Abstract
Sensory neurons innervating the skin can release neuropeptides that are believed to modulate cellular proliferation, wound healing, pigmentation, and keratinocyte innate immune responses. While the ability of neuropeptides to stimulate keratinocyte production of inflammatory mediators has been demonstrated, there is no information concerning the mechanism(s) by which neuropeptide activation of keratinocyte cell surface receptors ultimately leads to the up-regulation of mediator production. In this study we used a keratinocyte cell line to identify the presence of substance P (SP) and calcitonin gene-related peptide (CGRP) receptors on keratinocytes and examined the effects of SP and CGRP stimulation on keratinocyte neuropeptide signaling, cell proliferation, and interleukin-1β (IL-1), interleukin-6 (IL-6), tumor necrosis factor α (TNF), and nerve growth factor (NGF) expression. Neuropeptide stimulation caused an up-regulation of neuropeptide receptor expression in keratinocytes and a dramatic increase in keratinocyte secretion of SP and CGRP, suggesting possible autocrine or paracrine stimulatory effects and amplification of neuropeptide signaling. Both SP and CGRP concentration-dependently stimulated cellular proliferation and the expression and secretion of inflammatory cytokines and NGF in keratinocytes. SP also activated all 3 families of mitogen activated protein kinases (MAPK) and nuclear factor κB (NFκB) in keratinocytes, while CGRP only activated p38 and extracellular signal related kinases1/2 (ERK1/2) MAPK. Neuropeptide stimulated inflammatory mediatory production in keratinocytes was reversed by ERK1/2 and JNK inhibitors. The current study is the first to observe; 1) that CGRP stimulates keratinocyte expression of CGRP and its receptor complex, 2) that SP and CGRP stimulate of IL-6 and TNF secretion in keratinocytes, 3) that SP activated all three MAPK families and the NFκB transcriptional signaling pathway in keratinocytes, and 4) that SP and CGRP stimulated inflammatory mediator production in keratinocytes is dependent on ERK1/2 and JNK activation. These studies provide evidence suggesting that disruption of ERK1/2 and JNK signaling may potentially be an effective therapy for inflammatory skin diseases and pain syndromes mediated by exaggerated sensory neuron-keratinocyte signaling.
Keywords: substance P, calcitonin gene-related peptide, keratinocyte, cytokines, nerve growth factor, mitogen activated protein kinases
1. Introduction
Skin is densely innervated by sensory neurons that express and release neuropeptides such as substance P (SP) and calcitonin gene-related peptide (CGRP), thereby modulating cellular proliferation, wound healing, and pigmentation in the skin[1, 2]. Substance P and CGRP signaling can also stimulate keratinocytes to produce inflammatory mediators such as interleukin-1β (IL-1) and nerve growth factor (NGF) [3-5], and it has been proposed that up-regulated keratinocyte expression of inflammatory mediators such as IL-1, interleukin-6 (IL-6), tumor necrosis factor α (TNF), and NGF contribute to the development of psoriasis, atopic dermatitis, contact dematitis, post-incisional pain, and complex regional pain syndrome (CRPS) [2, 6-9]. Furthermore, these conditions are associated with the proliferation of cutaneous neuropeptide containing sensory neurons and neuropetide receptors, as well as exaggerated neurogenic inflammatory signaling in the affected skin, data suggesting that dysregulated neurocutaneous signaling could cause over-stimulation of the keratinocyte innate immune responses mediating these disease processes [2, 10, 11].
While the ability of neuropeptides to stimulate keratinocyte production of inflammatory mediators has been demonstrated, there is no information concerning the mechanism(s) by which neuropeptide activation of keratinocyte cell surface receptors ultimately leads to the up-regulation of mediator production. In other cell types SP can induce the phosphorylation of members of the mitogen activated protein kinases (MAPK) family (the extracellular signal related kinases (ERK1/2) and p38) and activate NF-κB. These MAPKs are activated by extracellular stimuli triggering a phosphorylation cascade resulting in nuclear transcription factor activation and inflammatory protein expression. Specific inhibitors of the MAPK signaling pathways have been reported to inhibit SP induced NF-κB activation and chemokine production in macrophages [12], inhibit SP induced IL-6 production in astrocytoma cells [13] and dental pulp cells [14], block SP induced TNF expression in mast cells [15], and prevent SP induced TNF production in human skin slices [16]. NF-κB is a “rapid-acting” transcription factor that is normally sequestered in an inactive state in the cell cytoplasm, but when stimulated the p50-p65 NF-κB heterodimer is rapidly translocated from the cytoplasm to the nucleus where the p65 NF-κB subunit induces the expression of specific genes involved in inflammation and innate immunity, cell proliferation, response to stress, and apoptosis. Inhibition of NF-κB activation has been reported to block SP induced TNF and IL6 production in mast cells [17]. Furthermore, CGRP has been reported to activate MAPK signaling in keratinocytes [18]. Collectively, these results suggest that the MAPK and NF-κB pathways could be involved in the transcriptional regulation of cytokine and NGF over-expression in SP and CGRP stimulated keratinocytes.
In the present study we first attempted to determine whether the neuropeptides SP and CGRP, could amplify their own neurocutaneous signal by up-regulating expression of their receptors or even by stimulating keratinocyte expression of these neuropeptides. We also evaluated the stimulatory effects of SP and CGRP signaling on keratinocyte proliferation, and the expression and secretion of proinflammatory cytokines and NGF. We went on to evaluate the roles of the MAPK and NF-κB signaling pathways in supporting neuropeptide stimulated inflammatory mediator responses.
2. Materials and Methods
2.1 Cell Culture
The rat epidermal keratinocyte cell line [19-23] was generously provided by Dr. Howard Baden (Massachussetts General Hospital, Boston, MA) and cultured as we have previously described [5]. In brief, cells were plated at 1X104 cells per 60mm dish and were cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen), supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, 0.4 ug/mL hydrocortisone, and 0.75 mM aminoguanidine. On reaching approximately 80% confluence, the cells were starved with the above culture medium supplemented with only 1% FBS overnight. Cells were then washed twice with fresh DMEM before the addition of SP or CGRP (Sigma) and various cell-permeable intracellular signaling inhibitors (SN50, which blocks NFkB nuclear translocation; PD98059, a selective upstream inhibitor of ERK1/2 MAPK phosphorylation; SB203580, a selective inhibitor of p38 MAPK phosphorylation; and SP600125, a selective inhibitor of c-Jun N-terminal kinases (JNK) phosphorylation (all inhibitors from Sigma)). In other experiments the SP NK1 receptor antagonist LY303870 (Lilly) or the CGRP receptor antagonist CCRP8-37 (Sigma) were added with their respective agonists.
2.2 Keratinocyte proliferation assay
Proliferation of the cultured cells was assessed by 5-bromo-2’-deoxy-uridine (BrdU) labeling. The keratinocytes were plated in 96 well microplates at a density of 3000 cells per well and cultured overnight. Cells were washed twice with phosphate-buffered saline and the medium was replaced with DMEM containing 1% bovine serum albumin (Sigma) supplemented with SP or CGRP in various concentrations. The cells were then incubated for 24 h and 100μM of BrdU was added to the culture medium 4 h prior to performing the BrdU assay. Incorporation of BrdU was determined using a cell proliferating ELISA kit (Roche) according to the manufacturer's instructions. All results were confirmed by repeating the experiment 3 times.
2.3 Immunofluorescence confocal microscopy
For antibody staining, cells cultured on cover slips were washed in phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde for 20 minutes at room temperature and then permeabilized with ice-cold ethanol for 5 minutes. The keratinocytes were incubated with 5% FBS plus 1% bovine serum albumin at room temperature for 1h to reduce background staining, then treated with primary antibodies against the mouse SP neurokinin 1 receptor (NK1, 1:50, SC14115, Santa Cruz Biotechnology) and the CGRP receptor dimer complex of the calcitonin receptor-like receptor (CRLR, 1:50, SC31569, Santa Cruz Biotechnology) and the receptor activity-modifying protein (RAMP1, 1:50, SC11379, Santa Cruz Biotechnology) for overnight at 4°C. Cells were then treated with the Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) in 1:450 dilution for 1h at room temperature. Control slides were stained with just the secondary antibody alone. Immunostained cells were visualized with a Zeiss LSM 510 META laser scanning confocal microscope and the confocal software was used for acquisition of the data and merging of the digital images.
