Abstract
Key components of atherosclerotic plaque known to drive disease progression are macrophages and cholesterol. It has been widely understood, and bolstered by recent evidence, that the efflux of cholesterol from macrophage foam cells quells disease progression or even to promote regression. Following macrophage cholesterol efflux, cholesterol loaded onto HDL must be removed from the plaque environment. Here, we focus on recent evidence that the lymphatic vasculature is critical for the removal of cholesterol, likely as a component of HDL, from tissues including skin and the artery wall. We discuss the possibility that progression of atherosclerosis might in part be linked to sluggish removal of cholesterol from the plaque.
Keywords: inflammation, cardiovascular disease, reverse cholesterol transport, macrophage
Introduction
Despite significant advances in reducing coronary events stemming from atherosclerosis, atherosclerotic complications remain a leading cause of morbidity and mortality in the world. Significant gains have been recently demonstrated by rigorous adherence to the Mediterranean diet [1], whereas the advances in therapeutic beyond the resounding success of statin therapy to modulate circulating cholesterol has moved forward much more slowly. In particular, enormous effort has been focused on increasing HDL in the circulation, driven by the concept that raising HDL would provide benefit alongside lowering LDL. In the past decade, pre-clinical and clinical trials have aimed to decrease cardiovascular morbidity and mortality in patients with coronary heart disease by increasing plasma HDL level. Despite some promising preliminary data, several studies, including analysis of nicotinic acid-based therapies or cholesteryl ester transport protein (CETP) inhibitors, have been discontinued because of failure to demonstrate benefit (HPS-2thrive, AIM-HIGH) or even because of increased mortality [2–5]. Furthermore, recent Mendelian randomization analyses have also challenged the concept that raising of plasma HDL cholesterol translates into reduced risk of myocardial infarction (MI) [6].
These unexpected outcomes have left the field with a modified view: that what really counts is cholesterol efflux from macrophages, an activity that requires HDL to serve as an acceptor of cholesterol. However, this revised view takes into account that not all HDL is the same and not all is equally capable of supporting robust cholesterol efflux. In support of this concept, the use of patient serum depleted of apolipoprotein B showed varying abilities to support cholesterol efflux from macrophages. Serum that promoted more prominent efflux correlated with reduced cardiovascular risk for the patient, and support of efflux was not simply a consequence of increased circulating HDL cholesterol levels [7]. This study assumes that the cholesterol-accepting components of plasma appropriately reflect the cholesterol-accepting components of extravascular tissue, including the environment of the macrophage within an atherosclerotic plaque. However, there is likely additional complexity to consider when one takes into account elements of cholesterol transport that go beyond the interaction of the macrophage with plasma components. In this review, we highlight recent studies by our group and others that lymphatic vessels participate critically in cholesterol transport, including from atherosclerotic plaques. We discuss these findings in light of what is known about how HDL matures as it passes through tissue. Indeed, interstitial fluid, only partially similar to plasma/serum, is the critical fluid interface that brings in HDL and other components that support cholesterol efflux from macrophage.
The lymphatic vasculature and the intake of fats that promote atherosclerosis
The structure of the lymphatic vasculature and even its potential relationship to cardiovascular disease has been expertly reviewed recently [8]. The lymphatic vasculature is organized as blind-ended lymphatic capillaries in all organs that then coalesce into vessels with a muscular wall, capable of pumping lymph. These vessels are referred to as collecting vessels. They run to (afferent) and from (efferent) lymph nodes and then drain back into the blood circulation where the lymphatic thoracic duct converges with the left brachiocephalic vein or the right lymphatic duct, which drains the right thoracic cavity and right upper body, converges with the right subclavian vein. The lymphatic vasculature within intestinal villi serves as the crucial portals for absorption of chylomicrons packaged and released on the basolateral surface of the intestine. The lymphatic capillaries within villi are called lacteals. The fenestrated blood supply within the villus is fenestrated and appears capable of absorbing most small molecules. It also appears that they leak small molecular tracers [9], but these tracers do not enter the lymph unless the formidable regiment of highly endocytic macrophages and dendritic cells in the intestinal villi surrounding blood and lymphatics are removed, leaving the lymphatic vasculature to take up these small molecules as well. Although the regulation of chylomicron absorption is understudied, these tracer studies suggest that molecular entry into the lacteal may be passive but typically highly impeded by both the fenestrated blood supply and endocytic scavengers of the immune system. A recent report indicated that absorption of cholesterol is independent of scavenger receptor B1 (SR-B1) [10]. It seems possible that chylomicrons enter the lacteal because they are passively excluded, perhaps because of their relatively large size, from the fenestrated blood supply.
