Abstract
Previous reports have shown that activation of N-methyl-D-aspartate (NMDA) receptors potentiates responses to activation of the group I metabotropic glutamate receptor mGluR5 by reversing PKC-mediated desensitization of this receptor. NMDA-induced reversal of mGluR5 desensitization is dependent on activation of protein phosphatases. However, the specific protein phosphatase involved and the precise mechanism by which NMDA receptor activation reduces mGluR desensitization are not known. We have performed a series of molecular, biochemical, and genetic studies to show that NMDA-induced regulation of mGluR5 is dependent on activation of calcium-dependent protein phosphatase 2B/calcineurin (PP2B/CaN). Furthermore, we report that purified calcineurin directly dephosphorylates the C-terminal tail of mGluR5 at sites that are phosphorylated by PKC. Finally, immunoprecipitation and GST fusion protein pull-down experiments reveal that calcineurin interacts with mGluR5, suggesting that these proteins could be colocalized in a signaling complex. Taken together with previous studies, these data suggest that activation of NMDA receptors leads to activation of calcineurin and that calcineurin modulates mGluR5 function by directly dephosphorylating mGluR5 at PKC sites that are involved in desensitization of this receptor. 2005 Elsevier Ltd. All rights reserved.
Keywords: Glutamate, Metabotropic, LTP, LTD, PKC, Plasticity
1. Introduction
Glutamate elicits synaptic responses by activation of both ionotropic receptors (iGluRs) and G protein-coupled metabotropic glutamate receptors (mGluRs). The iGluRs are ligand-gated cation channels that are classified into the N-methyl-D-aspartate (NMDA), kainate, and [RS]-α-amino-3-hydroxy-5-methyl-isoxazole-propionic acid (AMPA) receptor subtypes (Dingledine et al., 1999). The mGluRs are members of family III G protein-coupled receptors (GPCRs). To date, eight mGluR subtypes (mGluR1-mGluR8) have been cloned from mammalian brain (see Conn and Pin, 1997 for review). These mGluR subtypes can be further classified into three groups. Group I mGluRs consist of mGluRs 1 and 5 and are coupled to Gq and activation of phospholipase C. Group II (mGluRs 2 and 3) and group III (mGluRs 4, 6–8) mGluRs couple to Gi/Go and associated effectors.
One prominent effect of mGluR activation in many neuronal populations is an enhancement of agonist-evoked currents through NMDA receptor channels (Aniksztejn et al., 1991; Jones and Headley, 1995; Bleakman et al., 1992; Harvey and Collingridge, 1993; Fitzjohn et al., 1996; Pisani et al., 1997; Awad et al., 2000). In each of these cases, mGluR-induced potentiation of NMDA receptor currents is mediated by a group I mGluR. However, the specific group I mGluR subtype involved in eliciting this effect can vary in different neuronal populations. For instance, recent pharmacological studies reveal that potentiation of NMDA-evoked currents is mediated by mGluR5 in hippocampal pyramidal cells (Doherty et al., 1997) (Mannaioni et al., 2001) and in neurons in the subthalamic nucleus (Awad et al., 2000), whereas this response is mediated by mGluR1 in cortical cells (Heidinger et al., 2002).
Interestingly, the interaction between NMDA receptors and group I mGluRs is bi-directional. For instance, low concentrations of NMDA can enhance mGluR-mediated increases in phosphoinositide hydrolysis in rat cortex (Challiss et al., 1994; Alagarsamy et al., 1999b). Furthermore, NMDA receptor activation potentiates an inward current induced by 1S,3R-ACPD in hippocampal pyramidal cells (Luthi et al., 1994; Alagarsamy et al., 1999a). We recently reported that NMDA-induced potentiation of group I mGluR responses in recombinant systems and in the hippocampus is mediated by reversal of PKC-induced mGluR desensitization. Furthermore, we found that this response is dependent on activation of a protein phosphatase. Previous reports suggest that PKC desensitizes mGluR5 by phosphorylation of Ser and Thr residues on the C-terminal tail and first and second intracellular loops of the receptor (Gereau and Heinemann, 1998). Taken together, these findings raise the possibility that activation of NMDA receptors induces activation of a protein phosphatase that directly dephosphorylates mGluR5 at sites responsible for desensitization. The studies reported here suggest that NMDA-induced modulation ofmGluR5 is dependent on activation of the calcium-dependent protein phosphatase 2B (PP2B), calcineurin. Furthermore, we provide evidence that calcineurin may exist in a signaling complex with mGluR5 in several brain areas and that this phosphatase can directly dephosphorylate mGluR5 at sites that are phosphorylated by PKC on the C-terminal tail of the receptor.
2. Methods
2.1. Cell culture and transfection
Chinese hamster ovary (CHO) cells were cultured in DMEM supplemented with 10% fetal bovine serum. The cells were grown to ~80% confluency in 24-well plates. For expression of the receptors and a constitutively active mutant of calcineurin (CaNa) (Friday et al., 2000), the plasmids were transfected into the CHO cell line, using a calcium phosphate technique. Each plasmid (1 μg) was added to 50 μl CaCl (0.25 mM) and 50 μl BES (5 mM, pH 7.4) into wells containing cells and 1 ml media. After an overnight incubation, the CaPO4–DNA medium was replaced by fresh medium. Experiments were conducted 2–3 days following transfection.
2.2. Measurement of phosphoinositide hydrolysis
Phosphoinositide hydrolysis in cortical and hippocampal slices was measured as previously described (Desai and Conn, 1991; Conn and Wilson, 1991). Briefly, 300 μm cross-chopped slices were labeled with [3H]-inositol and agonist-induced accumulation of radioactivity in inositol phosphates during a 15-min incubation was measured. Lithium chloride (10 mM) and inhibitors or antagonists were added 20 min prior to addition of agonists. Samples were extracted with chloroform/methanol and [3H]-inositol monophosphate fraction was separated by anion exchange chromatography (Dowex 1 × 4400) using increasing amounts of ammonium formate. [3H]-IP content was assessed by liquid scintillation spectrometry and data are presented as percent of no-agonist control.