2.4 Quantitative real time PCR
Three hours after neuropeptide treatment the total RNA from keratinocytes grown in 60mm culture dishes was extracted, the cDNA was synthesized, and real time PCR reactions were conducted as we have previously described [5]. The primer sequences used in these experiments are listed in Table 1. The data from real time PCR experiments were analyzed by the comparative CT method as described in the manufacturer's manual. All results were confirmed by repeating the experiment 3 times.
Table 1.
Primers used in these studies
| Gene | GenBank Accession # | Forward primer | Reverse primer | Product Size(bp) |
|---|---|---|---|---|
| TAC1 | NM_012666 | tttgcagaggaaatcggtgccaac | ggcattgcctccttgatttggtca | 83 |
| TACR-1 | NM_012667 | ctggaaagaggagccttgtg | ctgagacggaaaggaacagc | 205 |
| CALCA | NM_017338 | agaagagatcctgcaacactgcca | ggcacaaagttgtccttcaccaca | 94 |
| CALCB | NM_138513 | cccagaagagatcctgcaac | agttcctcagacccgaaggt | 158 |
| CALCRL | NM_012717 | tcattgtggtggctgtgttt | aatgggaccatggatgatgt | 176 |
| RAMP1 | NM_031645 | ggcaaacaagattggctgtt | aatggggagcacaatgaaag | 154 |
| TNF-α | NM_012675 | ctcccagaaaagcaagcaac | cgagcaggaatgagaagagg | 210 |
| IL-1β | NM_031512 | agtctgcacagttccccaac | agacctgacttggcagagga | 230 |
| IL-6 | NM_012589 | ccggagaggagacttcacag | acagtgcatcatcgctgttc | 161 |
| NGF | XM_227525 | acctcttcggacactctgga | gtccgtggctgtggtcttat | 168 |
| 18S | X01117 | cgcggttctattttgttggt | agtcggcatcgtttatggtc | 219 |
2.5 Cytokine and NGF levels
At 24 hours after adding neuropeptide to the culture medium the inflammatory mediators secreted by the keratinocytes were assayed from the conditioned cell culture medium in duplicate using TNF, IL-1 and IL-6 ELISA kits (R&D Systems) and a ChemiKine NGF ELISA kit (Millipore) according to the manufacturer's instructions. All results were confirmed by repeating the experiment 3-4 times.
2.6 SP and CGRP levels
At 3 hours after adding neuropeptide to the culture medium the medium was removed and the keratinocytes were washed 3 times with fresh medium, then the cells were placed in fresh cell culture medium for another 21 hours. After 21 hours the neuropeptides secreted by the keratinocytes were assayed from the cell culture medium in duplicate using SP and CGRP ELISA kits (Cayman Chemical) according to the manufacturer's instructions. All results were confirmed by repeating the experiment 3-4 times.
2.7 Western blot
Western blot analysis was performed as previously described[5]. At 24 hours after neuropeptide treatment the keratinocytes were harvested and homogenized. Equal amounts of cell protein were size fractionated by sodium dodecyl sulfate/polyacrylamide (SDS-PAGE) gel electrophoresis and transferred onto a polyvinylidene difluorided membrane. The blot was blocked for overnight in 5% non-fat dry milk in Tris-buffered saline with 0.5% Tween-20 (TBST), and incubated at 4°C for 24h with primary antibody for detecting NK1(1:500), CRLR(1:5000) (Santa Cruz Biotechnology), RAMP1(1:5000) (Novus Biologicals), total p38 (1:1000), phosphorylated p38 (1:1000) (Cell Signaling Technology), total ERK1/2 (1:10000), phosphorylated ERK1/2 (1:200), total JNK(1:200) (Santa Cruz Biotechnology), phosphorylated JNK(1:500) (Cell Signaling Technology), or mouse NF-κB p65 (1:200) (Santa Cruz Biotechnology). For western blot analysis of NF-κB p65, the nuclear extract was prepared as we have previously described[24]. After washing in TBST, the blots were incubated in horseradish peroxidase conjugated anti-rabbit or anti-mouse antibody (1:5000)(Santa Cruz Biotechnology) for 1h at room temperature. The membrane was then washed again and exposed to film following chemiluminescence reagent treatment with the ECL plus western blotting reagents (Amersham). Bands were quantified using densitometry of digitalized images. Each blot was then stripped and re-probed with anti-β-actin antibodies, thus allowing normalization of expression between samples. The results of all assays were confirmed by repeating the experiment 3-4 times.
2.8 Statistical analysis
Statistical analysis was done using Prism 4.02 (GraphPad Software). All data was evaluated using an analysis of variance (ANOVA) followed by Bonferroni post hoc testing. Data are presented as the mean ± standard error of the mean (SEM) and P< 0.05 was considered statistically significant.
3. Results
3.1 Exaggerated neuropeptide signaling in keratinocytes exposed to neuropeptides
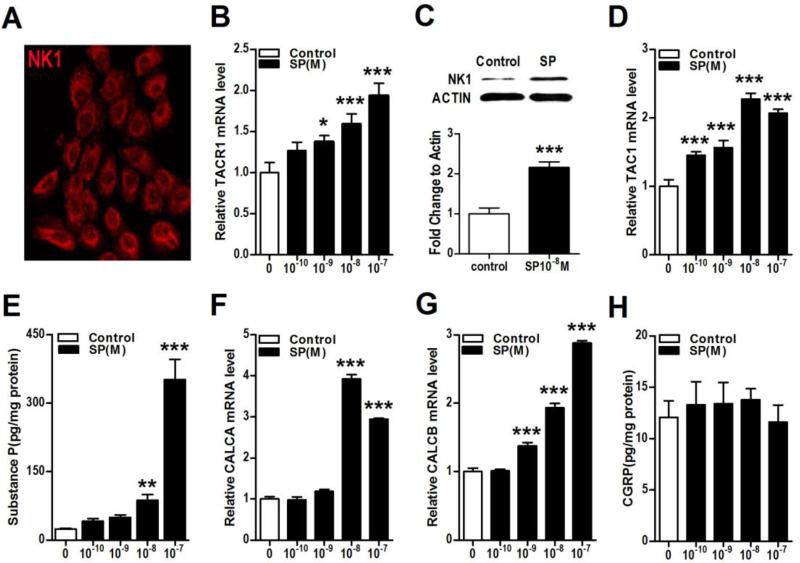
The stimulatory effects of SP and CGRP on neuropeptide signaling were tested in an epidermal keratinocyte cell line. Figure 1A illustrates that SP NK1 receptors are expressed on both the cell membrane and to a degree in the cytoplasm. Furthermore, SP treatment stimulated keratinocyte NK1 receptor expression at both the mRNA (TACR1 gene, Fig. 1B) and protein level, as measured by western immunoblot assay (Fig. 1C). Evidence that SP stimulation of NK1 receptor expression was mediated by MAPK ERK1/2 phosphorylation was obtained using the selective ERK1/2 antagonist PD98059, which concentration-dependently inhibited SP (10−8M) stimulation of TACR1 mRNA in keratinocytes (complete inhibition at 30 uM, data not shown). Substance P treatment also induced SP TAC1 gene up-regulation in cultured keratinocytes (Fig. 1D) and evoked a dramatic increase in keratinocyte SP secretion into the culture media (Fig. 1E). Adding SP to the keratinocyte culture media for 3 hours increased the secretion of SP 15-fold over the ensuing 21 hours, compared to the low basal levels of SP observed prior to stimulation. Substance P treatment also increased keratinocyte CGRP gene expression (CALCA and CALCB, Figs. 1 E,F), but had no effect on CGRP protein secretion into culture media (Fig. 1H). These data suggest that SP stimulated keratinocytes could secrete significant levels of SP capable of inducing further autocrine or paracrine keratinocyte activation with subsequent pro-inflammatory effects in the skin.
Figure 1. Substance P (SP) treatment increased keratinocyte NK1 receptor expression and secretion of SP.
