Abstract
This review presents the need for replacing gels in 2D separations for proteomics, where speed, high-throughput, and the ability to characterize trace level proteins or small samples are the current desires. The theme of the review is isoelectric focusing, which is a valuable tool because it pre-concentrates proteins in addition to separating with high peak capacity. The review traces the technological progress from gel IEF to cIEF to packed capillaries with immobilized gradients for cIEF. Multiple capillary techniques are progressing toward meeting the current desires, providing extremely high sensitivity with regard to concentration and to small samples, integrated automation, and high peak capacity from multiple dimensions of separation. Capillaries with immobilized pH gradients for cIEF are emerging, which will alleviate interference from ampholytes and improve reproducibility in separation times when this valuable technique can be used as one of the dimensions.
1. Introduction
Proteomics has produced an enormous number of biomarker candidates for cancer, but this effort has not been accompanied by an increase in validated biomarkers [1–3]. New technology is needed for biomarker discovery that incorporates at least some validation by being higher in throughput than conventional methods. This review paper is about an emerging technology, which is the replacement of gels by packed capillaries and channels. Gels are not amenable to the high throughput needed for biomarker discovery because they resist automation and cannot be miniaturized. It is inevitable that gels will eventually be replaced by new materials by advances in nanotechnology. The new materials could potentially be silica-based because silica has been so successful in liquid chromatography. We discuss advances in using packed capillaries and packed channels with silica-based materials. These media are still in the research stage, rather than being commercially available, therefore, applications to proteomics are in the future. We describe the advances in the context of advances in capillary techniques, particularly isoelectric focusing, and point to the trajectory for proteomic applications.
When the word electrophoresis is used, it commonly means sieving electrophoresis, where an electric field is used to separate ions based on charge and size, each of which affects the speed of migration. Gels were almost exclusively the media for electrophoresis for decades. The introduction of capillaries in the early 1980’s greatly increased the speed and efficiency of electrophoresis [4–5]. The speed is increased because thin capillaries reduce the thermal broadening from Joule heating, which allows higher electric fields. The peaks sharpen because there is less time for molecular diffusion. When a soluble polymer is used in the capillary as a sieving medium, the technique is called capillary sieving electrophoresis, and sized-based DNA and protein selectivity rivals that of gels. This technique is termed capillary sieving electrophoresis, and it replaced gels in DNA analysis to accelerate progress in sequencing the human genome by virtue of its ability to be automated [6]. Crime labs, which used to rely on gel electrophoresis for DNA fingerprinting, now use capillary sieving electrophoresis to gain reproducibility and speed [7]. The impact of translating electrophoresis from gels to capillaries for DNA analysis has been enormous. Capillaries replaced gels in DNA electrophoresis and they are headed in that direction for proteins.
The power of gels in protein separations is their 2D format, where thousands of proteins can potentially be separated by using isoelectric focusing (IEF) in one dimension, followed by sieving in the second dimension. The separations take more than a day, and the analysis is labor-intensive because the gels need to be handled for staining and readout. Also, many applications are just not amenable to analysis by gels. For example, only the relatively high abundance proteins can be detected from serum. This is a problem in biomarker discovery because the concentration range of proteins in serum spans 10 orders of magnitude [8], whereas the concentrations of proteins currently used as cancer biomarkers are at trace levels in serum [9]. New cancer biomarkers will likely also be at trace levels. Further, it might be a particular isoform or pattern of isoforms for a protein that makes it a biomarker, and these constituents are at an even lower concentration. For example, consider the problem of analyzing the glycoform pattern of prostate specific antigen, which shows promise in improving the selectivity for prostate cancer [10]. One can use immunobeads to selectively extract the targeted protein from serum, and pre-concentrate the protein. But gels are thick, requiring higher volumes of sample than capillaries or channels, therefore, samples cannot be pre-concentrated to the same degree to gain the very high sensitivity needed. The total amount of prostate specific antigen would be as low as 4 ng/mL for the analysis, with the glycoforms being at even lower levels. This is not feasible for gels. Capillaries can be made much thinner, allowing the same number of moles to be released into a smaller volume for higher pre-concentration. Capillaries thus offer needed advantages of speed and sensitivity, but not the 2D format.
The sheet format of gels can be mimicked by using a pair of capillaries, the first with a high resolution separation, and the second with a fast-scanning lower resolution separation that is complementary to the first. The power of this approach is demonstrated by a 2D capillary application in single-cell proteomics [11], illustrated in Figure 1. This shows a 2D electropherogram from a single cell, a mouse embryo in this case. The first dimension used capillary sieving electrophoresis and the second dimension used micellar electrokinetic chromatography (MEKC), which separates based on hydrophobicity. The important differences between this and a 2D gel electropherogram are several. First, the sample was only one cell, which would be prohibitively minute for gel, yet many electropherograms could be generated from this same small sample using capillaries. Second, the 2D electropherogram was obtained in only 1.25 hr, which is about 20 times faster than a 2D gel electropherogram would be obtained. Third, positions of the peaks were shown to be reproducible within about twice the spot size, which is far superior to gels. Fourth, the two dimensions were integrated together by automation, representing another advantage over gels. The peak capacity was 380, which is beginning to get competitive with gels. These capabilities make capillaries a tantalizing prospect for proteomics. The drawback is that these media described thus far are incompatible with mass spectrometry. The substances giving rise to the separation, which are the surfactant and the sieving polymer, preclude the use of mass spectrometry for characterizing the intact proteins. Use of packed capillaries would allow these materials to remain inside the capillaries to allow mass spectrometry of intact proteins.
Figure 1.

Illustration of data from two replicate 2D separations of proteins obtained from lysing a single cell, where a pair of capillaries was used in tandem for the two dimensions. The marks (⊕) indicate positions of 50 proteins used to demonstrate the high reproducibility of the separations. Figure is adapted from Harwood et al. [11].