Phosphoinositide hydrolysis was measured in cultured cells using a modified version of the method described by Chung et al. (1997). In brief, transfected cells were incubated overnight in glutamine-free DMEM containing [3H] myoinositol (5 μCi/ml). The following morning, cells were washed and incubated in Krebs bicarbonate buffer supplemented with 10 mM LiCl. Agonists were added after 30 min of incubation in the KRB, and cells were allowed to incubate for an additional 45 min. The reaction was terminated by the addition of 2N HCl and cells were frozen at −80 °C for 15 min. After thawing, a plastic policeman was used to scrape and transfer cells to labeled test tubes. Chloroform/methanol extraction and subsequent steps were as described above.
2.3. Xenopus oocyte recordings
Xenopus oocytes were injected and recorded from as previously described (Alagarsamy et al., 1999a). Briefly, oocytes were injected with in vitro transcribed mRNA encoding mGluR5a alone or in the presence of mRNA for a constitutively active mutant form of calcineurin (CaNa) (Friday et al., 2000). Control oocytes were injected with an appropriate volume of water. Dual-electrode voltage clamp recordings were performed in Barth’s medium containing 88 mM NaCl, 1 mM KCl, 2.4 mM CaCl2, 1.2 mM MgCl2, 0.33 mM CaNO3 and 10 mM HEPES.
2.4. Generation of glutathione-S-transferase-fusion proteins
The C-terminal tail of mGluR5a (amino acids 824–1171) was amplified by PCR using directional primers engineered with restriction sites 5′ proximal to the end of the oligomer. The PCR product was then digested with EcoR1 and XhoI and subcloned in-frame into the polylinker region of pGex6P3. Subcloned DNA was transformed into BL21 Eschericia coli (Stratagene) and plated onto LB medium plus ampicillin agar plates. Single colonies were grown overnight in LB medium plus ampicillin, and plasmid DNA was isolated using Qiagen DNA kits. Correct DNA sequence was verified by restriction enzyme analysis and DNA sequence analysis, and the predicted amino acid sequence was determined by computer analysis.
Large-scale preparation of bacterial sonicates for the purification of the mGluR5a-GST fusion protein was performed according to the manufacturer’s protocol (Pharmacia). In brief, a single colony of E. coli cells containing the recombinant pGex6P3 plasmid was grown overnight and used to inoculate 2YT medium plus ampicillin (1:100 dilution). The cells were grown at 37 °C with shaking to an A600 of 1–2, then incubated for an additional 3 h in 0.1 mM IPTG to induce protein expression. The cells were sedimented, sonicated, solubilized in 1X PBS/1% Triton X-100, then bound to glutathione sepharose-4B (Pharmacia Biotech). The glutathione-fusion protein matrix was washed three times in 10 bed volumes of 1X PBS, assayed for protein content, and analyzed by Coommassie gel. A portion of the proteins were eluted from the beads according to the manufacturer’s protocol with buffer containing 10 mM reduced glutathione in 50 mM Tris–HCl, pH 8.0. Elution buffer (1.0 ml) was used per 10 ml bead volume and the supernatant was collected following a 10 min RT incubation.
2.5. Pull-down assays and immunoblots
Frozen rat brains were homogenized by 15–20 strokes of a dounce homogenizer in ice-cold 1X HEN (50 mM HEPES, 10 mM EDTA, 5 mM NaCl) plus protease inhibitors set (Boehringer-Mannheim). The homogenate was solubilized in 1% Triton X-100, 0.1% SDS by gentle shaking at 4 °C for 30 min and then was centrifuged at 14,600 × g for 15 min at 4 °C. The supernatant was transferred to a new tube and a 1:100 dilution of glutathione sepharose-4B beads was added to preclear the lysate. After gently shaking for 30 min at 4 °C, the sample was again centrifuged at 14,600 × g for 15 min at 4 °C. The supernatant was then transferred to a new tube and assayed for protein content. The purified mGluR5a-GST fusion protein (200 μl, 50% slurry) was then added to the homogenate (60 mg protein) and incubated for 1.5 h at 4 °C with gentle shaking. The beads were centrifuged at 3000 RPM for 5 min and washed three times in 10 bed volumes of ice-cold PBS. SDS loading buffer was added to 1X concentrate, the samples were heated to 95 °C for 5 min, centrifuged at 14,000 × g and separated by SDS-polyacrylamide gel electrophoresis. The proteins were transferred onto PVDF membrane that was then blocked in 1X TBS, 5% milk overnight at 4 °C. The membrane was subsequently incubated with 1 μg/ml anti-protein phosphatase 2B mouse monoclonal antibody (Upstate Biotechnology), then 1:10,000 goat-anti mouse antibody conjugated to horseradish peroxidase (BioRad), then processed by ECL (Amersham).
2.6. In vitro phosphorylation
Aliquots (50 μl) of theGST fusion proteins were stored at −20 °C. On the day of phosphorylation an aliquot was thawed and centrifuged for 10 min at 4 °C. The pellet was resuspended in buffer containing 25 mM Tris, 0.5 mM EDTA, 1 mM Na4P2O7, 1.5 mM CaCl2, 20 mM ATP, 10 μCi 32-ATP, 1 μM purified PKC, 1 μM 3-sn-phosphatidyl-L-serine, 1 μM diacylglycerol (DAG) (Calbiochem) and 5 mM sodium orthovanadate. The reaction was incubated for 30 min at 37 °C. Purified PP2B/CaN (100 units, Sigma) and calmodulin (100 nM) was added in buffer containing 40 mM Tris–HCl (pH 7.4), 100 mM NaCl, 6 mM MgCl2, 0.1 mM CaCl2, 0.1 mg/ml BSA and 0.5 mM dithiothreitol and incubated for an additional 30 min (Minakami et al., 1997). The reaction was stopped by adding 100 μl sample buffer containing 100 mM Tris–HCl, pH 6.8, 3.0% SDS, 12% glycerol, 0.2 M dithiothreitol, 1% β-mercaptoethanol and 0.005% bromophenol blue. The samples were heated for 2 min at 100 °C and separated by SDS-PAGE. Gels were dried and exposed to a phosphoscreen and appropriate bands were quantitated on a phosphorimager (Molecular Dynamics) and expressed as percent of no-agonist control.
