Abstract
Objective
An important area concerning morbidity among infants with congenital heart defects (CHD) is related to feeding problems. Our objectives were to characterize the evolution of feeding milestones related to transition to per oral feeding among infants with CHD, and to identify associated variables impacting the feeding abilities. Specifically, we differentiated the feeding characteristics in neonates with acyanotic vs cyanotic CHD.
Study Design
Feeding progress was tracked during the first hospitalization in a retrospective chart review study involving 76 infants (29 acyanotic, 47 cyanotic CHD). The ages at which the following milestones attained were recorded: first feeds, maximum gavage feeds, first nipple feeds and maximum nipple feeds, in addition to the length of hospital stay. Effects of perinatal factors, duration of respiratory support, vasopressor and narcotic use and use of cardiopulmonary bypass on the feeding milestones were also evaluated. ANOVA, t-test, and stepwise linear regression analysis were applied as appropriate. Data stated as mean±s.e.m., or %; P<0.05 was considered significant.
Result
Prenatal and birth characteristics were similar (P = NS) between the neonates with acyanotic and cyanotic CHD. Cyanotic CHD required three times prolonged use of ventilation, narcotics and vasopressor use (all P<0.05, compared to the acyanotic group). In the acyanotic group, prolonged respiratory support correlated linearly with time to attain maximal gavage feeds and nippling (both, R2 = 0.8). In the cyanotic group, delayed initiation of gavage feeds and prolonged respiratory support both correlated linearly with time to attain maximal gavage feeds and nippling (both, R2 = 0.8). Age at first gavage feed correlated with maximum gavage feeds among neonates with cyanotic CHD, and first nipple feed correlated with maximum nipple feeds among all groups (P<0.01). Use of cardiopulmonary bypass in cyanotic CHD delayed the feeding milestones and prolonged the length of stay (both, P<0.05 vs non-bypass group); similar findings were not seen in the acyanotic group.
Conclusion
In contrast to neonates with acyanotic CHD, cyanotic CHD group had significant delays with (a) feeding readiness, (b) successful gastric feeding, (c) oromotor readiness and (d) successful oromotor skills. Co-morbid factors that may directly influence the delay in feeding milestones include the (a) duration of respiratory support and (b) use of cardiopulmonary bypass. Delays in achieving maximum gavage and maximum nippling may suggest foregut dysmotility and oropharyngeal dysphagia.
Keywords: feeding problems, congenital heart defects, infant
Introduction
The incidence of congenital heart defects (CHD) is about 8 per 1000 live births, and the defects are generally classified into cyanotic and acyanotic CHD.1 Both these groups have different pathophysiology, medical-surgical needs and also varying morbidity related to nutrition and growth failure.2 Furthermore, recent advances in fetal diagnosis, perinatal care, cardiovascular anesthesiology and surgery in neonates with CHD have improved life expectancy.1,3 However, more infants are now surviving with special needs; these requirements include the need for parenteral nutrition, modified enteral feeding strategies or prolonged respiratory support.2,4,5 Poor nutritional status resulting from inadequate feeding capabilities in neonates with CHD often leads to an imbalance of energy intake, thus resulting in growth failure. Malnutrition is a recognized problem in this patient population, and often affects the subsequent stages of cardiovascular surgery depending on the etiology.6,7 The nature of feeding-related disabilities or the timeline for acquisition of feeding abilities in neonates with CHD is not well understood. Knowledge of these facts will help with counseling and also with improving the feeding practice strategies prospectively.
Swallowing is present in the fetus by 16 weeks gestation, and these swallowing and feeding functions are expected to be functional at birth in full-term healthy neonates.8,9 Therefore, it is also a hope among parents of high-risk infants to expect achievement of feeding skills in their infants. However, in the neonate with CHD, the development of swallowing- and feeding-related difficulties are frequent concerns. This is more so after correction of CHD, and an estimated incidence of dysphagia varies depending on the risk factors including preoperative acuity, duration of intubation, nature of CHD, vocal cord injury, weight characteristics or type of surgical procedures.10–12 For example, laryngopharyngeal dysfunction is reported after Norwood procedure in about 48% of patients, and these effects are characterized as dysphagia, aspiration, left recurrent laryngeal nerve injury.12 Alternatively, feeding difficulties can also be due to respiratory compromise or due to vocal cord paralysis or may follow underlying neurological sequelae.
