Abstract
Introduction
Hematoma associated with epidural catheterization is rare, but the diagnosis might be suspected relatively frequently. We sought to estimate the incidence of suspected epidural hematoma after epidural catheterization, and to determine the associated cost of excluding or diagnosing an epidural hematoma through radiologic imaging.
Methods
We conducted an electronic retrospective chart review of 43,200 patient charts using 4 distinct search strategies and cost analysis, all from a single academic institution from 2001 through 2009. Charts were reviewed for use of radiological imaging studies to identify patients with suspected and confirmed epidural hematomas. Costs for imaging to exclude or confirm the diagnosis were related to the entire cohort.
Results
In our analysis, over a 9-year period that included 43,200 epidural catheterizations, 102 patients (1:430) underwent further imaging studies to exclude or confirm the presence of an epidural hematoma—revealing 6 confirmed cases and an overall incidence (per 10,000 epidural blocks) of epidural hematoma of 1.38 (95% CI 0, 0.002). Among our patients, 207 imaging studies, primarily lumbar spine MRI, were performed. Integrating Medicare cost expenditure data, the estimated additional cost over a 9-year period for imaging and hospital charges related to identifying epidural hematomas nets to approximately $232,000 or an additional $5.37 per epidural.
Discussion
About 1 in 430 epidural catheterization patients will be suspected to have an epidural hematoma. The cost of excluding the diagnosis, when suspected, is relatively low when allocated across all epidural catheterization patients.
INTRODUCTION
Epidural hematoma (hemorrhage into the epidural space) is a rare but potentially devastating complication associated with epidural catheterization. It is thought to occur when a vascular structure is punctured by a needle and/or catheter. Signs and symptoms vary and generally include some combination of weakness, numbness, and pain. Diagnosis is complicated by instillation of local anesthetic into the epidural space as part of planned therapy. Neurological deficits (reduced function of limbs, lasting numbness, or permanent disability and paralysis) are common results, even after prompt recognition and management.1-3 Epidural hematomas present with a wide range of symptoms, on variable timelines, and in modern practice are best diagnosed by a high-resolution MRI. The process from symptom presentation to diagnosis is quite unpredictable and creates a myriad of diagnostic challenges for physicians. Treatment options range from conservative waiting, drug therapy, or the more aggressive approach of a laminectomy procedure.3
Previous studies have presented widely varying incidence rates1-4,12 and several at-risk populations have been identified, notably patients receiving anticoagulation therapy and the elderly. Practice guidelines often provide particular consideration on anticoagulation treatments.1,3 Regardless of the risks associated with epidural catheterization, there are substantial benefits that balance those risks: from reduced blood loss and transfusion needs, increased joint mobility for orthopedic surgery, improved analgesia, earlier discharge, and reduced morbidity.5-12
Outcomes research in anesthesia is often hindered by the rare incidence of adverse events, such as epidural hematomas, which may be difficult to detect with either manual chart reviews or registry-based approaches.13-16 In studies published before the widespread availability of electronic medical records, rates of epidural hematomas caused by epidural catheterization were low,17,18,20,22 and yet recent estimates have shown a much higher range,3,26-28 likely due to the increasing adoption of AIMS (Anesthesia Information Management Systems) and other sources of electronic records. However, because few hospitals in the United States publically report their complication rates, the true incidences of epidural hematoma, and, importantly for informing patients, suspected epidural hematoma requiring additional diagnostic procedures, remain elusive. Also, in an increasingly cost-conscious environment, the allocated cost of radiologic studies over all epidural catheterizations to rule out epidural hematoma in cases where it is suspected is unknown. This knowledge gap makes weighing the risk-to-benefit ratio of procedures such as inserting an epidural catheter and the process of providing truly informed consent less robust.
The Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital (MGH) performs roughly 5,000 epidural catheterizations annually, and maintains several large-scale electronic medical record databases, making it possible to quantify the incidence of epidural hematomas as well as the cost of radiological imaging associated with cases of suspected epidural hematomas. Moreover, we recently led a large-scale multicenter effort to determine the incidence of actual epidural hematoma requiring laminectomy3. This prior work used surgical laminectomy as the case-finding indicator for epidural hematoma. In the present study, we developed a novel approach to retrospectively determine the incidence of suspected and actual epidural hematoma following epidural catheterization. We also calculated the incidence rate for patients in whom there was suspicion of an epidural hematoma that was further investigated by imaging, and provide an analysis of the readily quantifiable costs associated with investigating these lesions—focusing on the cost of imaging in particular.