The second dimension used for gels, which is IEF, has also been extensively developed for capillaries. IEF is the slow step in 2D gel separations, hence the attractiveness for using capillaries.
2. Principles of isoelectric focusing
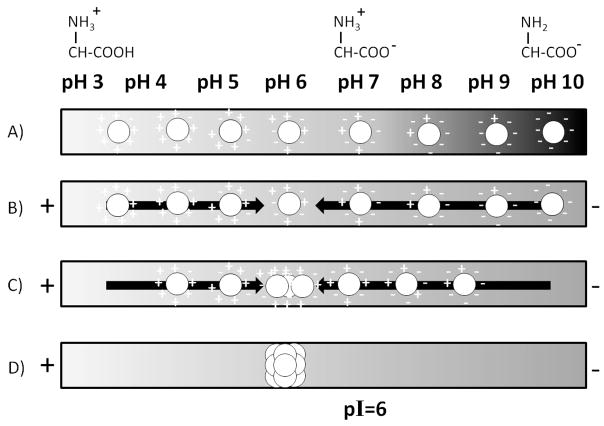
The principle of IEF with gels is illustrated in Figure 2. A gel having an immobilized pH gradient is used, and protein solution is placed on top of the gel. Overnight, the gel is rehydrated by the protein solution, distributing the proteins uniformly across the depth and breadth of the channel, as depicted in Figure 2A. Proteins at the high pH end adopt a net negative charge, and proteins at the low pH end adopt a net positive charge. When a voltage is applied, as shown in Figure 2B, positively charged proteins move from left to right, and vice versa for negatively charged proteins. As Figure 2C illustrates, migration continues, and it take hours to reach steady-state, indicated in Figure 2D. Migration stops when each protein reaches the pH at which its charge is zero, which is called its isoelectric point, or pI. For the illustration in Figure 2, pI=6. Steady-state is reached because proteins are still able to diffuse away from the equilibrium pH, but they then pick up a charge and the electric field drives them back to their positions of pH=pI [12].
Figure 2.
Illustration of how isoelectric focusing separates proteins by isoelectric point. A) Protein mixture is distributed randomly throughout the medium. B,C) In the presence of an electric field, proteins at any pH away from their pI migrate toward the electrode of opposite charge. C) The proteins stop when they reach the position where pH matches their pI. pI=6 is used as an illustration to explain that pI need not be neutral. The result is a concentrated protein band at the pI for that protein.
Equation 1 describes the resolution, Rs, in isoelectric focusing. The terms F, E, R and T
| (1) |
are Faraday’s constant, electric field, the gas constant and temperature, respectively [12]. The equation shows that resolution is proportional to the difference in pI between two proteins. Resolution also depends on how much the charge changes with pH, which is expressed by the term dz/dpH. This is what makes isoelectric focusing particularly good for large molecules, such as proteins, which have many charged groups. The diffusion coefficient is not in this expression because the friction coefficient affecting diffusion has a counterbalancing effect on the rate of the electromigration back to the position of pH=pI. The remaining terms are under experimental control. Resolution increases with a shallower pH gradient, ΔpH/Δx, i.e., choosing a pH gradient from 5–8 units would give somewhat better resolution than one from 3–10 units. Since this term is within the square-root sign, the choice of pH range is not a large factor; e. g., decreasing the pH ranges from 3–10 to 5–8 would only improve the resolution by 50%. People often use Equation 1 to express what minimum value of ΔpI can be separated with a resolution of unity, and this is also referred to as resolution, but with the distinction that it is expressed in pH units.
Typically for gels, resolution is sufficient to separate proteins differing in pI by as little as 0.01 pH. A ten-fold higher resolution, separating proteins differing by only 0.001 pH, was demonstrated using the extreme condition of a pH range of only 0.2 pH over 20 cm of gel [13]. Equation 1 shows that resolution increases with the square root of the electric field, E. The electric field is proportional to the applied voltage, E=V/ΔX, where V is the applied voltage and ΔX is the length of the medium. In gels, electric field is ramped over the course of the separation, which takes place over the course of an hour. The electric field must be ramped because initially the current is high from the many migrating proteins, causing electrical heating, which can damage the gel or the proteins. The current later tapers off to a constant value as focusing is reached. The final E after ramping is the value of E that determines resolution in Equation 1. By replacing gels with inorganic matrices, high electric fields will likely be possible, giving higher resolution.
The realities of IEF using commercial gels with immobilized gradients are illustrated in Figure 3, which shows an image for a protein separation using a commercial gel with an immobilized pH gradient from 4 to 7. The protein is bovine serum albumin, which is over-labeled with a dye to create a charge ladder, giving about a dozen bands. Each band differs from the adjacent band by one unit of charge, and the separation reveals that dz/dpH=8/0.5=16. One can calculate the expected resolution in pI units: setting Rs=1, assuming a linear gradient, dpH/dx=30 m−1 and using E=800 V/cm, one gets ΔpI=0.003. The actual pI resolution is closer to 0.06 pH. Protein variants, gel inhomogeneity, thermal gradients, broadening during scanning, and slow equilibration in focusing can all contribute to reducing the actual resolution. Gel inhomogeneity is evident in the image, particularly within a given zone, which varies in intensity from top to bottom.
Figure 3.
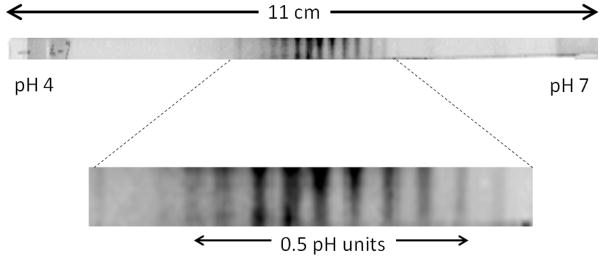
Image of separated proteins in a gel strip having an immobilized pH gradient. The pH gradient is 4–7 and the strip is 11 cm long. The protein, bovine serum albumin, was over-labeled with dye to give a charge ladder. The separation is shown on an enlarged image to detail the bands from each protein form. Wirth group, unpublished image.
