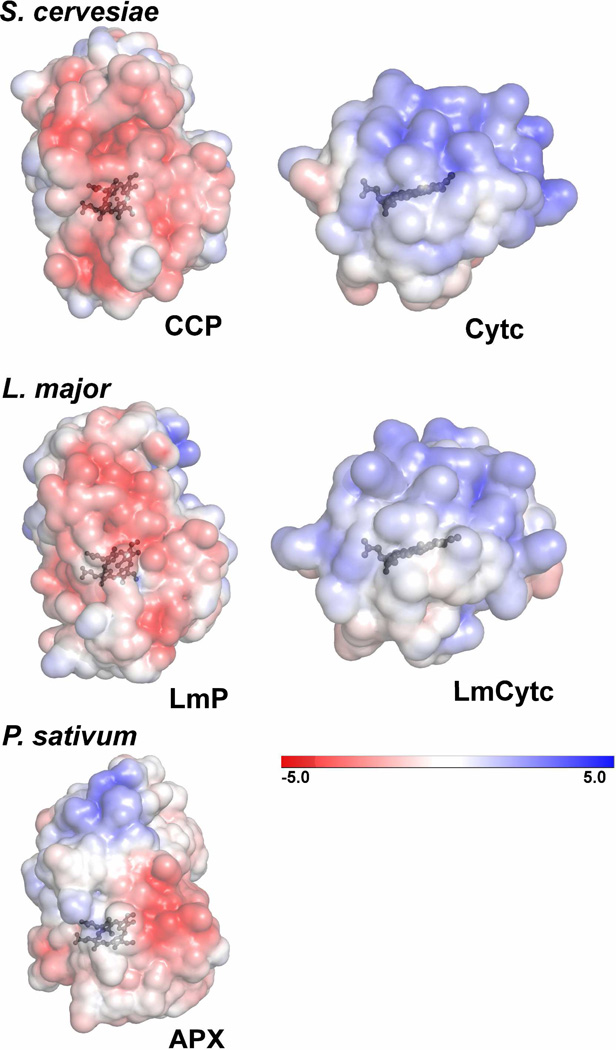
Figure 5.
Electrostatic surface representations (±5kT) of S. cervesiae CCP and Cytc, L. major LmP and LmCytc and as a control, cytosolic pea ascorbate peroxidase (APX). These surfaces are the same that would be involved in substrate binding as determined by the co-crystal structure of the S. cervesiae system (PDB: 2PCC). Both the S. cervesiae and L. major heme peroxidases have an overall distribution of negative charge at the Cytc binding interface while the respective Cytc substrates have a clearly positive distribution. P. sativum APX does not have a strong distribution of negative charge on the same surface. These figures were generated with the Adaptive Poisson-Boltzmann Solver (APBS) plug-in using PyMOL (34).