Abstract
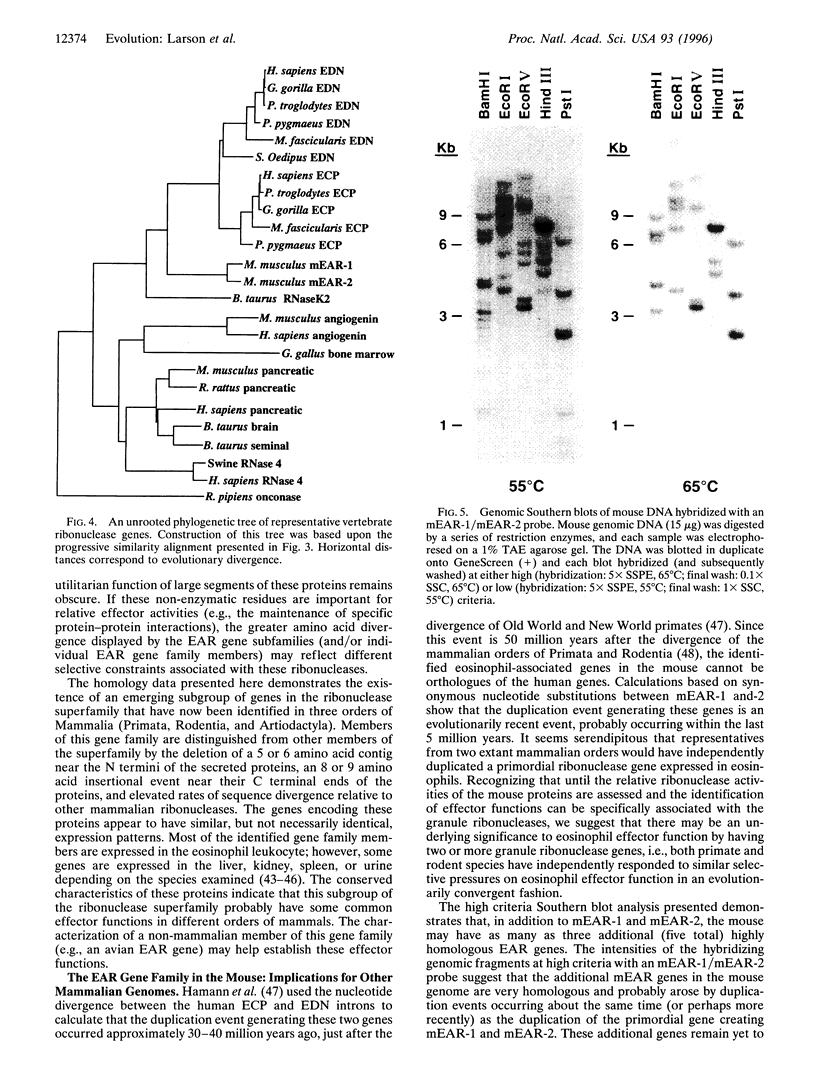
Two putative ribonucleases have been isolated from the secondary granules of mouse eosinophils. Degenerate oligonucleotide primers inferred from peptide sequence data were used in reverse transcriptase-PCR reactions of bone marrow-derived cDNA. The resulting PCR product was used to screen a C57BL/6J bone marrow cDNA library, and comparisons of representative clones showed that these genes and encoded proteins are highly homologous (96% identity at the nucleotide level; 92/94% identical/similar at the amino acid level). The mouse proteins are only weakly homologous (approximately 50% amino acid identity) with the human eosinophil-associated ribonucleases (i.e., eosinophil-derived neurotoxin and eosinophil cationic protein) and show no sequence bias toward either human protein. Phylogenetic analyses established that the human and mouse loci shared an ancestral gene, but that independent duplication events have occurred since the divergence of primates and rodents. The duplication event generating the mouse genes was estimated to have occurred < 5 x 10(6) years ago (versus 30 to 40 x 10(6) years ago in primates). The identification of independent duplication events in two extant mammalian orders suggests a selective advantage to having multiple eosinophil granule ribonucleases. Southern blot analyses in the mouse demonstrated the existence of three additional highly homologous genes (i.e., five genes total) as well as several more divergent family members. The potential significance of this observation is the implication of a larger gene subfamily in primates (i.e., humans).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson M. B., Schaefer K. Conjunctivitis of allergic origin: immunologic mechanisms and current approaches to therapy. Surv Ophthalmol. 1993 Jul-Aug;38 (Suppl):115–132. doi: 10.1016/0039-6257(93)90036-7. [DOI] [PubMed] [Google Scholar]
- Abu-Ghazaleh R. I., Kita H., Gleich G. J. Eosinophil activation and function in health and disease. Immunol Ser. 1992;57:137–167. [PubMed] [Google Scholar]
- Ackerman S. J., Gleich G. J., Loegering D. A., Richardson B. A., Butterworth A. E. Comparative toxicity of purified human eosinophil granule cationic proteins for schistosomula of Schistosoma mansoni. Am J Trop Med Hyg. 1985 Jul;34(4):735–745. doi: 10.4269/ajtmh.1985.34.735. [DOI] [PubMed] [Google Scholar]
- Barker R. L., Loegering D. A., Ten R. M., Hamann K. J., Pease L. R., Gleich G. J. Eosinophil cationic protein cDNA. Comparison with other toxic cationic proteins and ribonucleases. J Immunol. 1989 Aug 1;143(3):952–955. [PubMed] [Google Scholar]
- Beintema J. J., Hofsteenge J., Iwama M., Morita T., Ohgi K., Irie M., Sugiyama R. H., Schieven G. L., Dekker C. A., Glitz D. G. Amino acid sequence of the nonsecretory ribonuclease of human urine. Biochemistry. 1988 Jun 14;27(12):4530–4538. doi: 10.1021/bi00412a046. [DOI] [PubMed] [Google Scholar]