There has long been awareness that one point for control of cholesterol flux is to prevent absorption of chylomicrons. Despite a fair number of promising clinical analysis comparing the effect of a combination of absorption blockers like ezetimibe coadministered with statins, to conventional statin monotherapy in patients with metabolic syndrome, hypercholesterolemia, or acute coronary syndrome, the efficacy in of the combination therapy remains unclear [11]. The possibility of off-target effects has been raised [11]. Taking those observations into account, other recent data might be consequently very relevant here. Diets rich in cholesterol are also those typically rich in phosphatidylcholine and L-carnitine as well. These dietary components can be metabolized by the gut microbiota to generate trimethylamine-N-oxide (TMAO) locally in the intestine [12–14]. Thus, the molecular structure of TMAO suggests that it would likely be absorbed directly into the portal venous system rather than in chylomicrons that require absorption through the lymphatic vasculature, potentially explaining how the dietary intake of cholesterol-rich foods could support atherosclerosis even when the cholesterol itself is not absorbed.
Macrophage efflux of cholesterol is linked in vivo to inflammation in atherosclerosis
Cholesterol that accumulates in peripheral tissues, whether from dietary or synthetic sources, needs to be brought back to intestine for fecal excretion. The process of such removal is called reverse cholesterol transport (RCT), and when it concerns the removal of cholesterol from macrophage foam cells in plaque, the term macrophage RCT is applied. In brief, cholesterol that is stored as esters within macrophages is de-esterified and free cholesterol is passed to HDL as the primary cholesterol acceptor. Key ABC transporters that facilitate this transfer are ABCA1 and ABCG1 [15]. It has been argued from studies in vitro that if macrophages cannot efflux cholesterol, they upregulate inflammatory genes more strongly than macrophages that can efflux when both are exposed to stimulants such as TLR ligands [16]. Recent work that eliminated ABCG1 and ABCA1 in Lys-M-Cre positive cells—macrophages, neutrophils, but only a minority of dendritic cells [17]—shows that loss of these transporters in LysM-expressing cell lineages (most likely macrophages) exacerbates atherosclerosis [18]. Altogether, these investigations clearly highlight the importance of an adequate capacity of cholesterol to efflux from macrophages. However, once cholesterol has been loaded onto cholesterol acceptors in the periphery, what is the fate of the cholesterol-loaded lipoproteins before reaching the blood?
Linking RCT to the lymphatic vasculature
The role of ABCA1 and ABCG1 on macrophages is critical to RCT. In general, while much remains to be learned about many aspects of RCT from peripheral tissues, particularly little attention has been paid to how cholesterol-loaded HDL gets into the plasma from extravascular peripheral sites. The lymphatic vasculature has been established in humans to carry HDL out of tissues, as presented and comprehensively discussed by Nanjee et al. [19–21]. Recently, our work in mouse models revealed that lymphatic vessels are not only carriers of HDL out of tissues, but RCT is quantitatively dependent upon the lymphatic vasculature [22].
