1. Introduction
Ovarian cancer is the most lethal gynecologic malignancy, with 15,500 women expected to die from the disease in 20121. The standard therapy for women with advanced ovarian cancer has been cytoreductive surgery followed by platinum-based chemotherapy 2. Although this standard platinum-based chemotherapy results in high response rates (~80%), more than 70% of patients with advanced disease will experience recurrence within 5 years and develop drug resistance3. These patients with platinum-resistant disease receive other chemotherapeutic agents such as metronomic paclitaxel, docetaxel, topotecan, liposomal-doxorubicin, etoposide or gemcitabine, but response rates in this setting are low (10–25%) and short lived (2.5–5 months on the median). Therefore, novel treatment strategies to increase primary efficacy, decrease recurrence after primary treatment, and improve the response rate for recurrent disease are needed, and various molecular targeting therapies, such as those disrupting tumor angiogenesis, inhibiting Poly-(ADP) Ribose Polymerase (PARP) function and growth factors such as the insulin-like growth factor (IGF), are being pursued4.
Anti-angiogenic therapy, which is based on the theory that blocking angiogenesis in tumor will retard its growth and progression, is widely appealing5. Bevacizumab became the first anti-VEGF agent to be approved by the US Food and Drag Administration (FDA) for cancer patients in 20046. So far, several therapeutic agents targeting angiogenesis have been approved by FDA, sorafenib for hepatocellular carcinoma and kidney cancer, sunitinib for kidney cancer and neuroendocrine tumor, everolimus for kidney cancer, neuroendocrine tumor and breast cancer, pazopanib for kidney cancer and soft tissue sarcoma, and axitinib for renal cancer, albeit none in ovarian cancer7, 8. Phase II trials of anti-VEGF, such as sorafenib, sunitinib, cediranib, pazopanib and BIB1120, have been done and phase III trials of pazopanib and BIBF1120 are currently underway9–12.
2. Angiogenesis and VEGF in cancer
Angiogenesis, the formation of new blood vessels from existing vasculature, is an important promoter for solid tumor growth, invasion and metastasis13. This process is regulated by a number of growth factor receptor pathways and cytokines, such as vascular endothelial growth factors (VEGFs), fibroblast growth factor (FGF), angiopoietin, platelet-derived growth factors (PDGF), tumor necrosis factor (TNF) and interleukins14.
Much attention has been focused on the VEGF family and the receptor tyrosine kinases that mediate their proangiogenic effects 15, 16. The VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF) ligands. The major mediator of tumor angiogenesis is VEGF-A, usually called VEGF, and the VEGF receptor family includes VEGFR-1, 2 and 3, with VEGFR-2 functioning as the major signaling receptor in angiogenesis17.
The binding of VEGF to VEGFR2 leads to dimerization of the receptor, followed by intracellular activation of the Raf-MEK-MAPK pathway and subsequent initiation of DNA synthesis and cell growth, whereas activation of the PI3K-Akt pathway leads to increased endothelial cell survival. Subsequently, the proangiogenic signaling pathways, such as endothelial cell proliferation, migration, survival and differentiation are activated, while VEGF also increases vascular permeability and vasodilation18, 19. Overexpression of VEGF and VEGFR is often observed in several solid tumors, and has been associated with increased risk of tumor metastasis and poor survival including ovarian cancer20. Moreover, high expression of VEGFR is seen in patients whose cancer is resistant to platinum-based chemotherapy21. These observations have provided the rationale for clinical development of anti-angiogenesis agents, particularly those targeting the VEGF ligand, receptor and downstream signaling. The broad appeal of anti-angiogenesis therapy in solid tumors and its efficacy has prompted several reviews on the topic22, 23.
3. Bevacizumab
Bevacizumab is a recombinant, fully humanized monoclonal IgG antibody (rhuMAb) that binds and inactivates the biologic activity of VEGF-A. It suppresses tumor growth and inhibits metastatic tumor progression by inhibition of neovascularization, resulting in regression of existing microvessels 24, 25. Furthermore, bevacizumab is believed to directly affect the structure and function of tumor vessels, which become disordered and morphologically altered in the cancer microenvironment. Observed functional changes in response to VEGF ligand targeting are decreased interstitial fluid pressure, increased tumor oxygenation and improved penetration of drugs into tumors; restoration of fluid dynamics in tumor may lead to improvement of drug delivery, particularly chemotherapeutics 26. Bevacizumab was the first anti-angiogenesis agent approved by FDA for the treatment of solid tumors. It is currently labeled for treatment in patients with metastatic colon and renal cell, glioblastoma and non-small cell lung cancer. Single agent activity has also been documented in patients with ovarian, endometrial and cervix cancer. 27–31
3.1. Phase II trials (Table 1)
Table 1.
Phase II trials of bevacizumab
| GOG | Cannistra et al. | McGonigle et al. | Carmen et al. | Garcia et al. | |
|---|---|---|---|---|---|
| Setting/design | Recurrent platinum- sensitive/resistant | Recurrent platinum- resistant | Recurrent platinum- resistant | Recurrent platinum- sensitive | Recurrent platinum- sensitive/resistant |
| Bev dosage | 15mg/kg/q3w | 15mg/kg/q3w | 10mg/kg/q2w | 10mg/kg/q2w | 10mg/kg** |
| Treatment | Monotherapy | Monotherapy | Combination with TOP | Combination with PLD and CBDCA | Combination with cyclophosphamide |
| Treatment cycle (days) | 21 | 21 | 28 | 28 | 28 |
| Number of patients | 62 | 44 | 40 | 54 | 70 |
| Efficacy | |||||
| ORR | 21.0% | 15.9% | 25.0% | 72.2% | 24% |
| CR | 2 (3%) | 0 | 0 | 8 (15%) | 0 |
| PR | 11 (18%) | 7 (15.9%) | 10 (25%) | 31 (57%) | 17 (24%) |
| SD | 32 (52%) | 27 (25%) | 14 (35%) | 11 (20%) | 44 (63%) |
| Median PFS (range) (months) | 4.7 (2.7–12.9*) | 4.4 (3.1–5.5) | 7.8 (3.0–9.4) | 13.9 (11.4– 16.0) | 7.2 (5.3–8.7) |
| Median OS (range) (months) | 16.9 (9.1–32.4*) | 10.7 | 16.6 (12.8– 22.9) | N/A | 16.9 (11.4–25.2) |
| Adverse events (selected) | |||||
| Hypertension (>grade3) | 6 (9.7%) | 4 (9.1%) | 8 (20%) | 6 (11.1%) | 11 (15%) |
| GI perforation | 0 | 5 (11%) | 0 | 1 (1.9%) | 3 (4%) |
| Proteinuria (>grade3) | 1 (1.6%) | 0 | 1 (3%) | 6 (11.1%) | 3 (4%) |
| VTE | 2 (3.2%) | 1 (2.3%) | 1 (3%) | 2 (3.7%) | 3 (4%) |
GOG, gynecologic oncology group; Bev, bevacizumab; TOP, topotecan; PLD, pegylated liposomal doxorubicin; CBDCA, carboplatin; ORR, overall response rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PFS, progression free survival; OS, overall survival; GI, gastrointestinal; VTE, venous thromboembolism
first and third quartiles
every week for first 3 weeks of treatment followed by administration every 2 weeks
Two phase II trials of bevacizumab monotherapy in persistent or recurrent ovarian cancer or primary peritoneal carcinoma and two more phase II trials of bevacizumab in combination with other cytotoxic chemotherapy for recurrent ovarian cancer have been conducted.
