Abstract
Generalized tonic-clonic seizures (GTCS) are the commonest seizure type associated with Sudden Unexplained Death in Epilepsy (SUDEP). This study examines semiological and electroencephalographic differences (EEG) in the GTCS of adults as compared to children. The rationale lies in epidemiological observations that have noted a ten-fold higher incidence of SUDEP in adults. We analyzed video-EEG data of 105 GTCS in 61 consecutive patients (12 children, 23 seizures and 49 adults, 82 seizures) recruited from the Epilepsy Monitoring Unit. Semiological, EEG and 3-channel EKG features were studied. Peri-ictal seizure phase durations were analyzed including tonic, clonic, total seizure, post-ictal EEG suppression (PGES) and recovery phases. Heart rate variability (HRV) measures including RMSSD (root mean square successive difference of R-R intervals), SDNN (standard deviation of NN intervals) and SDSD (standard deviation of differences) were analyzed (including low frequency/high frequency power ratios) during pre-ictal baseline, ictal and post-ictal phases. Generalized estimating equations (GEE) were used to find associations between electro-clinical features. Separate subgroup analyses were carried out on adult and pediatric age groups as well as medication groups (no anti-epileptic medication cessation versus unchanged or reduced medication) during admission. Major differences were seen in adult and pediatric seizures with total seizure duration, tonic phase, PGES and recovery phases being significantly shorter in children (p<0.01). GEE analysis using tonic phase duration as the dependent variable, found age to correlate significantly (p<0.001) and this remained significant during subgroup analysis (adults and children) such that each 0.12 second increase in tonic phase duration correlated with a 1 second increase in PGES duration. PGES durations were on average 28 seconds shorter in children. With cessation of medication, total seizure duration was significantly increased by a mean value of 8 seconds in children and 11 seconds in adults (p<0.05). Tonic phase duration also significantly increased with medication cessation and although PGES durations increased, this was not significant. RMSSD was negatively correlated with PGES duration (longer PGES durations were associated with decreased vagally mediated heart rate variability; p<0.05) but not with tonic phase duration. This study clearly points out identifiable electro-clinical differences between adult and pediatric GTCS that may be relevant in explaining lower SUDEP risk in children. The findings suggest that some prolonged seizure phases and prolonged PGES duration may be electro-clinical markers of SUDEP risk and merit further study.
Keywords: Generalized tonic-clonic seizures, Age-specific, SUDEP, PGES
Introduction
The risk of Sudden Unexplained Death in Epilepsy (SUDEP) in children is up to tenfold less than that of adults, comparable to general population rates, varying between 1.1 and 3.4/10000 patient years (Donner et al 2001, Milroy 2011, Nickels et al 2012). Pediatric SUDEP may be phenomenologically different from adult SUDEP (Donner et al 2001, Rodriguez et al 2012). Generalized tonic clonic seizures (GTCS) are the seizure type most strongly associated with SUDEP (Hesdorffer et al 2011, Hesdorffer et al 2012, Langan et al 2005, Nilsson et al 1999, Shorvon & Tomson 2011). Carefully analyzed video-EEG studies have shown that typical GTCS are rare in children under 3 years of age (Hamer et al 1999, Korff & Nordli 2005). Post-ictal EEG Suppression (PGES) is an EEG phenomenon linked to the tonic phase of GTCS (Tao et al 2012) and has been proposed as a risk marker for SUDEP (Lhatoo et al 2010), which in the vast majority of cases is an ictal or post-ictal phenomenon (Shorvon & Tomson 2011, Surges & Sander 2012). Other studies have pointed out an association between GTCS and PGES (Semmelroch et al 2012, Seyal et al 2012a, Seyal et al 2012b, Surges et al 2011) as well as post-ictal impairment of respiratory function and arousal (Semmelroch et al 2012, Seyal et al 2012a, Surges et al 2011). We set out to examine and compare these peri-ictal clinical (semiological) and electroencephalographic differences between adults and childhood in a population of patients with refractory GTCS, a high-risk group for SUDEP.
Methodology
We analyzed video-EEG data on refractory epilepsy patients from the Epilepsy Monitoring Units at Rainbow Babies and Children's Hospital and University Hospitals Case Medical Center, Cleveland USA, monitored during a 9-year period up to January 2012 after obtaining IRB approval. We included all patients >1 month in age who had at least one GTCS during monitoring. The pediatric group comprised patients <16 years whereas older patients were considered adults. Data on age, sex, epilepsy onset, seizure frequency, type of epilepsy, co-morbidities, etiology, learning disabilities, MRI findings, localization of the putative epileptogenic zone, current and past anti-epileptic drugs (AEDs) and AED status during monitoring (unchanged, reduction, withdrawal). EEGs were recorded on Nihon Kohden EEG acquisition software with a 1000Hz sampling rate using conventional bipolar and common average referenced 10-20 montages.
