Abstract
Mice with collecting duct-specific deletion of endothelin-1 are hypertensive and have impaired Na excretion. Since endothelin-1 activates nitric oxide synthase (NOS) in collecting duct, we hypothesized that impaired renal NO production in knockout mice exacerbates the hypertensive state. Control and knockout mice were treated chronically with L-NAME, and arterial pressure (BP) and urinary nitrate/nitrite (NOx) excretion assessed. On a normal Na diet, knockout systolic BP was 18 mmHg greater than controls. L-NAME increased BP in control mice by 30 mmHg and 10 mmHg in CD ET-1 KO mice, thereby abolishing the difference in systolic BP between the groups. A high Na diet increased BP similarly in both groups. Urinary NOx excretion was lower in knockout mice than in controls on normal or high Na intake. In separate experiments, renal perfusion pressure was adjusted in anesthetized mice, and urinary NOx and Na excretion determined. Similar elevations of BP increased urinary Na and NOx excretion in control mice, but to a significantly lesser extent in knockouts. Isoform-specific NOS activity and expression were determined in renal inner medulla homogenates from control and knockout mice. NOS1 and NOS3 activities were lower in knockouts than in controls given normal or high Na diets. However, NOS1 or NOS3 protein expression were similar in both groups on normal or high Na intake. These data demonstrate that collecting duct-derived endothelin-1 is important in: 1) chronic L-NAME-induced hypertension; 2) full expression of pressure-dependent changes in sodium excretion; and 3) control of inner medullary NOS1 and NOS3 activity.
Keywords: ET-1, nitric oxide, Na, natriuresis, diuresis, blood pressure
INTRODUCTION
Collecting duct (CD)-derived endothelin (ET-1) is an important regulator of arterial pressure (BP) and renal Na excretion1. ET-1 inhibits distal nephron Na/K ATPase and epithelial Na channel activity2, 3, while CD-specific knockout of ET-1 causes hypertension and impaired Na excretion1. The natriuretic effect of ET-1 is due, at least partially, to activation of CD ETB receptors since CD-specific deletion of ETB receptors causes hypertension and reduced Na excretion4. How ET-1 modulates renal Na excretion and BP is unknown, however the nitric oxide (NO) pathway may be involved. ET-1 stimulates NO production in inner medullary CD via the ETB receptor and NO synthase 1 (NOS1) (also known as nNOS)5. Inner medullary NOS1 and NOS3 (latter known as eNOS) activity is reduced in rats with dysfunctional ETB receptors6, 7. In thick ascending limbs, ET-1 enhances NO production through increased NOS3 activity with resultant inhibition of chloride transport8, 9. NO also inhibits Na transport in isolated cortical CD10. Taken together, these studies suggest that NO is an important mediator of the natriuretic and antihypertensive effects of CD-derived ET-1.
One condition under which the CD ET-1/NO pathway may be of particular importance is increased Na excretion in response to elevations of renal perfusion pressure11. Intrarenal NO generation and urinary excretion of NO metabolites are increased during pressure-dependent changes in sodium excretion12, while NOS inhibition attenuates the pressure natriuretic response13–15. Furthermore, while the proximal tubule plays a role in pressure natriuresis, the CD may also be involved16.
Based on the above considerations, the current study tested the hypothesis that absence of CD-derived ET-1 decreases NOS activity. We examined urinary NO excretion and isoform-specific NOS activity in inner medullae of CD ET-1 KO and control mice. To assess the functional consequences of CD-specific ET-1 KO on renal NO production, we examined urinary NO metabolite excretion and Na excretion during changes in renal perfusion pressure, as well as the effect of NOS inhibition on systemic BP.
METHODS
Animals
All experiments were performed with approval from the Institutional Animal Care and Use Committees at the Medical College of Georgia and the University of Utah. CD ET-1 KO mice and littermate control mice were generated as previously described1, 4. Mice were studied at 3–4 months of age and fed either a normal (0.44% NaCl) or a high (4% NaCl) salt rodent diet, with free access to drinking water.
Chronic BP and NOx excretion experiments
A catheter was inserted into the right carotid artery, tunneled subcutaneously, and the attached radiotransmitter localized to the back. Continuous recording of arterial pressure and heart rate was performed by telemetry (Data Sciences International, Arden Hills, MN). Two days after the surgery, values were recorded for 2 days on a normal salt diet. Subsequently, L-NAME (1 mg/ml, NG-nitro-L-arginine methyl ester, Alexis Biochemicals, San Diego) was added to the drinking water, and BP and heart rate recorded for the next 3 days. Mice were then switched to a high salt diet and drinking water containing L-NAME, and telemetry data recorded for five days.