2.7. Coimmunoprecipitation
Immunoprecipitation experiments and Western blots were performed as previously described (Alagarsamy et al., 1999a) with some modifications. Hippocampal slices were used instead of cortical cultures, radioactive labeling was omitted and supernatants of homogenized samples were immunoprecipitated with anti-mGluR5 (4 μg; Upstate) were probed with either anti-mGluR5 or anti-calcineurin (4 μg; Upstate) for Western blots. Conversely, samples immunoprecipitated with anticalcineurin were probed with both antibodies for the Western blots. Immunoprecipitated samples were run on 7.5% SDS-polyacrylamide gels and transferred to PVDF membranes. Membranes were incubated overnight with primary antibody (1:10,000), 1.5 h with secondary antibody (1:10,000) and bands were visualized by chemiluminescence.
3. Materials
The constitutively active calcineurin construct was a kind gift from Dr. Grace Pavlath of Emory University, antibodies were obtained from Upstate Biotechnology, Lake Placid, NY, and cell culture reagents were obtained from Gibco, Invitrogen, Carlsbad, CA. All other reagents, unless otherwise noted, were obtained from Sigma, St. Louis, MO.
4. Results
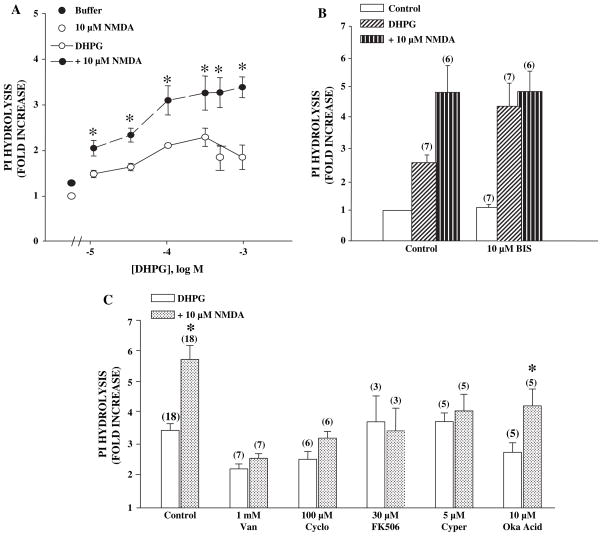
We previously reported that N-methyl-D-aspartate (NMDA) potentiates mGluR5 function by reversing PKC-mediated desensitization of the receptor. Furthermore, studies with protein phosphatase inhibitors suggested that this effect is dependent on activation of a phosphatase. In order to perform more detailed pharmacological studies to gain insight into the phosphatase responsible for this effect of NMDA receptor activation, we determined the effect of NMDA on DHPG-induced phosphoinositide hydrolysis in rat cortex. Since these experiments require agonist incubation times of 45 min compared to less than 45 s required for the physiology experiments, we first confirmed that this assay was suitable for studying the pharmacology of NMDA-induced potentiation of mGluR5-mediated responses. Previous studies suggest that PKC-mediated desensitization greatly reduces the PI response measured in response to these prolonged agonist applications (Schoepp and Johnson, 1988). If NMDA attenuates PKC-mediated desensitization of mGluR5, NMDA should induce an increase in DHPG-induced phosphoinositide hydrolysis. Furthermore, inhibitors of PKC should increase this response and occlude any further potentiation by NMDA. Consistent with this, NMDA (10 μM) potentiates the DHPG-induced increase in PI hydrolysis (Fig. 1A). Furthermore, preincubation for 20 min with the PKC inhibitor bisindolyl maleamide I (BIS) produced a similar increase in DHPG-induced PI hydrolysis in cortical slices. Finally, NMDA does not further potentiate agonist-induced responses in the presence of BIS (Fig. 1B), suggesting that NMDA does not potentiate mGluR5-mediated responses in the absence of PKC-mediated desensitization. In control experiments, BIS has no effect on slices treated with NMDA alone (data not shown).
Fig. 1.
(A) DHPG dose-dependently increases phosphoinositide hydrolysis and NMDA increases this response in slices from adult rat cortex. Data are represented as fold of no drug control and are means ± S.E.M. of 3 experiments done in triplicate. An asterisk (*) indicates statistical difference ( p < 0.05, Student’s t-test) from DHPG response without 10 μM NMDA. (B) PKC inhibitor bisindolylmaleimide (BIS) potentiates the DHPG-induced phosphoinositide hydrolysis and prevents NMDA from causing further potentiation. Data are represented as described for panel A and are the means ± S.E.M. of (n) independent experiments done in triplicate. NMDA-induced a significant potentiation of the response to DHPG in control conditions ( p < 0.05, Student’s t-test) but not in the presence of BIS ( p > 0.05, Student’s t-test). Also BIS induced a significant potentiation of the DHPG response ( p < 0.05 of DHPG control, Student’s t-test). All groups, except BIS control, are statistically different from untreated control, p < 0.01). (C) Phosphatase inhibitors block the NMDA-induced potentiation of DHPG-induced phosphoinositide hydrolysis. The non-specific and calcineurin/PP2B-specific phosphatase inhibitors block the NMDA-induced potentiation of the DHPG response while okadaic acid had no effect. Data represent (n) experiments done in triplicate and are calculated as described above. An asterisk (*) indicates significant difference from each DHPG control, p < 0.05 or less.