Clinical Analysis
GTCS were defined as seizures resulting in tonic and clonic motor phenomena, regardless of sequence, involving all four limbs and with complete loss of consciousness. Seizure type, lateralizing signs, clinical onset, and duration of tonic and clonic phases of each seizure were studied. Where there was more than one tonic or clonic phase, the sum of both phases was used in statistical analysis. Onset of the tonic phase was defined as the point where there was clear bilateral tonicity and included the “vibratory” or “jittery” phase described by Gastaut (8Hz EMG artifact) (Gastaut 1963). The onset of the clonic phase was defined as the end of the “vibratory period” (where EMG artifact slowed to 4Hz). Seizure end was defined as cessation of all clinical manifestations and/or EEG paroxysmal activity.
Electrophysiological Analysis
EEG recordings of GTCS were analyzed. PGES was defined as the immediate postictal (within 30 seconds), generalized absence of EEG activity >10 microV in amplitude, allowing for muscle, movement, breathing, and electrode artifacts (Lhatoo et al 2010, Semmelroch et al 2012, Seyal et al 2012a, Seyal et al 2012b, Surges et al 2011). We extended EEG analysis to the “recovery phase”, defined as the period beginning from the end of continuous PGES until normal background resumed. Three channel electrocardiographic recordings were considered in automatic R-Wave detection and results of detection visually validated. Then heart rate variability (HRV) measures including RMSSD (root mean square successive difference of R-R intervals), SDNN (standard deviation of RR intervals), SDSD (standard deviation of differences) (NASPE 1996) and standard Poincare′ parameters (Fishman et al 2012) were computed (short term variability SD1, long term variability SD2 and the short-term to long-term ratio SD1/SD2) and analyzed during the pre-ictal baseline, ictal and post-ictal (to 5 minutes) phases using an in-house validated and automated MATLAB™ HRV program.
Statistical analysis
All data were analyzed using STATA 10 for Windows. T-test mean values and analogous two-sample t-test were used to report means, which correspond to nonparametric tests but are more robust to normality violations. Generalized estimating equation (GEE) model analysis using linear regression models were used to find associations between electro-clinical features. GEE models were employed to account for correlation between more than one seizure event in a single subject.
Results
A total of 105 seizure events fulfilled study criteria (12 children with 23 seizures and 49 adults with 82 seizures). Clinical characteristics of subjects and seizures are presented in Table 1. Mean ages (with standard deviations) were 11.1 ± 3.4 years for the pediatric population and 35.1 ± 12.2 years for adults. All study children were >5 years old at the time of assessment. No gender differences were found.
Table 1. Patient Characteristics.
| CHILDREN (Seizures; N=12) | ADULTS (Seizures; N=49) | |
|---|---|---|
|
| ||
| Mean Age with Std Deviation at assessment | 11.1 ± 3.4 years | 35.1 ± 12.2 |
| Etiology: | ||
| Unknown/Cryptogenic | 6 (50%) | 30 (62%) |
| Remote Stroke | 1 (8.33%) | 1 (2%) |
| Meningoencephalitis | 1 (8.33%) | 2 (4%) |
| Cortical Dysplasia | 1 (8.33%) | 0 |
| Posterior leukomalacia | 1 (8.33%) | 0 |
| Gliosis caused by previous abscess | 1 (8.33%) | 3 (6%) |
| Mesial temporal sclerosis | 0 | 7 (14%) |
| Encephalomalacia (unexplained) | 0 | 2 (4%) |
| Post Traumatic brain injury | 0 | 1 (2%) |
| Low grade glioma | 0 | 1 (2%) |
| Cavernoma | 0 | 1 (2%) |
| Genetic generalized | 1 (8.33%) | 1 (2%) |
| Epilepsy Syndrome | ||
| Left temporal lobe | 1 (8.33%) | 16 (32%) |
| Right temporal lobe | 1 (8.33%) | 4 (8%) |
| Bitemporal lobe | 0 | 11 (23%) |
| Left frontal lobe | 1 (8.33%) | 6 (13%) |
| Right frontal lobe | 0 | 4 (8%) |
| Left parietal | 1 (8.33%) | 0 |
| Right occipital | 0 | 2 (4%) |
| Right hemisphere | 0 | 1 (2%) |
| Genetic generalized | 6 (50%) | 4 (8%) |
| Multi-focal | 1 (8.33%) | 1 (2%) |
| Left insular | 1 (8.33%) | 0 |
| Number of Antiepileptic Medications at the time of seizure(23 child and 82 adult seizures): | ||
| One or none | 4 (18%) | 18 (22%) |
| Two | 12 (52%) | 44 (54%) |
| Three | 6 (26%) | 17 (21%) |
| Four | 1 (4%) | 3 (3%) |
Peri-ictal seizure phases
Adult and pediatric seizures were different. PGES was present in 13/23 (57%) pediatric GTCS in 5/12 (42%) patients whereas it was present in 77/82 (94%) seizures in 44/49 (90%) adult patients. Using the independent samples Mann-Whitney U test, total seizure, tonic phase, PGES, and recovery phase durations were all found to be significantly shorter in children (Figure 1, Table 2). In terms of means, PGES duration was 8 times longer in adults and recovery duration twice as long. A peri-ictal seizure phase versus time plot was constructed to compare groups. In adults, the PGES and recovery phases contributed to almost three quarters of the peri-ictal period (Figure 2).