METHODS
After receiving approval from the Partners Healthcare Human Research Committee, we developed a novel strategy relative to our prior approach to retrospectively determine our local incidence of epidural hematoma using MGH enterprise-wide electronic databases. In order to expand upon our previous work and gain a comprehensive understanding of cases, several clinical databases were queried to find both (1) suspected and (2) actual cases of epidural hematomas at MGH during the 9-year study period (2001–2009). This new approach utilized 3 distinct databases to maximize the probability of accurate and complete case finding of all patients suspected to have a hematoma: (1) our Anesthesia Information Management System (AIMS), which contains clinical records for every anesthetic procedure performed in both the obstetrics and main operating rooms as well as billing information; (2) the MGH Quality Assurance (QA) database, which contains a list of self-reported complications; and (3) the Partners Research Patient Data Registry (RPDR), which is an enterprise-wide data repository containing patient diagnosis (ICD-9 codes), procedures (CPT codes), radiology reports, surgery notes, and other clinical and demographic data.
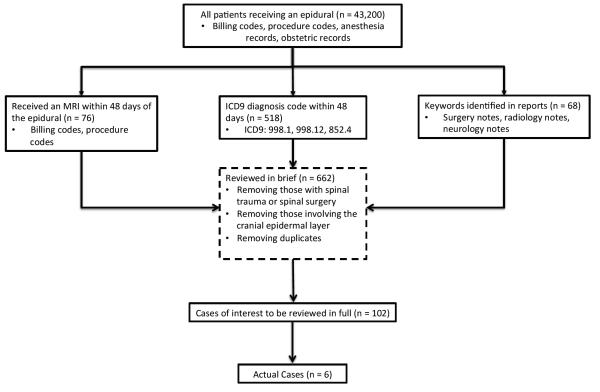
To compile a list of potential cases of suspected epidural hematoma after epidural catheterization, we queried the AIMS database and RPDR to obtain a list of all patients who received an epidural catheter at MGH, including all MGH obstetric epidural catheterizations. Any electronic radiology, surgery, and neurology notes for these patients were then obtained and reviewed by a trained researcher for specific key words that would suggest the presence or consideration of an epidural hematoma. The keywords used were general cause, diagnosis, or symptom based, and when used in combination helped limit the total set of cases to a smaller subset of cases of interest. We iteratively refined the search by identifying new key words from found cases and adding these new terms to our search criteria until no further cases were identified through the databases. Key words used included “epidural,” “hematoma,” “catheter,” “placement,” “s/p,” “numbness,” “weakness,” “paralysis,” and “cord compression.” After identification, our research team manually reviewed the patient record from each case of interest to identify cases of suspected epidural hematoma.
A second search was run in the RPDR to find all patients who underwent epidural catheterization, and had any type of MRI or CT imaging performed within 48 days after the epidural catheterization. A third search was run in RPDR matching all patients who received an epidural catheter and had a diagnosis code of a “hematoma” (any hematoma) in their record within 48 days. These 2 temporal-order driven searches resulted in a second set of cases of interest, which were manually reviewed.
Finally, the MGH QA database was searched for any patient having a reported complication of an epidural hematoma. Combination of these 4 searches led to a list of all patients with suspected epidural hematoma that underwent imaging to exclude or confirm the diagnosis.
Once a comprehensive list of potential cases of suspected epidural hematoma had been generated, the hospital chart for each unique case was reviewed to determine whether the case represented an actual epidural hematoma by 2 members of the of the research team (JME, JPH). The actual diagnosed cases were then analyzed a second time by reviewing the medical records (radiology reports, operation reports, progress notes, and neurology reports) in order to verify the presence of an epidural hematoma, as well as identify potential risk factors, symptoms, diagnosis timeline, and to document the treatment method and outcome.