We determined the effect of several phosphatase inhibitors on NMDA-induced potentiation of DHPG-induced PI hydrolysis in cortical slices. The effect of NMDA on 500 μM DHPG was inhibited by the non-selective phosphatase inhibitor, vanadate, as well as, several selective calcineurin inhibitors, including FK506 (Torii et al., 1995), cypermethrin (Fakata et al., 1998) and cyclosporin A (Ho et al., 1996) (Fig. 1C). In contrast, analogs of these compounds that do not inhibit calcineurin did not affect the NMDA response (as percent of NMDA response: rapamycin (10 μM), 82 ± 14%; n = 4 and permethrin (10 μM), 106 ± 11%; n = 5). Furthermore, okadaic acid, an inhibitor of phosphatase 1 and 2A at the concentrations used, failed to block the response to NMDA. Additionally, key experiments using vanadate and cyclosporine A were repeated using hippocampal slices. Results followed similar patterns as described above (data not shown) suggesting a general mechanism of mGluR5-NMDA interaction. Interestingly, there was some variation in DHPG response between the various treatment conditions. This variation is not surprising since many of these compounds were highly insoluble and required long incubation times to penetrate the tissue minces (300 μm cross-chopped) and have effect. There may have been some non-selective effects such as tissue toxicity or inhibition not involving phosphatases that are responsible for the observed variation. Indeed, apparent inhibition was generally much more pronounced when we used non-selective inhibitors compared to treatments with PP2B-selective inhibitors. Although, okadiac acid does not inhibit PP2B activity, it is not a selective phosphatase inhibitor inhibiting both PP1 and PP2A, thus many non-mechanism based effects are possible. Despite this possibility, there was not a significant reduction in DHPG response (3.49 ± 0.21, control 2.79 ± 0.29, okadaic acid, p = 0.11, t-test) with okadaic acid and there is no difference in fold potentiation with NMDA (1.66 ± 0.08, control 1.54 ± 0.06, okadaic acid, p = 0.45). Thus, the pharmacological profile of the NMDA-induced potentiation of DHPG-stimulated PI hydrolysis response suggests that this response is dependent on activation of PP2B, calcineurin.
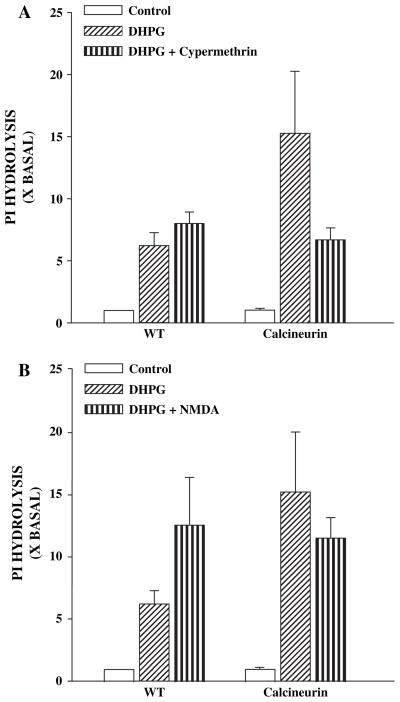
To confirm that increases in calcineurin activity can increase group I mGluR-mediated responses we determined the effect of 500 μM DHPG on PI hydrolysis in mice that have confirmed expression of a constitutively active form of calcineurin in the hippocampus (Mansuy et al., 1998). DHPG-induced PI hydrolysis was significantly higher in hippocampal slices from mice expressing the constitutively active form of calcineurin than the responses measured in slices made from wildtype animals (Fig. 2A). Furthermore, preincubation of the slices for 20 min with the calcineurin inhibitor, cypermethrin (5 μM), reduced DHPG-induced PI hydrolysis in mutant mice but not in WT animals (Fig. 2A), suggesting that the increase in the PI hydrolysis response is mediated by increased calcineurin activity. Finally, NMDA (3 μM) increased DHPG-induced PI hydrolysis in hippocampal slices obtained from WT mice, but failed to induce an increase in DHPG-induced PI hydrolysis in hippocampal slices obtained from the mutant mice (Fig. 2B). Taken together, these data suggest that increased calcineurin activity can increase mGluR5-mediated responses in hippocampal slices and occludes any further response to NMDA.
Fig. 2.
(A) Phosphoinositide hydrolysis in hippocampal slices made from wild-type (WT) mice that overexpress a constitutively active mutant of calcineurin (CAL). The agonist-induced responses are higher in tissue obtained from the mutant mice and this increase in response is reduced to WT levels in the presence of the calcineurin inhibitor, cypermethrin (cyper). (B) In contrast to slices made from WT mice, NMDA does not further potentiate the agonist response in the mutant mice. Data represented are pooled data from 12 mice and are expressed as percent of no drug control and are means ± S.E.M. of experiments done in triplicate.
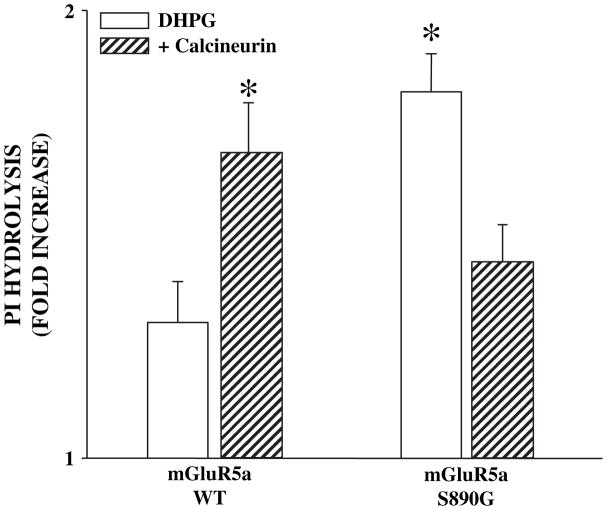
We employed recombinant approaches to further test the hypothesis that calcineurin potentiates mGluR5 by reducing desensitization. First, we transiently transfected Chinese hamster ovary (CHO) cells with cDNA encoding wild-type mGluR5 or a mutant of mGluR5 in which a major PKC phosphorylation site responsible for mGluR5 desensitization has been eliminated (mGluR5-S890G). While multiple sites on mGluR5 can be phosphorylated by PKC and may contribute to desensitization, this mGluR5 mutant has been shown to display little or no PKC-mediated desensitization (Gereau and Heinemann, 1998). WT or mutant mGluR5 was expressed in the presence or absence of a construct encoding a constitutively active form of calcineurin termed CaNa (Friday et al., 2000). CaNa is a truncated variant of calcineurin A which was modified by removal of the carboxy-terminus region containing the autoinhibitory domain and a portion of the calmodulin-binding domain (O’Keefe et al., 1992). Consistent with the hypothesis that calcineurin reverses mGluR5 desensitization, cells co-transfected with the WT mGluR5 and constitutively active CaNa displayed greater PI hydrolysis in response to 45 min stimulation with 500 μM DHPG when compared to the cells expressing WT mGluR5a alone (Fig. 3). Similarly, cells transfected with the non-desensitizing mutant mGluR5-S890G, displayed a higher agonist-induced PI response when compared to cells expressing wild-type mGluR5 alone. Interestingly, cells transfected with mGluR5-S890G and CaNa produced responses that were significantly reduced compared to cells expressing mGluR5-S890G alone. Although unexpected, this reduction in response suggests that CaNa-induced potentiation of mGluR5-mediated responses is dependent on the presence of the S890 PKC phosphorylation site.
