Abstract
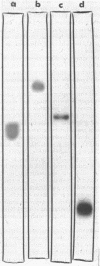
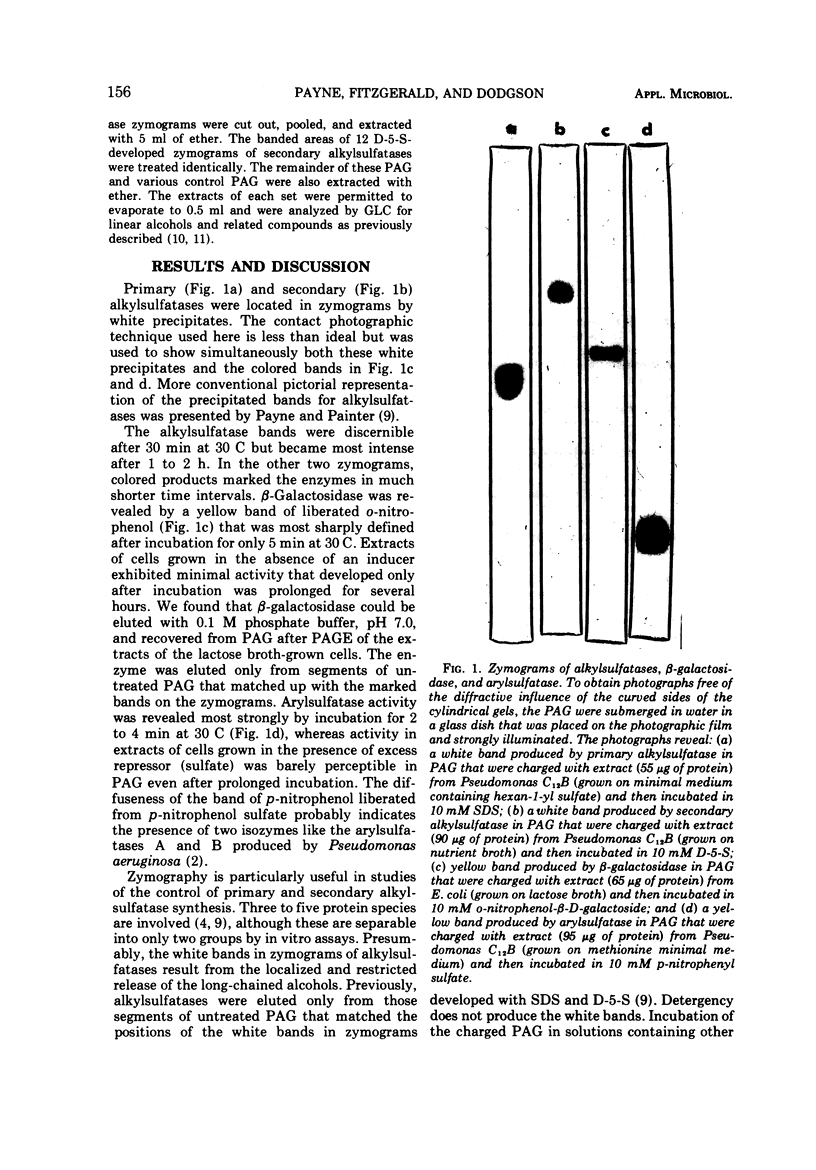
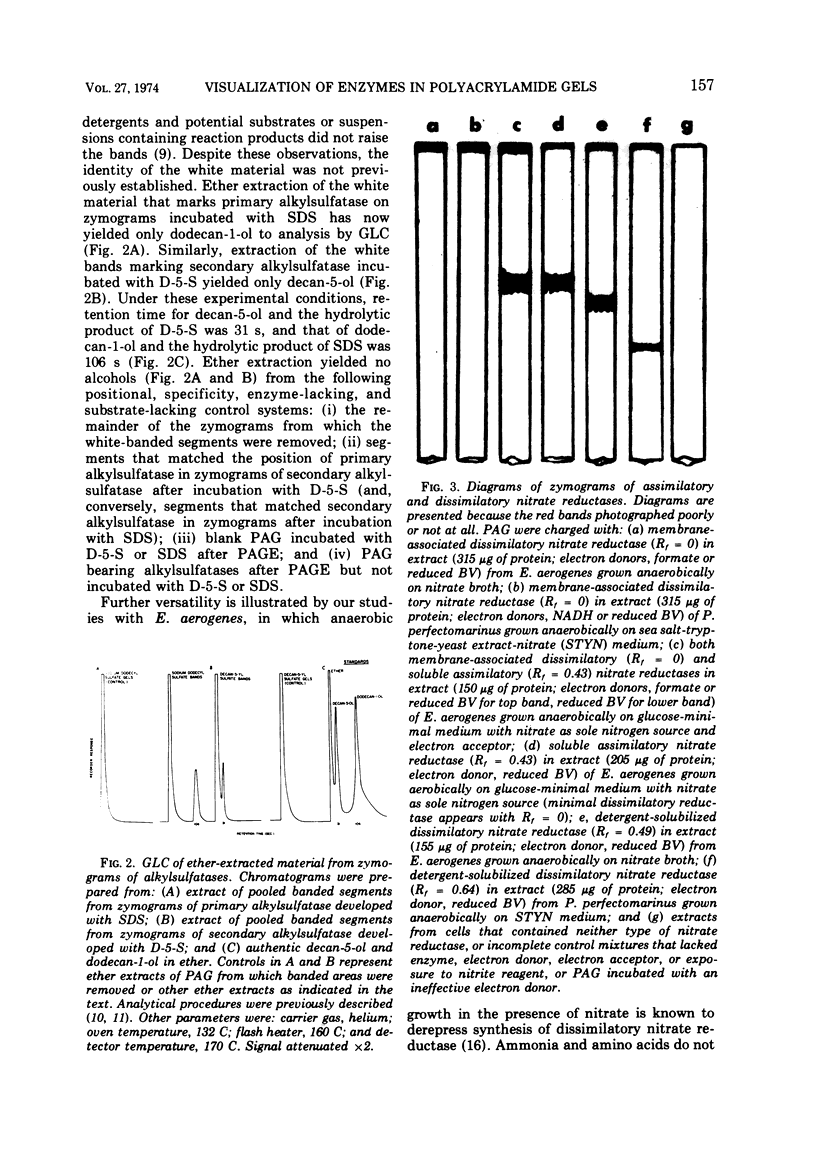
White bands resulting from precipitation of dodecan-1-ol liberated by hydrolysis of sodium dodecyl sulfate and decan-5-ol released by hydrolysis of decan-5-yl sulfate produced zymograms of the primary and secondary alkylsulfatases from Pseudomonas C12B. Gas-liquid chromatographic analyses of ether extracts of the precipitate-containing segments of the zymograms confirmed the identity of the alcohols which were not discerned in extracts of segments of the gels other than those containing precipitates. β-Galactosidase from Escherichia coli was marked on zymograms by the liberation of o-nitrophenol from o-nitrophenyl-β-D-galactoside, and arylsulfatase from Pseudomonas C12B was marked in gels by liberation of p-nitrophenol from p-nitrophenyl sulfate. Membrane-associated dissimilatory nitrate reductases from a nitrate respirer (Enterobacter aerogenes) and a denitrifier (Pseudomonas perfectomarinus) did not penetrate either 6.8 or 3% polyacrylamide gel but were demonstrable at the top of the gels. In the membrane-bound state, formate served as electron donor for nitrate reductase from E. aerogenes, and reduced nicotinamide adenine dinucleotide (NADH) served as donor for nitrate reductase from P. perfectomarinus. Both enzymes reduced nitrate at the expense of reduced benzyl viologen as well. Assimilatory nitrate reductase from E. aerogenes moved easily into the 6.8% gels (Rf = 0.43 under the conditions of these experiments). The reduced dye served as electron donor for the assimilatory reductase, but formate and NADH did not. Incubation of the membrane-associated nitrate reductases with 2% Triton X-100 solubilized the enzymes and removed the capacity of formate and NADH to serve as electron donors. Both retained the ability to reduce nitrate at the expense of reduced benzyl viologen. The solubilized dissimilatory reductase from E. aerogenes moved further in the gels (Rf = 0.49) than the soluble assimilatory reductase; the solubilized dissimilatory reductase from the denitrifier, P. perfectomarinus, moved further in the gels (Rf = 0.64) than either of the enzymes from E. aerogenes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 't Riet J van, Stouthamer A. H., Planta R. J. Regulation of nitrate assimilation and nitrate respiration in Aerobacter aerogenes. J Bacteriol. 