Our rationale for reporting the characteristics of feeding abilities among acyanotic and cyanotic CHD are obviously due to the developmental heterogeneity of the CHD, and also due to the subsequent variabilities in management strategies as required by their respective pathophysiological needs. For example, the treatment and intensive care support strategies depend on the type and severity of the lesion, as neonates with acyanotic CHD receive fewer interventions than those with cyanotic CHD. This may reflect in the duration of respiratory support, narcotic usage, vasopressor usage, the need for cardiopulmonary bypass or enteral tube feeding strategies. Any or all of these may influence feeding milestones. Therefore, our primary objective was to characterize the feeding milestones in neonates with CHD and identify the associated variables. We tested the hypothesis that neonates with acyanotic CHD achieve feeding milestones earlier than neonates with cyanotic CHD, and evaluated the role of associated factors.
Methods
Subjects
The Institutional Review Board of Children’s Hospital of Wisconsin and the Human Research Review Committee of Medical College of Wisconsin, at Milwaukee, WI, approved this study. Neonates with CHD admitted to the neonatal intensive care unit at Children’s Hospital of Wisconsin from January 1998 to December 1999 were evaluated in a retrospective chart review. Because this study pertains to feeding abilities, data from 76 CHD neonates that have received feeds were evaluated. Infants with chromosomal syndromes were excluded. Although CHD is heterogeneous, for simplicity, CHD was classified into the acyanotic and cyanotic groups. The acyanotic group included infants with aortic stenosis, coarctation of the aorta or atrioventricular canal defect, and received intensive care for aortoplasty, repair of coarctations and septal defects or pulmonary artery banding. The cyanotic group included infants with transposition of the great arteries, tetralogy of Fallot, total anomalous pulmonary venous return or hypoplastic left heart syndrome who underwent procedures such as arterial switch operation, Blalock-Taussig shunt or Norwood procedures.
Feeding practices and measurements
It was the nursery practice that the feeding management was carried on by the attending neonatologist, cardiologist and the ICU team, based on the physiological stability. In the nursery, there was in general a consensus on initiating oral feedings when infants were stable and showed oromotor cues. As this is a retrospective study, data were collected from the patient clinical records as to the timing of feeding initiation, maximal feeds and the feeding methods (oral or gavage). The changes in feeding methods with respect to initiation, gavage, nippling and assisted feeding methods were recorded.
A predesigned form was filled in for each infant by the investigators (SRJ, ASV). Existing data collected included demographics, antenatal care, feeding and hospital course during the newborn hospitalization. Variables such as the duration of ventilation, narcotic use, vasopressor support and the need for cardiopulmonary bypass were recorded. Feeding methods and changes in feeding methods with respect to initiation, gavage, nippling and assisted feeding methods were recorded.
For the purpose of this paper, oromotor readiness was defined as ability to take first oral feed; and successful oromotor skills were defined as an ability to take maximal oral feeds. For the purpose of measuring a fixed milestone in all categories, maximal feeds, whether by gavage or nippling routes was defined as an ability to tolerate a volume of at least 120 ml kg−1 day−1. We recorded the age at which these milestones were attained with respect to first gavage feed, maximum gavage feeds, first nipple feeds and maximal nipple feeds. The records were tracked until the end point, which was discharge or death, whichever occurred earlier.
Statistical analysis
One-way ANOVA with Tukey/Kramer post hoc tests and unpaired t-tests analyzed inter-group differences within the outcome variables. P<0.05 was considered significant. Data are presented as mean±s.e.m. or percentages (%). Data on feeding milestones are reported along with the number of infants with each statistical comparison. Multivariate analysis concerning feeding milestones with respect to initial feeding milestones and confounding variables utilized stepwise linear regression to examine the relationship between the variables. Variables that were highly inter-correlated (R2>0.80) were excluded because of colinearity.13,14 Resultant data are presented as correlation coefficients (β) ±standard errors and regression values (adjusted R2 values). Statistical analysis used StatView for Windows software (StatView 5.0.1, SAS Institute, Cary, NC, USA).