The substance P (SP) receptor NK1 was expressed in keratinocytes in vitro, and this expression was up-regulated after SP treatment. SP treatment also increased the gene and protein expression of SP and the gene expression of calcitonin gene-related peptide (CGRP) keratinocytes. Immunostaining demonstrated NK1 protein (red fluorescence) present in the cytoplasm and on the membrane of keratinocytes (A). SP concentration-dependently increased expression of its NK1 receptor gene (TACR1) in keratinocytes at 3 h after treatment, as measured by real time PCR (B). Western blot demonstrates that SP (10−8M) treatment increased NK1 protein levels in keratinocytes at 24 h after treatment (C). SP treatment also concentration-dependently increased the expression of the SP TAC1 gene in keratinocytes at 3h, as measured by real time PCR (D). When keratinocytes were treated with various concentrations of SP for 3h, then washed 3 times in fresh medium, and then incubated in fresh medium for 21h, there was a 15-fold increase in medium SP levels, as measured by EIA (E). SP treatment also increased expression of the CGRP genes CALCA (F) and CALCB (G) in keratinocytes at 3 h. When keratinocytes were treated with various concentrations of SP for 3h, then washed 3 times in medium, and then incubated in fresh medium for 21h, there was no increase in medium levels of CGRP, as measured by EIA (H). Values are means ± SE. * P<0.05, ** P<0.01, and *** P<0.001 vs vehicle treated control cells. All experiments were repeated 3-4 times.
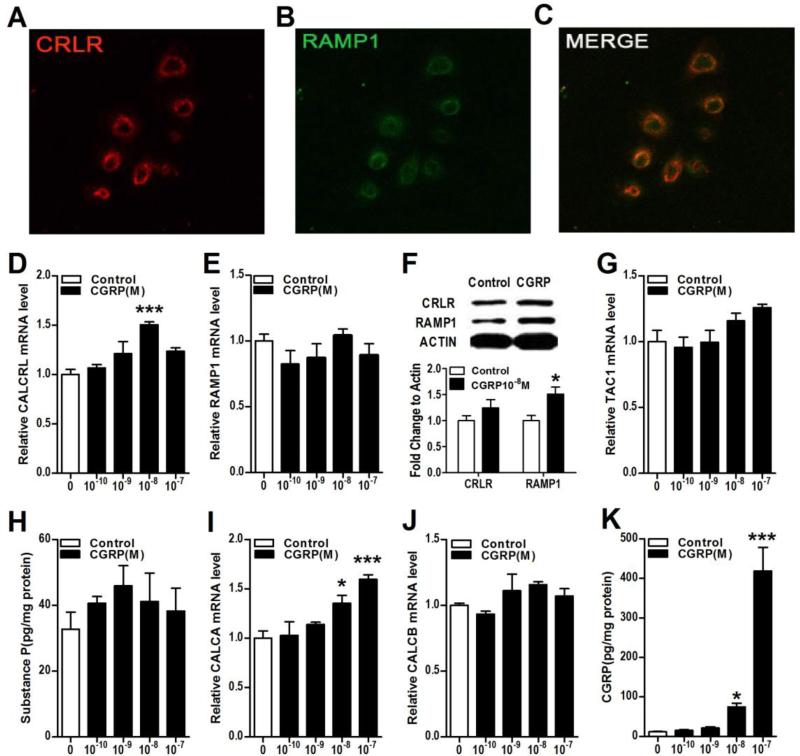
The CGRP receptor CRLR and its receptor activity-modifying protein RAMP1 were co-expressed on the keratinocyte cell membrane (Fig. 2A-C). CGRP treatment stimulated keratinocyte CLRL gene expression (Fig. 2D, CALCRL), but had no effect on CLRL protein levels (Fig. 2F). CGRP treatment did not stimulate keratinocyte RAMP1 gene expression (Fig. 2E), but did increase RAMP1 protein levels (Fig. 2F). The addition of CGRP to keratinocyte cell cultures did not increase SP expression at the mRNA level (TAC1, Fig. 2G) or protein level (Fig. 2H). In sensory nerves there are actually two different calcitonin gene-related peptides, the αCGRP peptide, which is generated by alternative splicing of the calcitonin gene CALCA, and βCGRP, which is derived from a separate gene, termed CALCB. The sequence homology between αCGRP and βCGRP is over 90% and they both activate the same CGRP receptor complex. CGRP did stimulate expression of the CALCA gene, but not the CALCB gene (Fig. 2I,J). Three hours of CGRP treatment caused a 44-fold increase in keratinocyte CGRP secretion over the ensuing 21 hours, relative to the low basal levels of CGRP observed without stimulation (Fig. 2K). These results support the hypothesis that CGRP stimulated keratinocytes release additional CGRP that acts in an autocrine or paracrine fashion, causes further keratinocyte activation with subsequent cutaneous inflammation.
Figure 2. CGRP treatment increased keratinocyte RAMP1 expression and secretion of CGRP.
The CGRP receptor components calcitonin receptor-like receptor (CRLR) and receptor activity-modifying protein 1 (RAMP1) are expressed in keratinocytes in vitro and this expression is up-regulated by CGRP treatment. CGRP treatment had no effect on SP gene or protein levels but did stimulate CGRP gene expression and protein secretion in keratinocytes. Immunostaining demonstrated CRLR protein (red) and RAMP1 protein (green) co-expressing in the cytoplasm and on the membrane of keratinocytes (A-C). CGRP treatment increased expression of its CRLR receptor gene (CALCRL) but not its RAMP1 receptor gene (RAMP1) at 3 h after treatment, as measured by real time PCR (D, E). Western blot demonstrates that CGRP (10−8M) increased RAMP1 protein level in keratinocytes at 24 h after treatment (F). CGRP treatment did not change the expression of the SP TAC1 gene at 3h, as measured by real time PCR (G). When keratinocytes were treated with various concentrations of CGRP for 3h, then washed 3 times in fresh medium, and then incubated in fresh medium for 21h, there was no increase in medium SP levels, as measured by EIA (H). CGRP treatment increased the expression of the CGRP gene CALCA (I), but not CALCB (J) at 3 h. When keratinocytes were treated with various concentrations of CGRP for 3h, then washed 3 times in fresh medium, and then incubated in fresh medium for 21h, there was a 44-fold increase in medium CGRP levels, as measured by EIA (K). Values are means ± SE. * P<0.05, ** P<0.01, and *** P<0.001 vs vehicle treated control cells. All experiments were repeated 3-4 times.