In separate experiments, mice were placed into Nalgene metabolic cages (Rochester, NY) and acclimated for 3 days on a normal salt diet. Mice were continued on the normal salt diet and urine collected on the second day after the conclusion of the acclimation period. Mice were then switched to a high salt diet and urine collected on the third day of the high salt intake. Urine NOx concentration was determined using Griess reagent and measuring fluorescence at 540 nm on a microplate reader17.
Pressure-excretion experiments
After the induction of anesthesia, the right jugular vein was cannulated, and bovine serum albumin (1%) and 0.75% fluorescein isothiocyanate (FITC)-inulin (Sigma-Aldrich, St. Louis, MO) were administered in saline at 0.4 µl/min/g body weight throughout the study. The left carotid artery was cannulated and connected to a pressure transducer for BP and heart rate measurements. The bladder was catheterized for urine collections. Ligatures were placed loosely around the celiac and the mesenteric arteries, and around the infrarenal aorta.
After 30 minutes of equilibration, baseline urine was collected for 30 minutes ("low" BP period). The ligatures around the celiac and mesenteric arteries were then tightened ("medium" BP period) and urine collected for 30 minutes. Thereafter, the ligature around the aorta was closed ("high" BP period) and urine was collected for 30 minutes. The animals were sacrificed, blood collected, and decapsulated kidney weight determined. For more details of the procedures, please see http://hyper.ahajournals.org.
Plasma and urinary FITC concentrations were determined by measuring fluorescence with a microplate reader. Urinary Na and K concentrations were analyzed using ion-sensitive electrodes (Synchron EL-ISE, Beckman Instruments, Brea, CAL). Urinary immunoreactive ET-1 concentrations were measured by radioimmunoassay (Amersham, Arlington Heights, IL), and urinary NOx concentrations were determined by chemiluminescence (Sievers 280, Nitric Oxide Analyzer, GE Instruments, Boulder, CO).
Isoform-specific NOS activity and expression
Mice were fed normal or high salt diets for 7 days, inner medullary tissue dissected, rapidly frozen, and homogenized as previously described18. Protein concentrations were determined by the Bradford assay (Bio-Rad; Hercules, CA).
NOS activity was assessed by the conversion of [3H]arginine-to-[3H]citrulline in the presence of optimal concentrations of cofactors as previously described18. Total NOS activity was determined using L-NNA (1 mM). N5-(1-imino-3-butenyl)-L-ornithine (VNIO, 1 µmol/L; Cayman Chemicals, Ann Arbor, MI) was used to assess NOS 1-specific activity, and 1400W•dihydrochloride (1400W, 100 nmol/L; Cayman) was used to assess NOS 2-specific activity. NOS 3-specific activity was calculated as total NOS activity - (NOS 1-specific activity + NOS 2-specific activity). The inhibitory constants of VNIO for NOS 1, NOS 2 and NOS 3 are 0.1, 60 and 12 µM, respectively19. The inhibitory constants of 1400W for NOS 1, NOS 2 and NOS 3 are 2 µM, 7 nM and 50 µM, respectively20. NOS activity was normalized to protein and reaction time. NOS isoform expression was determined by Western blotting with isoform-specific antibodies as previously described, with minor modifications6. For additional details of NOS activity and expression, please see http://hyper.ahajournals.org.
Statistics
All data are presented as mean ± standard error. The effect of mouse genotype on renal function during varying levels of BP, salt intake, or L-NAME administration was analyzed by 2-way ANOVA. Baseline parameters between genotypes were analyzed by unpaired student t-tests. The effect of mouse genotype and salt diet on NOS activity and expression was analyzed by 1-way ANOVA. Post-hoc comparison of individual means was by Neuman-Keuls Multiple Comparison Test. A value of P<0.05 was considered statistically significant.