IEF has one especially important advantage over sieving electrophoresis in that it actually concentrates analytes. A protein spread over the entire 11 cm of gel will be concentrated in a zone that is only a few mm, giving an increase in concentration by a factor of 50–100. IEF has the particular disadvantage of being extremely slow, requiring times on the order of 20 hours for rehydration and separation, and does not deliver the theoretically achievable resolution. In replacing gels with capillaries for isoelectric focusing, one would expect similar gains as those achieved in sieving electrophoresis: higher electric fields to give shorter run times, and ease of automation, in addition to the smaller sample volumes that allow for greater pre-concentration. Achievable resolution could be higher because there is no gel to impart inhomogeneity.
3. Principles of cIEF
Hjerten and Zhu translated gel IEF into cIEF almost 30 years ago [14], and Thormann et al. were investigating cIEF on the same time frame [15]. Since that time, cIEF has been developed into a widely used tool for protein separations, confirming vastly higher speed, as well as higher resolution, smaller sample volumes, and in-line integration with secondary separation modes and mass spectrometry.
cIEF, as it is most often practiced, uses an open capillary, which precludes the use of an immobilized pH gradient. Instead, the pH gradient is supplied by using a mixture of mobile carrier ampholytes having a distribution of isoelectric points, which self-organize in an electric field to create the pH gradient. By mixing the proteins into the carrier ampholyte solution, there is no time required for introducing proteins into the capillaries. This avoids the overnight wait required in the case of gels. The pH gradient is formed with carrier ampholytes by applying the negative electrode in basic solution at one end of the capillary, and the positive electrode in acidic solution at the other end. Mechanistic studies detail the gradient formation and its imperfections [16]. One problem that arises is that the acidic and basic solutions gradually encroach into the capillary to compress the pH gradient. Another problem is that electro-osmotic flow causes the pH gradient to shift gradually. Electro-osmotic flow is reduced by using a hydrophilic coating on the capillary wall [17–20], but is never entirely eliminated. Commercial carrier ampholytes have been rigorously characterized, and some are shown to be much better than others [21]. The inability to form a stable pH gradient is the biggest drawback of cIEF with carrier ampholytes because one can no longer strictly relate position to pH. This problem is typically circumvented by adding pI standards to the sample [22], allowing the speed and automation of capillaries to be used to advantage.
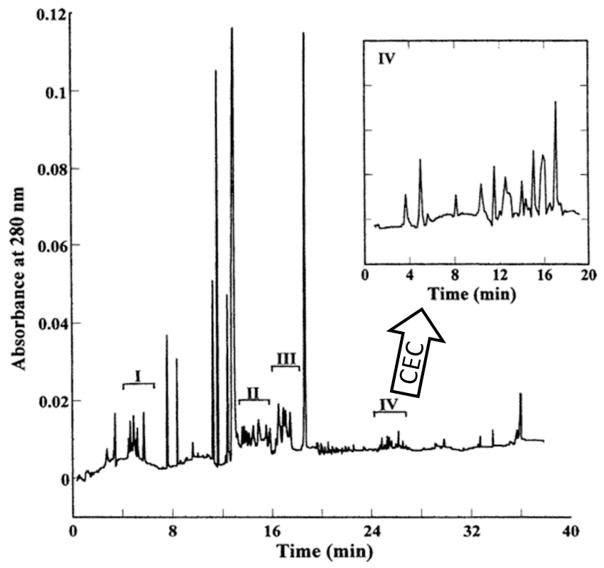
An example of a protein separation by cIEF is shown in Figure 4 for human serum depleted of serum albumin [23]. A pH gradient from 3–10 over 38 cm of capillary was used with an electric field of 400 V/cm. The time axis is from remobilization by gravity, where one end of the capillary was tilted to give flow. During this remobilization, the electric field maintains focusing. The net resolution is calculated to be 0.04 pH, which rivals a gel for the same electric field strength. The speed of focusing was much higher than for gels: 15 min. The remobilization was used to enable a second dimension of separation. The inset shows the electropherogram resulting from capillary electrochromatography of the fraction labeled as IV. Injecting fractions rather than individual peaks into the second dimension saves time but reduces peak capacity. The total theoretical peak capacity of the system exceeds 50,000 proteins, but it would take a full week to achieve this. It is the slow speed of chromatography that makes this 2D method so time consuming. Peak capacity per time, which makes for a better comparison with gels, is still an order of magnitude higher than for gels.
Figure 4.
Electropherogram from cIEF and remobilization for albumin-depleted human serum. Each of the four fractions indicated, I to IV, was injected into a second dimension for separation by capillary electrochromatography, the chromatogram for fraction IV is shown in the inset. Figure is adapted from Zhang et al. [23].
4. Optical detection in cIEF
Gels require staining for detection. A greater variety of methods are used for cIEF. Excellent reviews cover development of detection methods for cIEF [24–25]. Fluorescence imaging remains important [26]. Unlike for gels, the free excess dye need not be removed because it is unlikely to interfere: most dyes do not even focus because they have only one possible charge. Many dyes impart a different charge to the protein, such as fluorescein isothiocyanate and the Alexa Fluor dyes. Dyes that conserve charge are most useful, such as Chromeo-P40 or the Cy-dyes designed for differential in-gel electrophoresis, which are brighter. While the latter dyes are expensive, the lower volumes of capillaries make these dyes more cost-effective. On-column imaging can gain some of the information normally obtained from a 2D gel separation by allowing proteins to diffuse, adding size information to the pI information [27].
In addition to fluorescence, two optical detection methods that avoid labeling are refractive index imaging and UV absorbance imaging [24]. These avoid any changes that labeling might impart. UV detection is particularly suited to studies of protein-protein interactions because these studies do not require the higher sensitivity of fluorescence detection, and the detection technique avoids a dye label that would otherwise perturb these interactions [28]. The commercialization of a UV- imaging cIEF instrument has led to many applications, which have been reviewed [29].