Figure 1.
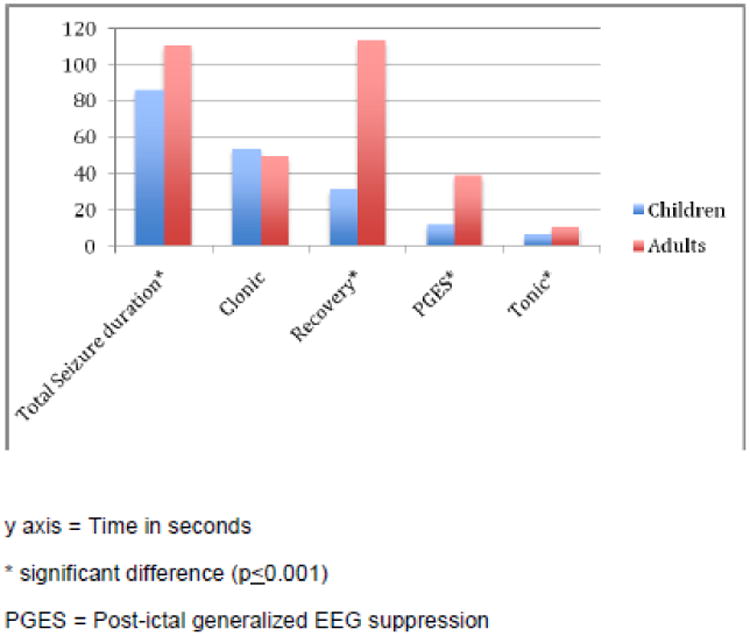
Comparison of durations in seizure phases, postictal generalized EEG suppression (PGES) and the recovery phase in children and adults.
Table 2.
Comparison of mean durations of seizure phases (in seconds), post-ictal generalized EEG suppression (PGES) and the recovery phase in children and adults.
| PHASE (in seconds) | CHILDREN N =23 | ADULTS N=82 | SIGNIFICANCE |
|---|---|---|---|
| Total seizure duration | 86.2 ±48.5 | 110.5 ±53.6 | 0.001* |
| Tonic | 6.5 ±2.2 | 10.5 ±3.6 | 0.00* |
| Clonic | 53.5 ±33.6 | 49.2 ± 26.8 | 0.464 |
| PGES | 11.7 ±14.4 | 38.8 ±24.4 | 0.00* |
| Recovery | 31.5 ±42.1 | 113.7±77.3 | 0.00* |
= Significant finding p<0.05
PGES = Post-ictal generalized EEG suppression
Figure 2.
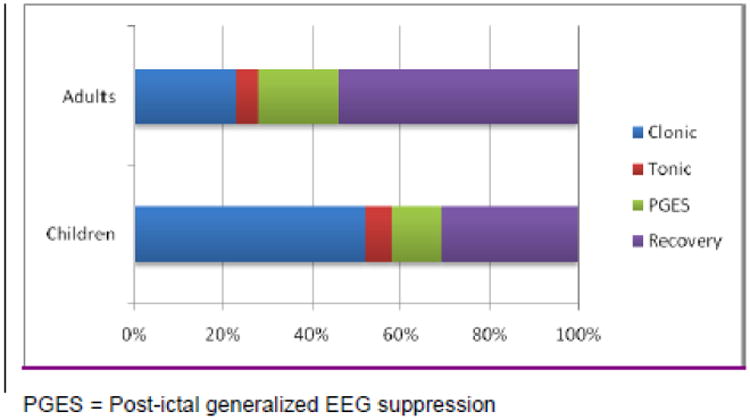
Peri-ictal seizure phases showing differences between children and adults.
Primary versus Secondary GTCS
Since 50% of the children had genetic generalized epilepsy, while only 8% of adults had this diagnosis, we looked at phase durations in the primary GTCS of genetic generalized epilepsies and the secondary GTCS of focal epilepsies to clarify whether seizure types were significantly different. We found that (Table 3) total seizure duration, tonic, PGES and recovery phases were significantly longer in secondary GTCS as compared to primary GTCS.
Table 3.