3.1.1 Phase II trial of bevacizumab monotherapy
Following a case report demonstrating clinical response in a patient who was heavily pretreated, the Gynecologic Oncology Group (GOG) conducted an open-label phase II clinical trial of bevacizumab monotherapy27, 32. The trial enrolled sixty-two women with measurable recurrent or persistent ovarian or primary peritoneal carcinoma. All were treated with one or more cytotoxic regimens, one of which was platinum-based. Bevacizumab was administered at 15 mg/kg, intravenously every 21 days and patients were treated until progression or prohibitive toxicity. Overall, a median 7 cycles (range, one to 35 cycles) per patient was administered. Objective clinical response (co-primary endpoint) was observed in 13 patients (21%), including complete response (CR) in 2 patients, and median response duration was 10.3 months. An additional 32 patients (52%) had stable disease. Twenty-five patients (40%) were progression-free for at least 6 months (co-primary endpoint). The median PFS and OS in this study population was 4.7 months and 16.9 months, respectively. A second phase II monotherapy trial enrolled forty-four women with previously treated platinum resistant ovarian or primary peritoneal carcinoma28. The treatment regimen was similar to the GOG study (15mg/kg by intravenous infusion every 21 days) although prior treatment with up to 3 regimens was allowed (48% of the cohort). Overall, 7 patients (16%) had a partial response (PR) and 11 (25%) had stable disease for at least 12 weeks. Patients received a median of 5 (range 2–16) cycles of therapy. Among those achieving a PR, the median response duration was 4.2 months. The median PFS and OS in this study was 4.4 months and 10.7 months, respectively.
In these phase II monotherapy trials, bevacizumab was considered to be effective therapeutic agent in recurrent ovarian cancer patients.
3.1.2 Phase II trial of bevacizumab combination therapy
In light of the non-overlapping toxicity profile and demonstration of enhanced activity of bevacizumab in combination with chemotherapy (both preclinically and clinically in other solid tumors), interest in combination studies rapidly followed. McGonigle and colleagues conducted a phase II trial of bevacizumab in combination with topotecan33. Forty patients with platinum- and taxane-resistant disease were enrolled. In an attempt to decrease the potential for GIP, the study population was restricted to patients who had received a maximum of 2 prior chemotherapy regimens. Treatment was administered in 28-day cycle, with bevacizumab 10 mg/kg intravenous infusion on days 1 and 15 and topotecan 4 mg/m2 intravenous infusion on days 1, 8 and 15. Patients received a median of 8 cycles (range, <1 to 32 cycles) of treatment. Ten patients (25%) experienced PR and 14 patients (35%) had stable disease (SD). All patients with PR and 86% of patients with SD had PFS longer than 6 months. The median PFS and OS were 7.8 months and 16.6 months, respectively. Eight patients (20%) had grade 3 or 4 hypertension, and no patients experienced a GIP. Carmen and colleagues conducted phase II trial of bevacizumab in combination with liposomal doxorubicin and carboplatin34. Fifty-four women with platinum-sensitive recurrent ovarian, fallopian tube or primary peritoneal carcinoma were enrolled. Treatment was administered in 28-day cycle, with pegylated liposomal doxorubicin (PLD) 30 mg/m2 and carboplatin area under the curve (AUC) 5 by intravenous infusion on day 1 and bevacizumab 10 mg/kg on days 1 and 15 for maximum of 10 cycles. The mean ± SD number of cycles administered was 5.9 ± 2.9. Objective response rate was 72% in which eight patients (15%) and 31 patients (57%) experienced CR and PR, respectively. The median PFS was 13.9 months (95% CI: 11.2 to 16.0). Garcia and colleagues conducted a phase II trial of bevacizumab in combination with low dose metronomic cyclophosphamide29. Seventy patients were enrolled, and forty percent of patients had platinum-resistant disease based on their initial therapy. Treatment was administered in 28-days cycle, with bevacizumab 10 mg/kg intravenous infusion every week during the first 3 weeks of treatment followed by administration every 2 weeks, and daily dose of 50 mg of oral cyclophosphamide. Patients received a median of 5 cycle (range, 1 to 31) of treatment. Seventeen patients (24%) experienced PR and 44 patients (63%) had SD. The median PFS and OS were 7.2 months and 16.9 months, respectively. Eleven patients (15%) had grade 3 hypertension and 3 patients (4%) experienced a GIP. The taxanes as a class, particularly when delivered in a semi-metronomic fashion have also been of interest for combination with an anti-angiogenesis agent because of their impact on alternate and complementary pro-angiogenesis pathways35. To explore this potential, Hurt and colleagues reviewed their retrospective experience with combination weekly paclitaxel and biweekly bevacizumab at a single institution36. Fifty-one patients with platinum-resistant recurrent ovarian cancer received at least two cycles of chemotherapy were evaluated. Treatment was administered in 28-day cycle, with