RESULTS
Chronic BP and NO metabolite excretion experiments
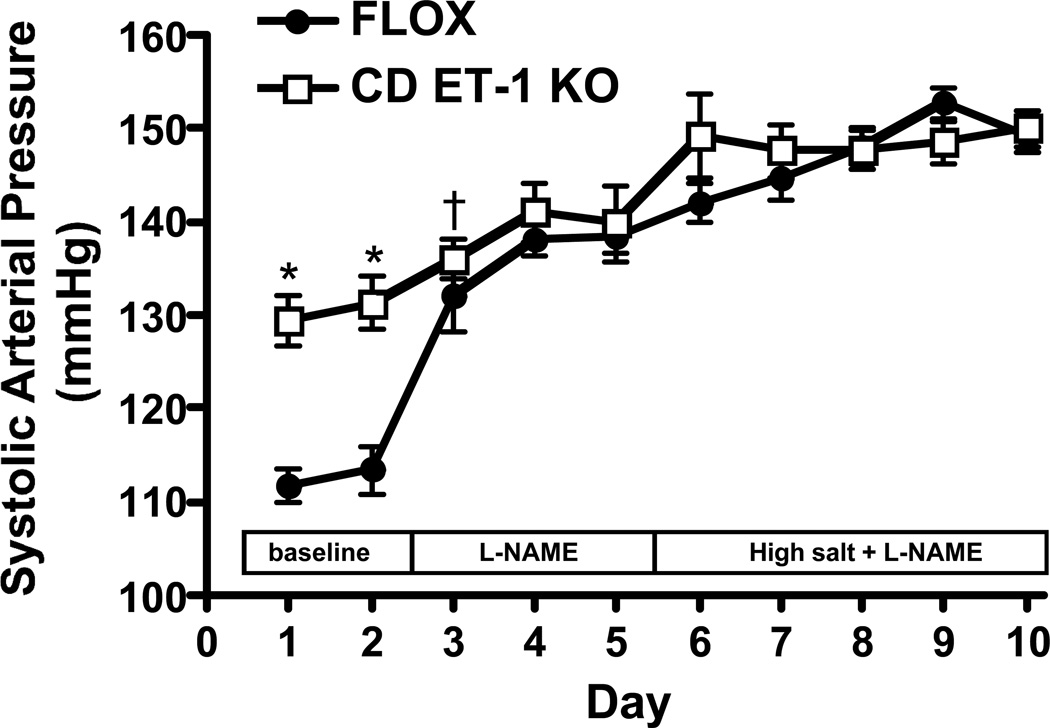
CD ET-1 KO mice had higher systolic BP than controls (Figure 1). Heart rate was not different between the two groups of mice (data not shown). Administration of L-NAME increased systolic BP in both groups, however the rise in systolic BP was greater in control than in CD ET-1 KO mice (Figure 1). After two days of L-NAME, systolic BP was similar in the two groups (Figure 1). Addition of a high Na diet + L-NAME increased systolic BP similarly in both groups (p<0.05 days 6–10 on high Na diet + L-NAME vs. days 4–5 L-NAME alone) (Figure 1). Mean and diastolic BP followed the same pattern as systolic BP, while pulse rate did not differ between the two groups after L-NAME treatment on a normal or high Na diet (data not shown). Food and water intake were similar between CD ET-1 KO and control mice on a normal or L-NAME diet; L-NAME plus high Na intake increased water intake in both groups, but to a comparable degree (data not shown).
Figure 1.
Effect of L-NAME (1 mg/ml in drinking water) on systolic arterial pressure in CD ET-1 KO and Flox control mice (n=6 each group) on a normal and high Na intake. Radiotelemetry devices were implanted in mice and arterial pressure assessed daily on the varying diets. *P<0.005 vs. flox control same day; †P<0.05 vs. flox control same day.
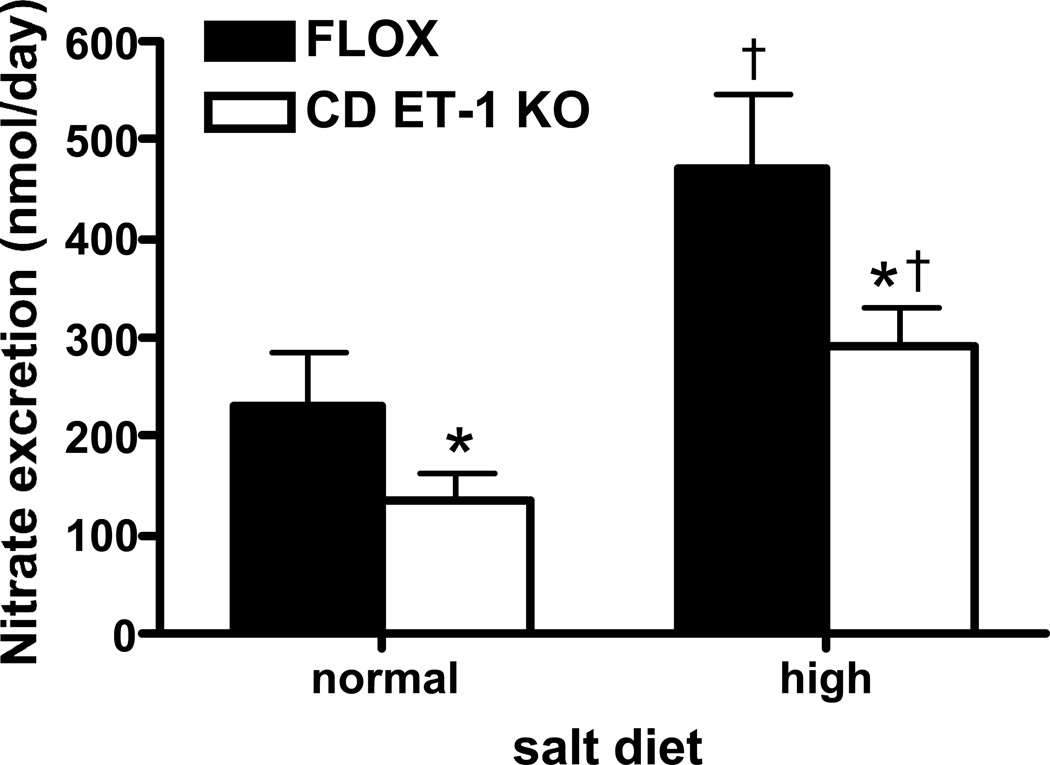