Comparison of mean durations of seizure phases (in seconds), post-ictal generalized EEG suppression and recovery phases in the primary GTCS of genetic generalized epilepsies and secondary GTCS of focal epilepsies.
| PHASE (in seconds) | PRIMARY GTCS (N=19) | SECONDARY GTCS (N=86) | SIGNIFICANCE |
|---|---|---|---|
| Total seizure duration | 76.2 ±37.94 | 111.62±54.2 | 0.008* |
| Tonic | 7.42±2.73 | 10.08±3.78 | 0.005* |
| Clonic | 53.16±37.56 | 49.48±26.02 | 0.611 |
| PGES | 16.26±21.72 | 36.51±24.55 | 0.001* |
| Recovery | 19.63±58.01 | 32.29±43.48 | 0.028* |
= Significant finding p<0.05
PGES = Post-ictal generalized EEG suppression
GTCS = generalized tonic-clonic seizures
Tonic phase duration
Using GEE models looking at parametric estimates and tonic phase duration as the dependent variable, we found age to be significantly associated (B=0.122, 95%CI 0.075-0.196; p<0.0001) (Table 4a). In sub-group (adult or child) analysis, this remained significant such that each year increase in age increased tonic phase duration by 0.12 seconds on average. Similarly, PGES duration was significantly increased in direct proportion to tonic phase duration (B=0.030, 95%CI0.002-0.058; p<0.05). Each second of PGES correlated with a 0.12 second increase in tonic phase duration. The effect of AED cessation was to significantly prolong the tonic phase (p<0.05) by an average of 1.7 seconds. In sub-analysis, this did not hold true in children (p=0.207).
Table 4.
Estimated generalized linear models using Generalized Estimating Equations and different dependent variables.
| a) Tonic Phase Duration in seconds as the dependent variable | ||||
|---|---|---|---|---|
|
| ||||
| Estimated B-value Estimate | Standard Error of Interval | 95% Wald Confidence | P value | |
| Total Sample (n=105 seizures) | ||||
| PGES duration | 0.030 | 0.0143 | 0.002-0.058 | 0.034* |
| Clonic phase duration | 0.003 | 0.024 | -0.36-0.043 | 0.864 |
| Total seizure duration | 0.006 | 0.0046 | -0.03-0.16 | 0.167 |
| Effect of medication | -1.704 | 0.7578 | 2.565-8.644 | 0.025* |
| Recovery phase | -0.004 | 0.0081 | -0.020-0.012 | 0.625 |
| RMSSD | 0.000 | 0.0001 | 0.000 to 0.000 | 0.159 |
| Intercept | 5.605 | 1.5507 | 2.565-8.644 | 0.000* |
| b) Post-ictal Generalized EEG Suppression Duration in seconds as the dependent variable | ||||
|
| ||||
| Tonic phase duration | 1.477 | 0.908 | -0.301-3.26 | 0.104 |
| Clonic phase duration | -0.155 | 0.0586 | -0.270- -0.041 | 0.008* |
| Total seizure duration | -0.016 | 0.0243 | -0.063-0.032 | 0.516 |
| Effect of medication reduction | 11.689 | 6.281 | -0.62-2400 | 0.063 |
| Recovery phase | 0.228 | 0.0300 | 0.169-0.287 | 0.000* |
| RMSSD | 0.002 | 0.0007 | 0.000-0.003 | 0.009* |
| Intercept | 45.472 | 12.037 | 2.1.88-69.07 | 0.000* |
| c) Recovery phase as the dependent variable | ||||
|
| ||||
| Tonic phase duration | -3.07 | 1.631 | -3.504-2.889 | 0.851 |
| Clonic phase duration | 0.214 | 0.2523 | -.0281-0.708 | 0.397 |
| Total seizure duration | -0.123 | 0.1789 | -0.474-0.228 | 0.492 |
| Effect of medication reduction | 13.9 | 13.878 | -13.3-41.1 | 0.317 |
| PGES | 2.581 | 0.2421 | 2.107-3.056 | 0.000* |
| RMSSD | 0.003 | 0.0016 | -1.983-0.006 | 0.051 |
| Intercept | -53.292 | 25.824 | -103.9- -2.678 | .039* |
= Significant finding p<0.05
PGES = Post-ictal generalized EEG suppression
Effect of Medication = No or some reduction vs no medication
RMSSD (root mean square successive difference of R-R intervals)
PGES Duration
With GEE analysis using PGES duration as dependent variable, children had PGES phases that were on average 28 seconds shorter. Each year of increase in age at the time of study was associated with a 0.6 second increase in PGES duration. Clonic phase duration was significantly and inversely proportional to PGES duration. Recovery phase duration and decreased HRV were significantly and directly proportional to PGES duration (Table 4b). In sub group analysis (adult or child), only recovery phase duration remained a significant association in children. Conversely, when recovery phase duration (Table 4c) was taken as the dependent variable, PGES duration increase was the only significant association.