RESULTS
Of the 43,200 total cases of epidural catheterization at MGH, 102 patients (0.24%) underwent further imaging due to suspected epidural hematoma, and 6 confirmed cases were identified (1:7,200 (95% CI 0, 0.0002), 0.014% of total cases, or 6% of imaged cases, Table 1). The most common symptoms cited for imaging orders, in order of decreasing frequency, were weakness (n=44), numbness (n=19), paralysis (n=15) and pain (n=12). These symptoms presented in combinations, and also in a mixed pattern in both location and severity as shown in Table 2.
Table 1. Patient Demographics and Case Cohort Data.
Patient and case cohort demographic description
| Male | 26,790 |
|---|---|
| Mean age (SD) | 50.0 (17) |
| ASA > 3 | 13,680 |
| Emergency Status | 2,850 |
| Mean case duration in minutes (SD) | 101 (93) |
| Total # cases (n) | 43,200 |
| Suspected epidural hematomas (n) | 102 |
| Imaging studies (n) | 207 |
| Confirmed epidural hematomas | 6 |
ASA: American Society of Anesthesiologists Physical Status Classification System
Table 2. Symptoms Presenting in Suspected Cases of Epidural Hematoma.
Symptoms present during suspected epidural hematomas.
| Suspected Epidural Hematomas | Confirmed Epidural Hematomas | |
|---|---|---|
|
| ||
| Cases (n): | 96 | 6 |
|
| ||
| Symptoms Presenting: (non-exclusive) |
Numbness: 19 | Numbness: 2 |
| Weakness: 44 | Weakness: 6 | |
| Paralysis: 15 | Paralysis: 2 | |
| Pain: 12 | Pain: 1 | |
| Unknown: 9 | Unknown: 0 | |
A detailed review of all 6 hematoma patients’ charts was performed to identify potential risk factors and evaluate the progression of symptoms and treatment timeline to better develop evidence-based guidelines for diagnosis/treatment. A review of the confirmed cases is presented in Table 3. A summary of case identification methodology is shown in Table 4, and a diagram representing the search methodology is shown in Figure 1.
Table 3. Case Series of Epidural Hematomas.
Confirmed cases of epidural hematomas during study period using unique case identification method of multiple search formats and chart review. EH = epidural hematoma, T12 = vertebral level, 17G = gauge of needle used, PT = prothrombin time, PTT = partial thrombplastin time, INR = international normalized ratio, PLT = platelet level, ASA = American Society of Anesthesiologists physical status classification, POD = postoperative day, NA = not available
| Case | Age (years) |
Gender | Procedure/Surgery | Epidural Placement | Risk Factor of EH | Symptoms | Symptom Onset | Labs at Symptoms |
Treatment | Residual deficit |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76 | M | Aortic Aneurysm Repair |
T12 epidural via 17G needle |
ASA 3E; Age>70; postop anticoagulation (heparin,aspirin) |
Bilateral lower extremity weakness & numbness |
55 hours (POD #4) |
PT = 15.5 PTT = 44 INR = 1.5 Plt = 127 |
Laminectomy (x2) |
Residual paralysis improved with rehab |
|
| ||||||||||
| 2 | 74 | F | Thoracoabdominal Aortic Replacement |
T10 epidural via 17G needle/ 5-french spinal drain |
ASA 3; Age>70; intraoperative anticoagulation (heparin); postoperative anticoagulation (fragmin) |
Left lower extremity weakness & lower back pain |
72 hours (POD #3) |
PT = 15 PTT = 37.6 INR = 1.4 Plt = 75 |
Laminectomy | Permanent strength loss |
|
| ||||||||||
| 3 | 81 | F | Thoracoabdominal Aortic Replacement |
Lumber epidural via 17G needle/ 5- french spinal drain |
ASA 3; Age>70; intraoperative anticoagulation (heparin); postoperative anticoagulation (aspirin) |
Bilateral lower extremity weakness |
214 hours (POD #9) |
PT = 13.2 PTT = 32.9 INR = N/A Plt = 373 |
Laminectomy | Permanent strength loss |
|
| ||||||||||
| 4 | 79 | M | Endovascular Aortic Aneurysm Repair |
L1 epidural | ASA 3; Age>70; intraoperative anticoagulation (heparin); postoperative anticoagulation (aspirin) |
Bilateral lower extremity weakness & numbness |
3 hours (POD #0) |
Not available |
Conservative | None |
|
| ||||||||||
| 5 | 78 | F | Lower Anterior Resection |
T8 epidural via 17G needle |
ASA 2; Age >70; preoperative anticoagulation (heparin); postoperative anticoagulatoin (heparin) |
Right lower extremity & hip weakness |
23 hours (POD #1) |
Not available |
Laminectomy | None |
|
| ||||||||||
| 6 | 72 | F | Whipple | T8 epidural via 17G needle |
ASA 3; Age>70; intraoperative anticoagulation (heparin) |
Bilateral lower extremity weakness |
44 hours (POD #2) |
Not available |
Laminectomy | None |
Table 4. Case Identification.