3.2 Neuropeptide stimulation of keratinocyte proliferation and inflammatory mediator expression
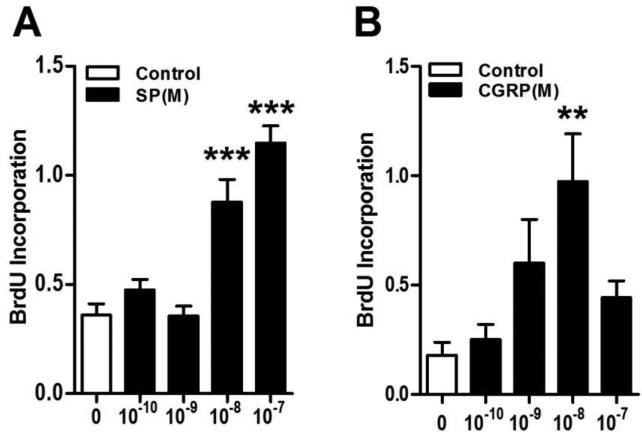
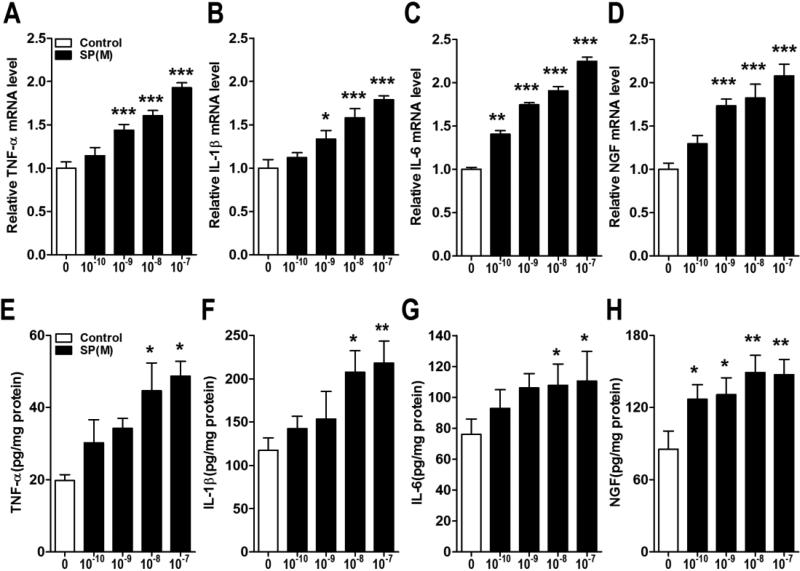
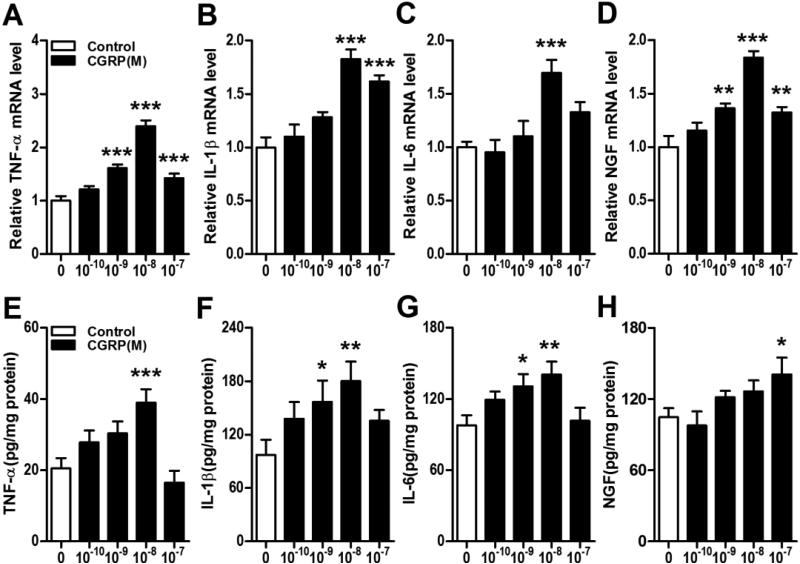
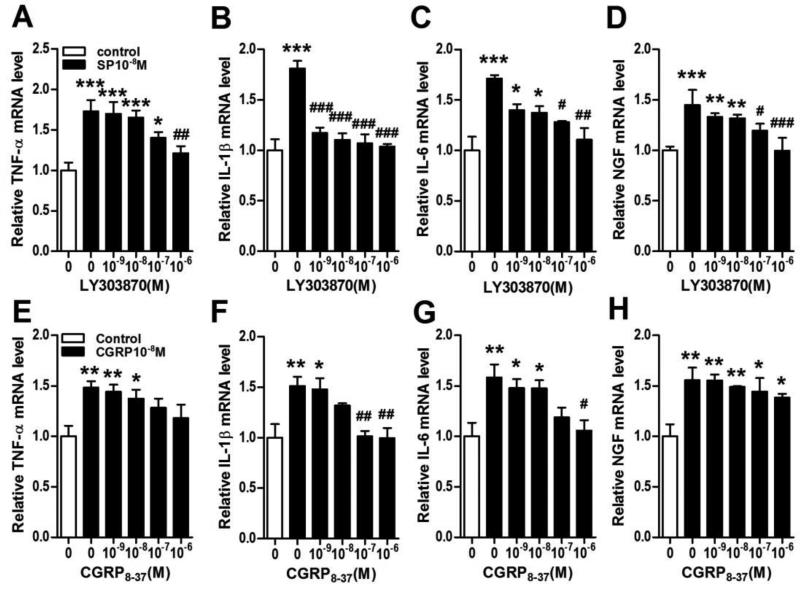
Using BrdU incorporation as an index of keratinocyte cell growth, both SP and CGRP concentration-dependently stimulated BrdU incorporation into keratinocytes (Fig. 3). Furthermore, both neuropeptides concentration-dependently stimulated TNF, IL-1, IL-6 and NGF inflammatory mediator expression in keratinocytes at the gene and protein levels (Figs. 4,5). LY303870, a selective SP NK1 receptor antagonist [25, 26], potently and concentration-dependently reduced SP (10−8M)-stimulated inflammatory mediator production, evidence that NK1 receptor activation accounts for the SP pro-inflammatory effects (Fig. 6A-D). CGRP8-37, a selective CGRP receptor antagonist, also concentration-dependently inhibited CGRP induced TNF, IL-1, IL-6 gene expression in keratinocytes, but had no effect on CGRP stimulated NGF gene expression, indicating that CGRP receptor activation is required for CGRP (10-8M) stimulatory effects on keratinocyte cytokine production (Fig. 6E-H).
Figure 3. SP and CGRP stimulated keratinocyte cellular proliferation.
SP (A) and CGRP (B) concentration-dependently stimulated cell proliferation in keratinocytes in vitro, as measured by BrdU incorporation over a 24 hour period. Values are means ± SE. * P<0.05, ** P<0.01 and *** P<0.001 and vs vehicle treated control cells. These experiments were repeated 3 times.
Figure 4. SP treatment stimulated keratinocyte expression of inflammatory mediators.
SP concentration-dependently up-regulated tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), interleukin 6 (IL-6), and nerve growth factor (NGF) gene expression in keratinocytes at 3 h, as measured by real time PCR (A-D) and similarly increased cytokine and NGF protein secretion into the media over 24 hours after SP treatment, as measured by EIA (E-H). Values are means ± SE. * P<0.05, ** P<0.01, and *** P<0.001 vs vehicle treated control cells. These experiments were repeated 3-4 times.
Figure 5. CGRP stimulated keratinocyte expression of inflammatory mediators.
CGRP concentration-dependently up-regulated TNF-α, IL-1, IL-6, and NGF gene expression in keratinocytes at 3 h, as measured by real time PCR (A-D) and similarly increased cytokine and NGF protein secretion into the media over 24 hours after SP treatment, by EIA (E-H). Values are means ± SE. * P<0.05, ** P<0.01, and *** P<0.001 vs vehicle treated control cells. These experiments were repeated 3-4 times.
Figure 6. Inflammatory mediator expression in keratinocytes required neuropeptide receptor activation.
SP (10−8M) induced up-regulation of cytokine and NGF gene expression in keratinocytes was concentration-dependently inhibited by the SP NK1 receptor antagonist LY303870, as measured by real time PCR at 3 h after SP treatment (A-D). Similarly, CGRP (10−8M) evoked up-regulation of cytokine gene expression in keratinocytes was concentration-dependently inhibited by the CGRP receptor antagonist CGRP8-37, as measured by real time PCR at 3 h after CGRP treatment (E-G). CGRP8-37 treatment had no significant effect on CGRP stimulated NGF gene expression in keratinocytes (H). Values are means ± SE. * P<0.05, ** P<0.01, and *** P<0.001 vs vehicle treated control cells. #P<0.05, ## P<0.01 and ### P<0.001 vs SP or CGRP treated cells. These experiments were repeated 3-4 times.