Fig. 3.
DHPG-induced phosphoinositide hydrolysis in Chinese hamster ovary (CHO) cells transiently transfected with mGluR5a wild-type (mGluR5a-WT) or mGluR5a-S890G DNA in the presence or absence of a calcineurin construct that is constitutively active (CaNa). There is a significant increase in response of cells transfected with mGluR5a-WT and CaNa and mGluR5a-S890G alone. As predicted, there is no further potentiation of responses in cells transfected with mGluR5a-S890G and CaNa. Data are represented as fold increase of no drug control measured 45 min after addition of 500 μM DHPG and are means ± S.E.M. of 5 experiments done in triplicate. An asterisk (*) indicates significant difference from response of cells transfected with mGluR5a-WT alone, p < 0.05 or less.
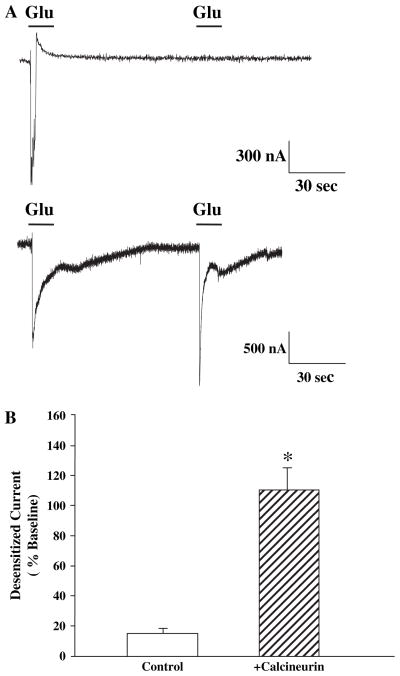
In order to more directly determine the effect of calcineurin on mGluR5 desensitization, we coexpressed mGluR5a and CaNa in Xenopus oocytes. We found that when mGluR5a is expressed alone, there is normal desensitization that can be measured as a dramatic reduction in the response to the second of two closely spaced sequential applications of agonist (Fig. 4). Glutamate was used in these experiments because there was only expression of one subtype (mGluR5) of glutamate receptor. However, if CaNa is coexpressed with mGluR5a, there is full recovery of the response to the second agonist application (Fig. 4). Furthermore, the duration of the response to the first agonist application is extended, suggesting that desensitization that occurs during the agonist application is removed. Duration of return to absolute baseline was variable between oocytes in these experiments, but mean duration of peak currents was 2.1 ± 0.23 fold larger than oocytes expressing mGluR5 alone. This is similar to our previous results showing that PKC inhibitors, which prevent desensitization, increase response duration in mGluR5-expressing oocytes (Gereau and Heinemann, 1998). Control oocytes injected with water or CaNa alone produced no measurable currents (data not shown).
Fig. 4.
Dual-electrode voltage clamp recordings from Xenopus oocytes injected with mRNA made from cDNA of mGluR5a alone (top trace) or mGluR5a plus constitutively active calcineurin (CaNa) (bottom trace). Those oocytes expressing both mGluR5 and CaNa show less mGluR5 desensitization. Bar graph shows mean data expressed as percentage of second current from nine oocytes in each group and an asterisk (*) indicates statistical significance from oocytes not expressing CaNa, p < 0.01.
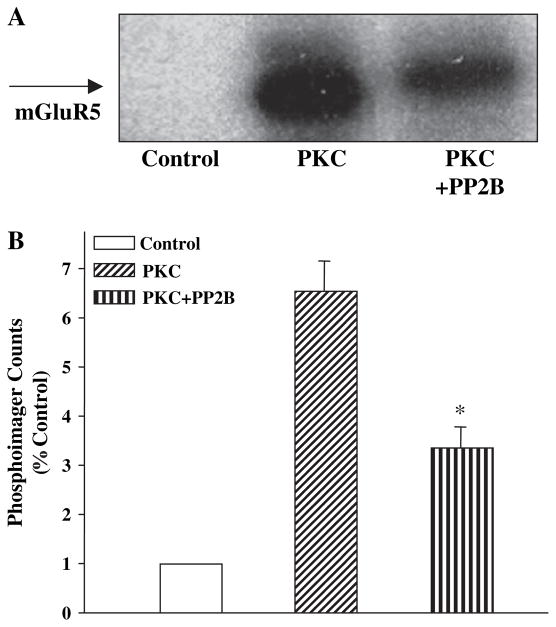
Taken together, these data suggest that activation of calcineurin reduces mGlu5 desensitization. Our previous studies suggest that PKC desensitizes mGluR5 by direct phosphorylation of the receptor. Thus, it is possible that calcineurin reverses mGluR5 desensitization by dephosphorylating this receptor on sites phosphorylated by PKC. We performed a series of biochemical studies to determine whether calcineurin dephosphorylates mGluR5 phosphorylated by PKC. To accomplish this, we constructed a glutathione-S-transferase (GST) fusion protein containing the sequence of the C-terminal tail of mGluR5 (GST-mGluR5aCTX). This fusion protein was incubated with purified PKC, Ca2+, 3-sn-phosphatidyl-L-serine and diacylglycerol (DAG) in the presence and absence of calcineurin/PP2B and 32P-ATP. PKC-induced a robust phosphorylation of GST-mGluR5aCTX that could be measured as an increase in incorporation of 32P into the protein (Fig. 5). Control reactions performed in the absence of PKC (Fig. 5) or with GST alone (data not shown) produced no measurable 32P-ATP incorporation. Incubation with calcineurin/PP2B following PKC-induced phosphorylation causes a significant decrease in the amount of 32P incorporated into GST-mGluR5aCTX. These data suggest that the intracellular C-terminal tail of mGluR5 is a substrate for calcineurin/PP2B and that calcineurin/PP2B directly dephosphorylates mGluR5 at sites phosphorylated by PKC.