paclitaxel 60–70mg/m2 on days 1, 8, 15 and 22, and bevacizumab 10–15mg/kg on days 1 and 15. The median PFS and OS was 7 months (range, 2 to 38) and 12 months (range, 2 to 38), respectively. Despite a heavily pretreated patient population (median number of prior regimens = 4, range, 2 to 12), the objective response rate (ORR) was 60%. To provide reference, O’Malley and colleagues compared the outcomes of this cohort to a contemporary cohort treating with weekly paclitaxel without bevacizumab 37. Twenty-nine patients treated with weekly paclitaxel and 41 patients treated with weekly paclitaxel and biweekly bevacizumab were reviewed. Improvement of PFS was seen in those treated with combination paclitaxel and bevacizumab in comparison to weekly paclitaxel alone (median PFS 6.2 vs. 13.2 months, p<0.01). Ongoing evaluation of bevacizumab in patients with primary is addressing its role in alternative infusion schedules of chemotherapy. Gonzalez-Martin and colleagues recently presented their safety and feasibility data from a phase II trial of weekly paclitaxel (80 mg/m2, IV, days 1, 8, 15), every 3 week carboplatin (AUC 5, IV, day 1) and bevacizumab (7.5 mg/kg, IV, day 1) (OCTAVIA)38. One cycle was 3 weeks. Patients were allowed to receive up to 8 cycles of combination therapy and continue alone at the same dose for a total of up to 17 cycles (1 year) . Patients were allowed be stage I–IIa as long as they had grade 3 or clear cell carcinoma (20% of the population), or any grade stage IIb–IV (80%). One hundred eighty-nine patients were enrolled; 168 (89%) went into maintenance bevacizumab and 135 (71%) completed 1 year of therapy. Bevacizumab was discontinued in 12% for adverse events and for progression in 10%. The most common grade 3–4 toxicity was neutropenia, anemia and thrombocytopenia. Thromboembolic events occurred in 6%, and 1 patients had a GIP. PFS (primary endpoint) and OS were not mature at the time of the report. These observations provide important rationale for future phase III clinical trial development in both primary and recurrent disease.
3.2 Phase III trials
Four phase III randomized trials of bevacizumab in combination with cytotoxic chemotherapy have been conducted to date including two as primary adjuvant therapy following surgical debulking/staging and two in women with recurrent disease.
3.2.1 Primary adjuvant therapy
GOG-218 (NCT00262847) enrolled 1873 women who had previously untreated epithelial ovarian, primary peritoneal or fallopian tube carcinoma, with macroscopic residual stage III or stage IV 39. This trial was designed as a randomized, double-blind placebo controlled phase III study, and had three arms; control standard therapy group had combination chemotherapy with carboplatin AUC6 and paclitaxel 175mg/m2 every 3 weeks for 6 cycles plus placebo added in cycles 2 through 22; bevacizumab initiation therapy group had the same regimen of combination chemotherapy with bevacizumab (15mg/kg) added in cycles 2 through 6 and placebo added in cycles 7 through 22; bevacizumab-throughout therapy group had the same regimen of combination chemotherapy with bevacizumab added in cycles 2 through 22. While bevacizumab-initiation group showed no statistical significant difference in progression free survival (hazard ratio (HR) for progression or death in bevacizumab-initiation group, 0.908; 95% CI, 0.795 to 1.040; p=0.16) in this trial, bevacizumab-throughout group showed significantly longer progression free survival than standard therapy group (hazard ratio for progression or death in bevacizumab-throughout group, 0.717; 95% CI, 0.625 to 0.824; p<0.001). At the time of analysis, no statistical difference was reported in overall survival.
The second phase III trial (ICON7, NCT00483782) randomized 1528 women with high-risk early stage or advanced epithelial ovarian, primary peritoneal or fallopian tube carcinoma to either combination chemotherapy with carboplatin (AUC 5 or 6) and paclitaxel (175 mg/m2) intravenously every 3 weeks for 6 cycles or to the same chemotherapy regimen with bevacizumab (7.5 mg/kg body weight) for 5 or 6 cycles with a planned maintenance for 12 additional cycles of single agent bevacizumab at the same dose and frequency40. With median follow up of 28 months, the bevacizumab-containing arm showed longer progression free survival than the standard therapy group (HR for progression or death in the bevacizumab group, 0.87; 95% CI, 0.77 to 0.99; p=0.04), the effect of bevacizumab was approximately 2 months at the median. Analysis of overall survival is immature with final results expected in 2013. A comparison of key features between the two studies is presented in Table 2. Since ICON7 additionally recruited early stage patients, an exploratory analysis of a “high-risk” subgroup (suboptimal cytoreduced stage III and stage IV) was conducted to examine the consistency of treatment effects between the two studies. This analysis demonstrated an improvement in both PFS (median: 10.5 vs 15.9 months; HR: 0.68 95%CI: 0.55–0.85) and overall survival (median: 28.8 vs 36.6 months; HR: 0.64 95%CI: 0.48–0.85) when bevacizumab was added to chemotherapy.
Table 2.