In skin, we investigated two models—one surgical and one genetic—to examine the importance of lymphatics in RCT after macrophages loaded with radioactive cholesterol were injected [22]. Then, we utilized a model of aortic vessel transplant to sever the lymphatic vasculature and then utilized neutralizing mAbs to prevent reestablishment of the lymphatic vasculature. To track cholesterol, we loaded the apoE−/− transplant donor with deuterium-labeled cholesterol quantified by mass spectrometry. This step indicated that RCT from the aortic wall affected by atherosclerosis is also dependent upon the lymphatic vasculature [22] (Fig. 1). We believe this work is just the beginning of a new direction and that it ushers in the need to better understand underlying processes such as how apoA-1 crosses the vasculature to enter tissues [23, 24] and, as HDL exits plaque, how it traverses the formidable medial layer of arteries [25–27]. Though it remains extremely challenging, it will be important to investigate the role of lymphatic vessels in RCT from atheromata and from nondiseased artery walls in non-surgical models that will rely on the locally established lymphatic vasculature.
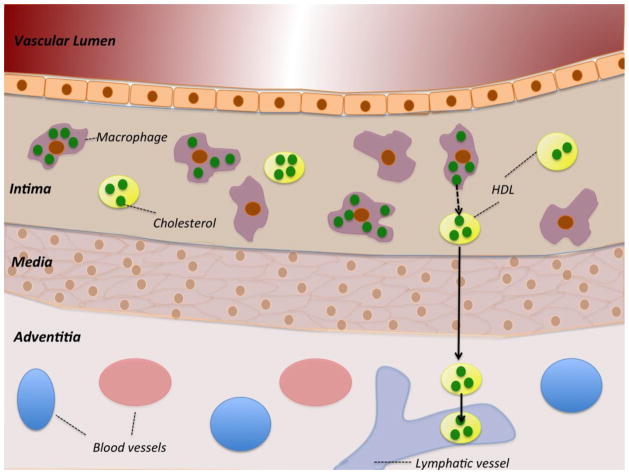
Figure 1.
Schematic depiction of a macrophage-rich atherosclerotic lesion in cross-section. Cholesterol is shown in green within either macrophages or loaded onto HDL particles. During RCT, macrophages efflux cholesterol to HDL which then moves through the lymphatic vasculature to leave plaques.
Another recent study that utilized a fluorescent analog to trace cholesterol transport out of skin also concluded that RCT from skin was dependent upon the lymphatic vasculature [28]. In this study, the authors went on to argue that cholesterol accesses the lymphatic vasculature in a selective manner, through SR-B1 mediated uptake. This latter result is intriguing, since the elegant structure of the lymphatic capillary system would seemingly be capable of allowing HDL into the lymphatic capillary in a nonselective manner, like most other macromolecules, including nonspecific tracers like dextran. If SR-B1 drives RCT from the periphery, its role there would thus differ from chylomicron transport in the intestine that is SR-B1 independent [10].
Lymphatic vessels in atherosclerotic plaques and other cardiovascular pathologies
Recent work has revealed the presence of neo-lymphatic vessels in atherosclerotic plaque [29–31] and in aortic valve stenosis [32]. Aortic valve stenosis occurs when the heart’s aortic valve narrows, thus preventing the valve from opening fully and consequently obstructing blood flow. Intensive calcification, extracellular matrix remodelling and valvular accumulation of lipids and inflammatory cells characterize its progression [33–37]. Syväranta et al. find that lymphangiogenesis is induced as aortic valve stenosis advances [32]. They observe lymphangiogenic growth factors VEGF-C and VEGF-D and the receptor for these factors VEGFR-3 in the aortic valve, along with expression of the lymphatic vessel marker LYVE-1. The biological consequence of this lymphangiogenesis is unknown.
Kholova et al. have described the structure of the lymphatic network in human heart and relationship to some pathological conditions [29]. They concur with earlier work showing that lymphatic vessels are found in the peri-adventitial space of large arteries [38, 39] and reveal that lymphatics in hearts and coronary arteries of children and adults invade atherosclerotic plaques as they become advanced. The vessels are also expanded in ischemic and inflamed hearts. Furthermore, Drozdz et al. have characterized the lymphatic network in the adventitia of large arteries affected by atherosclerosis in man using antibodies against LYVE-1 and podoplanin [30]. They observed a significant positive correlation between the number of adventitial lymphatic capillaries and disease progression, as assessed by intimal thickness.
