 19(4), 119–125 (2006). 10.1111/j.1749-0774.2006.00019.x [DOI] [PubMed] [Google Scholar]
- Akagi S., Takahashi S., Adachi N., Hasegawa K., Sugawara T., Tozuka Y., and Izaike Y., Cloning Stem Cells 5(2), 101–108 (2003). 10.1089/153623003322234696 [DOI] [PubMed] [Google Scholar]