Urinary NOx excretion was lower in CD ET-1 KO mice than in controls under baseline conditions (measured on day 2 of a normal Na diet) (Figure 2). Administration of a high Na diet increased urinary NOx excretion in both groups, although it remained lower in CD ET-1 KO animals (measured on day 2 of a high Na diet) (Figure 2).
Figure 2.
Urinary nitrate/nitrite excretion in Flox control and CD ET-1 KO mice (n=6 each group) on a normal and high Na diet. *P<0.05 vs. control on same diet; †P<0.05 vs. normal Na diet in same mouse genotype.
Pressure-excretion experiments
Flox control and CD ET-1 KO mice had similar body weights and sexes. There was no difference in hematocrit values measured at the end of the protocol (36.4 ± 1.7% Flox control vs. 36.8 ± 2.0% CD ET-1 KO). Total kidney weight (both kidneys) was similar between groups: 414 ± 30 mg Flox control vs. 419 ± 33 mg CD ET-1 KO.
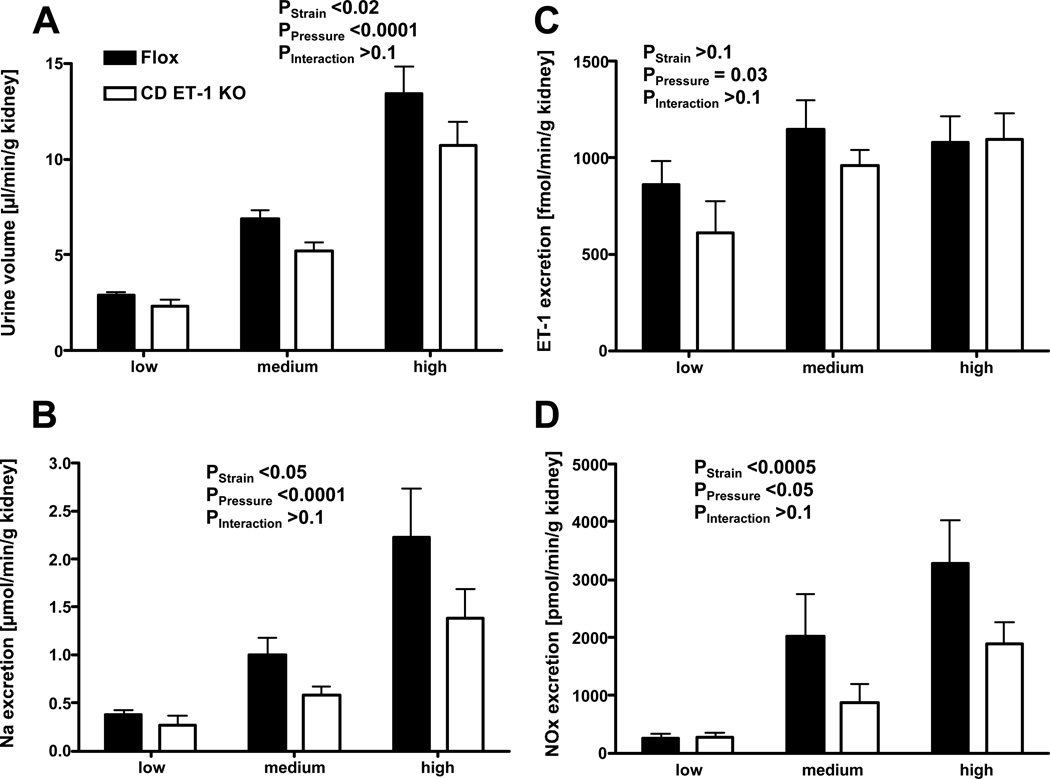
Mean BP increased after tying the celiac and mesenteric arteries, and increased further after tying off the abdominal aorta, with no difference between groups (Figure 3A). Heart rate decreased in both groups similarly (Figure 3B). GFR increased similarly in both groups during increases in renal perfusion pressure (Figure 3C). Urine flow rate also increased in parallel with pressure, however urinary flow rate during the high-pressure period was greater in the Flox controls than CD ET-1 KO. Urinary Na excretion was blunted in CD ET-1 KO mice compared to Flox controls (Figure 4B). During increases in renal perfusion pressure, ET-1 excretion rate increased similarly in both groups (Figure 4C). NOx excretion increased in both groups, but the increase was less in Flox controls (Figure 4D).