Fluorescence detection affords the highest sensitivity. The mass sensitivity is extremely high because the volumes of the focused zones are miniscule, e.g., for a 75 μm i.d. capillary and a 75 μm width of the focused zone, the zone occupies a volume of only 3 nL. Fluorescence detection for reasonable dyes routinely gives sensitivity on the nanomolar scale, therefore, attomole detection limits are expected, provided that components in the carrier phase are nonfluorescent and that the analyte is well separated from components of high concentration. Attomole (10−18) detection limits have been demonstrated [30], and zeptomole (10−21) detection limits of proteins has been achieved by photobleaching the ampholyte solution prior to cIEF [31]. This extreme mass sensitivity has promise for using cIEF in studies of single cells, which is an area that has recently been reviewed [32]. In addition to high sensitivity, fluorescence detection also enables ease of multiplexing to increase throughput. As an example, one laser beam can traverse 32 sheath-flow cuvettes, allowing detection as the proteins pass through the laser beam after remobilization with resolution of 0.002 pH [33]. This reinforces the ideas mentioned earlier that the powerful combination of high-throughput, high sensitivity and high resolution will significantly advance single-cell proteomics, as well as biomarker discovery and disease diagnostics, all of which will benefit from automated, multiplexed separations.
5. Mass spectrometric detection and applications for cIEF
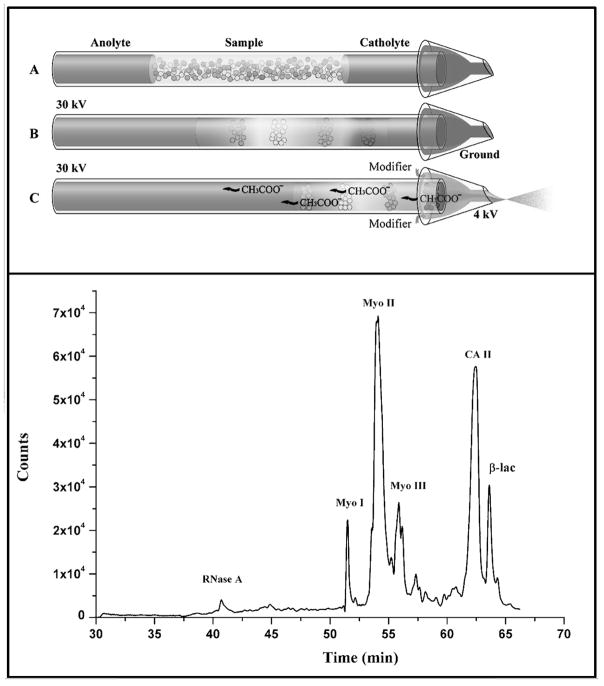
For proteomic separations, mass spectrometry is an essential tool. Integration of gels with mass spectrometry is cumbersome. After a 2D gels is run, the gel is stained to identify the positions of protein spots of interest, then individual spots are cut out of the gel, which is then de-stained, and then a protease is added to digest the protein into peptides. The peptides diffuse out of the gel, allowing LCMS for protein characterization. The goal with capillaries, as we have discussed, is seamless integration of separations to allow for automation. After cIEF, one can remobilize the proteins by using gravity-induced flow, electro-osmotic flow, or by replacing the basic reservoir with an acidic one (or vice versa), which imparts a charge to the proteins to give electromigration. One interesting advance is the direct integration of cIEF with mass spectrometry by Zhong et al., where the catholyte end of the capillary is in a microvial, as illustrated in Figure 5 [34]. After focusing, the voltage is switched for electrospray, giving the total-ion electropherogram of intact proteins that is also shown in Figure 5. Carrier ampholytes tend to suppress ionization [35]. 2D instrumentation includes cIEF coupled to LC to remove the ampholytes, enabling efficient nanospray ionization [36–40]. cIEF-MS of intact proteins has recently been reviewed, including capillaries and channels, and ionization methods of MALDI and ESI [41].
Figure 5.
Illustration of direct coupling of cIEF to electrospray for mass spectrometry. Top diagram depicts scheme for switching from cIEF to electrospray. Bottom shows a total ion electropherogram from cIEF for a handful of intact proteins. Figures are adapted from Zhong et al. [37].
The irreproducibility of the pH gradient in cIEF is the biggest drawback to 2D separations because it makes it difficult to systematically select the same peaks for the second dimension from one run to the next. Efforts to make stable pH gradients by using packed capillaries with immobilized gradients are directed to solving this problem. First, the issue of using packed capillaries with mobile carrier ampholytes needs to be addressed.
6. cIEF in packed capillaries and channels
There are many ifs that spring to mind when considering the use of packed capillaries for cIEF. The first is that the medium might be adsorptive to proteins. The second potential problem is speed because the packing would necessarily slow the focusing since mobility scales with the free volume fraction. The third potential problem is that the smaller free volume of a packed capillary would lower the sensitivity because fewer moles of analyte would occupy a given detection volume. These problems turn out not to be significant limitations. If adsorption of proteins in packed media were really a limitation, there would be no such thing as size-exclusion chromatography. The free volume is indeed smaller, but it is only smaller by a factor of three or four, which is not a major factor. E.g., instead of focusing in 2 minutes, it might take 5 minutes, which is still considerably faster than the 16 hour analysis time for gels. Also, the slightly lower sensitivity can probably be recouped by higher resolution from higher thermal conductivity of a solid. Finally, when immobilized gradients become advantageous for capillaries, it would be valuable to remobilize by pressure driven flow because one can avoid the imprecision of using electric field and its associated electro-osmotic flow. This raises the question of whether flow can effectively remobilize proteins in the absence of an electric field without excessive peak broadening. In short, the idea of packed capillaries for cIEF cannot be dispelled at the outset, but questions remain.
