Abstract
Objective
To assess whether oral ketamine aids is is safe at higher dosages for sedating children and whether it may be an option for control of chronic pain in children. Study design A prospective study was performed on 12 children with chronic pain to identify the maximum tolerated dosage of oral ketamine. Participants were given 14 days of oral ketamine, three times daily, at dosages ranging from 0.25–1.5 mg/kg/dose. Participants were assessed for toxicity and for pain severity at baseline and on day 14 of treatment.
Results
Two participants, both treated at 1.5 mg/kg/dose, experienced dose-limiting toxicities (sedation and anorexia). One participant, treated at 1 mg/kg/dose, opted to stop ketamine treatment due to new pain on treatment. Nine participants completed their course of ketamine treatment. Of these 12 children, 5 experienced improvement in their pain scores, two with complete resolution of pain, lasting for more than 4 weeks off ketamine treatment.
Conclusion
Oral ketamine at dosages of 0.25–1 mg/kg/dose appears to be safe when given for 14 days to children with chronic pain.
Chronic and recurrent pain are common in children[1, 2]. Although traditional pain medications such as acetaminophen, non-steroidal anti-inflammatories, and morphine are regarded as safe and effective for pain control in children, few approved alternative therapies are available when these medications are insufficient.
Ketamine was first described in 1962[3] and has many mechanisms of action. It is an NMDA-R (N-methyl-D-aspartate-receptor) antagonist[4], a mu opioid receptor agonist[5], an inducer of nitric oxide release[6], and it interacts with multiple ion channels[7]. It has dissociative amnestic and analgesic effects[3, 8]. Ketamine is particularly successful as a dissociative amnestic for children in emergency settings as it has little respiratory or cardiac impact, a short half-life, and fewer psychotomimetic effects in children than in adults[3]. However, anesthesia is not the only use of ketamine. It has been evaluated as an analgesic for patients with chronic pain that is not resolved with narcotics and/or adjuvant medications. There are a number of case reports and small case series that suggest that ketamine is a useful medication for control of chronic pain in adults[9–12]. Additionally, there are case studies that describe lasting (12 week) pain control in adults after 4–10 days of ketamine treatment[13, 14]. To date, however, there are few data that aid a pediatrician in determining if ketamine is a safe and effective option for children with chronic pain.
There are few proven safe and effective medications for use in chronic pain in children. Ketamine has a well-understood safety profile when given intravenously and for short periods of time. An important question that remains to be answered is whether ketamine is a safe option for chronic use in children, whose brains are immature and whose metabolism is different compared with adults. The major aims of this study were to describe the short-term safety profile and explore the maximum tolerated dosage of oral ketamine in children with chronic pain.
Methods
This study was approved by the Research Subjects Review Board of the University of Rochester. It was registered on ClinicalTrials.gov before the first participant was enrolled (NCT01369680). The study conformed to the Food and Drug Administration (FDA) guidelines for human subjects protection (IND#110,951).
Participants were recruited by multiple subspecialists, including palliative care, orthopedic surgery, and rheumatology. An informed consent process was completed for all. This consisted of signed informed consent for participants 18 years of age or older, and a joint signed parental permission and verbal or signed child assent for those younger than 18 years. Children were eligible for enrollment if they were aged 8–21 years, had pain for > 3 months[1], were able to cooperate with neuro-cognitive testing, and had an average Numerical Rating Scale (NRS) pain score[15] greater than 4 (NRS scale anchored at 0 and 10) in the past 24 hours. Exclusion criteria consisted of a positive history of known or suspected drug dependence or addiction, a diagnosis of depression, schizophrenia, or bipolar disorder, a history of hypertension, chronic pain related to chronic abdominal pain syndrome, known liver disease (or AST or ALT greater than 3 times the upper limit of normal on screening), a previous intolerance or allergic reaction to ketamine, exposure to CYP3A4 inhibitors or inducers (including grapefruit products) within 2 weeks prior to enrollment, the inability to avoid these products during ketamine administration, or pregnancy. All menstruating females had to have a negative urine pregnancy test before baseline and had to take appropriate precautions not to become pregnant (e.g., abstinence or hormonal contraceptives) while on ketamine.