Figure 3.
Panel A: Mean arterial blood pressure in Flox control (n=7) and CD ET-1 KO mice (n=6) during baseline (‘low’ pressure), after ligation of celiac and mesenteric arteries (‘medium’) and after ligation of the abdominal aorta below the renal arteries (‘high’). Panel B: Heart rate in Flox control and CD ET-1 KO mice during periods of low, medium, and high blood pressure. Panel C: Glomerular filtration rate (GFR) in Flox control and CD ET-1 KO mice during periods of low, medium, and high blood pressure.
Figure 4.
Panel A: Urinary excretion volumes in Flox control (n=7) and CD ET-1 KO mice (n=6) during periods of low, medium, and high blood pressure. Panel B: Urinary Na excretion in Flox control mice and CD ET-1 KO mice during periods of low, medium, and high blood pressure. Panel C: Urinary ET-1 excretion in Flox control and CD ET-1 KO mice during periods of low, medium, and high blood pressure. Panel D: Urinary nitrate/nitrite (NOx) excretion in Flox control and CD ET-1 KO mice during periods of low, medium, and high blood pressure.
Renal Inner Medullary NOS activity and expression
During normal salt intake, total NOS activity in inner medulla was lower in CD ET-1 KO mice versus Flox controls (Figure 5A). Both NOS1 and NOS3 activities were blunted in homogenates from the CD ET-1 KO compared to Flox controls. High salt intake increased NOS activity in inner medulla from both groups (Figure 5A) when compared to mice on a normal salt diet. Medullary NOS1 and NOS3 activity increased similarly in both groups, although activity in CD ET-1 KO mice remained lower than in Flox controls (Figure 5C–D). In contrast to activity, NOS1 and NOS3 protein expression in inner medulla were similar in Flox control and CD ET-1 KO mice on a normal or high salt diet (please see http://hyper.ahajournals.org).
Figure 5.
Panel A: Isoform-specific NOS activity in renal inner medullary homogenates from Flox control (n=8) and CD ET-1 KO mice (n=8) during normal salt (NS) and high salt (HS) diets. *P<0.05 vs. Flox control on the same diet; †P<0.05 vs. normal Na diet in same mouse genotype.
DISCUSSION
Key findings of this study are: 1) pressure-dependent changes in Na, water and NO excretion are impaired in CD ET-1 KO mice; 2) renal NO production is reduced in CD ET-1 KO mice associated with decreased renal inner medullary NOS1 and NOS3 activity; 3) the difference in BP between CD ET-1 KO and control mice is abolished by NOS blockade; and, 4) the hypertensive effect of L-NAME in control mice is severely attenuated in CD ET-1 KO mice. These results indicate that CD-derived ET-1 regulates the pressure-induced changes in natriuresis and diuresis. CD-derived ET-1 effects are largely due to activation of medullary NO. Finally, CD-derived ET-1-dependent NO production is a major factor in chronic NOS inhibition-induced hypertension.
Pressure-dependent changes in urinary Na excretion are partially dependent upon renal NO production11–15. Our findings indicate that CD-derived ET-1, likely through NO, modulates the pressure-natriuresis relationship. Because ET-1 acts in an autocrine/paracrine fashion, absence of CD-derived ET-1 presumably decreases local NO generation during pressure natriuresis. Potential sites of ET-1-regulated NO generation are the CD5 or neighboring cells, such as interstitial21 or endothelial cells22. Nephron segments proximal to the collecting duct also participate in pressure-natriuresis; the involvement of ET-1 and NO in Na reabsorption in these regions needs investigation.