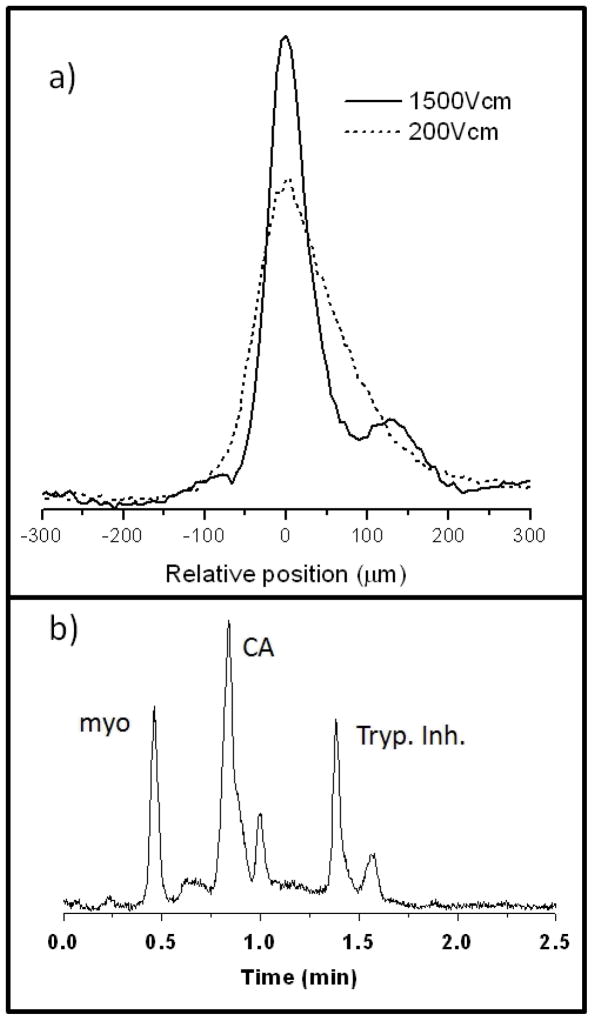
Our own group investigated the influence of a packing material on cIEF and pressure-driven remobilization [42]. We used capillaries packed with nonporous 1 μm diameter silica spheres bearing a covalently monolayer of linear polyacrylamide chains. Focusing was shown to work better than in open capillaries, allowing electric fields at least as high as 1500 V/cm. This is illustrated in Figure 6a, where the band for trypsin inhibitor looks like it might be tailing when 200 V/cm is used, but use of 1500 V/cm reveals that it is comprised of two bands. Such a high electric field has never been approached with cIEF in open capillaries or gels to our knowledge. We attribute the ability to use higher voltages to better thermal conductivity of solids. The high voltage compensates for the lower free volume to give the same sensitivity as one would have in an open capillary, while providing the higher resolution. Figure 6b shows the electropherogram of a simple mixture of proteins after field-free, pressure-driven remobilization. A pH gradient of 3–10 was used over a distance of only 2 cm, and the remobilized proteins span a range of 3 pH units. Broadening from remobilization originated both from diffusion and from slight inhomogeneity of the medium. The inhomogeneity corresponds to a plate height of only 0.6 μm. We have shown in other work that it is possible for capillaries to be packed so uniformly that the packing imparts only a few nm of plate height [43]. The ability to focus at very high electric fields combined with the ability to remobilize with minimal broadening are potentially valuable features for the eventuality of immobilized gradients in cIEF.
Figure 6.
cIEF in a capillary packed with micrometer-sized silica particles with polyacrylamide coatings, using carrier ampholytes. a) Image data for a focused zone of trypsin inhibitor, showing increased resolution at very high electric field. b) Remobilized protein peaks after cIEF using pressure driven flow in the absence of electric field. Figures are adapted from Hua et al. [45].
7. cIEF with immobilized pH gradients
There have been few papers on immobilized gradients in packed capillary/channels for cIEF [44–49]. None has yet resulted in a resolution as high as that obtained using carrier ampholytes in cIEF, but the field is progressing. The early results will guide new ideas to lead to success.
The most widely used method for immobilizing a pH gradient thus far has been to form a polymer monolith that includes groups reactive toward amino groups, then focusing ampholytes in an electric field to create the pH gradient. The amino groups then react with the monolith to become immobilized. The first report of an immobilized pH gradient in cIEF was by Yang et al. in 2004, where they also included a linker between ampholyte and epoxide [50]. The resolution for proteins was approximately 0.2 pH units. Liang et al. in 2009 [44], Yang et al. in 2010 [47], and Wang et al. in 2010 [46] used variations of this approach. The potential problem with this approach is that the reaction between amino and glycidoxyl groups is pH dependent, working best at high pH and requiring overnight reaction times at neutral pH. The ampholytes diffuse during this time, which can potentially wash out the pH gradient. Using a long capillary, and relying on the fact that obstructed diffusion in liquids is quite slow, a good pH gradient can nonetheless be obtained. Yang et al. in 2010 used a different approach, where a few ampholytes with vinyl groups were included in the mixture to be polymerized, and an electric field was applied before polymerization to form a pH gradient [47]. This allowed for a faster polymerization reaction to immobilize the gradient, but gave lower resolution.
Most recently, Liang et al. used UV photografting to polymerize ampholytes, immobilizing the ampholytes in 20 min to give a stable and well defined gradient [51]. Figure 7a shows the electropherogram of a mixture of proteins after remobilization, ranging in pI from 5.1 to 8.8. Figure 7b shows the linearity in the relation between pI and remobilization time, which demonstrates the reliability in determining the pI of the protein. The resolution is 0.3 pH for this 24 cm long capillary and an electric field of 400 V/cm. It is possible that the initial resolution iside the capillary was high but that the peaks were broadened during remobilization because it is challenging to form a homogeneous monolith inside of a capillary. Recent advances in the packing of silica particles in capillaries could virtually eliminate broadening from inhomogeneity [52–53], which could perhaps give rise to packed capillaries that have the advantages of both gels and capillaries.