Medication Effects
Medication profiles in terms of AED numbers in both groups were similar (Table 1). With medication as the dependent variable using GEE, no differences in tonic, clonic, PGES or recovery phases between medication groups were seen. However, in sub-analysis, total seizure duration with cessation of medication was significantly increased by a mean value of 8 seconds in children and 11 seconds (p<0.05) in adults. The effect of medication cessation was to significantly increase PGES duration in adults (p<0.0001) but not in children (p=0.385). Similar significances were not seen when clonic phase duration was the dependent variable. The differences in subjects on different classes of AEDs were not analyzed because the numbers were too small for any meaningful analysis.
Heart rate variability
GEE models looking at RMSSD (to 5 minutes post-ictally), SDSD and SDNN with seizure phase, PGES and recovery durations did not show any significant results except for RMSSD (at 2 min) which had a negative correlation with PGES duration (longer PGES durations were associated with decreased vagally mediated heart rate variability; p<0.05) when tonic phase or PGES duration were dependent variables (Table 4).
Discussion
SUDEP is rare in children. One study, assuming a pediatric epilepsy prevalence of 0.59% over a 10-year period, estimated an incidence of 27/138,620 person-years of epilepsy (2 per 10,000 person-years). In comparison to predominantly adult SUDEP estimates of 1 to 2 per 1,000 person-years (Derby et al 1996, Tennis et al 1995), this represents a 10-fold lower rate of SUDEP in children (Donner et al 2001). Whether this is due to age-related syndromic, etiologic, electro-clinical or other factors is not clear.
GTCS are the commonest seizure type associated with SUDEP. Semiological analyses in pediatric seizures indicate differences from adult seizures (Hamer et al 1999, Korff & Nordli 2005, Loddenkemper et al 2004) that may be relevant in agonal SUDEP phenomenology. One analysis of 109 seizures in 77 infants did not find a single typical GTCS (Korff & Nordli 2005). Another study of 296 seizures in 76 children up to the age of three years similarly reported complete absence of GTCS (Hamer et al 1999), suggesting that this is a rare seizure type in children. Few studies have compared electro-clinical seizure phases (Lhatoo et al 2010) and none have done so comparing adults and pediatric GTCS. In our study, only 23 children in >500 patients monitored over 9 years, had true GTCS and none were under the age of 5 years. Dravet's syndrome on the other hand, is a pediatric epilepsy syndrome strongly associated with SUDEP. In an analysis of 623 patients with Dravet's, 59 deaths were examined (a proportional mortality rate of >10%), of which 53% were sudden death cases (Sakauchi et al 2011). GTCS are frequently observed in this syndrome (Bureau & Dalla Bernardina 2011, Dravet 2011) in contrast to non-Dravet patients (Hamer et al 1999, Korff & Nordli 2005) and may at least in part explain the relatively lower incidence of SUDEP in non-Dravet children as compared to those with Dravet's and to adults. These observations are of interest because of the consistently strong association between GTCS and SUDEP in case-control and epidemiological studies in both adults and children (Hesdorffer et al 2011, Hesdorffer et al 2012, Langan et al 2005, Nilsson et al 1999, Shorvon & Tomson 2011).
In our study, we found several age-related electro-clinical differences in GTCS. Total seizure and tonic phase durations were significantly longer in adults and the effect of AED cessation during monitoring was to further prolong these in this higher SUDEP-risk population. Children appear to have a shorter tonic phase that is relatively unaffected by absence of medication. Why childhood GTCS are semiologically different from adult GTCS is unclear. This has been attributed to relative immaturity and lack of organization of developing brains, characterized by variable neuronal excitability, imperfect myelination, and incomplete inter-hemispheric connections (Arzimanoglou et al 2004, Hamer et al 1999, Korff & Nordli 2005). Partial seizures in humans are attributed to forebrain seizure circuitry (Jobe et al 1999) although some phases of GTCS may be driven by brainstem seizure circuitry (Coffey et al 1996, Jobe & Browning 2006). In animals, GTCS can be induced by electrical stimulation of the brainstem reticular core, despite removal of the forebrain. Although there is no described mechanism to connect brainstem driven GTCS phenomena in humans with post-ictal autonomic and cardio-respiratory compromise, it is tempting to speculate that in adults, prolonged tonic phases may conceivably drive pontomedullary autonomic network dysfunction and increase SUDEP risk. The shorter tonic phase in children may reflect immature, poorly established sub-cortical seizure networks and lesser post-ictal autonomic dysregulation.