While Kholova et al. argue that preventing lymphangiogenesis might be beneficial, we argue based on recent findings on the role of lymphatics in RCT that the new vessels likely have a beneficial role in facilitating cholesterol removal from advanced plaque, as once lymphatic vessels have invaded the plaque, the need for cholesterol to mobilize across the medial wall to gain access to the lymphatic vasculature is removed, perhaps improving RCT. This viewpoint fits with the older concept that absence or obstruction of lymphatics may accelerate intimal inflammation [38, 39].
Clinical studies in support of the concept that the lymphatic vasculature may be linked to cardiovascular disease
A question that comes to mind in relation to the claim that the lymphatic vasculature mediates RCT is whether RCT is impaired or cardiovascular disease is worsened in patients with known mutations in lymphatics. We are not aware of specific studies examining directly this issue. However, a few papers in the literature may provide hints of a connection (Table 1). First, the transcription factor GATA2 that plays a role in directing the fate of the hemangioblast [10] has been linked with familial early-onset of coronary artery disease (CAD) [40], primary (hereditary) lymphedema in Emberger syndrome [41], as well as hematological diseases characterized by altered myeloid cell differentiation [41]. It is completely unclear if its role in lymphatic biology relates to its role in cardiovascular disease, but this possibility warrants consideration.
Table 1.
Potential links between lymphatic function and cardiovascular diseases in clinical settings
| Genes involved | Implication in lymphatic function | Implication in cardiovascular disease |
|---|---|---|
| GATA2 | Linked with primary (hereditary) lymphedema in Emberger syndrome [41] | Linked with familial early- onset of coronary artery disease [40] Linked with hematological diseases characterized by altered myeloid cell differentiation [41] |
| VEGF-C and its receptor, VEGFR-3 | Mutation of the VEGFR-3 gene causes primary lymphedema [8] | A polymorphism in the gene encoding VEGF-C, causing lower levels of circulating VEGF-C, is associated with CAD [48] VEGF-C levels in plasma correlate with plasma mass of Lp-PLA2 [49] VEGF-C levels in plasma correlate with metabolic parameters and dyslipidemia [50] |
| FOXC2/PROX-1 | Key genes involved in the process of lymphatic vessel formation [53] Transcription factors highly expressed from the onset of valve formation and collecting vessel maturation [43] |
Familial combined hyperlipidaemia (FCHL) is associated with decreased PROX-1 and / or FOXC2 expression in adipose tissue [44] Genetic linkage shows an association between FOXC2 and FCHL [45] |
FOXC2 is a transcription factor expressed in adipocytes [42] but also in lymphatic vessels, where it mediates the maturation of lymphatic collecting vessels that transport lymph [43]. It and another transcription factor associated both with metabolism and the lymphatic vasculature, Prox-1, appear to be poorly expressed in subcutaneous fat of patients with familial combined hyperlipidaemia (FCHL) [44]. FOXC2 in particular has been suggested to be causal in giving rise to a low-HDL trait [45], and it has been experimentally implicated in metabolic disorders of hypertriglyceridemia [42]. The role of lymphatic transport in these scenarios remains to be investigated.
Development and regulation of the lymphatic system is at least partially dependent upon VEGF-C and the receptor for VEGF-C, VEGFR3 (FLT4) [8]. Futhermore, binding of VEGF-C to VEGFR3 acts on existing lymphatic collecting vessels to support increased pumping [46], suggesting that VEGF-C maintains and amplifies the transport function of the existing lymphatic network. Mutation of the VEGFR-3 gene in humans is a fundamental cause of primary lymphedema [8] and mice bearing a mutation in this receptor that impairs signaling do not develop the full lymphatic vasculature [47]. A polymorphism in the gene encoding VEGF-C that leads to lower levels of circulating VEGF-C is associated with coronary atherosclerosis in humans [48]. In the Framingham Heart Study, VEGF-C levels in plasma correlated with plasma mass of Lp-PLA2 [49], long regarded as a biomarker of atherosclerosis. In another study VEGF-C levels in human plasma were elevated in correlation with dyslipidemia and mouse models of atherosclerosis showed a similar pattern in plasma and plaque [50]. While VEGF-C may have multiple roles in disease, we wonder if VEGF-C increases as a mechanism to combat disease progression, in this case acting to improve lymphatic transport of cholesterol from plaque, much as titers of CXCL5 [51] or innate IgM against oxidized LDL [52] rise with disease activity and provide a counter-measure against disease progression.