Figure 7.
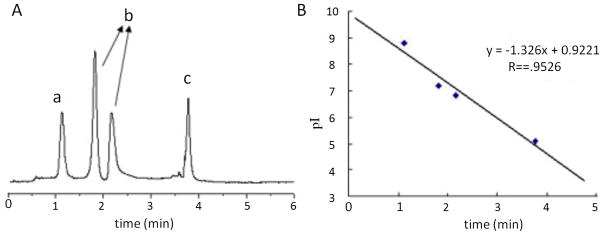
cIEF data for a packed capillary with an immobilized pH gradient. A) Protein peaks from remobilization: a) ribonuclease B, b) myoglobin, and c) β-lactoglobulin. B) Linearity between protein pI with remobilization time. Figures are adapted from Liang et al. [54].
There has been development of 2D instrumentation in this budding area of packed cIEF with immobilized gradients. Wang et al. combined cIEF with CE to separate proteins [45], and they redesigned the instrument to add an immobilized trypsin reactor after the cIEF and separate the peptides by CE [46]. They also coupled the cIEF/microreactor to nano-reversed phase LC and mass spectrometry [54]. Zhang et al. have demonstrated coupling of immobilized cIEF to MALDI MS for neuropeptide analysis [48]. The advances thus far represent an important step: a pH gradient is immobilized in capillaries to give resolution that may eventually rival that of gels while allowing for the advantages of capillaries: fast sample introduction, fast focusing, and integration with detection, and coupling a secondary separation and mass spectrometry.
8. Future prospects
Applications proliferate when tools become commercially available. The immobilized pH gradient for capillaries is still in the invention stage, and still must be made by each researcher. The approaches have been different from how immobilized gradients in gels are made because the method used for gels has not been easily adaptable to capillaries. But it could be soon. Commercial strips for gel IEF make use of gradient mixers. The surge in gradient nanoLC instrumentation is likely to give rise to immobilized gradients in capillaries that are similarly made, and eventually commercialized. Capillaries can be packed with extraordinary uniformity homogeneity [43], as mentioned earlier, and these can be used with nanoLC gradient mixers. Combining the packing uniformity with an immobilized pH gradient is only a matter of time. Once this happens and becomes commercialized, the greater reproducibility, facile remobilization, and the absence of ampholytes will facilitate applications in proteomics. The result will be high speed and the ability to routinely analyze small samples, such as single-cells, small tumors, and trace proteins in pre-concentrated serum extracts.
Acknowledgments
This work was supported by NIH under grant R01 GM65980 and R21CA139108.
Footnotes
Conflict of interest
The authors have stated that they have no conflict of interest to declare.
References
- 1.Aebersold R, Anderson L, Caprioli R, Druker B, et al. Perspective: A program to improve protein biomarker discovery for cancer. Journal of Proteome Research. 2005;4:1104–1109. doi: 10.1021/pr050027n. [DOI] [PubMed] [Google Scholar]
- 2.Poste G. Bring on the biomarkers. Nature. 2011;469:156–157. doi: 10.1038/469156a. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P. Proteomics retrenches. Nature Biotechnology. 2010;28:665–670. doi: 10.1038/nbt0710-665. [DOI] [PubMed] [Google Scholar]
- 4.Jorgenson JW, Lukacs KD. Zone electrophoresis in open-tubular glass-capillaries. Analytical Chemistry. 1981;53:1298–1302. [PubMed] [Google Scholar]
- 5.Jorgenson JW, Lukacs KD. Capillary zone electrophoresis. Science. 1983;222:266–272. doi: 10.1126/science.6623076. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JZ, Fang Y, Hou JY, Ren HJ, et al. Use of non-cross-linked polyacrylamide for 4-color dna-sequencing by capillary electrophoresis separation of fragments up to 640 bases in length in 2 hours. Analytical Chemistry. 1995;67:4589–4593. doi: 10.1021/ac00120a026. [DOI] [PubMed] [Google Scholar]
- 7.Butler JM, Buel E, Crivellente F, McCord BR. Forensic DNA typing by capillary electrophoresis using the ABI Prism 310 and 3100 genetic analyzers for STR analysis. Electrophoresis. 2004;25:1397–1412. doi: 10.1002/elps.200305822. [DOI] [PubMed] [Google Scholar]
- 8.Anderson NL, Anderson NG. The human plasma proteome - History, character, and diagnostic prospects. Molecular & Cellular Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 9.Polanski M, Anderson NL. A list of candidate cancer biomarkers for targeted proteomics. Biomarker Insights. 2006;2:1–48. [PMC free article] [PubMed] [Google Scholar]
- 10.Sarrats A, Comet J, Tabares G, Ramirez M, et al. Differential Percentage of Serum Prostate-Specific Antigen Subforms Suggests a New Way to Improve Prostate Cancer Diagnosis. Prostate. 2010;70:1–9. doi: 10.1002/pros.21031. [DOI] [PubMed] [Google Scholar]
- 11.Harwood MM, Christians ES, Fazal MA, Dovichi NJ. Single-cell protein analysis of a single mouse embryo by two-dimensional capillary electrophoresis. Journal of Chromatography A. 2006;1130:190–194. doi: 10.1016/j.chroma.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 12.Giddings JC. Unified Separation Science. John Wiley & Sons, Inc; New York: 1991. [Google Scholar]
- 13.Righetti PG, Gianazza E, Gelfi C, Chiari M, Sinha PK. Isoelectric-focusing in immobilized pH gradients. Analytical Chemistry. 1989;61:1602–1612. [Google Scholar]
- 14.Hjerten S, Zhu MD. Adaptation of the equipment for high-performance electrophoresis to isoelectric-focusing. Journal of Chromatography. 1985;346:265–270. [Google Scholar]