This single-institution phase 1 trial was designed to estimate the maximum tolerated dosage of oral ketamine in children with chronic pain. Oral ketamine was compounded and made available by investigational drug services of the University of Rochester. Intravenous ketamine was diluted to a concentration of 10 mg/mL in sterile water[16]. This bitter solution was added to ½-1 ounce of soda or orange juice (participant's preference) [17]. Participants were enrolled in three-person cohorts. Each cohort completed ketamine treatment and adverse event assessments before the next cohort could be enrolled. Participants were instructed to continue their current pain regimen and to record any changes in pain medication administration, which were allowed to avoid an unethical restriction of pain control in a vulnerable participant population[1].
The study used a modified continual reassessment method (CRM) trial design with a total enrollment of 12 participants. Such designs were first described by O'Quigley et al[18] and were modified by Goodman et al[19] to enhance their safety. In this study, the first dosage to be given was 0.25 mg/kg TID with possible dosages of 0.5, 1.0, and 1.5 mg/kg TID in subsequent cohorts (no dosage skipping was allowed when escalating the dosage). The dosage for the subsequent cohort depended on the numbers of dose limiting toxicities (defined below) observed in previous cohorts and was determined by the CRM[19]. The maximum tolerated dosage was defined as the dosage above which at least 30% of the subjects would experience a dose limiting toxicity. The sample size of 12 subjects was determined based on feasibility constraints rather than statistical considerations.
Each participant received ketamine orally three times a day for 12 consecutive days (days 0–11) at the dosage established for the cohort. The following 2 days (days 12–13), participants were given 50% of their original dosage of ketamine to minimize withdrawal. Ketamine was discontinued on Day 14. Participants were evaluated at baseline (the first day of ketamine treatment), Day 7, and Day 14 for rating of Numerical Rating Scale (NRS) pain scores, Douleur Neuropathique 4 questions (DN4), an assessment of neuropathic pain[20], report of adverse events, and a physical exam. Participants were instructed to report their NRS score “right now”[21] and relevant symptoms for the past 24 hours for the DN4 score during clinic evaluations. Participants were also contacted twice weekly while they were on ketamine for telephone report of adverse events and medication compliance.
Participants were also asked to consent separately for blood draws after 1 week of oral ketamine administration for pharmacokinetics. These participants underwent ketamine and norketamine blood draws at 0, 15, 30, 60, 150, and 300 minutes after the first oral dose on Day 7 of ketamine therapy.
Evaluation of Outcomes
The primary outcome measure was the occurrence of a dose-limiting toxicity (DLT). DLTs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0[22, 23] by the principal investigator and discussed with the study clinical monitor. Any adverse event with a grade ≥ 3 was considered a DLT. Additionally, the following grade 2 adverse events were considered to be dose limiting toxicities: hallucinations, delirium, confusion, mania, anxiety, amnesia, insomnia, agitation, dizziness, depressed level of consciousness, hypertension (adjusted for age), nausea, and anorexia.
The secondary outcome measures were derived from pharmacokinetic testing. Ketamine and norketamine levels were analyzed in plasma with the first morning ketamine dose. The antecedent bedtime dose was a minimum of 8 hours prior to the morning blood measurements. Blood was collected in EDTA tubes, and plasma was sent at ambient temperature to ARUP (Salt Lake City, Utah) for pharmacokinetics. These specimens are stable at ambient temperature for 1 week prior to testing. Ketamine and norketamine levels were measured by mass spectroscopy with a lower limit of detection of 20 ng/dL. Non-compartmental pharmacokinetic analysis was performed using the linear trapezoidal rule to estimate the area under the plasma concentration-time curve (AUC). The maximum (Cmax) and minimum (Cmin) concentrations were also calculated. A time 0 draw (the pre-dose plasma sample) was planned to estimate Cmin in order to minimize hospitalization time for participants (from 8 hours to 5 hours).
The final outcome measure was the change from baseline to day 14 in the NRS pain score, which was analyzed descriptively.