The directly proportional relationship between tonic phase duration (when tonic phase was the dependent variable) and PGES duration seen in our study confirms the findings of one recent report (Tao et al 2012); the chronology of these phenomena seem to suggest that prolonged tonic phases are reflected in greater disturbances of cortical function in our patients, regardless of age. The recovery phase was not similarly affected, suggesting that the tonic phase's main effect is on the early post-ictal period when the patient is presumed most vulnerable to SUDEP. Prolonged PGES has been shown to indicate increased SUDEP risk in refractory epilepsy in one study (Lhatoo et al 2010) where it was significantly longer in the GTCS of SUDEP patients. With PGES durations of >50 seconds, SUDEP odds were significantly increased, with a quadrupled risk with PGES >80 seconds. Another study which examined the EEG records of 17 SUDEP cases and matched controls questioned this association although this may be explained by methodological differences (Surges et al 2011). Patients were predominantly temporal lobe epilepsy patients undergoing pre-surgical evaluations for temporal lobectomy. The matched surviving controls are likely to have become seizure free with surgery and the risk of SUDEP artificially removed, but in essence were potentially biologically indistinct from cases. PGES also appears to inversely correlate with clonic phase duration, regardless of tonic phase and total seizure duration. This effect is difficult to explain unless seizures with long clonic phases result in less obtundation. It may be relevant that in clinical practice, generalized clonic seizures (without a tonic component) sometimes occur without loss of consciousness. Overall, PGES was three times longer in adults.
PGES occurs in between 8% of pediatric seizure patients (Kim et al 2006) to 65% or more of adult patients with GTCS (Lhatoo et al 2010) and has been reported in several monitored SUDEP/near SUDEP cases (Bateman et al 2010, Bird 1997, Lhatoo et al 2010, McLean & Wimalaratna 2007, So et al 2000, Tao et al 2010) where some authors have used the term “cerebral shutdown” (McLean & Wimalaratna 2007). The increased incidence and duration of PGES in adults is noteworthy as they are a higher risk population than children. Several studies highlight the possible significance of PGES. In one study of 48 patients, those with PGES were significantly more likely to be motionless post-ictally and to have simple resuscitative interventions (Semmelroch et al 2012). These observations are indirectly corroborated in another study that analyzed 21 GTCS with no peri-ictal interventions and 84 with interventions. Earlier interventions were associated with briefer hypoxia and shorter PGES duration (Seyal et al 2012a). Another study compared secondary GTCS with and without PGES, and found that oxygen desaturation duration and extent as well as peak end-tidal CO2 elevation was more marked in PGES patients (Seyal et al 2012b). Thus PGES appears to indicate a greater degree of post-ictal obtundation and vulnerability to respiratory compromise. The high incidence of PGES in our patients possibly reflects a high rate of medication cessation during monitoring. PGES duration appears to directly correlate with recovery phase duration suggesting a continuum of recovery processes in the post-ictal period.
In common with at least one more study (Surges et al 2011), we found no correlation between HRV measures and electro-clinical seizure variables, with one exception. RMSSD measures at 2 minutes post-ictally were negatively correlated to PGES suggesting that PGES may be associated with the reduced vagal tone observed in some patients (Poh et al 2012).
The effect of AED cessation during monitoring (usually done to induce seizures as part of pre-surgical assessment), is interesting as this artificially amplifies the refractoriness of a patient's epilepsy, or creates a situation akin to non-compliance, another risk factor for SUDEP (Surges & Sander 2012). Total seizure durations were significantly increased by a mean value of 8 seconds and 11 seconds respectively in children and adults, and both PGES and tonic phase durations significantly increased in adults when medication was stopped. This indirectly appears to corroborate literature suggesting greater SUDEP risk in refractory epilepsy patients and in particular those who are non-compliant with medication, where seizure frequency and severity can be expected to be worse than on treatment.
Our study has limitations. Patient records were retrospectively analyzed with its attendant biases. A much smaller number of pediatric GTCS reflects the relative rarity of this seizure type in this age group and limits statistical power. The medication tapering protocols and total duration of hospital stay is, in general, shorter in children. AEDs have different half-lives which may influence the seizure duration but our AED groups are too small to look for these differences. We also considered the adult population to be >16 years of age rather than the > 20 years figure used in some SUDEP studies, for pragmatic reasons. All 4 patients in the 16 - 20 year bracket were aged 19 years and in biological terms, were more suited to be analyzed as adults. Additionally, we did not have respiratory measurements to determine the presence and influence of hypoxia, bradypnea and apnea, phenomena that are potential SUDEP mechanisms and that are known to occur in pediatric seizures (Singh et al 2013).
Since there is no forward surveillance of patients, the true incidence of SUDEP in both groups cannot be known and hence there is no gold standard for validation of the observed results. However, our data clearly points out identifiable electro-clinical differences between the adult and pediatric population which may at least in part explain differences in SUDEP incidence. It also highlights the importance of careful characterization of seizure semiology and EEG, particularly PGES. There is a gathering body of evidence that PGES is an important post-ictal phenomenon; its patho-physiology requires further, careful elucidation. Overall however, prolonged PGES may be best seen as a potential risk “marker” of SUDEP rather than a risk “factor”; the latter implies a causal role which is as yet uncharacterized and unproven.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Highlights.