Fig. 5.
(A) Representative autoradiograph of mGluR5aCTX-GST fusion protein phosphorylated in vitro with purified protein kinase C (PKC) and appropriate activators in the presence and absence of purified calcineurin/PP2B (PP2B). PKC causes a significant increase in 32P incorporation into mGluR5aCTX-GST fusion proteins over samples without PKC while addition of PP2B decreases the amount. (B) The bands from three independent experiments were quantitated using a phosphoimager and are represented in the bar graph as fold increase over samples containing no PKC. An asterisk (*) indicates significant difference from the PKC effect, p < 0.05.
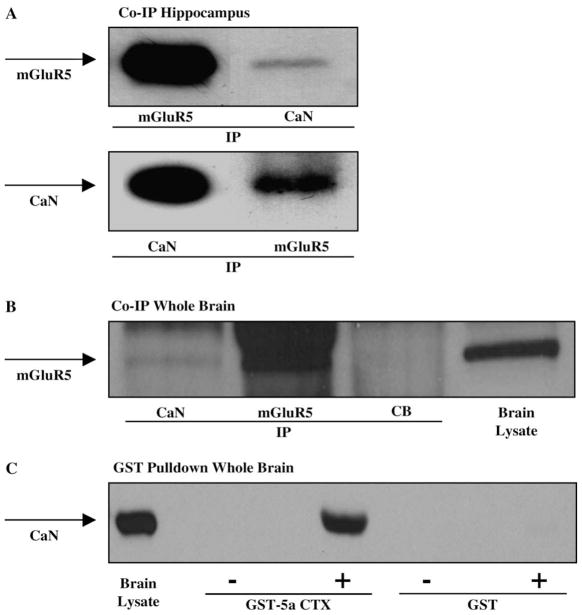
Several recent studies suggest that phosphatases can exist in large signaling complexes present in the postsynaptic density (see Kennedy, 1999 for review). Furthermore, mGluR5 is physically connected to other proteins in the post-synaptic density via the interacting proteins Homer and Shank (Naisbitt et al., 1999; Tu et al., 1999). Therefore, we hypothesized that mGluR5 and calcineurin might be connected as components of a signaling complex. We employed immunoprecipitation techniques to determine whether mGluR5 and calcineurin interact in native systems. Fig. 6A and B show immunoblot analysis of mGluR5 and calcineurin coimmunoprecipitations in rat hippocampal lysates or whole brain lysates, respectively. Fig. 6A shows that mGluR5 coimmunoprecipitates with CaN, and that CaN coimmunoprecipitates with mGluR5 in hippocampus. Fig. 6B shows that mGluR5 coimmunoprecipitates with CaN but not with a non-specific antibody, cascade blue, in whole brain lysates. These data suggest that mGluR5 and calcineurin are physically associated in hippocampal or whole brain homogenates, either directly or through intermediate proteins.
Fig. 6.
(A) Coimmunoprecipitation of mGluR5 and calcineurin. Immunoblots of rat hippocampal homogenates were immunoprecipitated with either mGluR5 or calcineurin (CaN) antibodies, and probed with mGluR5 and CaN antibodies. The blots show that mGluR5 and CaN are immunoprecipitated from hippocampal lysates, and that CaN coimmunoprecipitates with mGluR5, and mGluR5 coimmunoprecipitates with CaN. (B) Immunoblot of rat whole brain lysates immunoprecipitated with CaN, mGluR5, or a negative control cascade blue antibody, and probed for mGluR5. The blot shows that mGluR5 is detected in total brain lysate and in the mGluR5 immunoprecipitate, and that CaN is also detected as a coimmunoprecipitate of mGluR5, however, there is no mGluR5 detected in the negative control (CB). (C) Pull-down assay of mGluR5a and CaN. Rat whole brain homogenates were incubated with GST fusion protein or GST-mGluR5a carboxy terminal domain (GST-5aCTX) and probed with CaN antibody. The results show no CaN detected in GST alone, GST incubated with rat brain lysate, or GST-5aCTX alone, but CaN is detected in total brain lysate and GST-5aCTX incubated with rat brain lysate.
Further evidence to support an interaction between mGluR5a and calcineurin comes from pull-down assays using GST fusion proteins (Fig. 6C). Whole brain lysates were incubated with GST alone or GST-mGluR5aCTX, and the resultant protein blots were incubated with antibody to CaN. Consistent with the immunoprecipitation studies, CaN is detected in the GST-mGluR5aCTX samples, but not in the GST alone, nor in the negative controls where GST and GSTmGluR5aCTX were incubated with buffer alone. These data also suggest that mGluR5 and CaN interact in rat brain lysates, directly or through intermediate proteins.