Phase III trials of bevacizumab
| Trial | GOG-0218 | ICON7 | OCEANS | AURELIA |
|---|---|---|---|---|
| Setting/design | Primary adjuvant | Primary adjuvant | Recurrent platinum- sensitive | Recurrent platinum- resistant |
| Double-blinded, placebo-controlled | Open-label | Double-blinded, placebo-controlled | Randomized physician-specified, cohort constrained (see text) | |
| Three-arm study | Two-arm study | Two-arm study | Two-arm study | |
| Bev for 15 months | Bev for 12 months | Bev/placebo until PD or unacceptable toxicity | Bev until PD or unacceptable toxicity | |
| Bev dosage | 15 mg/kg/q3w | 7.5 mg/kg/q3w | 15mg/kg/q3w | 10mg/kg/q2w or 15mg/kg/q3w (schedule depended on chemotherapy partner) |
| Treatment | Combination with CBDCA and PAC | Combination with CBDCA and PAC | Combination with CBDCA and GEM | Combination with PLD, TOP or weekly PAC |
| Treatment cycle (days) | 21 | 21 | 21 | 21 or 28 |
| Patient populations |
|
|
Measurable platinum- sensitive recurrent disease | Measurable and assessable (GCIG) Platinum-resistant recurrent disease |
| Primary Endpoint | PFS | PFS | PFS | PFS |
| Additional endpoint | OS analysis (formal testing at time of PFS) | Defined final OS analysis (pending) | ORR, OS and DOR | ORR, OS safety and QOL |
| IRC | No IRC | IRC | No IRC |
Bev, bevacizumab; CBDCA, carboplatin; PAC, paclitaxel; GEM, gemcitabine; PLD, pegylated liposomal doxorubicin; TOP, topotecan; PFS, progression-free survival; OS, overall survival; ORR, objective response rate; DOR, duration of response; QOL, quality of life; IRC, independent radiologic review committee; GCIG, Gynecologic Cancer InterGroup
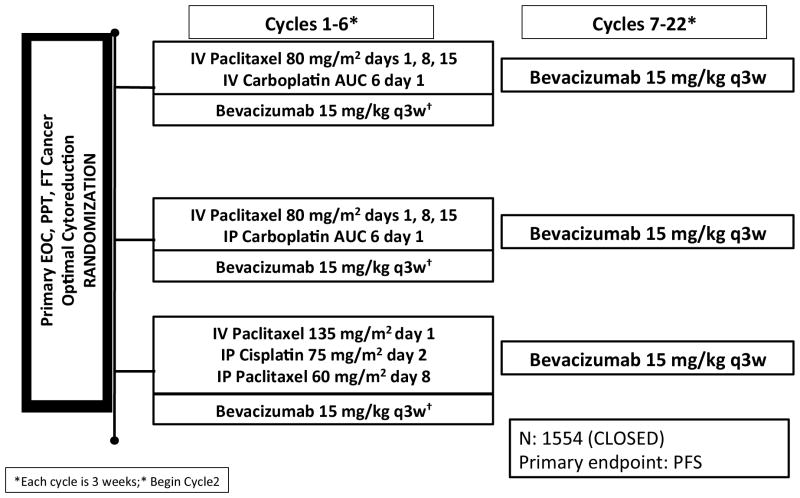
The GOG has enrolled 2 trials (GOG 252, NCT00951496 and GOG 262, NCT01167712) studying at alternative chemotherapy infusion schedules including dose-dense paclitaxel and intraperitoneal chemotherapy. Figures 1 and 2 show the schema and patient populations for these two trials.
FIGURE 1.
Schema for GOG 252. This trial was initially opened for patients with both optimal (≤1 cm postoperative tumor residuum) or suboptimal cytoreduction surgery. When GOG 262 became available, only optimal patients were allowed to accrue on this trial. It met it’s primary accrual target of 1520 patients.
FIGURE 2.
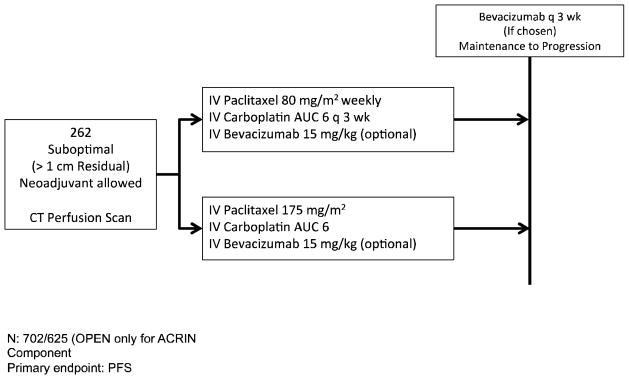
Schema for GOG 262. This trial was initially open for patients with suboptimal disease (> 1cm postoperative tumor residuum). Major amendments were enacted to allow patients undergoing neoadjuvant chemotherapy followed by surgery, and upon the enrollment completion of GOG 252, patients with optimal tumor cytoreduction. The trial has met its accrual target but is still enrolling patients who are participating on the perfusion imaging sub-study (ACRIN 6695). This trial, as opposed to GOG 252, 218, ICON7 and OCTAVIA allows for bevacizumab to be administered until progression or unacceptable toxicity in both arms (See text).
3.2.2 Recurrent Disease: Phase III trial of bevacizumab for platinum-sensitive recurrent ovarian cancer patients
The OCEANS (Ovarian Cancer Study Comparing Efficacy and Safety of Chemotherapy and Anti-Angiogenic Therapy in Platinum-Sensitive Recurrent Disease, NCT00434642) trial was initially designed to test the safety, particularly GI toxicity, of combination therapy in women with recurrent platinum-sensitive disease41. Following a satisfactory safety assessment, the trial was expanded to further address an efficacy endpoint, progression-free survival. In this phase III trial 484 patients were equally randomized into two groups; a control group, where gemcitabine (1000mg/m2 on days 1 and 8) and carboplatin (AUC 4 on day 1) was administered every 21 days with placebo for six to 10 cycles. In the experimental group, patients received the same chemotherapy in combination with bevacizumab (15 mg/kg on day 1). In the absence of progression, placebo or bevacizumab was to be continued until PD or unacceptable toxicity. With the median follow up of 24 months, patients receiving bevacizumab showed statistically significant increase in PFS compared with placebo group (hazard ratio for progression or death in the bevacizumab group, 0.484; 95% CI, 0.388 to 0.605). The median PFS was 8.4 and 12.4 months for the control and bevacizumab groups, respectively. There was also a statistically significant improvement in objective response rate (ORR) of 21% in the bevacizumab group (ORR, 79% [190 of 242] v 57% [139 of 242], p<0.0001). In the 3rd interim OS analysis, there was no difference in OS between the group, median OS was 33.7 months in the control group and 33.4 months in the experimental group42.