3.3 Neuropeptide activation of keratinocyte inflammatory mediator transcription pathways
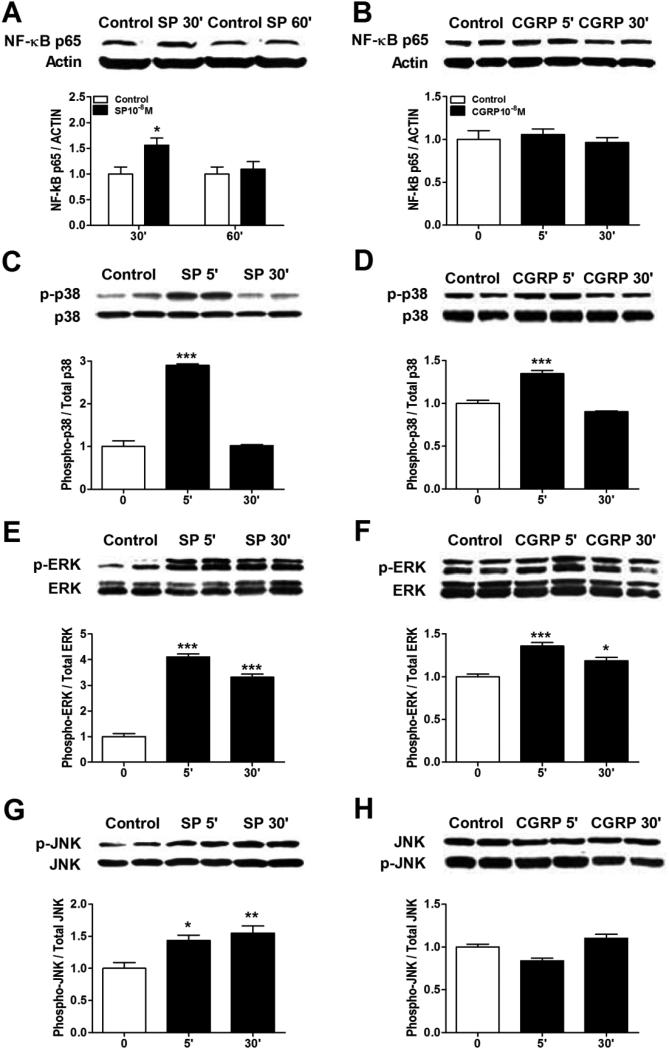
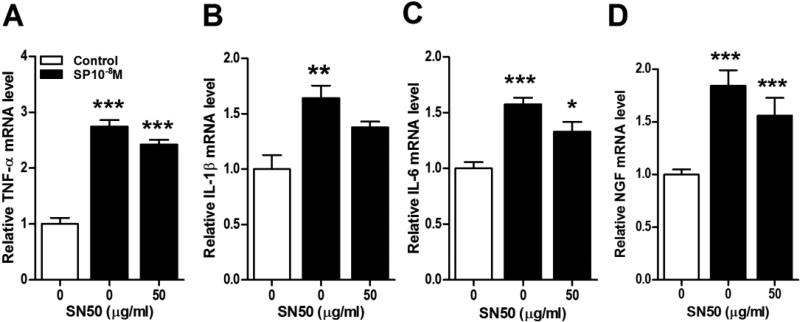
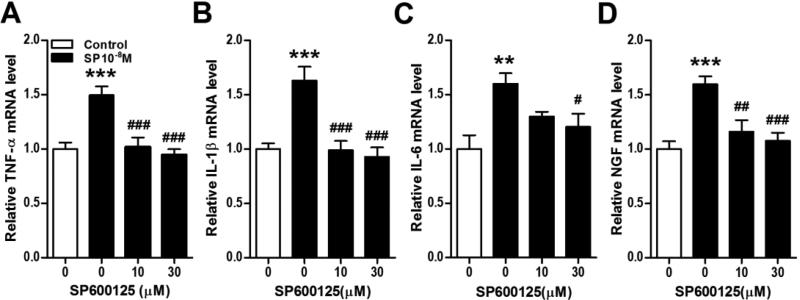
Next we focused on intracellular signaling pathways potentially linking SP induced NK-1 receptor activation to cytokine and NGF production. Figure 7A illustrates that SP (10−8M) caused rapid NF-κB p65 activation and nuclear translocation in keratinocytes. Under the same conditions, however, the addition of the NF-κB inhibitor SN50 had relatively little effect on SP stimulation of cytokine or NGF production (Fig. 8A-D). The same concentration of SN50 (50 ug/ml) that was ineffective in blocking SP stimulation of cytokine and NGF expression completely blocked SP stimulated NF-κB p65 nuclear translocation (data not shown), suggesting that other transcription pathways are responsible for SP induced inflammatory mediator expression. Possible pro-inflammatory roles for components of the MAPK signaling systems were also investigated. SP rapidly, but transiently caused the phosphorylation of p38 MAPK in keratinocytes (Fig. 7C). The selective p38 inhibitor SB203580 failed to reduce SP-induced cytokine and NGF production, suggesting the involvement of other protein kinases or even signaling pathways were involved in SP pro-inflammatory effects (Fig. 9A-D). SP also caused rapid and prolonged phosphorylation of the ERK kinases in keratinocytes (Fig.7E). Moreover, co-incubation of keratinocyte cell cultures with SP and the selective ERK inhibitor PD989059 concentration-dependently reversed SP-induced cytokine and NGF up-regulation (Fig. 10A-D). At the highest dose of inhibitor (30uM), SP (10−8M) stimulatory effects on TNF, IL-1, and NGF expression were completely blocked and stimulation of IL-6 was reduced by 41%. SP also induced moderate and prolonged phosphorylation of the JNK MAPK (Fig. 7G), and the JNK inhibitor SP600125 completely reversed SP-induced cytokine and NGF production in keratinocytes (Fig. 11).
Figure 7. Neuropeptides induced NF-κB and MAPK activation in keratinocytes.
Western blot was used to evaluate the stimulatory effects of SP and CGRP treatment on various transcriptional pathways in keratinocytes. SP (10−8M) induced NF-κB p65 nuclear translocation in keratinocytes at 30 min after treatment (A), but CGRP (10−8M) had no effect on NF-κB nuclear translocation (B). Both SP (C) and CGRP (D) caused the rapid phosphorylation of p38 mitogen activated protein kinase (MAPK) at 5 min, but CGRP had a less robust effect. Similarly, Both SP (E) and CGRP (F) caused the rapid phosphorylation of extracellular signal related kinases 1/2 (ERK1/2) MAPK at 5 and 30 min, but again CGRP had a less robust effect. SP (G) treatment induced moderate phosphorylation of c-Jun N-terminal kinases (JNK) MAPK at 5 and 30 min after treatment, but CGRP (H) had no effect. These experiments were repeated 3-4 times.
Figure 8. NF-κB activation was not required for SP stimulation of keratinocyte inflammatory mediator expression.
The NF-kB inhibitor SN50 (50 ug/ml) did not inhibit SP (10−8M) stimulated TNF (A), IL-1 (B), IL-6 (C), and NGF (D) gene expression in keratinocytes at 3 h, measured by real time PCR. The same concentration of SN50 (50 ug/ml) that was ineffective in blocking SP stimulation of cytokine and NGF expression completely blocked SP stimulated NF-κB p65 nuclear translocation (data not shown), suggesting that other transcription pathways are responsible for SP induced inflammatory mediator expression. There were no significant differences between the SP and SP + SN50 groups (black bars) for any inflammatory mediator. CGRP treatment did not induce NF-κB nuclear translocation (Fig. 7), thus CGRP was not tested against SN50. Values are means ± SE. * P<0.05, ** P<0.01, and *** P<0.001 vs vehicle treated control cells.
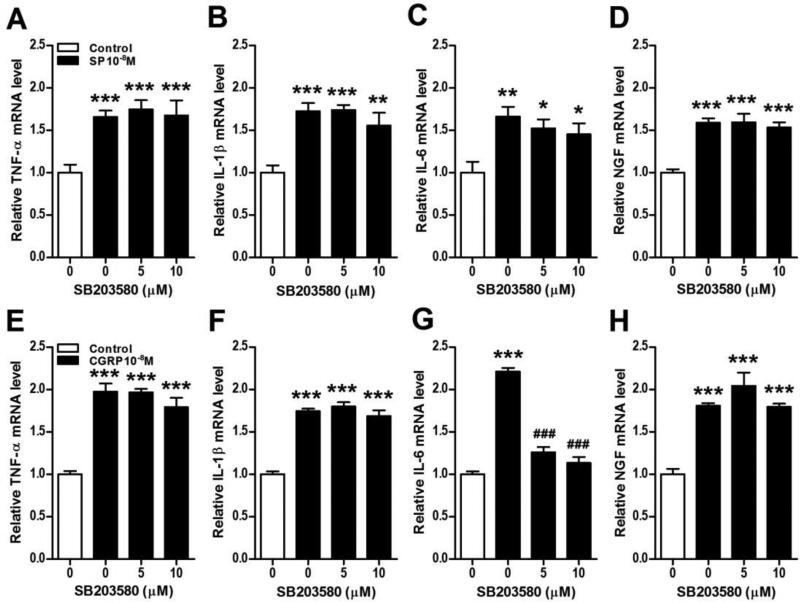
Figure 9. Neuropeptide stimulation of keratinocyte inflammatory mediator expression does not require p38 MAPK activation.
The p38 MAPK inhibitor SB203580 was used to demonstrate that SP and CGRP induced cytokine and NGF expression in keratinocytes does not require p38 activation. SB203580 had no effect on SP (10−8M, black bars) stimulation of TNF (A), IL-1 (B), IL-6 (C), and NGF (D) gene expression in keratinocytes at 3 h, measured by real time PCR. Similarly, SB203580 had no effect on CGRP (10−8M, black bars) stimulation of TNF (E), IL-1 (F), and NGF (H) gene expression in keratinocytes at 3 h, measured by real time PCR. SB203580 did inhibit CGRP stimulation of IL-6 (G) expression, suggesting that the p38 kinase activation may contribute to CGRP stimulation of IL-6 expression in keratinocytes. Values are means ± SE. * P<0.05, ** P<0.01, and *** P<0.001vs vehicle treated control cells. # P<0.05, ## P<0.01, and ### P<0.001 vs SP or CGRP treated cells. These experiments were repeated 3-4 times.