Conclusion
Inflammation in atherosclerotic plaque is driven at least in part by cholesterol-loaded macrophages in an ABCA1- and ABCG1-dependent manner, arguing that at least in the context of the plaque environment, cholesterol unloading by macrophages is essential to reversing disease. While it has been thought that raising HDL within the circulation might provide clinical benefit, clinical trials testing the effect of CETP inhibitors do not show efficacy. A key issue is likely how well cholesterol is cleared from tissues, particularly the atherosclerotic plaque. We discuss recent data from our laboratory and others that illustrates the important role that lymphatic vessels play in mediating cholesterol clearance from peripheral sites, including the artery wall. More work is needed in this area, but the possibility that cholesterol-driven inflammation in atherosclerotic plaque may be enhanced by poor lymphatic clearance of cholesterol at these sites, or that therapies designed to enhance lymphatic transport following or in concert with macrophage cholesterol efflux, deserves attention.
Footnotes
Conflict of Interest
Catherine Martel and Gwendalyn J. Randolph declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1*.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. Extremely effective impact of the Mediterranean diet in preventing cardiovascular disease in a very well-monitored study. [Google Scholar]
- 2.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 3.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GG, Olsson AG, Ballantyne CM, Barter PJ, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Shah PK, et al. Rationale and design of the dal-OUTCOMES trial: efficacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am Heart J. 2009;158(6):896–901. e893. doi: 10.1016/j.ahj.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17 (11):1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 9.Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang HS, O’Keeffe M, Liao G, Karp CL, et al. Circulatory antigen processing by mucosal dendritic cells controls CD8(+) T cell activation. Immunity. 2013;38(1):153–165. doi: 10.1016/j.immuni.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bura KS, Lord C, Marshall S, McDaniel A, Thomas G, Warrier M, Zhang J, Davis MA, Sawyer JK, Shah R, et al. Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice. J Lipid Res. 2013;54(6):1567–1577. doi: 10.1194/jlr.M034454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor AJ, Villines TC, Stanek EJ. Paradoxical progression of atherosclerosis related to low-density lipoprotein reduction and exposure to ezetimibe. Eur Heart J. 2012;33(23):2939–2945. doi: 10.1093/eurheartj/ehs105. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. This paper is the first in a series by the Hazen laboratory that forges new links between cardiovascular disease and the intestinal microbial environment. The work illustrates how dietary components promote the generation of compounds produced by microbiota that amplify cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19 (5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7(5):365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51(11):3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205(12):2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, et al. Deficiency of ABCA1 and ABCG1 in Macrophages Increases Inflammation and Accelerates Atherosclerosis in Mice. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301086. the most specific evidence to date that macrophage loss of ABCA1 and ABCG1, rather than loss of these transporters in other cell types, drives atherogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanjee MN, Cooke CJ, Wong JS, Hamilton RL, Olszewski WL, Miller NE. Composition and ultrastructure of size subclasses of normal human peripheral lymph lipoproteins: quantification of cholesterol uptake by HDL in tissue fluids. J Lipid Res. 2001;42(4):639–648. [PubMed] [Google Scholar]