3.2.3 Phase III trial of bevacizumab for platinum-resistant recurrent ovarian cancer patients
AURELIA (A Multi-center, Open-label, Randomized, Two-arm Phase III Trial of the Effect on Progression Free Survival of Bevacizumab Plus Chemotherapy Versus Chemotherapy Alone in Patients With Platinum-resistant, Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer, NCT00976911), the first randomized trial of bevacizumab in platinum-resistant ovarian cancer patients was recently reported43. 361 patients were enrolled and randomized to two groups; chemotherapy alone (CT) or chemotherapy plus bevacizumab (CT+BEV). The regimen of chemotherapy was selected by investigator, and included liposomal doxorubicin (40 mg/m2 28-day cycle), topotecan (2 schedules: weekly 4 mg/m2 day 1, 8, 15, 28-day cycle or 1.25 mg/m2 daily for 5 days, 21-day cycle) or weekly paclitaxel (80 mg/m2 days 1, 8, 15, 22, 28-day cycle). For CT+BEV group, bevacizumab was given 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks. With a median follow up of 13.5 months, CT+BEV group showed statistical improvement of PFS compared with CT group (hazard ratio for progression or death in the CT-BEV, 0.48; 95% CI, 0.38 to 0.60; p<0.001) and improvement of ORR of 18% in CT-BEV (ORR, 31% v 13%).
3.3 Ongoing clinical trials of bevacizumab
Substantial interest in leveraging anti-VEGF targeting in ovarian cancer persists particularly as strategic therapeutic partners are discovered. Currently, several ongoing trials are addressing the impact of biological combinations targeting other aspects angiogenesis drivers such as vascular disrupting agents, PI3K inhibitors, angiopoeitin inhibitors and Poly-(ADP) ribose polymerase (PARP) inhibitors (GOG9923, NCT00989651), vascular disrupting agents (GOG-186I, NCT01305213), mTOR inhibitors (GOG-186G, NCT00886691), as well as other chemotherapy backbones, such as pemetrexed and nab-paclitaxel44, 45. Understanding the impact and safety of this additional targeting as well as understanding the mechanisms of emergent resistance will provide new therapeutic angles in future treatment paradigms.
3.4 Biomarkers for predicting efficacy of bevacizumab treatment
First two phase III trials (GOG-218 and ICON7) and each of these ongoing trials is studying biomarkers, which might identify sub-populations most likely to or not benefit from therapy. Plasma vascular endothelial growth factor (VEGF)-A has shown to be potential prognostic and predictive value for bevacizumab efficacy in breast, pancreatic and gastric cancers, and plasma VEGF receptor (VEGFR)-2 has shown to be potential prognostic predictive value in breast and pancreatic cancer46–48. In the analysis of GOG-218, plasma VEGF-A and VEGFR-2 were used as biomarkers for PFS and OS49. In contrast to other tumor types, analysis using the median value as the cut-off revealed no clear predictive effect of plasma VEGF-A for PFS and the effect of bevacizumab treatment was more pronounced in patients with low VEGF-A. No predictive value was seen for either PFS or OS when median VEGFR-2 level at baseline was used as the cut-off. However, exploratory analysis with other cut-offs are hypothesis generating for potential predictive (VEGF-A and VEGFR-2) or prognostic (VEGF-A: OS) value. There may be differences between tumor types in the biological effects and/or abundance of various VEGF-A isoforms. Additional candidate biomarkers in tumor tissue and blood DNA are being analyzed. Garcia et al. also reported the levels of plasma VEGF, E-selectin and TSP-1 in phase II trial of bevacizumab with metronomic cyclophosphamide29. In their report, levels of VEGF and TSP-1 decreased over time, but there were no significant associations between any of these markers and clinical outcome. Additionally, GOG262 is studying with subset patients population whether non-invasive radiographic CT perfusion scanning can aid in identifying patients likely, or not, to respond. This is predicated on the observations of other VEGF/VEGFR agents, such as sunitinib and aflibercept, on short-term tumor perfusion later response to therapy (add the aflibercept reference: Phase 1–2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Coleman RL, Duska LR, Ramirez PT, Heymach JV, Kamat AA, Modesitt SC, Schmeler KM, Iyer RB, Garcia ME, Miller DL, Jackson EF, Ng CS, Kundra V, Jaffe R, Sood AK. Lancet Oncol. 2011 Nov;12(12):1109–17. Epub 2011 Oct 10.
3.5 Adverse events of bevacizumab
Although the use of bevacizumab for ovarian cancer has led to improved progression-free survival and palliation of cancer related symptoms, it has been associated with an increased risk of serious adverse events such as intestinal perforation, hemorrhage and delayed wound healing. Prescribing information for bevacizumab notes a GIP incidence of 0.3% to 2.4% across all studies50. However, concern arose in ovarian cancer patients following a report of a phase II single-agent trial where 5 of 44 patients (11%) experienced GIP, 51 to 178 days after starting bevacizumab, and all of five patients who experienced GIP had radiographic evidence of bowel involvement at study entry28. In addition, all 5 patients had received three or more prior chemotherapy regimens and compared to 0 of the 23 patients who had received two prior chemotherapy regimens in this study. These events led to the early termination of the trial and raised awareness of the potential risks for GIP with bevacizumab therapy in patients with recurrent ovarian cancer. Although not definitive, exposure appeared to be associated with the development of GIP particularly when there was pre-existing bowel compromise. In ICON7, 10 of 745 (1%) patients experienced GIP in bevacizumab group, compared to 3 of 753 (<1%) patients in the control group40. In GOG-218, the rates of GIP in the two bevacizumab groups were almost twice those in the control group (1.2%, 2.8% and 2.6% in the control group, bevacizumab initiation group and bevacizumab throughout group, respectively) but was not statistically increased39. All but one GIP occurred during receipt of chemotherapy, not the maintenance phase, and the highest risk of GIP was those with large bowel resection and inflammatory bowel disease. Of note, in the two phase III trials of bevacizumab and chemotherapy for recurrent disease where eligibility was restricted to 2 or fewer chemotherapy regimens, the rates of GIP in platinum sensitive patients was 0% (OCEANS) and in 2% in patients with platinum-resistant disease (AURELIA)41, 43. These results from phase III trials suggest that the risk of GIP seems to be elevated but in line with prescribing information when the number of prior chemotherapy regimens was restricted to 2 or fewer.