Results
Demographic characteristics and outcomes
The demographic characteristics were separated out under acyanotic and cyanotic CHD categories (Table 1). Out of 76 infants with CHD, survival to discharge was 100% among neonates with acyanotic CHD (n = 29), and 85% in the cyanotic CHD group (n = 40/47). Among the neonatal survivors, 93% in both the acyanotic CHD (n = 27/29) and cyanotic CHD (n = 37/40) achieved maximal nipple feeds at discharge. The remaining survivors required gavage feeds at discharge. The length of hospital stay was 12.2±1.4 vs 24±1.7 days for the neonates with acyanotic vs cyanotic CHD, respectively (P<0.0001). The duration from first feed to discharge was 7±1 vs 17.5±2.1 days for the neonates with acyanotic vs cyanotic group, respectively, (P<0.01). The postmenstrual age at discharge was 41.4±0.4 vs 42.3±0.4 weeks for the neonates with acyanotic vs cyanotic group, respectively (P = NS).
Table 1.
Demographic characteristics
| Characteristics | Acyanotic CHD (n = 29) | Cyanotic CHD (n = 47) |
|---|---|---|
| Birth weight, kg, (range) | 3.4±0.1 (1.2–5.1) | 3.2±0.1 (1.5–4.8) |
| Gestational age, week, (range) | 39±0.4 (31–43) | 38±0.3 (30–41) |
| Median APGAR score at 1 min, (range) | 8 (5–9) | 8 (3–9) |
| Median APGAR score at 5 min, (range) | 9 (8–10) | 9 (5–9) |
| Cardiovascular surgery, #(%) | 18 (62) | 46 (98)a |
| Day of life, at surgery | 7.7±1.4 | 7.4±0.7 |
Abbrevation: CHD, congenital heart defects.
Values shown as mean±s.e.m., unless otherwise noted.
One infant expired before surgery.
Number.
All seven infants with cyanotic CHD who died had feeding experiences at several stages. Four died before reaching full gavage feeds, two died after achieving full gavage feeds but before reaching first nipple feed and one died after reaching maximal nipple feeds.
Early morbidity characteristics
Twenty-two of the 29 (76%) acyanotic and 46 out of 47 (98%) cyanotic neonates required ventilation (P<0.002, X2 = 9) for 6.2±1 vs 13.6±1.8 days, respectively (P<0.01). Eleven acyanotic and 43 cyanotic neonates received narcotics (P<0.0001, X2 = 25) for 7.2±1.5 vs 12±1.2 days, respectively (P = 0.07). Eleven acyanotic and 44 cyanotic neonates (P<0.0001, X2 = 28) required vasopressor support for 4.7±1.2 vs 7.6±1.1 days, respectively (P = NS). Ten acyanotic and 38 cyanotic neonates required cardiopulmonary bypass (P<0.0001, X2 = 17). One acyanotic (3.4%) and nine cyanotic (19%) neonates (all requiring cardiopulmonary bypass) had an intracranial abnormality (intraventricular or intraparenchymal bleeding and infarcts, P<0.05, X2 = 4); among whom four died (44%), one (11%) was discharged on gavage feeds and four (44%) achieved maximal nipple feeds.
Characteristics of feeding capabilities
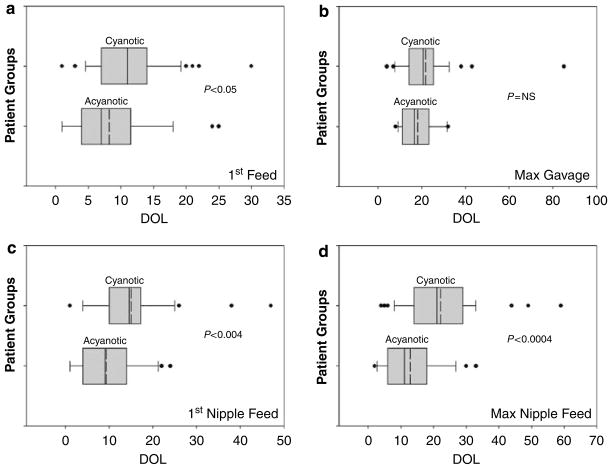
Timing of initiation and maximal gavage feeds are described in Figures 1a and b, respectively; and acquisition of initial and maximal nipple feeding skills are described in Figures 1c and d, respectively. The maturational ages (postmenstrual age) at which the timing of first feed postoperatively were compared between acyanotic and cyanotic neonates and were equivalent (40.4±0.4 vs 40±0.3 weeks, respectively, P = NS).
Figure 1.