Results
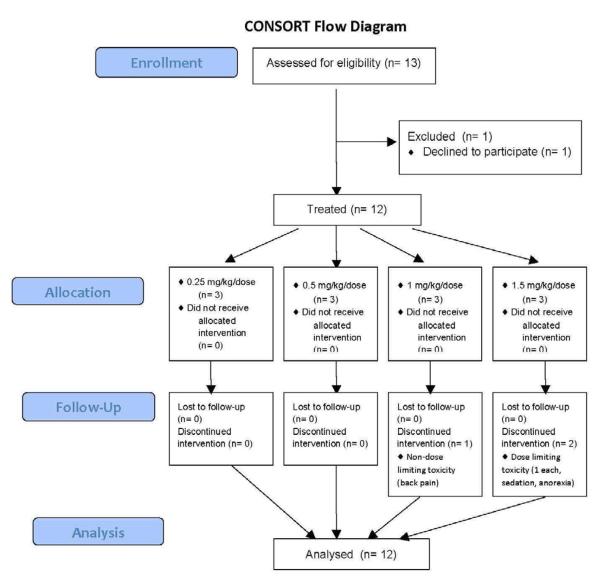
Twelve participants were enrolled (Figure 1; available at www.jpeds.com); demographic and clinical characteristics are described in Table I. Pain diagnoses included chronic pancreatitis, Crohn's disease, esophageal spasm, scoliosis, spina bifida, headache (n=2), hypermobility, joint pain not otherwise specified (NOS) (n=2), bone pain NOS, and amplified musculoskeletal pain syndrome. One participant who was originally diagnosed with headache and joint pain NOS was later diagnosed with complex regional pain syndrome. All participants were evaluable at the end of their ketamine treatment for assessment of adverse events and pain response.
Figure 1.
CONSORT diagram of participants approached and treated.
Table 1.
Demographics of all participants at baseline.
| Age (years) | |
| Range | 11–19 |
| Median | 16 |
| Sex | |
| Female | 9 |
| Male | 3 |
| Race | |
| African-American | 1 |
| White | 10 |
| More than One Race | 1 (White and African-American) |
| Ethnicity | |
| Hispanic | 2 |
| Not Hispanic | 10 |
| Site of Pain | |
| Gastrointestinal pain | 3 |
| Joint/musculoskeletal pain | 7† |
| Headache | 3† |
| Skin | 1 |
| Other therapies | |
| Opioid therapy | 1 |
| ≥ 2 previous non-opioid therapies | 12 |
Some participants had multiple pain sites.
Toxicity
All participants were administered their first dose of oral ketamine under observation for a minimum of 4 hours. Participants did not experience respiratory depression, hypoxemia, alterations in heart rate, or alterations in blood pressure during the inpatient observation. Many participants did experience fatigue or somnolence after their first dose of oral ketamine, which resolved in all cases within 1–2 hours. All participants denied experiencing similar fatigue after subsequent administrations of ketamine. Delayed sedation was not reported by any participant, though delayed dysphoria (e.g., confusion, dizziness) was experienced by 7 of 12 participants.
The first 3 dosage cohorts (0.25, 0.5, and 1 mg/kg/dose) were treated without experiencing any DLTs. Two participants, however, in the final dosage cohort of 1.5 mg/kg/dose (participants 1010 and 1012; Table II) did experience DLTs (anorexia and sedation). Participant 1012 also experienced grade 2 dysuria, though without hematuria. This was concerning due to the possibility of dysuria preceding the development of hemorrhagic cystitis due to ketamine exposure. The dysuria resolved within 2 days of ketamine discontinuation. Both of these participants were wheelchair bound. One other participant who was wheelchair bound was in the first dosage cohort (0.25 mg/kg/dose) and did not experience a DLT. Adverse events were generally mild and are shown in Table II. Participant 1003 had both spina bifida and cystic fibrosis and was treated after a vesicostomy, which later became infected. In the weeks after ketamine treatment, though still during study observations, the participant experienced 2 more infections (Table II). These infections were not considered related to treatment due to the long period of time (11 weeks) between infection and treatment.
Table 2.