- Beintema J. J., Schüller C., Irie M., Carsana A. Molecular evolution of the ribonuclease superfamily. Prog Biophys Mol Biol. 1988;51(3):165–192. doi: 10.1016/0079-6107(88)90001-6. [DOI] [PubMed] [Google Scholar]
- Britten R. J. Rates of DNA sequence evolution differ between taxonomic groups. Science. 1986 Mar 21;231(4744):1393–1398. doi: 10.1126/science.3082006. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E. Cell-mediated damage to helminths. Adv Parasitol. 1984;23:143–235. doi: 10.1016/s0065-308x(08)60287-0. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Ackerman S. J., Loegering D. A., Gleich G. J. Purification of human eosinophil-derived neurotoxin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5165–5169. doi: 10.1073/pnas.78.8.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson M., Glitz D. G. Characterization of a ribonuclease from bovine brain. Biochemistry. 1975 Apr 8;14(7):1471–1476. doi: 10.1021/bi00678a019. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive alignment and phylogenetic tree construction of protein sequences. Methods Enzymol. 1990;183:375–387. doi: 10.1016/0076-6879(90)83025-5. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Adolphson C. R., Leiferman K. M. The biology of the eosinophilic leukocyte. Annu Rev Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Jacoby D. B., Fryer A. D. Eosinophil-associated inflammation in bronchial asthma: a connection to the nervous system. Int Arch Allergy Immunol. 1995 May-Jun;107(1-3):205–207. doi: 10.1159/000236978. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Loegering D. A., Bell M. P., Checkel J. L., Ackerman S. J., McKean D. J. Biochemical and functional similarities between human eosinophil-derived neurotoxin and eosinophil cationic protein: homology with ribonuclease. Proc Natl Acad Sci U S A. 1986 May;83(10):3146–3150. doi: 10.1073/pnas.83.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada A., Greene B. M. Clq enhancement of IgG-dependent eosinophil-mediated killing of schistosomula in vitro. J Immunol. 1987 Feb 15;138(4):1240–1245. [PubMed] [Google Scholar]
- Hamann K. J., Barker R. L., Loegering D. A., Gleich G. J. Comparative toxicity of purified human eosinophil granule proteins for newborn larvae of Trichinella spiralis. J Parasitol. 1987 Jun;73(3):523–529. [PubMed] [Google Scholar]
- Hamann K. J., Barker R. L., Loegering D. A., Pease L. R., Gleich G. J. Sequence of human eosinophil-derived neurotoxin cDNA: identity of deduced amino acid sequence with human nonsecretory ribonucleases. Gene. 1989 Nov 15;83(1):161–167. doi: 10.1016/0378-1119(89)90414-9. [DOI] [PubMed] [Google Scholar]