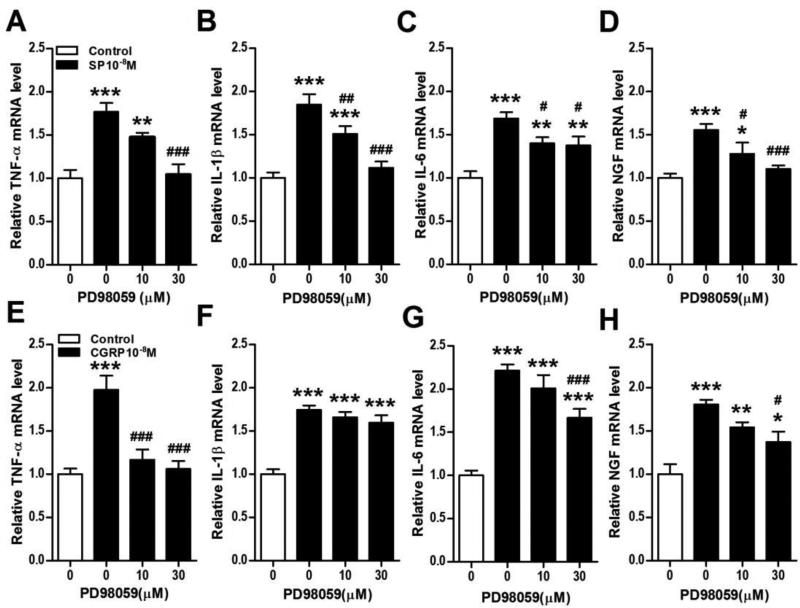
Figure 10. Neuropeptide stimulation of keratinocyte inflammatory mediator expression requires ERK MAPK activation.
The ERK1/2 MAPK inhibitor PD98059 was used to demonstrate that SP and CGRP induced cytokine and NGF expression in keratinocytes required ERK1/2 activation. PD98059 concentration-dependently inhibited SP (10−8M, black bars) stimulation of TNF (A), IL-1 (B), IL-6 (C), and NGF (D) gene expression in keratinocytes at 3 h, measured by real time PCR. Similarly, PD98059 concentration-dependently inhibited CGRP (10−8M, black bars) stimulation of TNF (E), IL-6 (G), and NGF (H) gene expression in keratinocytes at 3 h, measured by real time PCR. There was no PD98059 inhibition of CGRP stimulation of IL-1 (F) expression, suggesting that other protein kinases or signaling pathways may mediate CGRP stimulation of IL-1 expression in keratinocytes. Values are means ±SE. * P<0.05, ** P<0.01, and *** P<0.001 vs vehicle treated control cells. # P<0.05, ## P<0.01, and ### P<0.001 vs SP or CGRP treated cells. These experiments were repeated 3-4 times.
Figure 11. SP stimulation of keratinocyte inflammatory mediator expression requires ERK MAPK activation.
The JNK MAPK inhibitor SP600125 was used to demonstrate that SP induced cytokine and NGF expression in keratinocytes required JNK activation. SP600125 completely blocked SP (10−8M, black bars) stimulation of TNF (A), IL-1 (B), IL-6 (C), and NGF (D) gene expression in keratinocytes at 3 h, measured by real time PCR. CGRP treatment did not induce JNK activation (Fig. 7), thus CGRP was not tested against a SP600125. Values are means ± SE. * P<0.05, ** P<0.01, and *** P<0.001 vs vehicle treated control cells. # P<0.05, ## P<0.01, and ### P<0.001 vs SP treated cells. These experiments were repeated 3-4 times.
We also attempted to identify the intracellular signaling pathways in mediating CGRP-induced cytokine and NGF production in keratinocytes. CRGP failed to induce NF-kB activation or nuclear translocation (Fig. 7B). There was a modest and transient CGRP phosphorylation effect on p38 MAPK (Fig. 7D), but the selective p38 inhibitor SB203580 failed to significantly reduce CGRP-induced cytokine or NGF production, except for IL-6 expression, which was completely inhibited (Fig. 9E-H). CGRP also induced moderate and prolonged phosphorylation the ERK kinases in keratinocytes (Fig.7F) and co-incubation of keratinocyte cell cultures with CGRP and the selective ERK inhibitor PD989059 reversed CGRP-induced cytokine and NGF up-regulation, except for IL-1 (Fig. 10E-H). PD98059 treatment only partially reversed CGRP stimulatory effects on IL-6 expression (Fig. 10G). CGRP treatment had no effect on JNK MAPK phosphorylation in keratinocytes (Fig. 7H).
4. Discussion
Figures 1 and 2 illustrate that under normal culture conditions keratinocytes synthesized and secreted low levels of the neurotransmitters SP and CGRP and expressed their cognate receptors. Furthermore, physiologic concentrations these neuropeptides stimulated keratinocyte expression of the SP and CGRP receptors, and dramatically increase keratinocyte secretion of SP and CGRP. Keratinocyte secreted neuropeptides could have autocrine or paracrine stimulatory effects, potentially amplifying neuropeptide-keratinocyte signaling and innate immune responses in the skin. These findings are supported by previous reports that SP (10−8M), and much less potently CGRP (10−8M), can stimulate SP expression and secretion in human keratinocytes [27]. In addition, it has been reported that keratinocytes express the SP NK1 receptor and that exposure to SP can increase the expression of NK1 receptors [28], although another group of investigators failed to observe this effect [29]. A recent study also observed CGRP expression in keratinocytes, both in vivo and in vitro, with increased keratinocyte CGRP expression in various rodent pain models and in postherpetic neuralgia and CRPS patient skin [30]. These investigators also identified mRNA for the CGRP receptor complex in keratinocyte cell cultures. The current study is the first to observe that CGRP dramatically stimulates keratinocyte expression and secretion of CGRP (Fig. 2I, J, K). We also observed that CGRP treatment modestly stimulated protein expression of the RAMP1 component of the CGRP receptor complex in keratinocytes (Fig. 2F), despite the lack of CGRP effect on keratinocyte RAMP1 mRNA levels (Fig. 2E). These results are in agreement with another recent study that failed to observe any CGRP stimulatory effects on mRNA levels for RAMP1 in keratinocytes [29].
Another finding of the current study was that both SP and CGRP concentration-dependently increased keratinocyte proliferation (Fig. 3), confirming previous reports that SP and CGRP stimulated cell proliferation in human and mouse keratinocyte cell lines [18, 29, 31, 32]. Furthermore, we observed that both SP and CGRP concentration-dependently stimulated keratinocyte IL-1, IL-6, TNF, and NGF expression and secretion (Figs. 4, 5). The bell shaped concentration-response curves observed for inflammatory mediator protein expression after treatment with the highest neuropeptide concentrations tested (10−7M, Fig. 5, and 10−6M, data not shown) may be attributable to receptor desensitization, internalization, uncoupling, or depletion of second messenger [33]. Maximal stimulatory effects on inflammatory mediator expression occurred at neuropeptide concentrations of 10−9 to 10−8M, similar to the SP (3.5 × 10−9M) and CGRP (1.0 × 10−8M) concentrations we observed in rat hindpaw skin using EIA assays (data not shown). The stimulatory effects of SP and CGRP on inflammatory mediator expression in keratinocytes were blocked by the selective NK1 receptor antagonist LY303870 and the selective CGRP receptor antagonist CGRP8-37, respectively (Fig. 6), indicating that neuropeptide induction of innate immune responses in keratinocytes required cell surface receptor activation. The results of the current study are novel with regards to SP and CGRP stimulation of IL-6 and TNF secretion in keratinocytes, and the IL-1 and NGF findings are consistent with previous reports from both our lab and from other groups that physiologic concentrations of SP and CGRP can stimulate IL-1 and NGF expression and secretion in human and rat keratinocytes [3-5].
One concern about the current study is that these experiments examined neuropeptide stimulation of a homogenous population of keratinocytes in a similar state of differentiation, as opposed to in vivo conditions in which there is a heterogeneous keratinocyte population distributed in the various layers of the epidermis. Our current in vitro results are in agreement with our previous in vivo findings that intradermal SP injection induced up-regulated epidermal keratinocyte expression of IL-1, IL-6, TNF, and NGF inflammatory proteins [34]. We have also previously demonstrated that post-traumatic up-regulated keratinocyte NGF expression stimulated sensory neurons to express and release SP and CGRP into the skin, further stimulating keratinocyte inflammatory mediator expression [8, 35-37]. Dysregulated neuropeptide-keratinocyte signaling caused by exaggerated keratinocyte neuropeptide and NGF expression could contribute to the development of inflammatory skin diseases and pain syndromes, thus it is physiologically important to maintain homeostasis in neural control of skin function. Amplified neuropeptide-keratinocyte signaling may be negatively regulated by the peripheral release of sensory neuropeptides such as vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating peptide (PACAP), and somatostatin, all of which exhibit anti-inflammatory effects in various types of immune cells [2].