- 20.Nanjee MN, Cooke CJ, Olszewski WL, Miller NE. Lipid and apolipoprotein concentrations in prenodal leg lymph of fasted humans. Associations with plasma concentrations in normal subjects, lipoprotein lipase deficiency, and LCAT deficiency. J Lipid Res. 2000;41(8):1317–1327. [PubMed] [Google Scholar]
- 21.Nanjee MN, Cooke CJ, Olszewski WL, Miller NE. Concentrations of electrophoretic and size subclasses of apolipoprotein A-I-containing particles in human peripheral lymph. Arterioscler Thromb Vasc Biol. 2000;20(9):2148–2155. doi: 10.1161/01.atv.20.9.2148. [DOI] [PubMed] [Google Scholar]
- 22*.Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. 2013;123(4):1571–1579. doi: 10.1172/JCI63685. illustrates the quantitative importance of the lymphatic vasculature in macrophage reverse cholesterol transport, including its transport from the atherosclerosis-affected artery wall. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohrer L, Cavelier C, Fuchs S, Schluter MA, Volker W, von Eckardstein A. Binding, internalization and transport of apolipoprotein A-I by vascular endothelial cells. Biochim Biophys Acta. 2006;1761(2):186–194. doi: 10.1016/j.bbalip.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Cavelier C, Ohnsorg PM, Rohrer L, von Eckardstein A. The beta-chain of cell surface F(0)F(1) ATPase modulates apoA-I and HDL transcytosis through aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32 (1):131–139. doi: 10.1161/ATVBAHA.111.238063. [DOI] [PubMed] [Google Scholar]
- 25.Kim WS, Tarbell JM. Macromolecular transport through the deformable porous media of an artery wall. J Biomech Eng. 1994;116(2):156–163. doi: 10.1115/1.2895714. [DOI] [PubMed] [Google Scholar]
- 26.Meyer G, Merval R, Tedgui A. Effects of pressure-induced stretch and convection on low-density lipoprotein and albumin uptake in the rabbit aortic wall. Circ Res. 1996;79(3):532–540. doi: 10.1161/01.res.79.3.532. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin AL, Wilson LM, Gradus-Pizlo I, Wilensky R, March K. Effect of atherosclerosis on transmural convection an arterial ultrastructure. Implications for local intravascular drug delivery. Arterioscler Thromb Vasc Biol. 1997;17(12):3365–3375. doi: 10.1161/01.atv.17.12.3365. [DOI] [PubMed] [Google Scholar]
- 28*.Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. Lymphatic Vessels Are Essential for the Removal of Cholesterol from Peripheral Tissues by SR-BI-Mediated Transport of HDL. Cell Metab. 2013;17(5):671–684. doi: 10.1016/j.cmet.2013.04.002. provides evidence that cholesterol trafficking through the lymphatic vasculature in skin depends on SR-B1. [DOI] [PubMed] [Google Scholar]
- 29.Kholova I, Dragneva G, Cermakova P, Laidinen S, Kaskenpaa N, Hazes T, Cermakova E, Steiner I, Yla-Herttuala S. Lymphatic vasculature is increased in heart valves, ischaemic and inflamed hearts and in cholesterol-rich and calcified atherosclerotic lesions. Eur J Clin Invest. 2011;41(5):487–497. doi: 10.1111/j.1365-2362.2010.02431.x. [DOI] [PubMed] [Google Scholar]
- 30.Drozdz K, Janczak D, Dziegiel P, Podhorska M, Piotrowska A, Patrzalek D, Andrzejak R, Szuba A. Adventitial lymphatics and atherosclerosis. Lymphology. 2012;45(1):26–33. [PubMed] [Google Scholar]
- 31.Hatakeyama K, Kaneko MK, Kato Y, Ishikawa T, Nishihira K, Tsujimoto Y, Shibata Y, Ozaki Y, Asada Y. Podoplanin expression in advanced atherosclerotic lesions of human aortas. Thromb Res. 2012;129(4):e70–76. doi: 10.1016/j.thromres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Syvaranta S, Helske S, Lappalainen J, Kupari M, Kovanen PT. Lymphangiogenesis in aortic valve stenosis--novel regulatory roles for valvular myofibroblasts and mast cells. Atherosclerosis. 2012;221(2):366–374. doi: 10.1016/j.atherosclerosis.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 33.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103(11):1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92(8):2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 35.Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoc A, Kilic R, Brueckmann M, Lang S, Zahn I, Vahl C, et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. Journal of molecular and cellular cardiology. 2004;36 (1):57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90(2):844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 37.Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Ryden L, Nilsson J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol. 1994;23(5):1162–1170. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 38.Eliska O, Eliskova M, Miller AJ. The absence of lymphatics in normal and atherosclerotic coronary arteries in man: a morphologic study. Lymphology. 2006;39(2):76–83. [PubMed] [Google Scholar]
- 39.Miller AJ, DeBoer A, Palmer A. The role of the lymphatic system in coronary atherosclerosis. Med Hypotheses. 1992;37(1):31–36. doi: 10.1016/0306-9877(92)90009-2. [DOI] [PubMed] [Google Scholar]
- 40.Connelly JJ, Wang T, Cox JE, Haynes C, Wang L, Shah SH, Crosslin DR, Hale AB, Nelson S, Crossman DC, et al. GATA2 is associated with familial early-onset coronary artery disease. PLoS genetics. 2006;2 (8):e139. doi: 10.1371/journal.pgen.0020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyde RK, Liu PP. GATA2 mutations lead to MDS and AML. Nat Genet. 2011;43(10):926–927. doi: 10.1038/ng.949. [DOI] [PubMed] [Google Scholar]
- 42.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106(5):563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 43.Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10(9):974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 44.Horra A, Salazar J, Ferre R, Vallve JC, Guardiola M, Rosales R, Masana L, Ribalta J. Prox-1 and FOXC2 gene expression in adipose tissue: A potential contributory role of the lymphatic system to familial combined hyperlipidaemia. Atherosclerosis. 2009;206(2):343–345. doi: 10.1016/j.atherosclerosis.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 45.Pajukanta P, Allayee H, Krass KL, Kuraishy A, Soro A, Lilja HE, Mar R, Taskinen MR, Nuotio I, Laakso M, et al. Combined analysis of genome scans of dutch and finnish families reveals a susceptibility locus for high-density lipoprotein cholesterol on chromosome 16q. Am J Hum Genet. 2003;72(4):903–917. doi: 10.1086/374177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breslin JW, Gaudreault N, Watson KD, Reynoso R, Yuan SY, Wu MH. Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am J Physiol Heart Circ Physiol. 2007;293(1):H709–718. doi: 10.1152/ajpheart.00102.2007. [DOI] [PubMed] [Google Scholar]
- 47.Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7(2):199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 48.Guerzoni AR, Biselli PM, Godoy MF, Souza DR, Haddad R, Eberlin MN, Pavarino-Bertelli EC, Goloni-Bertollo EM. Homocysteine and MTHFR and VEGF gene polymorphisms: impact on coronary artery disease. Arq Bras Cardiol. 2009;92(4):263–268. doi: 10.1590/s0066-782x2009000400003. [DOI] [PubMed] [Google Scholar]
- 49.Schnabel R, Dupuis J, Larson MG, Lunetta KL, Robins SJ, Zhu Y, Rong J, Yin X, Stirnadel HA, Nelson JJ, et al. Clinical and genetic factors associated with lipoprotein-associated phospholipase A2 in the Framingham Heart Study. Atherosclerosis. 2009;204(2):601–607. doi: 10.1016/j.atherosclerosis.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wada H, Ura S, Kitaoka S, Satoh-Asahara N, Horie T, Ono K, Takaya T, Takanabe-Mori R, Akao M, Abe M, et al. Distinct characteristics of circulating vascular endothelial growth factor-a and C levels in human subjects. PLoS One. 2011;6(12):e29351. doi: 10.1371/journal.pone.0029351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rousselle A, Qadri F, Leukel L, Yilmaz R, Fontaine JF, Sihn G, Bader M, Ahluwalia A, Duchene J. CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest. 2013;123(3):1343–1347. doi: 10.1172/JCI66580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9(6):736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 53.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22(23):3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]