Box plots showing age when 10, 25, 50, 75 and 90 percentiles were achieved in each group for the studied milestones. Mean value is depicted as dotted line within the box. Only those that have achieved the milestones are included, hence individual infant numbers (n) vary. (a) Shows age at first feed among acyanotic (n = 29), and cyanotic (n = 47). (b) Shows age at maximum gavage feeding among all acyanotic (n = 29) and 34 cyanotic infants. Among cyanotic group, nine infants did not need gavage feeding, whereas four died before reaching maximum gavage feeding. (c) Shows age at first nipple feed among acyanotic (n = 27, 2 did not nipple feed), and cyanotic (n = 41, six died before beginning nippling). (d) Shows age at maximum nipple feeds among acyanotic (n = 27), cyanotic (n = 37, as seven died and three needed tube feeds).
First gavage feeds occurred on 8.2±1.2 days in the neonates with acyanotic CHD, while not started until 11.0±0.9 days in the cyanotic group (P<0.05). Fourteen out of 29 acyanotic CHD infants attained maximum gavage feeding ability by 18.1±2.0 days. The other 15 advanced to maximal nipple feeds before requiring maximum gavage feeding. In the cyanotic group, 34 out of 47 infants achieved the maximal gavage milestone by 21.6±2.4 days. Four infants died before reaching maximum gavage feeds, and nine others advanced to maximal nipple feeding, never requiring maximal gavage feeds.
Among those acyanotic infants who had some nippling experience, 27 out of 29 acyanotic infants began first nipple feeds by 9.3±1.3 days, only 2 days after starting gavage feedings. The other two infants never developed nippling skills. In contrast, 41 out of 47 cyanotic infants began first nipple feeds at 15±1.4 days (P<0.004 vs acyanotic CHD). The other six infants died receiving gavage feeds but before nippling were ever attempted.
The acyanotic group (n = 27) advanced to maximal nipple feeds by 12.9±1.6 days; this milestone was achieved sooner as 15 out of 27 infants did not need gavage as a route to support maximal feeding volume. Out of the 41 cyanotic CHD neonates, 37 achieved maximal nipple feeds by 22.1±1.9 days (P<0.0004 vs acyanotic CHD). Of the remaining four neonates in the cyanotic group, three never achieved independent nippling skills and one died before discharge.
Relationship between acute morbidity parameters and feeding milestones
We examined the relationship of comorbidity variables (the duration of vasopressor use, respiratory support, narcotic use) on two major feeding goals (maximal gavage and maximal nippling). Specifically, in both groups, correlation between independent variables (DOL (day of life) first gavage feed, DOL first nipple feed) and dependent variables (DOL at maximum gavage feeds, DOL at maximum nipple feeds) was sought using stepwise linear regression analysis. Correlation coefficients and regression values are described below as β±s.e.m. and adjusted R2. In the acyanotic group, age at first gavage feed did not correlate with age at maximum gavage feeds; however, the respiratory support duration influenced the age at which maximum gavage was achieved (β±s.e.m. = 1.1±0.2, R2 = 0.82). Similarly, the age at first nipple feed did not correlate with age at maximum nipple feeds; however, the respiratory support duration influenced the age at which maximum nipple feeds was achieved (β±s.e.m. = 1.2±0.2, R2 = 0.82). The duration of vasopressor or narcotic use had no influence on feeding milestones.
In the cyanotic group, age at first gavage feed (β±s.e.m. = 0.9±0.2) and the duration of respiratory support (β±s.e.m. = 0.8±0.1) influenced the DOL at maximum gavage feeds (R2 = 0.777), while duration of vasopressor or narcotic use did not influence. DOL at first nipple feeds (β±s.e.m. = 1.1±0.1) and the duration of respiratory support (β±s.e.m. = 0.5±0.1) influenced strongly with the age at maximum nipple feeds (R2 = 0.81). Again, duration of vasopressor or narcotic use had no influence.