We observed increased renal ET-1 excretion (which derives entirely from the kidney23) with increased renal perfusion pressure, suggesting that production and/or secretion of renal ET-1 is acutely modified by perfusion pressure. During the ‘low pressure’ period, urinary ET-1 excretion was less in the CD ET-1 KO mice, consistent with previous findings1. Urinary ET-1 excretion rose to comparable levels between the two groups when renal perfusion pressure increased, suggesting that urinary ET-1 excretion, under these conditions, reflects ET-1 production by sites other than the CD. However, the vast majority of CD ET-1 is secreted abluminally24, hence urinary ET-1 excretion is not likely a sensitive marker of CD-derived ET-1 release. How perfusion pressure increases CD ET-1 production is speculative, although one possibility is tubule flow rate; shear stress increases endothelial cell ET-1 production25, 26.
Two other aspects of the pressure-excretion studies deserve comment. First, the BP difference between CD ET-1 KO and control mice was abolished by isoflurane anesthesia. It is conceivable that the substantially lower BP during anesthesia obscures differences found in conscious animals. Importantly, during induction of natriuresis by increased renal perfusion pressure, BP rose similarly in the two groups, allowing the comparison of changes in renal parameters. Second, GFR values during the low-pressure period were lower than previously reported for anesthetized mice, possibly due to BP being at the lower end of the renal blood flow autoregulation range27. This could also explain why GFR was not tightly autoregulated when BP was increased during pressure natriuresis. However, GFR was similar between CD ET-1 KO and control mice throughout the pressure range.
Two major findings were observed during L-NAME administration. First, the BP difference between the two groups of mice was abolished by L-NAME, strongly implicating NO as an effector of CD-derived ET-1 actions. The reduction in urinary NOx excretion and NOS activity in CD ET-1 KO mice supports this conclusion. Second, L-NAME markedly increased BP in controls, but only modestly elevated BP in CD ET-1 KO mice. It is remarkable that NO which is dependent upon CD-derived ET-1, presumably from the CD and adjacent cells, greatly contributed to L-NAME-induced hypertension. This finding underscores the importance of the CD ET-1/NO pathway in control of systemic BP. Notably, another ET-1 regulated natriuretic factor, prostaglandin E2 (PGE2), is not involved in the hypertensive phenotype of CD ET-1 KO mice. ET-1 stimulates collecting duct PGE2 production, however blockade of cyclooxygenase does not alter BP in these mice30.
The finding that that CD-derived ET provides a tonic stimulatory signal to both NOS1 and NOS3 in the renal inner medulla agrees with previous studies. Rats deficient in ETB receptors have reduced inner medullary NOS1 and NOS3 activities6, 7. ET-1 induces NO release in CD cells via activation of NOS15, while ET-1 increases expression of NOS3 in CD cells28. High Na intake increases medullary NOS activity and ET-1 production29. High Na intake increased NOS1 and NOS3 activities in the inner medulla in both mouse genotypes similarly, suggesting that the high salt diet-induced NOS activity may not be via CD-derived ET. Yet, NOS activity from the CD ET-1 KO mice on a high salt diet was lower than in control mice. NOS1 and NOS3 expression in the medullary homogenates were similar in all groups, thus the decreased activity may be mediated by post-translational modification of the NOS isoforms. The cellular sources of the NOS isoforms were not determined in the current study; clearly collecting duct, endothelial, and interstitial cell sources are possibilities.
While these studies provide support for the CD ET/NO system in control of excretory function, there remains the question of hemodynamic versus tubular actions of ETB receptor dependent NO production. Several laboratories have shown that ETB receptor activation increases blood flow within the renal medulla,31,32 which could facilitate improved Na excretion. We have reported that a high-salt diet increases ETB receptor expression and vasodilator activity within the pre-glomerular vessels of juxtamedullary nephrons that feed the medullary circulation.33 The balance between hemodynamic and direct tubular actions will require further study.
PERSPECTIVES
This study highlights the physiological importance of the CD ET-1/NO axis in regulating BP. Our experiments demonstrate that pressure-dependent changes in Na and water excretion require CD-derived ET-1 activation of the NO pathway. This most likely involves activation of NOS1 and/or NOS3. These findings are particularly important in the context of hypertension where there is an altered relationship between BP and Na excretion. It is possible that defects in this pathway could account for elevations in BP in hypertension due to a variety of potential mechanisms, including reduced CD-dependent ET-1 synthesis, reduced NOS expression and/or activity, and others.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance of Jacquelyn Musall.
SOURCE OF FUNDING
This study was funded by the NIH (HL 64776 (DMP), HL 74167 (DMP/JSP), HL 60653 (JSP), DK96392 (DEK) and DK075362 (DEK)), the Deutsche Forschungsgemeinschaft (MPS; SCHN 769/1-1) and by AHA Established Investigator Awards (DMP, JSP).