- 15.Thormann W, Tsai A, Michaud JP, Mosher RA, Bier M. Capillary isoelectric-focusing - effects of capillary geometry, voltage gradient and addition of linear polymer. Journal of Chromatography. 1987;389:75–86. [Google Scholar]
- 16.Mosher RA, Thormann W. Experimental and theoretical dynamics of isoelectric-focusing .4. Cathodic, anodic and symmetrical drifts of the pH gradient. Electrophoresis. 1990;11:717–723. doi: 10.1002/elps.1150110908. [DOI] [PubMed] [Google Scholar]
- 17.Dolník V. Wall coating for capillary electrophoresis on microchips. Electrophoresis. 2004;25:3589–3601. doi: 10.1002/elps.200406113. [DOI] [PubMed] [Google Scholar]
- 18.Lucy CA, MacDonald AM, Gulcev MD. Non-covalent Capillary Coatings for Protein Separations in Capillary Electrophoresis. J Chromatogr A. 2008;1184:81–105. doi: 10.1016/j.chroma.2007.10.114. [DOI] [PubMed] [Google Scholar]
- 19.Doherty EAS. Microchannel Wall Coatings for Protein Separations by Capillary and Chip Electrophoresis. Electrophoresis. 2003;24:34–54. doi: 10.1002/elps.200390029. [DOI] [PubMed] [Google Scholar]
- 20.Ramsay LM, Cermak N, Dada OO, Dovichi NJ. Capillary isoelectric focusing with pH 9.7 cathode for the analysis of gastric biopsies. Anal Bioanal Chem. 2011;400:2025–2030. doi: 10.1007/s00216-011-4926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Righetti PG, Simo C, Sebastiano R, Citterio A. Carrier ampholytes for IEF, on their fortieth anniversary (1967–2007), brought to trial in court: The verdict. Electrophoresis. 2007;28:3799–3810. doi: 10.1002/elps.200700232. [DOI] [PubMed] [Google Scholar]
- 22.Jin Y, Luo GA, Oka T, Manabe T. Estimation of isoelectric points of human plasma proteins employing capillary isoelectric focusing and peptide isoelectric point markers. Electrophoresis. 2002;23:3385–3391. doi: 10.1002/1522-2683(200210)23:19<3385::AID-ELPS3385>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Zhang MQ, El Rassi Z. Two-dimensional microcolumn separation platform for proteomics consisting of on-line coupled capillary isoelectric focusing and capillary electrochromatography. 1. Evaluation of the capillary-based two-dimensional platform with proteins, peptides, and human serum. Journal of Proteome Research. 2006;5:2001–2008. doi: 10.1021/pr060185u. [DOI] [PubMed] [Google Scholar]
- 24.Mao QL, Pawliszyn J. Capillary isoelectric focusing with whole column imaging detection for analysis of proteins and peptides. Journal of Biochemical and Biophysical Methods. 1999;39:93–110. doi: 10.1016/s0165-022x(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 25.Shimura K. Recent advances in IEF in capillary tubes and microchips. Electrophoresis. 2009;30:11–28. doi: 10.1002/elps.200800615. [DOI] [PubMed] [Google Scholar]
- 26.Wu XZ, Wu JQ, Pawliszyn J. Fluorescence imaging detection for capillary isoelectric-focusing. Electrophoresis. 1995;16:1474–1478. doi: 10.1002/elps.11501601244. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Lemma T, Pawliszyn J. Capillary isoelectric focusing coupled with dynamic imaging detection: A one-dimensional separation for two-dimensional protein characterization. Journal of Proteome Research. 2006;5:1246–1251. doi: 10.1021/pr060023y. [DOI] [PubMed] [Google Scholar]
- 28.Bo T, Pawliszyn J. Characterization of bovine serum albumin-tryptophan interaction by capillary isoelectric focusing with whole column imaging detection. J Chromatogr A. 2006;1105:25–32. doi: 10.1016/j.chroma.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 29.Silvertand LHH, Torano JS, van Bennekom WP, de Jong GJ. Recent developments in capillary isoelectric focusing. J Chromatogr A. 2008;1204:157–170. doi: 10.1016/j.chroma.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay LM, Dickerson JA, Dovichi NJ. Attomole protein analysis by CIEF with LIF detection. Electrophoresis. 2009;30:297–302. doi: 10.1002/elps.200800498. [DOI] [PubMed] [Google Scholar]
- 31.Ramsay LM, Dickerson JA, Dada O, Dovichi NJ. Femtomolar Concentration Detection Limit and Zeptomole Mass Detection Limit for Protein Separation by Capillary Isoelectric Focusing and Laser-Induced Fluorescence Detection. Analytical Chemistry. 2009;81:1741–1746. doi: 10.1021/ac8025948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin YQ, Trouillon R, Safina G, Ewing AG. Chemical Analysis of Single Cells. Analytical Chemistry. 2011;83:4369–4392. doi: 10.1021/ac2009838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dada OO, Ramsay LM, Dickerson JA, Cermak N, et al. Capillary array isoelectric focusing with laser-induced fluorescence detection: milli-pH unit resolution and yoctomole mass detection limits in a 32-channel system. Analytical and Bioanalytical Chemistry. 2010;397:3305–3310. doi: 10.1007/s00216-010-3595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong XF, Maxwell EJ, Ratnayake C, Mack S, Chen DDY. Flow-Through Microvial Facilitating Interface of Capillary Isoelectric Focusing and Electrospray Ionization Mass Spectrometry. Analytical Chemistry. 2011;83:8748–8755. doi: 10.1021/ac202130f. [DOI] [PubMed] [Google Scholar]
- 35.Tang Q, Harrata AK, Lee CS. Capillary Isoelectric-Focusing Electrospray Mass-Spectrometry For Protein-Analysis. Analytical Chemistry. 1995;67:3515–3519. [Google Scholar]