- Hamann K. J., Ten R. M., Loegering D. A., Jenkins R. B., Heise M. T., Schad C. R., Pease L. R., Gleich G. J., Barker R. L. Structure and chromosome localization of the human eosinophil-derived neurotoxin and eosinophil cationic protein genes: evidence for intronless coding sequences in the ribonuclease gene superfamily. Genomics. 1990 Aug;7(4):535–546. doi: 10.1016/0888-7543(90)90197-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Henikoff J. G. Performance evaluation of amino acid substitution matrices. Proteins. 1993 Sep;17(1):49–61. doi: 10.1002/prot.340170108. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Larson K. A., Lee J. J., Lee N. A. Cloning of the murine eosinophil peroxidase gene (mEPO): characterization of a conserved subgroup of mammalian hematopoietic peroxidases. J Leukoc Biol. 1996 Aug;60(2):285–294. doi: 10.1002/jlb.60.2.285. [DOI] [PubMed] [Google Scholar]
- Irie M., Nitta R., Ohgi K., Niwata Y., Watanabe H., Iwama M., Beintema J. J., Sanda A., Takizawa Y. Primary structure of a non-secretory ribonuclease from bovine kidney. J Biochem. 1988 Aug;104(2):289–296. doi: 10.1093/oxfordjournals.jbchem.a122460. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Nicholas W. L., Metcalf D., McKenzie I. F., Mitchell G. F. Peritoneal cell population of mice infected with Mesocestoides corti as a source of eosinophils. Int Arch Allergy Appl Immunol. 1979;59(3):315–322. doi: 10.1159/000232275. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Corrigan C. J. Asthma. Eosinophils and neutrophils. Br Med Bull. 1992 Jan;48(1):51–64. doi: 10.1093/oxfordjournals.bmb.a072541. [DOI] [PubMed] [Google Scholar]
- Kroegel C., Virchow J. C., Jr, Kortsik C., Matthys H. Cytokines, platelet activating factor and eosinophils in asthma. Respir Med. 1992 Sep;86(5):375–389. doi: 10.1016/s0954-6111(06)80004-1. [DOI] [PubMed] [Google Scholar]
- Larson K. A., Horton M. A., Madden B. J., Gleich G. J., Lee N. A., Lee J. J. The identification and cloning of a murine major basic protein gene expressed in eosinophils. J Immunol. 1995 Sep 15;155(6):3002–3012. [PubMed] [Google Scholar]
- Lawrence C. W., Little P. A., Little B. W., Miller M. J., Bazel S., Alhadeff J. A. Human non-secretory ribonucleases. I. Purification, peptide mapping and lectin blotting analysis of the kidney, liver and spleen enzymes. Glycobiology. 1993 Jun;3(3):241–248. doi: 10.1093/glycob/3.3.241. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Szklarek D., Barton A., Ganz T., Hamann K. J., Gleich G. J. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989 Jun 15;142(12):4428–4434. [PubMed] [Google Scholar]
- Leiferman K. M., Peters M. S., Gleich G. J. The eosinophil and cutaneous edema. J Am Acad Dermatol. 1986 Sep;15(3):513–517. doi: 10.1016/s0190-9622(86)70203-x. [DOI] [PubMed] [Google Scholar]
- Molina H. A., Kierszenbaum F., Hamann K. J., Gleich G. J. Toxic effects produced or mediated by human eosinophil granule components on Trypanosoma cruzi. Am J Trop Med Hyg. 1988 Mar;38(2):327–334. doi: 10.4269/ajtmh.1988.38.327. [DOI] [PubMed] [Google Scholar]