The MAPK signaling system has been implicated in promoting IL-1, IL-6, and TNF cytokine production in many tissues, including sunburned skin [38-40]. Strong evidence also links inflammatory activation of the NF-κB system to cytokine production in various types of cells [41, 42]. While no prior studies have looked at SP activation of MAPK or NFκB transcription pathways in keratinocytes, CGRP activation of the three principle MAPK families in keratinocytes has been previously reported [18]. The current study demonstrates that SP stimulation of keratinocytes activated all three MAPK families and the NFκB transcriptional signaling pathway, both novel findings (Fig. 7). CGRP had a much weaker stimulatory effect on P38 and ERK1/2 activation in keratinocytes, and no effect on JNK phosphorylation or NF-κB nuclear translocation (Fig. 7). SP stimulated keratinocyte inflammatory mediator production was blocked by a selective ERK1/2 inhibitor (Fig. 10) and by a selective JNK inhibitor (Fig. 11), but not by a p38 MAPK (Fig. 9) or NFκB inhibitor (Fig. 8). CGRP stimulated TNF, IL-1, and NGF expression in keratinocytes was blocked or reduced by a selective ERK1/2 inhibitor (Fig. 10), and only CGRP-induced IL-6 expression was blocked by a p38 inhibitor (Fig. 9). These results indicate that the predominant MAPK transcription pathways for neuropeptide induced innate immunity responses in keratinocytes are the ERK1/2 and JNK signaling pathways for SP and the ERK1/2 pathway for CGRP, which are novel findings.
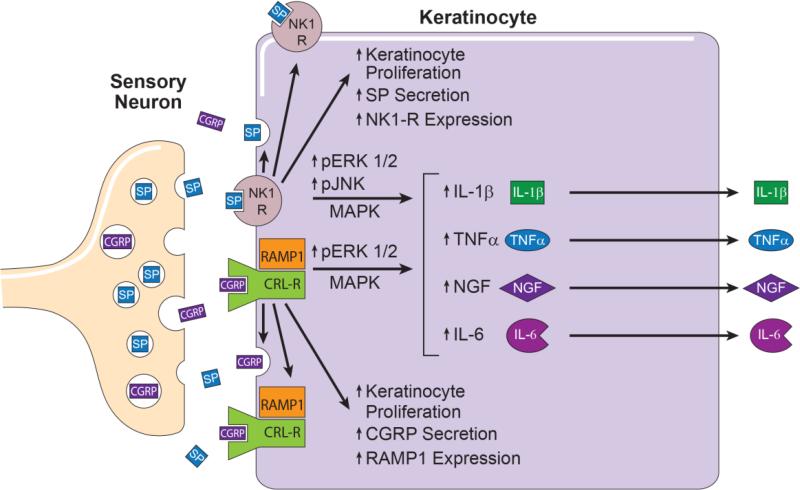
The results of the current study are schematically summarized in Figure 12. There is a large body of clinical and translational data indicating that up-regulated neuropeptide signaling and keratinocyte inflammatory mediator expression contribute to chronic inflammatory skin diseases such as psoriasis, atopic dermatitis, and contact dermatitis, and can contribute to the development of post-incisional pain and CRPS. These chronic neuro-inflammatory changes in the skin result from the disruption of the normally sustained and tightly regulated communication network between two different cell types; specifically SP and CGRP releasing peptidergic sensory afferent neurons and keratinocytes. This paper demonstrates that neuropeptide stimulated keratinocytes express neuropeptide receptors and synthesize and release large quantities of SP and CGRP, which can act in an autocrine or paracrine fashion to further stimulate keratinocytes. Both SP and CGRP signaling initiated keratinocyte proliferation and inflammatory mediator secretion via cell-surface receptor activation. After neuropeptide receptor activation on the keratinocyte cell surface, intracellular transcription factors shuttle information to the cell nucleus to initiate inflammatory mediator expression and secretion. Collectively, the results presented in this study indicate that both the ERK1/2 and JNK MAPK signal transduction pathways are crucial mediators of neuropeptide evoked inflammatory responses in keratinocytes and identifies these signaling pathways as a potential molecular targets for controlling inflammatory skin diseases, post-incisional pain, and CRPS.
Figure 12. This schematic summarizes the results of these experiments.
Cutaneous primary sensory afferents release SP and CGRP in the epidermis. These neuropeptides then diffuse through the interstitial space to bind and activate their cognate receptors on the keratinocyte cell surface, the SP NK1 receptor and the CGRP receptor dimer complex of CRLR and RAMP1. Keratinocyte NK1 receptor activation stimulates cellular proliferation, SP expression and secretion, NK1 receptor expression, and the phosphorylation and activation of ERK 1/2 and JNK MAPK intracellular transcription factors that stimulate TNFα, IL-1β, IL-6, and NGF expression and secretion. Similarly, activation of the keratinocyte CGRP receptor dimer complex stimulates keratinocyte proliferation, CGRP expression and secretion, RAMP1 receptor expression, and the phosphorylation and activation of ERK 1/2 MAPK, an intracellular transcription factor that up-regulates TNFα, IL-6, and NGF expression and secretion. Keratinocyte secreted inflammatory cytokines and NGF can directly activate their cognate receptors expressed on cutaneous sensory afferent neurons, with subsequent pain sensitization. Keratinocyte secreted inflammatory mediators also can activate various cellular components of the innate and adaptive immune systems, thus supporting the development of inflammatory skin diseases and nociceptive sensitization. CGRP, calcitonin gene-related peptide; SP, substance P; NK1-R, neurokinin 1 receptor; CRL-R, calcitonin receptor –like receptor; RAMP1 receptor activity-modifying protein; MAPK, mitogen activated protein kinases; ERK1/2; extracellular signal related kinases 1/2; JNK, c-Jun N-terminal kinases; IL, interleukin; TNF α, tumor necrosis factor α; NGF, nerve growth factor.
Highlights.
Neuropeptides stimulate keratinocytes to secrete SP and CGRP.
SP and CGRP stimulate keratinocytes to secrete inflammatory cytokines and NGF.
SP activates p38, ERK1/2, and JNK MAPKs and NFκB in keratinocytes.
CGRP activates p38 and ERK1/2 MAPKs in keratinocytes.
ERK1/2 and JNK signaling mediate keratinocyte inflammatory responses.