Adverse events by participant with dosage levels. All adverse events were coded according to the CTCAEv.4.0.
| Participant | Age (years) | Gender | Ketamine Dosage (mg/kg/dose) | Adverse Events | Pain Diagnosis at Enrollment | Pain Medications at Enrollment |
|---|---|---|---|---|---|---|
|
| ||||||
| Cohort 1 | ||||||
|
| ||||||
| 1001 | 19 | F | 0.25 | Grade 1 confusion | Chronic pancreatitis | Morphine |
| Grade 2 chills – narcotic withdrawal | ||||||
|
| ||||||
| 1002 | 11 | F | 0.25 | None | Bone pain NOS | Ibuprofen, naproxen, acetaminophen |
|
| ||||||
| 1003 | 19 | F | 0.25 | Grade 1 infection – post-op wound infection | Spina bifida | Ketorolac, naproxen |
| Grade 3 infection – line infection | ||||||
| Grade 4 infection – fungal lung infection | ||||||
| Cohort 2 | ||||||
|
| ||||||
| 1004 | 16 | F | 0.5 | Grade 1 headache | Hypermobility | Meloxicam, acetaminophen, lysine, osteo biflex |
|
| ||||||
| 1005 | 13 | F | 0.5 | Grade 1 headache | Joint pain NOS | Ibuprofen |
| Grade 1 dizziness | ||||||
| Grade 1 confusion | ||||||
| Grade 3 pain | ||||||
|
| ||||||
| 1006 | 15 | M | 0.5 | Grade 1 worsening headache | Headache | Topamax, verapamil, duloxetine, riboflavin |
| Cohort 3 | ||||||
|
| ||||||
| 1007 | 13 | F | 1 | Grade 1 confusion | Crohn's disease | 6-Mercaptopurine, adalimumab, celecoxib, mesalamine |
| Grade 1 memory impairment | ||||||
|
| ||||||
| 1008 | 11 | F | 1 | Grade 1 dizziness | Joint pain NOS | None |
| Grade 1 headache | ||||||
|
| ||||||
| 1009 | 17 | F | 1 | Grade 1 headache and sinus pressure | Headache | Naproxen |
| Grade 1 back pain | ||||||
| Grade 1 sore throat | ||||||
| Cohort 4 | ||||||
|
| ||||||
| 1010 | 16 | M | 1.5 | Grade 1 dizziness | Scoliosis | None |
| Grade 1 confusion | ||||||
| Grade 1 vomiting | ||||||
| Grade 1 chest pain – constipation | ||||||
| Grade 2 anorexia* | ||||||
| Grade 2 dysuria | ||||||
|
| ||||||
| 1011 | 16 | M | 1.5 | Grade 1 dizziness | Amplified musculoskeletal pain syndrome | Etanercept, methotrexate, sulfasalazine, sulindac |
|
| ||||||
| 1012 | 19 | F | 1.5 | Grade 2 depressed level of consciousness* | Esophageal spasm | Gabapentin, ranitidine, esomeprazole |
Grading of adverse events was performed using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Dose limiting toxicity
Pharmacokinetics
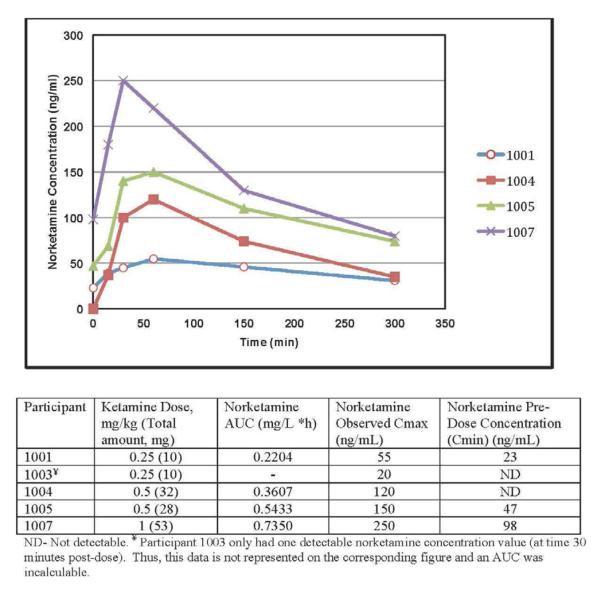
The pharmacokinetic blood draws were completed in 5 participants, ages 13–19 (Figure 2). Ketamine levels at every blood draw were below the limit of detection (20 ng/mL), except for participant 1007 at time 30 minutes (27 ng/mL). Therefore, the AUC of ketamine could not be estimated. Norketamine levels, however, were measurable and were used for describing the pharmacokinetic profiles. The norketamine concentration-time curves are shown in Figure 2. Of note, participants 1001, 1005, and 1007 had detectable levels of norketamine at time 0, indicating that norketamine accumulated in the plasma of these participants.