Impact of bypass vs non-bypass on feeding milestones
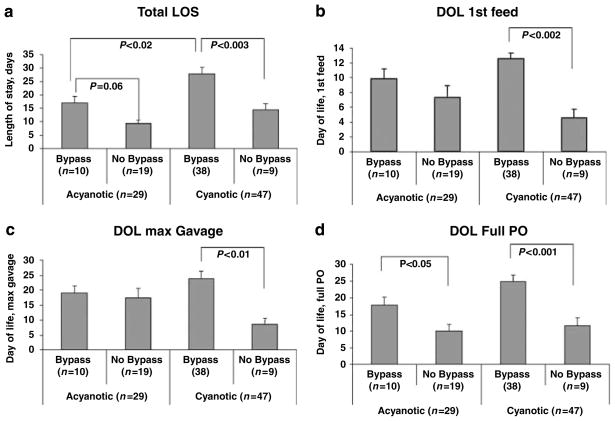
Outcomes for infants who underwent cardiopulmonary bypass were compared with those without the need for bypass within the cyanotic and acyanotic CHD groups (Figure 2). Within the acyanotic group, infants requiring bypass had a delay in achieving maximal nipple feedings (P<0.05, vs non-bypass group). Within the cyanotic group, those requiring bypass had significant delays in length of stay, first feeds, maximum gavage feeds and maximal nipple feeds (all P<0.05, vs non-bypass group).
Figure 2.
Comparison of length of stay and feeding milestones among infants who underwent cardiopulmonary bypass vs those who did not. Significant comparisons (mean±s.e.m.) with their non-bypass counterparts are shown. (a) Describes length of stay, (b) describes day of life (DOL) first feed, (c) describes age at maximum gavage feeds and (d) describes age at maximum nipple feeds.
Comparison of feeding milestones in neonates with hypoplastic left heart syndrome vs rest of the cyanotic CHD group
Some patients with single ventricle palliations may have metabolic stressors that beget poor feeding. Therefore, in a subset analysis, we tested if patients with hypoplastic left heart syndrome (n = 17) have differences in feeding capabilities vs rest of the cohort within the category of cyanotic CHD (n = 30). Between the groups, there were no differences in the following comorbidity variables (all P = NS): gestational age at birth, duration of ventilation, narcotic use or the length of hospital stay. The feeding milestones among hypoplastic left heart syndrome vs rest of the cyanotic CHD group, respectively are as follows: (a) days to first feed, 11.7±1.0 vs 10.6±1.2 days (P = NS), (b) days to maximum gavage, 20.7±1.4 vs 22.1±3.8 days (P = NS), (c) days to first nipple feeds, 16.9±2.3 vs 13.7±1.7 days (P = NS), (d) days to maximal nipple feeds, 24.9±3.0 vs 20.3±2.4 days (P = NS).
Discussion
A major conundrum of poor nutritional state in hospitalized infants with CHD is inadequate enteral intake, gastrointestinal morbidity and feeding problems.15 This is a significant cause of morbidity and affects growth and recovery, influencing short- and long-term outcomes.2,16 The optimal methods of enteral feeding or the timelines for the achievement of specific enteral feeding milestones have not been characterized in a representative cohort of neonates with CHD. In this retrospective study, we compared the impact of acyanotic and cyanotic CHD in neonates on the acquisition of enteral feeding milestones. Confirming our hypothesis, the neonates with cyanotic CHD group had (1) significant delays in time to initiate and achieve maximal gavage feeds, (2) significant delays in initiation and achieving maximal nipple feeds and (3) prolonged lengths of hospital stay. The encouraging outcome is the high percentage of infants in both groups who were able to eventually achieve maximal nipple feedings for discharge.
We examined the comorbid factors presumed to influence feeding outcomes in this study. The need for respiratory support or of its prolonged use and the prolonged duration of narcotic and vasopressor support have been proposed to alter gut motility or perfusion or airway protection mechanisms to permit safe feeding strategies.17–21 The only confounding variable that had an impact on feeding milestones was respiratory support, and this was consistent in both groups. The duration of respiratory support correlated strongly with feeding outcomes. Although it is not surprising that this variable should affect the time to initiate nipple feeds, it was also a factor in delayed acquisition of maximal gavage feeds and maximal nippling skills.
There are initial concerns about feeding infants with CHD before surgery, including the potential injury mechanisms to the intestines or cardiorespiratory instability. But what leads to the delay in achieving goals postoperatively in these infants is not well understood. There are essentially three organ systems that can be explored in determining this. The most obvious is the cardiorespiratory system. Infants at risk of significant congestive heart failure even with surgery, such as the hypoplastic left heart syndrome, may be simply breathing too fast for safe oral feedings or they may require fluid restrictions and high calorie food for their increased metabolic rate.18 Respiratory compromise and the negative effect on oral feeding can sometimes occur; however, most infants in the cohort were able to transition without showing any respiratory compromise associated with oral feeding. Among those who never achieved full oral feeds, the duration of prolonged respiratory support, the use of bypass and neurological concerns were contributory to limitations in oromotor feeding skills.