In a meta-analysis of 10,217 patients with a variety of advanced solid tumors from 16 randomized control trials, the overall incidence of fatal adverse events (FAE) with bevacizumab was 2.9%, and the most common causes of FAE were hemorrhage (23.5%), neutropenia (12.2%) and GIP (7.1%) 51. Although this meta-analysis did not include phase II and III trials for ovarian cancer patients, compared with chemotherapy alone, the addition of bevacizumab was associated with an increased risk of FAEs, with a relative risk of 1.3 (95% CI, 1.02 to 1.73; p=0.04), and this association varied significantly with chemotherapeutic agents but not with tumor types or bevacizumab doses. Interestingly, bevacizumab was associated with an increased risk of FAEs in patients receiving taxanes or platinum agents (RR, 3.49; 95% CI, 1.82 to 6.66).
In bevacizumab monotherapy trials, hypertension was the most common grade 3/4 adverse event. Burger et al. reported 6 of 62 patients (11.3%) had grade 3/4 hypertension, but these effects were manageable39. They also reported increased rate of grade 2 or more hypertension in bevacizumab initiation or throughout group than the control group in combination therapy (16.5%, 22.9% vs. 7.2%) in GOG-218. Although the risk of hypertension appeared to be cumulative, it was controlled with the use of medical therapy. In this trial, one reversible posterior leukoencephalopathy (RPLE) was reported in both bevacizumab initiation and throughout group, no RPLE was seen in the control group. There was no difference between the risk of venous and arterial thromboembolism in bevacizumab initiation and throughout, and the control group. The rates of severe neutropenia and febrile neutropenia in combination with chemotherapy were reported and, comparing to chemotherapy without bevacizumab, chemotherapy with bevacizumab showed no statistical difference of the rate of severe neutropenia or febrile neutropenia in three phase III trials (GOG-218, ICON-7 and OCEANS)39–41. For example, the rates of grade 4 or 5 neutropenia and febrile neutropenia were reported and were slightly higher in bevacizumab initiation or throughout group than the control group (grade 4 or 5 neutropenia; 63.3%, 63.3% vs. 57.7%, febrile neutropenia; 4.9%, 4.3% vs. 3.5%), but there was no significant difference in GOG-21839.
Aghajanian et al. reported the rate of proteinuria of grade 3 or higher was increased in bevacizumab group than the control group (8.5% vs. 0.9%), and the development of proteinuria tended to develop after more extended bevacizumab treatment; the median time to onset of grade 3 or higher proteinuria was 26.5 months41. Burger et al. reported the rate of proteinuria of grade 3 or higher (0.7%, 1.6% and 0.7% for bevacizumab initiation, throughout and control group, respectively) in GOG-218, and, unlike other adverse effects, proteinuria was more commonly reported during the extended therapy phase than the chemotherapy phase among patients in the bevacizumab throughout group39. The risk of proteinuria of grade 3 or higher also appeared to be cumulative.
Aghajanian et al. also reported an updated safety analysis of OCEANS trial and showed that proteinuria and hypertension resolved the majority of the time52. In this analysis, the risk of hypertension of grade 3 or higher was 17.8%, all grade hypertension resolved 79.4% of the time and grade 3 or higher hypertension resolved 72.7% of the time. The risk of proteinuria of grade 3 or higher was 9.7%, all grade proteinuria resolved 82% of the time and grade 3 or more proteinuria resolved 91.7% of the time.
3.6 Cost effectiveness of bevacizumab
A cost effectiveness analysis compared the three arms of GOG-218 study has been reported 53. In this analysis, estimated costs of treatment plus the potential costs of complications were established for each strategy. Incremental cost effectiveness ratio (ICER) and the costs per progression-free life-year saved (PF-LYS) was estimated, and ICER per PE-LYS was $479,712 for bevacizumab initiation therapy and $401,088 for bevacizumab throughout therapy. The addition of bevacizumab to standard chemotherapy in patients with advanced ovarian cancer was felt not be cost effective, and treatment with maintenance bevacizumab leads to improved progression free survival but is associated with both direct and indirect costs. The cost effectiveness of bevacizumab in the adjuvant treatment of ovarian cancer is primarily dependent on drug costs. However, this report was based on the estimated costs based on planned therapy (15 months), not actual treatment duration (closer to 9 months).
4. Expert opinion
Ovarian cancer is a disease without specific symptomatology reflective of early growth and lacking an effective screening system. In light of this, most patients present with advanced stage. Maximal surgical cytoreduction followed by platinum-based chemotherapy is the standard treatment for patients with advanced ovarian cancer. However, despite rising median survival statistics, recurrence is frequent and five-year survival rate has been essentially flat over the last 30 years.
Several therapeutic approaches have been explored in an attempt to alter these statistics, including intraperitoneal chemotherapy and dose-dense paclitaxel. Both strategies have documented significant effects on PFS and OS. Unfortunately, further attempts to impact survivorship with chemotherapy have reached a therapeutic ceiling. This has fostered the discovery and development of agents focused on mechanisms driving tumor biology. There are noted successes from this approach in hematological malignancies as well as, some solid tumors. However, recent analysis of the ovarian cancer genome suggests a “smoking gun” target will be elusive54. Nevertheless, principles guiding the growth and metastases of this disease are being elucidated and several targeted agents are available to impact these processes. The most deeply explored in ovarian cancer is angiogenesis, where bevacizumab, arguably the maturely developed agent, has demonstrated anti-tumor effect.