Case identification strategy using multiple search methodology and review
| Search Method | Cases of Interest | Actual Cases | Unique Cases |
|---|---|---|---|
| Keyword | 68 | 3 | Cases 1 - 3 |
| Epidural + MRI | 76 | 4 | Cases 1, 4-6 |
| Epidural + Hematoma | 518 | 5 | Cases 1, 3-6 |
| Quality Assessment Database | N/A | 2 | Cases 1 & 3 |
Figure 1.
We sought to determine the added cost of radiologic imaging for suspected cases in order to understand the cost burden. In the 102 suspected cases, a total of 207 imaging studies were performed. Total reimbursement for 207 imaging studies would have been approximately $232,000 (or $1,120/study) using the average Medicare payment for the Metropolitan Boston area for both the hospital cost and interpreting radiologist’s fee. Thus, applying the total imaging cost over the study period amongst the entire 43,200 procedures, this represents an added “cost” of $5.37 per epidural catheterization if Medicare reimbursement rates can be considered representative of costs.
DISCUSSION
Using a multidimensional search strategy for posthoc case identification at a single institution, we found an incidence rate of 1:7,200 for epidural hematomas resulting from a catheter insertion. This incidence rate is significantly higher than older reports, and consistent with more recently reported incidence rates.3 This may represent the presence of readily accessible electronic medical records, making identifying rare events more practical. Our previous report captured only the cases where surgical intervention occurred, and in this study, we discovered additional cases managed conservatively. Although the calculated and reported incidence rate here is higher than expected from older reports, we believe that our methodological approach of systematically combining four unique approaches more accurately captures suspected and confirmed cases of epidural hematomas.
Importantly, neither the list of suspected or actual cases of epidural hematomas was completely present in any of 1 of the 3 independent searches. Centers interested in determining their own incidence rates should therefore take into account the imperfections of clinical data sources, and seek to maximize the range of information considered. Of note, our hospital QA database contained only 2 of the 6 cases identified. This further supports the notion that adverse event prevalences are underrepresented by conventional self-reporting methods, and researchers should consider all sources of available information to accurately determine incidences.
We recognize that our approach is unique (relying on overlapping databases potentially not consistent across institutions) and is reported here as a single center study, and that this limits the generalizability of our results. A key limitation of our study and approach is that a sensitivity analysis with varying key words and terms was not conducted. The approach used of combining several databases with the described key term search is novel; however, the addition of other terms may have revealed a different patient cohort. Thus, our reported incidence rate may different than the actual rate based on both missed cases of epidural hematomas (numerator data) and total epidural utilization (denominator data). However, our current methodology uses broader search criteria for identifying possible cases of epidural hematomas enhancing sensitivity, whereas our prior approach favored specificity.3
Advanced age (>70 years) and invasive surgeries were themes for all cases. Lower extremity weakness appeared in all of the cases prior to diagnosis of epidural hematoma, as expected for epidural catheterization patients receiving local anesthetics. Lower-extremity weakness beyond that “expected” based on clinical intuition may provide a clinical factor influencing pursuit of the diagnosis but further analysis is needed to confirm this. Data on expected lower-extremity weakness for a given insertion level and drug dose might also be helpful in establishing expected norms of lower-extremity weakness.
The added cost per case allocated over all epidural catheterizations attributable to imaging alone in the absence of an epidural hematoma was $5.37, a negligible amount. The relatively low cost and high frequency of epidural catheterization, coupled with the significantly lower incidence of epidural hematoma and other adverse outcomes, allows for a large amount of variance in a clinician’s decision-making when it comes to ordering imaging studies. The cost analysis indicates that clinicians properly have a low threshold of indication when considering imaging studies. Our data do not allow any judgment to be made about the relative value of intervention vs conservative approaches, or about the timing of intervention. Our current result, along with our recent multicenter review3 does not reproduce a prior result suggesting that early laminectomy leads to a better neurologic outcome.