Footnotes
CONFLICT OF INTEREST
The authors of have no conflicts of interest to disclose.
REFERENCES
- 1.Ahn D, Ge Y, Stricklett P, Gill P, Taylor D, Hughes A, Yanagisawa M, Miller L, Nelson R, Kohan D. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J. Clin. Invest. 2004;114:504–511. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallego MS, Ling BN. Regulation of amiloride-sensitive Na+ channels by endothelin-1 in distal nephron cells. Am. J. Physiol Renal Physiol. 1996;271:F451–F460. doi: 10.1152/ajprenal.1996.271.2.F451. [DOI] [PubMed] [Google Scholar]
- 3.Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. Endothelin, a peptide inhibitor of Na+-K+-ATPase in intact tubular epithelial cells. Am J Physiol Cell Physiol. 1989;257:C1101–C1107. doi: 10.1152/ajpcell.1989.257.6.C1101. [DOI] [PubMed] [Google Scholar]
- 4.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291:F1274–F1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- 5.Stricklett PK, Hughes AK, Kohan DE. Endothelin-1 stimulates NO production and inhibits cAMP accumulation in rat inner medullary collecting duct through independent pathways. Am J Physiol. 2006;290:F1315–F1319. doi: 10.1152/ajprenal.00450.2005. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan J, Goodchild T, Cai Z, Pollock D, Pollock J. ETA and ETB receptor-mediated regulation of nitric oxide synthase 1 (NOS1) and NOS3 isoforms in the renal inner medulla. Acta Physiol. 2007;191:329–336. doi: 10.1111/j.1748-1716.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 7.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet. Hypertension. 2003;41:657–662. doi: 10.1161/01.HYP.0000048193.85814.78. [DOI] [PubMed] [Google Scholar]
- 8.Herrera M, Garvin J. Endothelin stimulates endothelial nitric oxide synthase expression in the thick ascending limb. Am J Physiol Renal Physiol. 2004;287:F231–F235. doi: 10.1152/ajprenal.00413.2003. [DOI] [PubMed] [Google Scholar]
- 9.Plato C, Pollock D, Garvin J. Endothelin inhibits thick ascending limb chloride flux via ETB receptor-mediated NO release. Am J Physiol Renal Physiol. 2000;279:F326–F333. doi: 10.1152/ajprenal.2000.279.2.F326. [DOI] [PubMed] [Google Scholar]
- 10.Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol. 1995;6:89–94. doi: 10.1681/ASN.V6189. [DOI] [PubMed] [Google Scholar]
- 11.Hall JE, Guyton AC, Coleman TG, Mizelle HL, Woods LL. Regulation of arterial pressure: role of pressure natriuresis and diuresis. Fed Proc. 1986;45:2897–2903. [PubMed] [Google Scholar]
- 12.Majid DS, Omoro SA, Chin SY, Navar LG. Intrarenal nitric oxide activity and pressure natriuresis in anesthetized dogs. Hypertension. 1998;32:266–272. doi: 10.1161/01.hyp.32.2.266. [DOI] [PubMed] [Google Scholar]
- 13.Ikenaga H, Suzuki H, Ishii N, Itoh H, Saruta T. Role of NO on pressure-natriuresis in Wistar-Kyoto and spontaneously hypertensive rats. Kidney Int. 1993;43:205–211. doi: 10.1038/ki.1993.33. [DOI] [PubMed] [Google Scholar]
- 14.Majid DS, Williams A, Navar LG. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am J Physiol Renal Physiol. 1993;264:F79–F87. doi: 10.1152/ajprenal.1993.264.1.F79. [DOI] [PubMed] [Google Scholar]
- 15.Salom MG, Lahera V, Miranda-Guardiola F, Romero JC. Blockade of pressure natriuresis induced by inhibition of renal synthesis of nitric oxide in dogs. Am J Physiol Renal Physiol. 1992;262:F718–F722. doi: 10.1152/ajprenal.1992.262.5.F718. [DOI] [PubMed] [Google Scholar]
- 16.Sonnenberg H, Honrath U, Wilson DR. Effects of increased perfusion pressure on medullary collecting duct function. Can J Physiol Pharmacol. 1990;68:402–407. doi: 10.1139/y90-056. [DOI] [PubMed] [Google Scholar]