Sudden Unexpected Death in Epilepsy (SUDEP), which is strongly associated with refractory generalized tonic-clonic seizures, is ten times less common in children
Prolonged post-ictal generalized EEG suppression may be a risk marker of SUDEP
The tonic phase of the generalized tonic clonic seizure and post-ictal generalized EEG suppression are significantly shorter in children
Long tonic phases and prolonged post-ictal generalized EEG suppression in children may identify those at particular risk of SUDEP
Acknowledgments
This study was supported in part by NINDS grant NS076965-01 - The Prevention and Risk Identification of SUDEP Mortality (PRISM) Project.
Footnotes
Disclosure: None of the authors has any conflict of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arzimanoglou A, Guerrini R, Aicardi J. Epilepsy in Infants. In: Arzimanoglou A, Guerrini R, Aicardi J, editors. Aicardi's Epilepsy in Children. Philadelphia: Lippincott and Williams; 2004. pp. 210–19. [Google Scholar]
- Bateman LM, Spitz M, Seyal M. Ictal hypoventilation contributes to cardiac arrhythmia and SUDEP: report on two deaths in video-EEG-monitored patients. Epilepsia. 2010;51:916–20. doi: 10.1111/j.1528-1167.2009.02513.x. [DOI] [PubMed] [Google Scholar]
- Bird JMD, KAT, Sandeman D, Butler S. Sudden Unexplained Death in Epilepsy. Epilepsia. 1997;38(Suppl 11):S52–S56. [Google Scholar]
- Bureau M, Dalla Bernardina B. Electroencephalographic characteristics of Dravet syndrome. Epilepsia. 2011;52(2):13–23. doi: 10.1111/j.1528-1167.2011.02996.x. [DOI] [PubMed] [Google Scholar]
- Coffey LL, Reith ME, Chen NH, Mishra PK, Jobe PC. Amygdala kindling of forebrain seizures and the occurrence of brainstem seizures in genetically epilepsy-prone rats. Epilepsia. 1996;37:188–97. doi: 10.1111/j.1528-1157.1996.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Derby LE, Tennis P, Jick H. Sudden unexplained death among subjects with refractory epilepsy. Epilepsia. 1996;37:931–5. doi: 10.1111/j.1528-1157.1996.tb00529.x. [DOI] [PubMed] [Google Scholar]
- Donner EJ, Smith CR, Snead OC., 3rd Sudden unexplained death in children with epilepsy. Neurology. 2001;57:430–4. doi: 10.1212/wnl.57.3.430. [DOI] [PubMed] [Google Scholar]
- Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(2):3–9. doi: 10.1111/j.1528-1167.2011.02994.x. [DOI] [PubMed] [Google Scholar]
- Fishman M, Jacono FJ, Park S, Jamasebi R, Thungtong A, et al. A method for analyzing temporal patterns of variability of a time series from Poincare plots. J Appl Physiol. 2012;113:297–306. doi: 10.1152/japplphysiol.01377.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaut H. Semiology and physiopathogenesis of generalized epileptic seizures. Helv Med Acta. 1963;30:319–37. [PubMed] [Google Scholar]
- Hamer HM, Wyllie E, Luders HO, Kotagal P, Acharya J. Symptomatology of epileptic seizures in the first three years of life. Epilepsia. 1999;40:837–44. doi: 10.1111/j.1528-1157.1999.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, et al. Combined analysis of risk factors for SUDEP. Epilepsia. 2011;52:1150–9. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, et al. Do antiepileptic drugs or generalized tonic-clonic seizure frequency increase SUDEP risk? A combined analysis. Epilepsia. 2012;53:249–52. doi: 10.1111/j.1528-1167.2011.03354.x. [DOI] [PubMed] [Google Scholar]
- Jobe PC, Browning RA. Mammalian models of genetic epilepsy characterized by sensory evoked seizures and generalized seizure susceptibility. In: Pitkanen A, Schwartzkroin PA, Moshe S, editors. Models of Seizures and Epilepsy. Amsterdam: Academic Press; 2006. pp. 261–71. [Google Scholar]
- Jobe PC, Mishra PK, Dailey JW, Ko KH, Reith MA. Genetic predisposition to partial (focal) seizures and to generalized tonic/clonic seizures: Interactions between seizure circuitry of the forebrain and brainstem. In: Berkovic SF, Genton P, Hirsch E, Picard F, editors. Genetics of Focal Epilesies. Avignon, France: John Libbey & Company Ltd; 1999. pp. 251–60. [Google Scholar]
- Kim AJ, Kuroda MM, Nordli DR., Jr Abruptly attenuated terminal ictal pattern in pediatrics. J Clin Neurophysiol. 2006;23:532–50. doi: 10.1097/01.wnp.0000229045.28725.5c. [DOI] [PubMed] [Google Scholar]
- Korff C, Nordli DR., Jr Do generalized tonic-clonic seizures in infancy exist? Neurology. 2005;65:1750–3. doi: 10.1212/01.wnl.0000187125.87414.f3. [DOI] [PubMed] [Google Scholar]
- Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology. 2005;64:1131–3. doi: 10.1212/01.WNL.0000156352.61328.CB. [DOI] [PubMed] [Google Scholar]
- Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010 doi: 10.1002/ana.22101. [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Wyllie E, Neme S, Kotagal P, Luders HO. Lateralizing signs during seizures in infants. J Neurol. 2004;251:1075–9. doi: 10.1007/s00415-004-0463-7. [DOI] [PubMed] [Google Scholar]
- McLean BN, Wimalaratna S. Sudden death in epilepsy recorded in ambulatory EEG. J Neurol Neurosurg Psychiatry. 2007;78:1395–7. doi: 10.1136/jnnp.2006.088492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milroy CM. Sudden unexpected death in epilepsy in childhood. Forensic Sci Med Pathol. 2011;7:336–40. doi: 10.1007/s12024-011-9245-6. [DOI] [PubMed] [Google Scholar]
- NASPE. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–81. [PubMed] [Google Scholar]
- Nickels KC, Grossardt BR, Wirrell EC. Epilepsy-related mortality is low in children: A 30-year population-based study in Olmsted County, MN. Epilepsia. 2012;53:2164–71. doi: 10.1111/j.1528-1167.2012.03661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case-control study. Lancet. 1999;353:888–93. doi: 10.1016/s0140-6736(98)05114-9. [DOI] [PubMed] [Google Scholar]
- Poh MZ, Loddenkemper T, Reinsberger C, Swenson NC, Goyal S, et al. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology. 2012;78:1868–76. doi: 10.1212/WNL.0b013e318258f7f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez ML, McMillan K, Crandall LA, Minter ME, Grafe MR, et al. Hippocampal asymmetry and sudden unexpected death in infancy: a case report. Forensic Sci Med Pathol. 2012;8:441–6. doi: 10.1007/s12024-012-9367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakauchi M, Oguni H, Kato I, Osawa M, Hirose S, et al. Mortality in Dravet syndrome: search for risk factors in Japanese patients. Epilepsia. 2011;52(2):50–4. doi: 10.1111/j.1528-1167.2011.03002.x. [DOI] [PubMed] [Google Scholar]
- Semmelroch M, Elwes RD, Lozsadi DA, Nashef L. Retrospective audit of postictal generalized EEG suppression in telemetry. Epilepsia. 2012;53:e21–4. doi: 10.1111/j.1528-1167.2011.03296.x. [DOI] [PubMed] [Google Scholar]
- Seyal M, Bateman LM, Li CS. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia. 2012a doi: 10.1111/j.1528-1167.2012.03691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyal M, Hardin KA, Bateman LM. Postictal generalized EEG suppression is linked to seizure-associated respiratory dysfunction but not postictal apnea. Epilepsia. 2012b;53:825–31. doi: 10.1111/j.1528-1167.2012.03443.x. [DOI] [PubMed] [Google Scholar]
- Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet. 2011;378:2028–38. doi: 10.1016/S0140-6736(11)60176-1. [DOI] [PubMed] [Google Scholar]
- Singh K, Katz ES, Zarowski M, Loddenkemper T, Llewellyn N, et al. Cardiopulmonary complications during pediatric seizures: a prelude to understanding SUDEP. Epilepsia. 2013;54:1083–91. doi: 10.1111/epi.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia. 2000;41:1494–7. doi: 10.1111/j.1528-1157.2000.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Surges R, Sander JW. Sudden unexpected death in epilepsy: mechanisms, prevalence, and prevention. Curr Opin Neurol. 2012;25:201–7. doi: 10.1097/WCO.0b013e3283506714. [DOI] [PubMed] [Google Scholar]
- Surges R, Strzelczyk A, Scott CA, Walker MC, Sander JW. Postictal generalized electroencephalographic suppression is associated with generalized seizures. Epilepsy Behav. 2011;21:271–4. doi: 10.1016/j.yebeh.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Tao J, Yung I, Lee A, Rose S, Jacobsen J, Ebersole JS. Tonic phase of a generalized convulsive seizure is an independent predictor of post-ictal generalized EEG suppression; Presented at American Epilepsy Society; San Diego, CA. 2012. [DOI] [PubMed] [Google Scholar]
- Tao JX, Qian S, Baldwin M, Chen XJ, Rose S, et al. SUDEP, suspected positional airway obstruction, and hypoventilation in postictal coma. Epilepsia. 2010;51:2344–7. doi: 10.1111/j.1528-1167.2010.02719.x. [DOI] [PubMed] [Google Scholar]
- Tennis P, Cole TB, Annegers JF, Leestma JE, McNutt M, Rajput A. Cohort study of incidence of sudden unexplained death in persons with seizure disorder treated with antiepileptic drugs in Saskatchewan, Canada. Epilepsia. 1995;36:29–36. doi: 10.1111/j.1528-1157.1995.tb01661.x. [DOI] [PubMed] [Google Scholar]