Acknowledgements
This work was supported by National Institutes of Health grant NS072168, and Department of Veterans Affairs, Rehabilitation Research and Development Merit grant F7137R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.da Silva L, Carvalho E, Cruz MT. Role of neuropeptides in skin inflammation and its involvement in diabetic wound healing. Expert Opin Biol Ther. 2010;10:1427–39. doi: 10.1517/14712598.2010.515207. [DOI] [PubMed] [Google Scholar]
- 2.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 3.Dallos A, Kiss M, Polyanka H, Dobozy A, Kemeny L, Husz S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides. 2006;40:251–63. doi: 10.1016/j.npep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Burbach GJ, Kim KH, Zivony AS, Kim A, Aranda J, Wright S, Naik SM, Caughman SW, Ansel JC, Armstrong CA. The neurosensory tachykinins substance P and neurokinin A directly induce keratinocyte nerve growth factor. J Invest Dermatol. 2001;117:1075–82. doi: 10.1046/j.0022-202x.2001.01498.x. [DOI] [PubMed] [Google Scholar]
- 5.Shi X, Wang L, Li X, Sahbaie P, Kingery WS, Clark JD. Neuropeptides contribute to peripheral nociceptive sensitization by regulating interleukin-1beta production in keratinocytes. Anesth Analg. 2011;113:175–83. doi: 10.1213/ANE.0b013e31821a0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho RF, Nilsson G, Harvima IT. Increased mast cell expression of PAR-2 in skin inflammatory diseases and release of IL-8 upon PAR-2 activation. Exp Dermatol. 2010;19:117–22. doi: 10.1111/j.1600-0625.2009.00998.x. [DOI] [PubMed] [Google Scholar]
- 7.Marinus J, Moseley GL, Birklein F, Baron R, Maihofner C, Kingery WS, van Hilten JJ. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10:637–48. doi: 10.1016/S1474-4422(11)70106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li WW, Guo TZ, Li XQ, Kingery WS, Clark JD. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain. 2010;151:843–52. doi: 10.1016/j.pain.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark JD, Shi X, Li X, Qiao Y, Liang D, Angst MS, Yeomans DC. Morphine reduces local cytokine expression and neutrophil infiltration after incision. Mol Pain. 2007;3:28. doi: 10.1186/1744-8069-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber M, Birklein F, Neundorfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91:251–7. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 11.Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Exp Neurol. 2003;183:197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 12.Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol. 2008;294:C1586–96. doi: 10.1152/ajpcell.00129.2008. [DOI] [PubMed] [Google Scholar]
- 13.Fiebich BL, Schleicher S, Butcher RD, Craig A, Lieb K. The neuropeptide substance P activates p38 mitogen-activated protein kinase resulting in IL-6 expression independently from NF-kappa B. Journal of immunology. 2000;165:5606–11. doi: 10.4049/jimmunol.165.10.5606. [DOI] [PubMed] [Google Scholar]
- 14.Tokuda M, Miyamoto R, Sakuta T, Nagaoka S, Torii M. Substance P activates p38 mitogen-activated protein kinase to promote IL-6 induction in human dental pulp fibroblasts. Connect Tissue Res. 2005;46:153–8. doi: 10.1080/03008200500182490. [DOI] [PubMed] [Google Scholar]
- 15.Azzolina A, Guarneri P, Lampiasi N. Involvement of p38 and JNK MAPKs pathways in Substance P-induced production of TNF-alpha by peritoneal mast cells. Cytokine. 2002;18:72–80. doi: 10.1006/cyto.2002.0879. [DOI] [PubMed] [Google Scholar]
- 16.Okabe T, Hide M, Koro O, Yamamoto S. Substance P induces tumor necrosis factor-alpha release from human skin via mitogen-activated protein kinase. Eur J Pharmacol. 2000;398:309–15. doi: 10.1016/s0014-2999(00)00304-6. [DOI] [PubMed] [Google Scholar]
- 17.Azzolina A, Bongiovanni A, Lampiasi N. Substance P induces TNF-alpha and IL-6 production through NF kappa B in peritoneal mast cells. Biochim Biophys Acta. 2003;1643:75–83. doi: 10.1016/j.bbamcr.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Yu XJ, Li CY, Xu YH, Chen LM, Zhou CL. Calcitonin gene-related peptide increases proliferation of human HaCaT keratinocytes by activation of MAP kinases. Cell Biol Int. 2009 doi: 10.1016/j.cellbi.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Presland RB, Fleckman P. Tetracycline-regulated gene expression in epidermal keratinocytes. Methods Mol Biol. 2005;289:273–86. doi: 10.1385/1-59259-830-7:273. [DOI] [PubMed] [Google Scholar]
- 20.Kuechle MK, Presland RB, Lewis SP, Fleckman P, Dale BA. Inducible expression of filaggrin increases keratinocyte susceptibility to apoptotic cell death. Cell Death Differ. 2000;7:566–73. doi: 10.1038/sj.cdd.4400687. [DOI] [PubMed] [Google Scholar]
- 21.Haydock PV, Blomquist C, Brumbaugh S, Dale BA, Holbrook KA, Fleckman P. Antisense profilaggrin RNA delays and decreases profilaggrin expression and alters in vitro differentiation of rat epidermal keratinocytes. J Invest Dermatol. 1993;101:118–26. doi: 10.1111/1523-1747.ep12363609. [DOI] [PubMed] [Google Scholar]
- 22.Baden HP, Kubilus J. The growth and differentiation of cultured newborn rat keratinocytes. J Invest Dermatol. 1983;80:124–30. doi: 10.1111/1523-1747.ep12532899. [DOI] [PubMed] [Google Scholar]
- 23.Presland RB, Kuechle MK, Lewis SP, Fleckman P, Dale BA. Regulated expression of human filaggrin in keratinocytes results in cytoskeletal disruption, loss of cell-cell adhesion, and cell cycle arrest. Exp Cell Res. 2001;270:199–213. doi: 10.1006/excr.2001.5348. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Zhao R, Shi X, Wei T, Halloran BP, Clark DJ, Jacobs CR, Kingery WS. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone. 2009;45:309–20. doi: 10.1016/j.bone.2009.04.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gitter BD, Bruns RF, Howbert JJ, Waters DC, Threlkeld PG, Cox LM, Nixon JA, Lobb KL, Mason NR, Stengel PW, Cockerham SL, Silbaugh SA, Gehlert DR, Schober DA, Iyengar S, Calligaro DO, Regoli D, Hipskind PA. Pharmacological characterization of LY303870: a novel, potent and selective nonpeptide substance P (neurokinin 1) receptor antagonist. J Pharmacol Exp Therapeut. 1995;275:737–44. [PubMed] [Google Scholar]
- 26.Iyengar S, Hipskind PA, Gehlert DR, Schober D, Lobb KL, Nixon JA, Helton DR, Kallman MJ, Boucher S, Couture R, Li DL, Simmons RMA. LY303870, a centrallyactive neurokinin-1 antagonist with a long duration of action. J Pharmacol Exp Ther. 1997;280:774–85. [PubMed] [Google Scholar]
- 27.Bae S, Matsunaga Y, Tanaka Y, Katayama I. Autocrine induction of substance P mRNA and peptide in cultured normal human keratinocytes. Biochem Biophys Res Commun. 1999;263:327–33. doi: 10.1006/bbrc.1999.1285. [DOI] [PubMed] [Google Scholar]
- 28.Liu JY, Hu JH, Zhu QG, Li FQ, Sun HJ. Substance P receptor expression in human skin keratinocytes and fibroblasts. Br J Dermatol. 2006;155:657–62. doi: 10.1111/j.1365-2133.2006.07408.x. [DOI] [PubMed] [Google Scholar]
- 29.Roggenkamp D, Kopnick S, Stab F, Wenck H, Schmelz M, Neufang G. Epidermal Nerve Fibers Modulate Keratinocyte Growth via Neuropeptide Signaling in an Innervated Skin Model. J Invest Dermatol. 2013 doi: 10.1038/jid.2012.464. [DOI] [PubMed] [Google Scholar]
- 30.Hou Q, Barr T, Gee L, Vickers J, Wymer J, Borsani E, Rodella L, Getsios S, Burdo T, Eisenberg E, Guha U, Lavker R, Kessler J, Chittur S, Fiorino D, Rice F, Albrecht P. Keratinocyte expression of calcitonin gene-related peptide beta: implications for neuropathic and inflammatory pain mechanisms. Pain. 2011;152:2036–51. doi: 10.1016/j.pain.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi K, Nakanishi S, Imamura S. Direct effects of cutaneous neuropeptides on adenylyl cyclase activity and proliferation in a keratinocyte cell line: stimulation of cyclic AMP formation by CGRP and VIP/PHM, and inhibition by NPY through G protein-coupled receptors. J Invest Dermatol. 1993;101:646–51. doi: 10.1111/1523-1747.ep12371670. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T, Danno K, Ikai K, Imamura S. Effects of substance P and substance K on the growth of cultured keratinocytes. J Invest Dermatol. 1988;90:399–401. doi: 10.1111/1523-1747.ep12456487. [DOI] [PubMed] [Google Scholar]
- 33.Honore P, Kamp EH, Rogers SD, Gebhart GF, Mantyh PW. Activation of lamina I spinal cord neurons that express the substance P receptor in visceral nociception and hyperalgesia. J Pain. 2002;3:3–11. doi: 10.1054/jpai.2002.27001. [DOI] [PubMed] [Google Scholar]
- 34.Wei T, Guo TZ, Li WW, Hou S, Kingery W, Clark JD. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. J Neuroinflammation. 2012;9:181. doi: 10.1186/1742-2094-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138:47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo TZ, Wei T, Shi X, Li WW, Hou S, Wang L, Tsujikawa K, Rice KC, Cheng K, Clark DJ, Kingery WS. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Mol Pain. 2012;8:85. doi: 10.1186/1744-8069-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2009;144:278–86. doi: 10.1016/j.pain.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–46. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 39.Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch Dermatol Res. 2010;302:5–17. doi: 10.1007/s00403-009-0994-y. [DOI] [PubMed] [Google Scholar]
- 40.Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67:909–16. doi: 10.1136/ard.2007.074278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 42.Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67:668–73. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]