- 17.Markewitz BA, Michael JR, Kohan DE. Cytokine-induced expression of a nitric oxide synthase in rat renal tubule cells. J Clin Invest. 1993;91:2138–2143. doi: 10.1172/JCI116439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D, Sasser J, Hobbs J, Boriskie A, Pollock D, Carmines P, Pollock J. Posttranslational regulation of NO synthase activity in the renal medulla of diabetic rats. Am J Physiol Renal Physiol. 2005;288:F82–F90. doi: 10.1152/ajprenal.00127.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babu B, Griffith O. N5-(1-imino-3-butenyl)-L-ornithine. A neuronal isoform selective mechanism-based inactivator of nitric oxide synthase. J Biol Chem. 1998;273:8882–8889. doi: 10.1074/jbc.273.15.8882. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen L, Scott J, Topley P, Knowles R, Keerle A, Frend A. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 1997;57:3300–3304. [PubMed] [Google Scholar]
- 21.Maric C, Aldred GP, Antoine AM, Eitle E, Dean RG, Williams DA, Harris PJ, Alcorn D. Actions of endothelin-1 on cultured rat renomedullary interstitial cells are modulated by nitric oxide. Clin Exp Pharmacol Physiol. 1999;26:392–398. doi: 10.1046/j.1440-1681.1999.03060.x. [DOI] [PubMed] [Google Scholar]
- 22.Schena M, Mulatero P, Schiavone D, Mengozzi G, Tesio L, Chiandussi L, Veglio F. Vasoactive hormones induce nitric oxide synthase mRNA expression and nitric oxide production in human endothelial cells and monocytes. Am J Hypertens. 1999;12:388–397. [PubMed] [Google Scholar]
- 23.Abassi ZA, Tate JE, Golomb E, Keiser HR. Role of neutral endopeptidase in the metabolism of endothelin. Hypertension. 1992;20:89–95. doi: 10.1161/01.hyp.20.1.89. [DOI] [PubMed] [Google Scholar]
- 24.Kohan DE, Padilla E. Endothelin-1 is an autocrine factor in rat inner medullary collecting ducts. Am. J. Physiol Renal Phsyiol. 1992;263:F607–F612. doi: 10.1152/ajprenal.1992.263.4.F607. [DOI] [PubMed] [Google Scholar]
- 25.Dancu MB, Berardi DE, Vanden Heuvel JP, Tarbell JM. Asynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:2088–2094. doi: 10.1161/01.ATV.0000143855.85343.0e. [DOI] [PubMed] [Google Scholar]
- 26.Walshe TE, Ferguson G, Connell P, O'Brien C, Cahill PA. Pulsatile flow increases the expression of eNOS, ET-1, and prostacyclin in a novel in vitro coculture model of the retinal vasculature. Invest Ophthalmol Vis Sci. 2005;46:375–382. doi: 10.1167/iovs.04-0806. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz JN. A practical guide to evaluating cardiovascular, renal, and pulmonary function in mice. Am J Physiol Regu Integ Compar Physiol. 2002;282:R1565–R1582. doi: 10.1152/ajpregu.00759.2001. [DOI] [PubMed] [Google Scholar]
- 28.Ye Q, Chen S, Gardner DG. Endothelin inhibits NPR-A and stimulates eNOS gene expression in rat IMCD cells. Hypertension. 2003;41:675–681. doi: 10.1161/01.HYP.0000047204.72286.34. [DOI] [PubMed] [Google Scholar]
- 29.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol. 2001;281:F144–F150. doi: 10.1152/ajprenal.2001.281.1.F144. [DOI] [PubMed] [Google Scholar]
- 30.Ge Y, Strait KA, Stricklett PK, Yang T, Kohan DE. Role of prostaglandins in collecting duct-derived endothelin-1 regulation of blood pressure and water excretion. Am J Physiol Renal Physiol. 2007;293:F1805–F1810. doi: 10.1152/ajprenal.00307.2007. [DOI] [PubMed] [Google Scholar]
- 31.Vassileva I, Mountain C, Pollock DM. Functional role of ETB receptors in the renal medulla. Hypertension. 2003;41:1359–1363. doi: 10.1161/01.HYP.0000070958.39174.7E. [DOI] [PubMed] [Google Scholar]
- 32.Gurbanov K, Rubinstein I, Hoffman A, Abassi Z, Better OS, Winaver J. Differential regulation of renal regional blood flow by endothelin-1. Am J Physiol Renal Physiol. 1996;271:F1166–F1172. doi: 10.1152/ajprenal.1996.271.6.F1166. [DOI] [PubMed] [Google Scholar]
- 33.Schneider MP, Inscho EW, Pollock DM. Attenuated vasoconstrictor responses to endothelin in afferent arterioles during a high-salt diet. Am J Physiol Renal Physiol. 2007;292:F1208–F1214. doi: 10.1152/ajprenal.00280.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.