5. Discussion
In total, the data presented here suggest that calcineurin and mGluR5 may exist in a signaling complex and that calcineurin is capable of potentiating mGluR5 function by direct dephosphorylation of the receptor at PKC phosphorylation sites. Our biochemical studies provide clear support for the hypothesis that mGluR5 desensitization is reversed by NMDA receptor-mediated activation of calcineurin. Functional studies, coupled with the finding that purified calcineurin dephosphorylates PKC sites on the C-terminal tail of the receptor suggests that calcineurin reverses this desensitization by directly dephosphorylating the Cterminal tail of mGluR5 on Ser/Thr residues phosphorylated by PKC. Finally, we provide evidence that mGluR5 and calcineurin are physically associated, either directly or through intermediate proteins and may therefore be part of an associated signaling complex in the post-synaptic density. One possible intermediate protein linking mGluR5 and calcineurin could be calmodulin, which is necessary for calcineurin activation and also binds to the C-terminus of mGluR5 (Minakami et al., 1997; Ishikawa et al., 1999).
Several findings from a combination of molecular, biochemical, and genetic studies clearly support the hypothesis that NMDA-induced potentiation of mGluR5 function is dependent on activation of calcium-dependent protein phosphatase 2B/calcineurin (PP2B/CaN). Furthermore, the finding that purified calcineurin directly dephosphorylates the C-terminal tail of mGluR5 at sites that are phosphorylated by PKC supports the view that this is mediated by direct dephosphorylation of the receptor. However, one surprising finding in the current studies was that expression of constitutively active form of calcineurin (CaNa) inhibited responses to a mutant form of mGluR5 in which a key PKC phosphorylation site was removed (mGluR5a-S890G). This occurred despite the fact that CaNa potentiated the response to activation of the WT receptor. These data suggest that CaN must have an effect elsewhere in the system that can actually inhibit the response to the non-desensitizing mutant. While this finding is not inconsistent with the primary conclusion that calcineurin reverses mGluR5 potentiation, it does suggest that this phosphatase may also have other actions in CHO cells that can be unmasked with the non-desensitizing mutant. CaN may have any number of effects in these cells that include dephosphorylation of another site on mGluR5 but also include dephosphorylation of any number of other proteins that participate in the phosphoinositide hydrolysis response. In future experiments, it will be important to characterize this effect of calcineurin on mGluR5 signaling and to determine whether this is specific to CHO cells or also occurs in native systems.
Recent evidence suggests that NMDA receptors and mGluR5 may be physically linked through a series of protein–protein interactions (Naisbitt et al., 1999; Tu et al., 1999; Ehlers, 1999). Therefore, these two glutamate receptor subtypes may function together as tightly associated signaling partners. The data presented here suggest that calcineurin may be an additional member of this signaling complex. This physical and functional interaction may have implications in several physiological and pathophysiological processes. For example, studies suggest that group I mGluRs modulate LTP and LTD in the hippocampus and participate in certain NMDA receptor-dependent forms of learning (Collingridge and Bliss, 1995; Riedel, 1996). Consistent with this hypothesis, mGluR5 knockout (KO) mice are deficient in NMDA receptor-dependent LTP and spatial learning (Lu et al., 1997; Jia et al., 1998). These effects may be mediated by PKC-mediated potentiation of NMDA receptor function by group I mGluRs (Kelso et al., 1992; Aniksztejn et al., 1991; Skeberdis et al., 2001).
Just as mGluR5 regulates NMDA receptor function, NMDA receptors are critical for mGluR5 function in many areas of the CNS. Previous studies have shown that NMDA potentiates mGluR5 responses (Challiss et al., 1994; Luthi et al., 1994). We have previously shown that a likely mechanism for this effect is via reversal of PKC-mediated desensitization of mGluR5 (Alagarsamy et al., 1999a). Our previous report and current data are consistent with the hypothesis that NMDA activates PP2B/calcineurin to mediate reversal of mGluR5 desensitization and that calcineurin acts by interaction with, and dephosphorylation of, the C-terminus of mGluR5. Thus, if NMDA receptors and mGluR5 are physically and functionally attached, PP2B/calcineurin may be an integral part of that signaling complex. Accordingly, PP2B/calcineurin has been implicated in several processes involving glutamatergic transmission. PP2B/calcineurin has been reported to be important for mechanisms of synaptic plasticity such as long-term depression (LTD) (Mulkey et al., 1993; O’Dell and Kandel, 1994) and depotentiation (Zhuo et al., 1999). Furthermore, PP2B/calcineurin has been shown to alter long-term potentiation (LTP) (Malleret et al., 2001; Winder et al., 1998; Mansuy et al., 1998). Thus, understanding mechanisms of PP2B/calcineurin regulation could provide valuable insight into regulation of glutamatergic signal transduction.
Acknowledgments
The authors would like to thank Dr. Grace Pavlath for her kind contribution of the constitutively active calcineurin construct and FK506. This work was supported by NIH NINDS grants (S.A. and P.J.C.) and NIMH grants (R.W.G).