The second system is the gastrointestinal system. For example, gastrointestinal functions may be impaired due to the presence of sluggish reflexes, decreased sensory input, hypoxemia, neurological insults or an ischemic injury to bowel, and therefore gut function may be poor, thus contributing to the morbidity risks.16–21 Patients undergoing hypothermic cardiopulmonary bypass often have hypotension, and the intestine becomes hypoperfused, thereby affecting absorption and increased gut permeability.19 Waiting for bowel recovery and function may be a reason to delay initiation, and may be a reason for further delay in achieving maximal gavage feedings. Patients requiring longer time on the ventilator and those undergoing bypass may have less availability for feeding exposure, and therefore a delayed transition to oral feeding can be expected. In this study, we found a significant delay in both these milestones (first feeds and maximal gavage feeds) among the neonates with cyanotic CHD who have undergone bypass, but not in the acyanotic group. However, those undergoing bypass in both the disease groups had significant delays in achieving maximal nippling skills.
Finally, neonates with CHD are at increased risk for neurological sequelae. Infants with CHD are at risk of central nervous system injury, such as bleeds or strokes, which we saw in our study. Indeed, feeding offers one of the first windows into central nervous system functioning; but most infants with CHD are known to have developmental disabilities that may be presenting with delayed feeds. The causes of these are speculated to theoretical risks of developmental abnormalities, to hypoxia or abnormal flow in utero or to events in the first months of life. Such neurological vulnerability could be from hypoxic or hypoperfused states or from the consequences of anesthetic and surgical manipulation, including recurrent laryngeal or vagal nerve neuropathies and laryngopharyngeal dysfunction.12,22,23 In the presence of inadequate oral skills, maintenance of appropriate growth is commonly achieved by the placement of long-term tube feeding strategies such as gastrostomy.24 However, the scientific rationale for feeding management or the central and peripheral mechanisms of dysphagia, or the timing of chronic tube feeding strategies is not clearly understood. Therefore, the development and acquisition of safe oral or enteral feeding milestones can be challenging; all such delays thus contribute to an increase in total length of stay. In this study, the total length of stay was significantly delayed, more so in the cyanotic group and the bypass group. Systematic prospective studies are needed to evaluate the mechanisms of airway and feeding dysfunction in vulnerable populations of interest.
Furthermore, the economic burden can be estimated to be greater due to delayed initiation and maintenance of feeds or in the presence of the associated covariates, such as durations of respiratory support, narcotic use, vasopressor support and cardiopulmonary bypass. Any or all of these factors may have influenced the outcome measures and feeding milestones in this study. This information is helpful for prognosis, feeding guidelines, estimation of length of stay, parental counseling and for prospective clinical trials. It is also valuable baseline information to see what clinical initiatives can improve earlier feeding and discharge home.25 For example, a trial of nipple feedings preoperatively in the stable infant could imprint swallowing skills related memory;26 as it has been recently shown that behavioral sensitization reflecting neuronal plasticity in the early developmental period may be a prelude to enhancement of expression of such feeding behaviors in the recovery phase of illness.27 On the other hand, the return of gastrointestinal functions under normal circumstances may therefore depend on the integrity of central and enteric neural functions.16,17,19,21 Thus, all these factors assume importance, as difficulty with feeding behaviors has been considered a sensitive indicator of central nervous system integrity in neonates, and preceded the diagnosis of delayed neurological development and cerebral palsy.15,24 For example, a normal healthy full-term infant is expected to have regular normal nipple feeding cycles. However, presence of heart disease at birth poses an increased need for alternate or assisted feeding methods.
Similar to any retrospective design, this study has certain limitations with respect to the following: (1) heterogeneity of CHD or severity of illness at admission may have resulted in the variability observed. Accepted scoring criteria for SNAP, CRIB, PRISM were not available from the retrospective reviews of charts. Such a determination would have controlled for the severity of CHD. (2) Infants were classified broadly as acyanotic and cyanotic groups, but individual variability within the conditions or variability in therapeutic interventions or effect of medications or variable severity of their illness may have resulted in the outcomes. (3) Feeding management was based on clinical evaluation and cues. One can assume a subjective bias; however, study from a single center with similar management approaches may minimize treatment bias. Instrumental swallowing exams or a structured swallowing skill assessment were not part of the routine care in all patients. (4) Premature infants with CHD were included in this study. Prematurity may have independent implications with regard to feeding. (5) Although this is a retrospective study of several years ago, the concepts and practices stay, and survival may have increased, so has the morbidity. All the above facts may be considered in future outcome-related studies.