- 36.Chen JZ, Lee CS, Shen YF, Smith RD, Baehrecke EH. Integration of capillary isoelectric focusing with capillary reversed-phase liquid chromatography for two-dimensional proteomics separation. Electrophoresis. 2002;23:3143–3148. doi: 10.1002/1522-2683(200209)23:18<3143::AID-ELPS3143>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Wang YJ, Balgley BM, Rudnick PA, Evans EL, et al. Integrated capillary isoelectric focusing/nano-reversed phase liquid chromatography coupled with ESI-MS for characterization of intact yeast proteins. Journal of Proteome Research. 2005;4:36–42. doi: 10.1021/pr049876l. [DOI] [PubMed] [Google Scholar]
- 38.Yang LY, Lee CS, Hofstadler SA, Pasa-Tolic L, Smith RD. Capillary isoelectric focusing-electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry for protein characterization. Analytical Chemistry. 1998;70:3235–3241. doi: 10.1021/ac980224o. [DOI] [PubMed] [Google Scholar]
- 39.Zhou F, Johnston MV. Protein characterization by on-line capillary lsoelectric focusing, reversed-phase liquid chromatography, and mass spectrometry. Analytical Chemistry. 2004;76:2734–2740. doi: 10.1021/ac035446n. [DOI] [PubMed] [Google Scholar]
- 40.Zhou F, Johnston MV. Protein profiling by capillary isoelectric focusing, reversed-phase liquid chromatography, and mass spectrometry. Electrophoresis. 2005;26:1383–1388. doi: 10.1002/elps.200410125. [DOI] [PubMed] [Google Scholar]
- 41.Haselberg R, de Jong GJ, Somsen GW. Capillary electrophoresis-mass spectrometry for the analysis of intact proteins 2007–2010. Electrophoresis. 2011;32:66–82. doi: 10.1002/elps.201000364. [DOI] [PubMed] [Google Scholar]
- 42.Hua Y, Koshel BM, Wirth MJ. Field-Free Remobilization of Proteins After Isoelectric Focusing in Packed Capillaries. Analytical Chemistry. 2010;21:8910–8915. doi: 10.1021/ac101680z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malkin D, Wei B, Fogiel A, Staats SL, Wirth MJ. Submicrometer Plate Heigths for Capillaries Packed with Silica Colloidal Crystals. Analytical Chemistry. 2010;82:2175–2177. doi: 10.1021/ac100062t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang Y, Cong YZ, Liang Z, Zhang LH, Zhang YK. Microchip isoelectric focusing with monolithic immobilized pH gradient materials for proteins separation. Electrophoresis. 2009;30:4034–4039. doi: 10.1002/elps.200900209. [DOI] [PubMed] [Google Scholar]
- 45.Wang TT, Ma JF, Wu SB, Sun LL, et al. On-line combination of monolithic immobilized pH gradient-based capillary isoelectric focusing and capillary zone electrophoresis via a partially etched porous interface for protein analysis. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2011;879:804–810. doi: 10.1016/j.jchromb.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Wang TT, Ma JF, Zhu GJ, Shan YC, et al. Integration of capillary isoelectric focusing with monolithic immobilized pH gradient, immobilized trypsin microreactor and capillary zone electrophoresis for on-line protein analysis. Journal of Separation Science. 2010;33:3194–3200. doi: 10.1002/jssc.201000324. [DOI] [PubMed] [Google Scholar]
- 47.Yang C, Wang SS, Chang CY, Wang Y, Hu XY. Capillary Isoelectric Focusing with an Open Tubular Immobilized pH Gradient. Analytical Chemistry. 2010;82:1580–1583. doi: 10.1021/ac902223y. [DOI] [PubMed] [Google Scholar]
- 48.Zhang ZC, Wang JH, Hui LM, Li LJ. Poly(glycidyl methacrylate-divinylbenzene) based immobilized pH gradient capillary isoelectric focusing coupling with MALDI mass spectrometry for enhanced neuropeptide analysis. Electrophoresis. 2012;33:661–665. doi: 10.1002/elps.201100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu GJ, Yuan HM, Zhaol P, Zhang LH, et al. Macroporous polyacrylamide-based monolithic column with immobilized pH gradient for protein analysis. Electrophoresis. 2006;27:3578–3583. doi: 10.1002/elps.200600189. [DOI] [PubMed] [Google Scholar]
- 50.Yang C, Zhu GJ, Zhang LH, Zhang WB, Zhang YK. Repeatedly usable immobilized pH gradient in a monolithic capillary column. Electrophoresis. 2004;25:1729–1734. doi: 10.1002/elps.200405916. [DOI] [PubMed] [Google Scholar]
- 51.Liang Y, Zhu GJ, Wang TT, Zhang XD, et al. Fast preparation of monolithic immobilized pH gradient column by photopolymerization and photografting techniques for isoelectric focusing separation of proteins. Electrophoresis. 2011;32:2911–2914. doi: 10.1002/elps.201100195. [DOI] [PubMed] [Google Scholar]
- 52.Malkin DS, Wei BC, Fogiel AJ, Staats SL, Wirth MJ. Submicrometer Plate Heights for Capillaries Packed with Silica Colloidal Crystals. Analytical Chemistry. 2010;82:2175–2177. doi: 10.1021/ac100062t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei B, Malkin DS, Wirth MJ. Plate Heights Below 50 nm for Protein Electrochromatography Using Silica Colloidal Crystals. Analytical Chemistry. 2010;82:10216–10221. doi: 10.1021/ac102438w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang TT, Ma JF, Wu SB, Yuan HM, et al. Integrated platform of capillary isoelectric focusing, trypsin immobilized enzyme microreactor and nanoreversed-phase liquid chromatography with mass spectrometry for online protein profiling. Electrophoresis. 2011;32:2848–2856. doi: 10.1002/elps.201100030. [DOI] [PubMed] [Google Scholar]