Figure 2.
Concentration-time curve and estimated pharmacokinetic parameters for norketamine by participant.
Pain Ratings
At the end of treatment, 5 of 12 participants reported an improvement in their pain scores. Two participants (1001, 1007) had complete resolution of pain on the NRS (Figure 3). An additional 3 participants (1002, 1003, 1008) had a 2-point or greater reduction in their pain scores. Participant 1005 experienced increased pain while on ketamine. This participant was later diagnosed with a somatization syndrome and subsequently with complex regional pain syndrome. Other participants had no change in their pain scores. Participant 1010 had a history of pain around 6–7 prior to treatment, but was in no pain at the time of the baseline and end of treatment visits. This participant's pain worsened throughout the course of most days and these visits were performed in the mornings.
Figure 3.
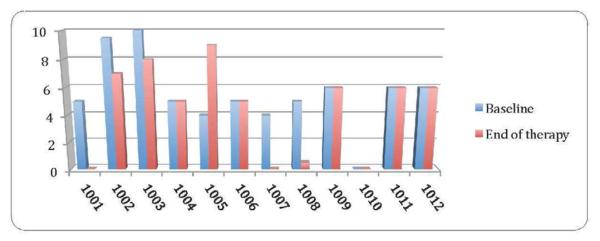
NRS pain scores at baseline and at the end of ketamine treatment for all participants. If the participant did not complete 14 days of treatment, their pain score at completion is noted (1009, 1010, 1012). Otherwise, the pain score is for the Day 14 visit.
A score of 4 or more on the DN4 is sensitive and specific for identifying neuropathic pain in adult patients[20]. Of the 12 participants tested, only 3 (1005, 1007, and 1011) met the required cutoff of 4 points indicating neuropathic pain at baseline. Of these 3 participants, one had a DN4 score that reduced to 0, but the other 2 participants had worsening or no change in their DN4 score.
Discussion
This study demonstrated that ketamine appears to be safe and tolerable in children and young adults at dosages ranging from 0.25 to 1 mg/kg/dose TID for a two-week period. A maximum tolerated dosage for oral ketamine in children and young adults with chronic pain has not been estimated. The data indicate that the maximum tolerated dosage may be between 1 and 1.5 mg/kg TID, though a precise estimate of the maximum tolerated dosage would require more testing in children. This dosage range is recommended for further study of oral ketamine in children with chronic pain. The small sample size in our study did not permit the CRM to converge at a maximum tolerated dosage.
It will also be important to eventually identify a maximally effective dosage of oral ketamine for children with chronic pain. It is possible that a lower dosage of ketamine may provide sufficient therapeutic benefit while avoiding potential toxicities related to increased dosages (e.g., sedation and dizziness appeared to be dosage-related in this small patient cohort). Given that one of the two participants with complete resolution of pain (who is also the participant who reported the maximal decrease in pain scores) on oral ketamine responded at the lowest tested dosage, it is possible that a maximally effective dosage of oral ketamine is not the highest dosage tested.
The pharmacokinetic data gathered in children with exposure to chronic, daily ketamine are interesting in that ketamine levels were rarely detected and were never sustained, even in the two participants with complete resolution of pain (participants 1001 and 1007), though norketamine levels were measurable in each participant tested.
One participant (participant 1003) had a much lower AUC than others. This participant's compliance was well documented, however, because the participant was in medical facilities during the entirety of care. One theoretical explanation for this difference is that the participant had an increase in the activity of their cytochrome P450 cyp3A4 enzyme, which is known to be the major enzyme that metabolizes ketamine[24]. Interindividual variation in cytochrome P450 activity has been linked to factors such as genetics, diet, physical activity, and inflammatory processes. For example, inflammation-induced regulation of major CYP enzymes has been studied in various animal models and through clinical observations, demonstrating that reduced CYP activity is related to elevated inflammatory markers (eg, IL-6, C-reactive protein, and Tumor Necrosis Factor-alpha)[25–27].