A review of the clinical data as presented suggests that measurable effects of anti-VEGF based therapy can be documented; this leads to symptom control in some patients (ascites) and many do achieve differential benefit from tumor resolution. Although there was no difference of OS in GOG-218 and OCEANS trials, each of the presented phase III trials met their primary endpoint. The lack of PFS preservation to an OS endpoint is not unexpected in diseases where post-progression survival (PPS) is long 55. The contamination of alternate therapy, cross-over therapy, off- and non-treatment effects, and lack of consistent treatment protocols are among the many reasons for this hypothesis. It is our opinion that in light of the long PPS frequently seen in ovarian, trials, PFS is a reasonable primary endpoint in clinical investigation, even when the endpoint is not associated with clinical symptomatology. However, the critical question in assessing the data is a balance of the risk: benefit ratio. Further complicating this evaluation is the difficulty in synthesizing or characterizing a benefit to response or progression-free survival in the absence of gains in overall survival. In the setting of a non-toxic agent, any substantive gains in a measured principle could be considered an advance. However, marginal benefits lose clinical impact when patients experience toxicity. Another confounding element in assessing the impact of these studies is the “cross-over” effect. Since ovarian cancer is somewhat unique in being characterized as having a post-progression survivorship equal to or exceeding it progression-free interval, marginal gains in intermediate endpoints can be “washed out” by post-progression treatment strategies and therapies. This is suggested when one reviews the post-progression use of bevacizumab for recurrent disease following participation in GOG-218 and ICON7. Use of bevacizumab in the latter was 1–3%, whereas, nearly a third of patients in the control arm of GOG-218 went on to receive the agent. Further evidence of this hypothesis will come with maturity of the OS analyses in the near future.
Globally, the evidence provided from these carefully conducted phase III trials has convinced some regulatory organizations, such as the European Medicines Agency (EMEA) to approve the use of bevacizumab for patients with primary ovarian cancer. The financial impact of the cost of care is generally divorced from the regulatory process. However, it continues to impact utilization via reimbursement quotients. Biomarker data of bevacizumab treatment have been collected. Future investigation will need to focus on improving clinical performance (via biomarkers of efficacy, early discontinuation, additional agents, etc.), as well as, more sensitive tools to assess direct patient impact. Tangible benefits achieved with prolongation of an intermediate endpoint will be necessary to provide convincing evidence that this strategy is an advance and worthy of support.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Mar 1;20(5):1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 3.Monk BJ, Coleman RL. Changing the paradigm in the treatment of platinum-sensitive recurrent ovarian cancer: from platinum doublets to nonplatinum doublets and adding antiangiogenesis compounds. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2009 Dec;19( Suppl 2):S63–7. doi: 10.1111/IGC.0b013e3181c104fa. [DOI] [PubMed] [Google Scholar]
- 4.Ziebarth AJ, Landen CN, Jr, Alvarez RD. Molecular/genetic therapies in ovarian cancer: future opportunities and challenges. Clinical obstetrics and gynecology. 2012 Mar;55(1):156–72. doi: 10.1097/GRF.0b013e31824b1699. [DOI] [PubMed] [Google Scholar]
- 5.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature medicine. 2011;17(11):1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nature clinical practice Oncology. 2006 Jan;3(1):24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011 May 19;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angiogenesis inhibitors. National Cancer Institute at the National Institutes of health; Bethesda, MD: [cited; Available from: http://www.cancer.gov/cancertopics/factsheet/Therapy/angiogenesis-inhibitors. [Google Scholar]
- 9.Matei D, Sill MW, Lankes HA, DeGeest K, Bristow RE, Mutch D, et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Jan 1;29(1):69–75. doi: 10.1200/JCO.2009.26.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biagi JJ, Oza AM, Chalchal HI, Grimshaw R, Ellard SL, Lee U, et al. A phase II study of sunitinib in patients with recurrent epithelial ovarian and primary peritoneal carcinoma: an NCIC Clinical Trials Group Study. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2011 Feb;22(2):335–40. doi: 10.1093/annonc/mdq357. [DOI] [PubMed] [Google Scholar]
- 11.Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Nov 20;27(33):5601–6. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedlander M, Hancock KC, Rischin D, Messing MJ, Stringer CA, Matthys GM, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecologic oncology. 2010 Oct;119(1):32–7. doi: 10.1016/j.ygyno.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Annals of surgery. 1972 Mar;175(3):409–16. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D, Christofori G, Naik P, Arbeit J. Transgenic mouse models of tumour angiogenesis: the angiogenic switch, its molecular controls, and prospects for preclinical therapeutic models. Eur J Cancer. 1996 Dec;32A(14):2386–93. doi: 10.1016/s0959-8049(96)00401-7. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nature reviews Cancer. 2002 Oct;2(10):795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 16.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Feb 10;23(5):1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 17.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Current opinion in cell biology. 2009 Apr;21(2):154–65. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nature reviews Molecular cell biology. 2011 Sep;12(9):551–64. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerbel RS. Tumor angiogenesis. The New England journal of medicine. 2008 May 8;358(19):2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nature reviews Cancer. 2002 Oct;2(10):727–39. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 21.Huang S, Robinson JB, Deguzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer research. 2000 Oct 1;60(19):5334–9. [PubMed] [Google Scholar]
- 22.Bamias A, Pignata S, Pujade-Lauraine E. Angiogenesis: A promising therapeutic target for ovarian cancer. Critical reviews in oncology/hematology. 2012 May 8; doi: 10.1016/j.critrevonc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Teoh D, Secord AA. Antiangiogenic agents in combination with chemotherapy for the treatment of epithelial ovarian cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2012 Mar;22(3):348–59. doi: 10.1097/IGC.0b013e31823c6efd. [DOI] [PubMed] [Google Scholar]
- 24.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer research. 2005 Feb 1;65(3):671–80. [PubMed] [Google Scholar]
- 25.Zondor SD, Medina PJ. Bevacizumab: an angiogenesis inhibitor with efficacy in colorectal and other malignancies. The Annals of pharmacotherapy. 2004 Jul-Aug;38(7–8):1258–64. doi: 10.1345/aph.1D470. [DOI] [PubMed] [Google Scholar]