In summary, we described the feeding milestones and effects of common variables on the short-term outcomes of neonates with CHD. This study focused on the descriptors of foregut functionality and feeding behaviors among infants with isolated CHD. Unlike measures of survival or length of stay as primary outcomes, feeding milestones may be included as a yardstick for comparison of feeding-related morbidity in prospective clinical trials. Feeding abilities provide indicators for integrated coordination of swallowing and aerodigestive reflexes, laryngo-pharyngeal functions and foregut peristaltic functions.28–30 In future studies, evaluation of aerodigestive protective reflexes and swallowing functions among those infants who do not have feeding capabilities may provide clues to the mechanisms of feeding dysfunction.31 Such approaches may lead towards the development of structured feeding strategies promoting better feeding skills. In addition, the data and comorbidity variables presented in this study may be appropriate to evaluate their effects on the outcomes.
Acknowledgments
We express our gratitude to Dr Raymond G Hoffmann, PhD, Department of Biostatistics, Medical College of Wisconsin, for advice with the statistical analysis; Dr P Sasidharan, MD, Section of Neonatology, and Dr Stu Berger, MD, Section of Cardiology, in the Department of Pediatrics, Medical College of Wisconsin, for their valuable advice, guidance and support for this initiative. Dr Jadcherla was supported in part by NIH Grant ROI DK 068158.
Footnotes
Location of work: Children’s Hospital of Wisconsin, Milwaukee, WI 53226, USA.
Conflict of interest
None.
References
- 1.www.americanheart.org.
- 2.Varan B, Tokel K, Yilmaz G. Malnutrition and growth failure in cyanotic and acyanotic congenital heart disease with and without pulmonary hypertension. Arch Dis Child. 1999;81:49–52. doi: 10.1136/adc.81.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kliegman RM. Neonatal technology, perinatal survival, social consequences, and the perinatal paradox. Am J of Public Health. 1995;85:909–913. doi: 10.2105/ajph.85.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Author: American Academy of Pediatrics, Committee on Children with Disabilities. Managed care and children with special health care needs. Pediatrics. 1998;102(3):657–660. doi: 10.1542/peds.102.3.657. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics, Committee on Fetus and Newborn. Hospital discharge of the high risk neonate: proposed guidelines. Pediatrics. 1998;102(2):411–417. [PubMed] [Google Scholar]
- 6.Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome after stage I Norwood procedure. Nutrition. 2006;22:237–244. doi: 10.1016/j.nut.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Sloper P, Turner S. Risk and resistance factors in the adaptation of parents of children with severe physical disability. J Child Psychol Pyschiatry. 1993;34:167–188. doi: 10.1111/j.1469-7610.1993.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 8.Sadler TW. Special embryology: Respiratory system and Digestive system. In: Sadler TW, editor. Langman’s medical embryology. 7. Williams and Wilkins; Baltimore, MD: 1995. pp. 232–271. [Google Scholar]
- 9.Bu’Lock F, Woolridge MW, Bairn JD. Development of coordination of sucking, swallowing, and breathing: ultrasound study of term and preterm infants. Dev Med Child Neurol. 1990;32:669–678. doi: 10.1111/j.1469-8749.1990.tb08427.x. [DOI] [PubMed] [Google Scholar]
- 10.Einarson KD, Arthur HM. Predictors of oral feeding difficulty in cardiac surgical infants. Pediatr Nurs. 2003;29:315–319. [PubMed] [Google Scholar]
- 11.Kogon BE, Ramaswamy V, Todd K, Plattner C, Kirshbom PM, Kanter KR, et al. Feeding difficulty in newborns following congenital heart surgery. Congenital Heart Dis. 2007;2:332–337. doi: 10.1111/j.1747-0803.2007.00121.x. [DOI] [PubMed] [Google Scholar]
- 12.Skinner ML, Halstead LA, Rubinstein CS, Atz AM, Andrews D, Bradley SM. Laryngopharyngeal dysfunction after Norwood procedure. J Thoracic Cardiovasc Surg. 2005;130:1293–1301. doi: 10.1016/j.jtcvs.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Armitage P, Berry G. Statistical methods in medical research. Blackwell Scientific Publications; Oxford: 1987. [Google Scholar]