- Newton D. L., Walbridge S., Mikulski S. M., Ardelt W., Shogen K., Ackerman S. J., Rybak S. M., Youle R. J. Toxicity of an antitumor ribonuclease to Purkinje neurons. J Neurosci. 1994 Feb;14(2):538–544. doi: 10.1523/JNEUROSCI.14-02-00538.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimert C. M., Minuva U., Kharazmi A., Bendtzen K. Eosinophil protein X/eosinophil derived neurotoxin (EPX/EDN). Detection by enzyme-linked immunosorbent assay and purification from normal human urine. J Immunol Methods. 1991 Jul 26;141(1):97–104. doi: 10.1016/0022-1759(91)90214-z. [DOI] [PubMed] [Google Scholar]
- Ring J., Bieber T., Vieluf D., Kunz B., Przybilla B. Atopic eczema, Langerhans cells and allergy. Int Arch Allergy Appl Immunol. 1991;94(1-4):194–201. doi: 10.1159/000235361. [DOI] [PubMed] [Google Scholar]
- Rosenberg H. F., Ackerman S. J., Tenen D. G. Human eosinophil cationic protein. Molecular cloning of a cytotoxin and helminthotoxin with ribonuclease activity. J Exp Med. 1989 Jul 1;170(1):163–176. doi: 10.1084/jem.170.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H. F., Dyer K. D., Tiffany H. L., Gonzalez M. Rapid evolution of a unique family of primate ribonuclease genes. Nat Genet. 1995 Jun;10(2):219–223. doi: 10.1038/ng0695-219. [DOI] [PubMed] [Google Scholar]
- Rosenberg H. F., Tenen D. G., Ackerman S. J. Molecular cloning of the human eosinophil-derived neurotoxin: a member of the ribonuclease gene family. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4460–4464. doi: 10.1073/pnas.86.12.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Interaction of human placental ribonuclease with placental ribonuclease inhibitor. Biochemistry. 1991 Feb 26;30(8):2246–2255. doi: 10.1021/bi00222a030. [DOI] [PubMed] [Google Scholar]
- Slifman N. R., Loegering D. A., McKean D. J., Gleich G. J. Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein. J Immunol. 1986 Nov 1;137(9):2913–2917. [PubMed] [Google Scholar]
- Sorrentino S., Glitz D. G., Hamann K. J., Loegering D. A., Checkel J. L., Gleich G. J. Eosinophil-derived neurotoxin and human liver ribonuclease. Identity of structure and linkage of neurotoxicity to nuclease activity. J Biol Chem. 1992 Jul 25;267(21):14859–14865. [PubMed] [Google Scholar]
- Sorrentino S., Glitz D. G. Ribonuclease activity and substrate preference of human eosinophil cationic protein (ECP). FEBS Lett. 1991 Aug 19;288(1-2):23–26. doi: 10.1016/0014-5793(91)80994-e. [DOI] [PubMed] [Google Scholar]
- Wallaert B., Desreumaux P., Copin M. C., Tillie I., Benard A., Colombel J. F., Gosselin B., Tonnel A. B., Janin A. Immunoreactivity for interleukin 3 and 5 and granulocyte/macrophage colony-stimulating factor of intestinal mucosa in bronchial asthma. J Exp Med. 1995 Dec 1;182(6):1897–1904. doi: 10.1084/jem.182.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe K. H., Sharp P. M. Mammalian gene evolution: nucleotide sequence divergence between mouse and rat. J Mol Evol. 1993 Oct;37(4):441–456. doi: 10.1007/BF00178874. [DOI] [PubMed] [Google Scholar]