The true cost generated by epidural catheterization incorporates many factors from the insertion of the epidural catheter itself to the diagnostic testing and physician services, imaging requirements and treatments, and, at times, extended length of stay and rehabilitation for patients with suspected or actual epidural hematoma. In addition to the direct morbidity associated with an epidural hematoma, this complication carries with it a significant cost. We focus on imaging, because its costs are readily apparent and are directly borne by the healthcare finance system. However, the added cost of imaging for suspected epidural hematoma is small when distributed over all epidural catheterizations.
By carefully assessing the risk of any procedure, and acknowledging the presence of “false-positives” for suspected complications, and finite cost associated with additional testing, it is possible for an institution to determine its own local incidence of suspected and actual epidural hematoma, as well as the costs for imaging to pursue a suspected diagnosis. This approach enables institutions to develop local evidence-based guidelines for patient education and for clinical justification for expensive and/or painful procedures. With regard to cases of epidural hematomas at MGH, we were able to develop an accurate local incidence of epidural hematomas to report to our patients. We also justified a low threshold for ordering imaging studies in future suspected cases of epidural hematoma. Finally, we are now able to accurately inform patients about the small possibility (1:400) of developing signs or symptoms suggestive of an epidural hematoma, and that this may be investigated with further diagnostic studies. This simple process for identifying the incidence and cost analysis would not have been feasible without electronic medical records, especially our AIMS and enterprise-wide clinical repository. Similar quality assessment and improvement would benefit from the creation of a reliable and internationally collaborated anesthesia outcomes database.3
Acknowledgments
Funding: Financial support for the preparation of this manuscript was provided 5T32GM007592 from the National Institute of Health, as well as by department funds of the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation) Reg Anesth Pain Med. 2003;28:172–197. doi: 10.1053/rapm.2003.50046. [DOI] [PubMed] [Google Scholar]
- 2.Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition) Reg Anesth Pain Med. 2010;35:64–101. doi: 10.1097/aap.0b013e3181c15c70. [DOI] [PubMed] [Google Scholar]
- 3.Bateman B, Mhyre JM, Ehrenfeld J, et al. Brief report: the risk and outcomes of epidural hematomas after perioperative and obstetric epidural catheterization: a report from the multicenter perioperative outcomes group research consortium. Anesth Analg. 2013;116:1380–1385. doi: 10.1213/ANE.0b013e318251daed. [DOI] [PubMed] [Google Scholar]
- 4.Green L, Machin SJ. Managing anticoagulated patients during neuraxial anaesthesia. Br J Haematol. 2010;149:195–208. doi: 10.1111/j.1365-2141.2010.08094.x. [DOI] [PubMed] [Google Scholar]
- 5.Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buggy DJ, Smith G. Epidural anaesthesia and analgesia: better outcome after major surgery? Growing evidence suggests so. BMJ. 1999;319:530–531. doi: 10.1136/bmj.319.7209.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballantyne JC, Carr DB, deFerranti S, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg. 1998;86:598–612. doi: 10.1097/00000539-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Tuman KJ, McCarthy RJ, March RJ, DeLaria GA, Patel RV, Ivankovich AD. Effects of epidural anesthesia and analgesia on coagulation and outcome after major vascular surgery. Anesth Analg. 1991;73:696–704. doi: 10.1213/00000539-199112000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Modig J. The role of lumbar epidural anaesthesia as antithrombotic prophylaxis in total hip replacement. Acta Chir Scand. 1985;151:589–594. [PubMed] [Google Scholar]