Norketamine is estimated to have approximately 1/3 the analgesic effect as its parent compound (ketamine). This difference in measurable levels of norketamine and ketamine indicates that there is a dramatic first pass metabolism of ketamine to norketamine when taken orally, which is primarily mediated by the CYP3A4 cytochrome p450 enzyme. The increase in norketamine levels and very low ketamine levels after oral administration corresponds to pharmacokinetic data in previous trials in adults[17] though the peak norketamine levels were uniformly lower in the children included in this study than those noted previously in adults. Also interesting is the preliminary evidence that some children taking chronic oral ketamine may accumulate norketamine in the bloodstream over time. There is not, to date, any suggestion of an association between response and norketamine accumulation, though there are not enough data presently to support or refute this.
Five participants reported decreased pain while on oral ketamine therapy. Three of these participants reported improved pain control that was sustained for up to 4 weeks after discontinuation of oral ketamine.
The limitations of this study include the fact that the NRS pain scores were measured in terms of pain “right now” rather than an average pain level for the previous 24 hours, which limits the ability to interpret the NRS pain score changes. This measurement is different from the previous 24 hours measured with the DN4 pain assessment. Other limits to the pain assessment in the study include the lack of blinding and the absence of an untreated control group. Furthermore, pain as a subjective problem is difficult to reliably estimate under the best of circumstances. There are, similarly, limitations to the pharmacokinetic assessments of oral ketamine. It was surprising that almost all levels of ketamine were undetectable, and that there was evidence of accumulation of norketamine in the plasma of chronically-exposed participants. Both of these intriguing findings will require further study with more sensitive ketamine assays and more intensive pharmacokinetic sampling for further clarification. In spite of these limitations, the anecdotal experience is intriguing, and oral ketamine therapy in children certainly warrants further study.
The next steps in development of oral ketamine for children could include a study to select an appropriate dosage from within the range established in this study (0.25–1 mg/kg/dose TID) to optimize pain control while minimizing adverse events. This would allow for safe outpatient administration for children without sedating adverse events. However, higher dosages of oral ketamine are not recommended for outpatient administration without careful observation due to concerns of sedation. Further investigation could also include examining the minimum duration of treatment required for a response in chronic pain, as those participants who had complete resolution of pain in this study did so within the first 2–5 days. The duration of the response also needs to be characterized.
The goal of providing children lasting relief from chronic pain remains elusive. This study is the first step in the development of ketamine for this purpose. Though this study does not formally establish a maximum tolerated dosage, it does describe a clinically relevant dosage range (0.25–1 mg/kg/dose TID for up to 14 days) for children with chronic pain.
Acknowledgments
The authors would like to thank Dr Karl Kieburtz for his crucial advice on the design of this trial.
Supported by a University of Rochester CTSA award (UL1 RR024160 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Registered with ClinicalTrials.gov: NCT01369680
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- [1].Berde CB, Walco GA, Krane EJ, Anand KJ, Aranda JV, Craig KD, et al. Pediatric analgesic clinical trial designs, measures, and extrapolation: report of an FDA scientific workshop. Pediatrics. 2012;129:354–64. doi: 10.1542/peds.2010-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–38. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- [3].Green SM, Cote CJ. Ketamine and neurotoxicity: clinical perspectives and implications for emergency medicine. Ann Emerg Med. 2009;54:181–90. doi: 10.1016/j.annemergmed.2008.10.003. [DOI] [PubMed] [Google Scholar]
- [4].Mehta AK, Halder S, Khanna N, Tandon OP, Sharma KK. Antagonism of stimulation-produced analgesia by naloxone and N-methyl-D-aspartate: role of opioid and N-methyl-D-aspartate receptors. Hum Exp Toxicol. 2012;31:51–6. doi: 10.1177/0960327111417908. [DOI] [PubMed] [Google Scholar]