- 14.Snedecor GW, Cochrann WG. Statistical methods. 7. The Iowa State University Press; Ames, Iowa: 1980. [Google Scholar]
- 15.Cameron JW, Rosenthal A, Olson AD. Malnutrition in hospitalized children with congenital heart disease. Arch Pediatr Adolesc Med. 1995;149:1098–1102. doi: 10.1001/archpedi.1995.02170230052007. [DOI] [PubMed] [Google Scholar]
- 16.Jeffries HE, Wells WJ, Starnes VA, Wetzel RC, Moromisato DY. Gastrointestinal morbidity after Norwood palliation for hypoplastic left heart syndrome. Ann Thorac Surg. 2006;81 (3):982–987. doi: 10.1016/j.athoracsur.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Glass P, Miller M, Short B. Morbidity for survivors of extra corporeal membrane oxygenation: neurodevelopmental outcome at 1 year of age. Pediatrics. 1989;83:72–78. [PubMed] [Google Scholar]
- 18.Pillo-Blocka F, Adatia I, Sharieff W, McCrindle BW, Zlotkin S. Rapid advancement to more concentrated formula in infants after surgery for congenital heart disease reduces duration of hospital stay: a randomized clinical trial. J Pediatr. 2004;145:761–766. doi: 10.1016/j.jpeds.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 19.Ohri SK, Somasundaram S, Koak Y, Macpherson A, Keogh BE, Taylor KM, et al. The effect of intestinal hypoperfusion on intestinal absorption and permeability during cardiopulmonary bypass. Gastroenterology. 1994;106:318–323. doi: 10.1016/0016-5085(94)90588-6. [DOI] [PubMed] [Google Scholar]
- 20.Jolley SG, McClelland KK, Mosesso-Ronssaeau M. Pharyngeal and swallowing disorders in infants. Semin Pediatr Surg. 1995;4(3):157–165. [PubMed] [Google Scholar]
- 21.Jadcherla SR, Berseth CL. Antroduodenal motility and feeding outcomes among neonatal extra corporeal membrane oxygenation survivors. J Pediatr Gastroenterol Nutr. 2005;41:347–350. doi: 10.1097/01.mpg.0000174331.00711.6d. [DOI] [PubMed] [Google Scholar]
- 22.Sachdeva R, Hussain E, Moss MM, Schmitz ML, Ray RM, Imamura M, et al. Vocal cord dysfunction and feeding difficulties after pediatric cardiovascular surgery. J Pediatr. 2007;151:312–315. doi: 10.1016/j.jpeds.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Berseth CL, McCoy HH. Birth asphyxia alters neonatal intestinal motility in term neonates. Pediatrics. 1992;90(5):669–673. [PubMed] [Google Scholar]
- 24.Hofner G, Behrens R, Koch A, Singer H, Hofbeck M. Enteral nutritional support by percutaneous endoscopic gastrostomy in children with congenital heart disease. Pediatr Cardiol. 2000;21:341–346. doi: 10.1007/s002460010076. [DOI] [PubMed] [Google Scholar]
- 25.Imms C. Feeding the infant with congenital heart disease: an occupational performance challenge. Am J Occup Ther. 2001;55(3):277–284. doi: 10.5014/ajot.55.3.277. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman HS, Stratton JW, Newby V. The control of feeding behavior by an imprinted stimulus. J Exp Anal Behav. 1969;12(6):847–860. doi: 10.1901/jeab.1969.12-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27(11):2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jadcherla SR, Gupta A, Stoner E, Fernandez S, Shaker R. Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. J Pediatr. 2007;151:597–603. doi: 10.1016/j.jpeds.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation on the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatr. 2006;141(1):77–82. doi: 10.1016/j.jpeds.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: a novel reflex elicited with concurrent manometry and ultrasonography. Am J Gastroenterol. 2007;102:2286–2293. doi: 10.1111/j.1572-0241.2007.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jadcherla SR, Stoner E, Gupta A, Bates DG, Fernandez S, Di Lorenzo C, et al. Evaluation and management of neonatal dysphagia: impact of pharyngo-esophageal motility studies and multidisciplinary feeding strategy. JPGN. 2008 doi: 10.1097/MPG.0b013e3181752ce7. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]