- 10.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Lynch EP, Welch KJ, Carabuena JM, Eberlein TJ. Thoracic epidural anesthesia improves outcome after breast surgery. Ann Surg. 1995;222:663–669. doi: 10.1097/00000658-199511000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology. 1995;82:1474–1506. doi: 10.1097/00000542-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Neal JM, Bernards CM, Hadzic A, et al. ASRA Practice Advisory on Neurologic Complications in Regional Anesthesia and Pain Medicine. Reg Anesth Pain Med. 2008;33:404–415. doi: 10.1016/j.rapm.2008.07.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu HP, Fan SW, Yang HL, Tang TS, Zhou F, Zhao X. Early diagnosis and treatment of acute or subacute spinal epidural hematoma. Chin Med J (Engl) 2007;120:1303–1308. [PubMed] [Google Scholar]
- 15.Braun P, Kazmi K, Nogués-Meléndez P, Mas-Estellés F, Aparici-Robles F. MRI findings in spinal subdural and epidural hematomas. Eur J Radiol. 2007;64:119–125. doi: 10.1016/j.ejrad.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mutair A, Bednar DA. Spinal epidural hematoma. J Am Acad Orthop Surg. 2010;18:494–502. doi: 10.5435/00124635-201008000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Scott DB, Hibbard BM. Serious non-fatal complications associated with extradural block in obstetric practice. Br J Anaesth. 1990;64:537–541. doi: 10.1093/bja/64.5.537. [DOI] [PubMed] [Google Scholar]
- 18.Tryba M. Epidural regional anesthesia and low molecular heparin: Pro. Anasthesiol Intensivmed Notfallmed Schmerzther. 1993;28:179–181. doi: 10.1055/s-2007-998902. [DOI] [PubMed] [Google Scholar]
- 19.Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950–959. doi: 10.1097/00000542-200410000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Wulf H. Epidural anaesthesia and spinal haematoma. Can J Anaesth. 1996;43:1260–1271. doi: 10.1007/BF03013437. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki M, Takasita M, Matsumoto H, Sonoda H, Tsumura H, Torisu T. Spinal epidural hematoma after removal of an epidural catheter: case report and review of the literature. J Spinal Disord Tech. 2005;18:547–551. doi: 10.1097/01.bsd.0000128692.44276.cf. [DOI] [PubMed] [Google Scholar]
- 22.Castillo J, Santiveri X, Escolano F, et al. Incidence in Catalonia of spinal cord compression due to spinal hematoma secondary to neuraxial anesthesia. Rev Esp Anestesiol Reanim. 2007;54:591–595. [PubMed] [Google Scholar]
- 23.Ruppen W, Derry S, McQuay H, Moore RA. Incidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology. 2006;105:394–399. doi: 10.1097/00000542-200608000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev. 2003;26:1–49. doi: 10.1007/s10143-002-0224-y. [DOI] [PubMed] [Google Scholar]
- 25.Bracco D, Hemmerling T. Epidural analgesia in cardiac surgery: an updated risk assessment. Heart Surg Forum. 2007;10:E334–337. doi: 10.1532/HSF98.20071077. [DOI] [PubMed] [Google Scholar]
- 26.Pöpping DM, Zahn PK, Van Aken HK, Dasch B, Boche R, Pogatzki-Zahn EM. Effectiveness and safety of postoperative pain management: a survey of 18 925 consecutive patients between 1998 and 2006 (2nd revision): a database analysis of prospectively raised data. Br J Anaesth. 2008;101:832–840. doi: 10.1093/bja/aen300. [DOI] [PubMed] [Google Scholar]
- 27.Cameron CM, Scott DA, McDonald WM, Davies MJ. A review of neuraxial epidural morbidity: experience of more than 8,000 cases at a single teaching hospital. Anesthesiology. 2007;106:997–1002. doi: 10.1097/01.anes.0000265160.32309.10. [DOI] [PubMed] [Google Scholar]
- 28.Christie IW, McCabe S. Major complications of epidural analgesia after surgery: results of a six-year survey. Anaesthesia. 2007;62:335–341. doi: 10.1111/j.1365-2044.2007.04992.x. [DOI] [PubMed] [Google Scholar]
- 29.Major complications in continuous epidural anesthesia. Chin Med J (Engl) 1980;93:194–200. [PubMed] [Google Scholar]
- 30.Parvizi J, Viscusi ER, Frank HG, Sharkey PF, Hozack WJ, Rothman RR. Can epidural anesthesia and warfarin be coadministered? Clin Orthop Relat Res. 2007;456:133–137. doi: 10.1097/01.blo.0000246548.25811.2d. [DOI] [PubMed] [Google Scholar]
- 31.Vandermeulen EP, Van Aken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79:1165–1177. doi: 10.1213/00000539-199412000-00024. [DOI] [PubMed] [Google Scholar]