References
- Alagarsamy S, Marino MJ, Rouse ST, Gereau RW, Heinemann SF, Conn PJ. Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci. 1999a;2:234–240. doi: 10.1038/6338. [DOI] [PubMed] [Google Scholar]
- Alagarsamy S, Rouse ST, Gereau RW, Heinemann SF, Smith Y, Conn PJ. Activation of N-methyl-D-aspartate receptors reverses desensitization of metabotropic glutamate receptor, mGluR5, in native and recombinant systems. Ann N Y Acad Sci. 1999b;868:526–530. doi: 10.1111/j.1749-6632.1999.tb11321.x. [DOI] [PubMed] [Google Scholar]
- Aniksztejn L, Bregestovski P, Ben Ari Y. Selective activation of quisqualate metabotropic receptor potentiates NMDA but not AMPA responses. Eur J Pharmacol. 1991;205:327–328. doi: 10.1016/0014-2999(91)90921-c. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakman D, Rusin KI, Chard PS, Glaum SR, Miller RJ. Metabotropic glutamate receptors potentiate ionotropic glutamate responses in the rat dorsal horn. Mol Pharmacol. 1992;42:192–196. [PubMed] [Google Scholar]
- Challiss RA, Mistry R, Gray DW, Nahorski SR. Modulatory effects of NMDA on phosphoinositide responses evoked by the metabotropic glutamate receptor agonist 1S,3R-ACPD in neonatal rat cerebral cortex. Br J Pharmacol. 1994;112:231–239. doi: 10.1111/j.1476-5381.1994.tb13057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DS, Traynelis SF, Murphy TJ, Conn PJ. 4-Methylhomoibotenic acid activates a novel metabotropic glutamate receptor coupled to phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1997;283:742–749. [PubMed] [Google Scholar]
- Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Wilson KM. Modifications to phosphoinositide hydrolysis. In: Wheal H, Chand J, editors. Cellular and Molecular Neurobiology: A Practical Approach. Macmillian Press; New York: 1991. pp. 115–133. [Google Scholar]
- Desai MA, Conn PJ. Excitatory effects of ACPD receptor activation in the hippocampus are mediated by direct effects on pyramidal cells and blockade of synaptic inhibition. J Neurophysiol. 1991;66:40–52. doi: 10.1152/jn.1991.66.1.40. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Ehlers MD. Synapse structure: glutamate receptors connected by the shanks. Curr Biol. 1999;9:R848–R850. doi: 10.1016/s0960-9822(00)80043-3. [DOI] [PubMed] [Google Scholar]
- Fakata KL, Swanson SA, Vorce RL, Stemmer PM. Pyrethroid insecticides as phosphatase inhibitors. Biochem Pharmacol. 1998;55:2017–2022. doi: 10.1016/s0006-2952(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Irving AJ, Palmer MJ, Harvey J, Lodge D, Collingridge GL. Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci Lett. 1996;203:211–213. doi: 10.1016/0304-3940(96)12301-6. [DOI] [PubMed] [Google Scholar]
- Friday BB, Horsley V, Pavlath GK. Calcineurin activity is required for the initiation of skeletal muscle difierentiation. J Cell Biol. 2000;149:657–666. doi: 10.1083/jcb.149.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereau RW, Heinemann SF. Role of protein kinase C phosphorylation in rapid desensitization of metabotropic glutamate receptor 5. Neuron. 1998;20:143–151. doi: 10.1016/s0896-6273(00)80442-0. [DOI] [PubMed] [Google Scholar]
- Harvey J, Collingridge GL. Signal transduction pathways involved in the acute potentiation of NMDA responses by 1S,3R-ACPD in rat hippocampal slices. Br J Pharmacol. 1993;109:1085–1090. doi: 10.1111/j.1476-5381.1993.tb13733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger V, Manzerra P, Wang XQ, Strasser U, Yu SP, Choi DW, Behrens MM. Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: mediation through the Pyk2/Src-family kinase pathway in cortical neurons. J Neurosci. 2002;22:5452–5461. doi: 10.1523/JNEUROSCI.22-13-05452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Nash SR, Nishimune A, Neki A, Kaneko S, Nakanishi S. Competitive interaction of seven in absentia homolog-1A and Ca2+/calmodulin with the cytoplasmic tail of group 1 metabotropic glutamate receptors. Genes Cells. 1999:381–390. doi: 10.1046/j.1365-2443.1999.00269.x. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Headley PM. Interactions between metabotropic and ionotropic glutamate receptor agonists in the rat spinal cord in vivo. Neuropharmacology. 1995;34:1025–1031. doi: 10.1016/0028-3908(95)00055-b. [DOI] [PubMed] [Google Scholar]
- Kelso SR, Nelson TE, Leonard JP. Protein kinase C-mediated enhancement of NMDA currents by metabotropic glutamate receptors in Xenopus oocytes. J Physiol. 1992;449:705–718. doi: 10.1113/jphysiol.1992.sp019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB. On beyond LTP. Long-term potentiation. Learn Mem. 1999;6:417–421. doi: 10.1101/lm.6.5.417. [DOI] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Gahwiler BH, Gerber U. Potentiation of a metabotropic glutamatergic response following NMDA receptor activation in rat hippocampus. Pflugers Arch. 1994;427:197–202. doi: 10.1007/BF00585965. [DOI] [PubMed] [Google Scholar]
- Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, Mansuy IM. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Minakami R, Jinnai N, Sugiyama H. Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. J Biol Chem. 1997;272:20291–20298. doi: 10.1074/jbc.272.32.20291. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschano J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- O’Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem. 1994;1:129–139. [PubMed] [Google Scholar]
- O’Keefe SJ, Tamura J, Kincaid RL, Tocci MJ, O’Neill EA. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Pisani A, Calabresi P, Centonze D, Bernardi G. Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Br J Pharmacol. 1997;120:1007–1014. doi: 10.1038/sj.bjp.0700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G. Function of metabotropic glutamate receptors in learning and memory. Trends Neurosci. 1996;19:219–224. doi: 10.1016/0166-2236(96)20012-8. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Johnson BG. Selective inhibition of excitatory amino acid-stimulated phosphoinositide hydrolysis in the rat hippocampus by activation of protein kinase C. Biochem Pharmacol. 1988;37:4299–4305. doi: 10.1016/0006-2952(88)90610-7. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Opitz T, Zheng X, Bennett MV, Zukin RS. mGluR1-mediated potentiation of NMDA receptors involves a rise in intracellular calcium and activation of protein kinase C. Neuropharmacology. 2001;40:856–865. doi: 10.1016/s0028-3908(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Torii N, Kamishita T, Otsu Y, Tsumoto T. An inhibitor for calcineurin, FK506, blocks induction of long-term depression in rat visual cortex. Neurosci Lett. 1995;185:1–4. doi: 10.1016/0304-3940(94)11210-a. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Winder DG, Mansuy IM, Osman M, Moallem TM, Kandel ER. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Zhang W, Son H, Mansuy I, Sobel RA, Seidman J, Kandel ER. A selective role of calcineurin aalpha in synaptic depotentiation in hippocampus. Proc Natl Acad Sci U S A. 1999;96:4650–4655. doi: 10.1073/pnas.96.8.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]