- [5].Smith DJ, Bouchal RL, deSanctis CA, Monroe PJ, Amedro JB, Perrotti JM, et al. Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology. 1987;26:1253–60. doi: 10.1016/0028-3908(87)90084-0. [DOI] [PubMed] [Google Scholar]
- [6].Romero TR, Galdino GS, Silva GC, Resende LC, Perez AC, Cortes SF, et al. Ketamine activates the L-arginine/Nitric oxide/cyclic guanosine monophosphate pathway to induce peripheral antinociception in rats. Anesth Analg. 2011;113:1254–9. doi: 10.1213/ANE.0b013e3182285dda. [DOI] [PubMed] [Google Scholar]
- [7].Hayashi Y, Kawaji K, Sun L, Zhang X, Koyano K, Yokoyama T, et al. Microglial Ca(2+)-activated K(+) channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. J Neurosci. 2011;31:17370–82. doi: 10.1523/JNEUROSCI.4152-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Okon T. Ketamine: an introduction for the pain and palliative medicine physician. Pain Physician. 2007;10:493–500. [PubMed] [Google Scholar]
- [9].Lauretti GR, Gomes JM, Reis MP, Pereira NL. Low doses of epidural ketamine or neostigmine, but not midazolam, improve morphine analgesia in epidural terminal cancer pain therapy. J Clin Anesth. 1999;11:663–8. doi: 10.1016/s0952-8180(99)00122-1. [DOI] [PubMed] [Google Scholar]
- [10].Lauretti GR, Lima IC, Reis MP, Prado WA, Pereira NL. Oral ketamine and transdermal nitroglycerin as analgesic adjuvants to oral morphine therapy for cancer pain management. Anesthesiology. 1999;90:1528–33. doi: 10.1097/00000542-199906000-00005. [DOI] [PubMed] [Google Scholar]
- [11].Mercadante S, Arcuri E, Tirelli W, Casuccio A. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: a randomized, controlled, double-blind, crossover, double-dose study. Journal of pain and symptom management. 2000;20:246–52. doi: 10.1016/s0885-3924(00)00194-9. [DOI] [PubMed] [Google Scholar]
- [12].Yang CY, Wong CS, Chang JY, Ho ST. Intrathecal ketamine reduces morphine requirements in patients with terminal cancer pain. Can J Anaesth. 1996;43:379–83. doi: 10.1007/BF03011718. [DOI] [PubMed] [Google Scholar]
- [13].Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain. 2009;147:107–15. doi: 10.1016/j.pain.2009.08.015. [DOI] [PubMed] [Google Scholar]
- [14].Sigtermans MJ, van Hilten JJ, Bauer MC, Arbous MS, Marinus J, Sarton EY, et al. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304–11. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- [15].von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children's self-reports of pain intensity. Pain. 2009;143:223–7. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- [16].Gupta VD. Stability of ketamine hydrochloride injection after reconsitution in water for injection and storage in 1-mL tuberculin polypropylene syringes for pediatric use. International Journal of Pharmaceutical Compounding. 2002:316–7. [PubMed] [Google Scholar]
- [17].Grant IS, Nimmo WS, Clements JA. Pharmacokinetics and analgesic effects of i.m. and oral ketamine. Br J Anaesth. 1981;53:805–10. doi: 10.1093/bja/53.8.805. [DOI] [PubMed] [Google Scholar]
- [18].O'Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- [19].Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med. 1995;14:1149–61. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- [20].Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- [21].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- [22].Health NIo, editor. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 2009. [Google Scholar]
- [23].Falchook GS, Duvic M, Hong DS, Wheler J, Naing A, Lim J, et al. Age-stratified phase I trial of a combination of bortezomib, gemcitabine, and liposomal doxorubicin in patients with advanced malignancies. Cancer chemotherapy and pharmacology. 2012;69:1117–26. doi: 10.1007/s00280-011-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hijazi Y, Boulieu R. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:853–8. doi: 10.1124/dmd.30.7.853. [DOI] [PubMed] [Google Scholar]
- [25].Barclay TB, Peters JM, Sewer MB, Ferrari L, Gonzalez FJ, Morgan ET. Modulation of cytochrome P-450 gene expression in endotoxemic mice is tissue specific and peroxisome proliferator-activated receptor-alpha dependent. The Journal of pharmacology and experimental therapeutics. 1999;290:1250–7. [PubMed] [Google Scholar]
- [26].Rivory LP, Slaviero KA, Clarke SJ. Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase response. British journal of cancer. 2002;87:277–80. doi: 10.1038/sj.bjc.6600448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen YL, Le Vraux V, Leneveu A, Dreyfus F, Stheneur A, Florentin I, et al. Acute-phase response, interleukin-6, and alteration of cyclosporine pharmacokinetics. Clinical pharmacology and therapeutics. 1994;55:649–60. doi: 10.1038/clpt.1994.82. [DOI] [PubMed] [Google Scholar]