1968 Nov;96(5):1455–1464. doi: 10.1128/jb.96.5.1455-1464.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J. M., Ashworth R. B. Disc electrophoresis. J Chem Educ. 1969 Jan;46(1):41–45. doi: 10.1021/ed046p41. [DOI] [PubMed] [Google Scholar]
- Delisle G. J., Milazzo F. H. Characterization of arylsulfatase isoenzymes from Pseudomonas aeruginosa. Can J Microbiol. 1972 May;18(5):561–568. doi: 10.1139/m72-089. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J. W., Payne W. J. Induction in a Pseudomonas species of sulphatases active on short chain alkylsulphates. Microbios. 1972 Mar-Apr;5(18):87–100. [PubMed] [Google Scholar]
- Fitzgerald J. W., Payne W. J. The regulation of arylsulphatase formation in Pseudomonas C 12 B. Microbios. 1972 Sep-Oct;6(22):147–156. [PubMed] [Google Scholar]
- Goldberg E. Lactic and Malic Dehydrogenases in Human Spermatozoa. Science. 1963 Feb 15;139(3555):602–603. doi: 10.1126/science.139.3555.602. [DOI] [PubMed] [Google Scholar]
- Kiszkiss D. F., Downey R. J. Localization and solubilization of the respiratory nitrate reductase of Bacillus stearothermophilus. J Bacteriol. 1972 Feb;109(2):803–810. doi: 10.1128/jb.109.2.803-810.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Payne W. J., Painter B. G. Resolution by acrylamide gel electrophoresis of alkyl sulphatases and alcohol dehydrogenase. Microbios. 1971 Apr;3(12):199–206. [PubMed] [Google Scholar]
- Payne W. J., Williams J. P., Mayberry W. R. Primary alcohol sulfatase in a Pseudomonas species. Appl Microbiol. 1965 Sep;13(5):698–701. doi: 10.1128/am.13.5.698-701.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichinoty F., Piéchaud M. Recherche des nitrate-réductases bactériennes A et B: méthodes. Ann Inst Pasteur (Paris) 1968 Jan;114(1):77–98. [PubMed] [Google Scholar]
- RICKENBERG H. V., YANOFSKY C., BONNER D. M. Enzymatic deadaptation. J Bacteriol. 1953 Dec;66(6):683–687. doi: 10.1128/jb.66.6.683-687.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]