- 26.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005 Jan 7;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 27.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Nov 20;25(33):5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 28.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Nov 20;25(33):5180–6. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 29.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Jan 1;26(1):76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 30.Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Jun 1;29(16):2259–65. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Mar 1;27(7):1069–74. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monk BJ, Choi DC, Pugmire G, Burger RA. Activity of bevacizumab (rhuMAB VEGF) in advanced refractory epithelial ovarian cancer. Gynecologic oncology. 2005 Mar;96(3):902–5. doi: 10.1016/j.ygyno.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 33.McGonigle KF, Muntz HG, Vuky J, Paley PJ, Veljovich DS, Greer BE, et al. Combined weekly topotecan and biweekly bevacizumab in women with platinum-resistant ovarian, peritoneal, or fallopian tube cancer: results of a phase 2 study. Cancer. 2011 Aug 15;117(16):3731–40. doi: 10.1002/cncr.25967. [DOI] [PubMed] [Google Scholar]
- 34.Del Carmen MG, Micha J, Small L, Street DG, Londhe A, McGowan T. A phase II clinical trial of pegylated liposomal doxorubicin and carboplatin plus bevacizumab in patients with platinum-sensitive recurrent ovarian, fallopian tube, or primary peritoneal cancer. Gynecologic oncology. 2012 Sep;126(3):369–74. doi: 10.1016/j.ygyno.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 35.Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, et al. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clinical cancer research : an official journal of the American Association for Cancer Research. 1996 Nov;2(11):1843–9. [PubMed] [Google Scholar]
- 36.Hurt JD, Richardson DL, Seamon LG, Fowler JF, Copeland LJ, Cohn DE, et al. Sustained progression-free survival with weekly paclitaxel and bevacizumab in recurrent ovarian cancer. Gynecologic oncology. 2009 Dec;115(3):396–400. doi: 10.1016/j.ygyno.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 37.O’Malley DM, Richardson DL, Rheaume PS, Salani R, Eisenhauer EL, McCann GA, et al. Addition of bevacizumab to weekly paclitaxel significantly improves progression-free survival in heavily pretreated recurrent epithelial ovarian cancer. Gynecologic oncology. 2011 May 1;121(2):269–72. doi: 10.1016/j.ygyno.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Martin A, Gladieff L, Tholander B, Stroyakovsky D, Gore ME, Segalla JGM, et al. Safety of front-line bevacizumab (BEV) combined with weekly paclitaxel (wPAC) and q3w carboplatin (C) for ovarian cancer (OC): Results from OCTAVIA. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(suppl; abstr 5017) [Google Scholar]
- 39.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England journal of medicine. 2011 Dec 29;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 40.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. The New England journal of medicine. 2011 Dec 29;365(26):2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- **41.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jun 10;30(17):2039–45. doi: 10.1200/JCO.2012.42.0505. These results suggest that the risk of GIP seems to be in line with prescribing information when the number of prior chemotherapy regimens is restricted to 2 or fewer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aghajanian C, Nycum LR, Goff B, Nguyen H, Husain A, Blank SV. Gynecological cancers/967OUpdated overall survival analysis in OCEANS, a randomized phase 3 trial of gemcitabine (G) + carboplatin (C) and bevacizumab (BV) or placebo (PL) followed by BV or PL in platinum-sensitive recurrent epithelial ovarian (ROC), primary peritoneal (PPC), or fallopian tube cancer (FTC) Annals of Oncology. 2012 Sep 1;23(suppl 9):ix319. [Google Scholar]
- **43.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. AURELIA: A randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(suppl; abstr LBA5002) doi: 10.1200/JCO.2013.51.4489. These results suggest that the risk of GIP seems to be in line with prescribing information when the number of prior chemotherapy regimens is restricted to 2 or fewer. [DOI] [PubMed] [Google Scholar]
- 44.Hagemann AR, Zighelboim I, Novetsky AP, Gao F, Massad LS, Thaker PH, et al. Phase II trial of bevacizumab and pemetrexed for recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(suppl; abstr 5013) doi: 10.1016/j.ygyno.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tillmanns TD, Lowe MP, Walker MS, Stepanski EJ, Schwartzberg LS. Phase II clinical trial of bevacizumab with albumin-bound paclitaxel in patients with recurrent, platinum-resistant primary epithelial ovarian or primary peritoneal carcinoma. Gynecologic oncology. 2012 Sep 5; doi: 10.1016/j.ygyno.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 46.Miles D, Haas SD, Dirix L, Chan A, Tomczak P, Provencher L, et al. Abstract P2-16-04: Plasma biomarker analysis in the AVADO phase III randomized study of first-line bevacizumab + docetaxel in patients with human epidermal growth factor receptor (HER) 2-negative metastatic breast cancer. Cancer research. 2010 Dec 15;70(24, Suppl 2) [Google Scholar]
- 47.Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jun 10;30(17):2119–27. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- 48.Lambrechts D, Claes B, Delmar P, Reumers J, Mazzone M, Yesilyurt BT, et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials. The lancet oncology. 2012 Jul;13(7):724–33. doi: 10.1016/S1470-2045(12)70231-0. [DOI] [PubMed] [Google Scholar]
- 49.Birrer MJ, Lankes H, Burger RA, Mannel R, Homesley H, Henschel V, et al. Biomarkers/198 Biomarker results from GOG-0218, a phase III trial of front-line bevacizumab plus chemotherapy for ovarian cancer. Annals of Oncology. 2012 Sep 1;23(suppl 9):ix81–ix82. This is the 1st biomarker analysis of bevacizumab trial in ovarian cancer patients. [Google Scholar]
- 50.Prescribing information of Avastin. Genentech, Inc; San Francisco, CA: 2012. [cited; Available from: http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf. [Google Scholar]
- 51.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA : the journal of the American Medical Association. 2011 Feb 2;305(5):487–94. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 52.Aghajanian C, Blank SV, Goff BA, Judson PL, Nycum LR, Sovak MA, et al. An updated safety analysis of OCEANS, a randomized, double-blind, phase III trial of gemcitabine (G) and carboplatin (C) with bevacizumab (BV) or placebo (PL) followed by BV or PL to disease progression (PD) in patients with platinum-sensitive (Plat-S) recurrent ovarian cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(suppl; abstr 5054) doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohn DE, Kim KH, Resnick KE, O’Malley DM, Straughn JM., Jr At what cost does a potential survival advantage of bevacizumab make sense for the primary treatment of ovarian cancer? A cost-effectiveness analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Apr 1;29(10):1247–51. doi: 10.1200/JCO.2010.32.1075. [DOI] [PubMed] [Google Scholar]
- 54.The Cancer Genome Atlas. National Cancer Institute at the National Institutes of Health; Bethesda, MD: [cited; Available from: http://cancergenome.nih.gov/ [Google Scholar]
- 55.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. Journal of the National Cancer Institute. 2009 Dec 2;101(23):1642–9. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]