Abstract
Ipilimumab, an anti-cytotoxic T-lymphocyte antigen 4 antibody, was the first therapy demonstrated to improve overall survival in melanoma. Since ipilimumab’s approval by the FDA in 2011, a wealth of data have amassed, helping clinicians to optimize its use. We have learned how to mitigate the adverse effects of ipilimumab, identified its effects in melanoma subpopulations such as those with brain metastases, uveal melanoma, and mucosal melanoma, discovered potential biomarkers of activity, and investigated its use in combination with other therapeutic modalities. These discoveries have paved the way for rapid development of second-generation immunomodulatory antibodies such as inhibitors of the programmed cell death 1 receptor axis. These new agents hold promise as monotherapy, but perhaps the greatest allure lies in the possibility of combining these agents in synergistic multidrug regimens.
Keywords: Ipilimumab, Checkpoint, Immunotherapy, Melanoma, Anti-programmed cell death 1
Introduction
Emerging novel immunotherapies have dramatically reshaped the treatment landscape for metastatic melanoma. Before 2010, the FDA-approved standards of care were dacarbazine and high-dose interleukin-2, neither of which had been shown to improve overall survival [1, 2]. Since then, two independent phase III trials have reported an improvement in overall survival with ipilimumab (Yervoy™, Bristol-Myers Squibb, New York, NY, USA), a fully human monoclonal antibody inhibitor of cytotoxic T-lymphocyte antigen 4 (CTLA-4) [3, 4]. Following these positive phase III studies, ipilimumab became a new standard of care, and was soon joined by vemurafenib, a selective B-Raf inhibitor that improves overall survival among patients with the activating V600E BRAF mutation [5].
Since ipilimumab’s FDA approval, it has become the prototypical immunomodulatory antibody, with which a wealth of clinical data have emerged. However, the past year alone has ushered in multiple second-generation immunomodulatory antibodies. Recently, both programmed cell death 1 (PD-1) and PD-1 ligand 1 (PD-L1) inhibitors have entered the spotlight, with recent phase I clinical trials reporting promising objective response rates with little toxicity [6, 7]. Trailing just behind, numerous other checkpoint agents are being explored in phase I clinical trials with exciting potential. This review will summarize the important updates in the treatment of melanoma with ipilimumab, describe the recent data published on PD-1 and PD-L1 inhibition, and finally, introduce future studies in checkpoint modulation.
Lessons Learned from Ipilimumab
Updated Ipilimumab Experience: Durability and Safety
The phase III registration trial compared ipilimumab at a dose of 3 mg/kg with or without the gp100 peptide vaccine versus gp100 peptide vaccine alone in patients with unresectable stage III or stage IV melanoma [3]. Median overall survival in the ipilimumab and ipilimumab plus gp100 cohorts was 10.1 and 10.0 months, respectively, compared with 6.4 months for the gp100 control arm (hazard ratio 0.68, p < 0.001). The subsequent first-line trial comparing dacarbazine plus placebo with dacarbazine plus ipilimumab at a dose of 10 mg/kg reported overall survival of 9.1 months for dacarbazine alone versus 11.2 months in the combination arm (hazard ratio 0.72, p < 0.001) [4].
The Kaplan–Meier survival curves in these trials illustrate several important points about ipilimumab therapy. First, the survival curves diverged after approximately 4 months. This suggests the benefit of ipilimumab can take some time to develop, and this differs from the survival curves seen in targeted therapy, where an early survival difference has been observed [5]. The curves also reached a plateau, indicating that a subset of patients experience long-term survival, observations underscored by the differences in overall survival at 1 year and 2 years after initiation of treatment.
In addition to improving overall survival, follow-up of these trials has also demonstrated preservation of quality of life while the patient is receiving treatment. Among patients treated in the registration trial, health-related quality of life was assessed at the baseline and at 12 weeks using the previously validated QLQ-C30 questionnaire [8]. With use of this measure, quality of life was not adversely affected by treatment with ipilimumab [9]. Thus, despite the low response rates, ipilimumab stands out as an effective treatment, improving overall survival and producing durable responses, with preservation of quality of life while the patient is receiving treatment.
Although long-term data from the ipilimumab registration studies continue to be analyzed, perhaps the longest-term follow-up data of ipilimumab’s effects are from an analysis of 177 patients treated in early studies of ipilimumab at the National Cancer Institute [10]. Median follow-up in these patients was 92, 84, and 71 months across the three early protocols reported, two evaluating ipilimumab in conjunction with gp100, and another evaluating ipilimumab with interleukin-2 [11–13]. A total of 15 patients experienced complete responses, with 14 of 15 patients experiencing durable complete responses that were ongoing after 54 to 99 months. Some patients who initially achieved a partial response ultimately went on to achieve a complete response. This reverberates the original message that, indeed, a proportion of patients achieve durable disease control, and that patients can experience benefit that may not be evident on first radiographic evaluation [14].
Dosing and Sequencing of Therapy
A randomized phase II study evaluated the influence of ipilimumab dose on response rate [15]. In that study, the best overall response rate (ORR) was 11.1% in the 10 mg/kg arm, versus 4.2% in the 3 mg/kg arm and 0% in the 0.3 mg/kg arm (p = 0.0015). However, the incidence of immune-related adverse events was also higher in the 10 mg/kg group, with 27% versus 10% of patients requiring discontinuation of treatment in the 10 mg/kg and 3 mg/kg arms, respectively. To definitively determine the optimal dose, a phase III randomized trial comparing the two doses (10 mg/kg versus 3 mg/kg) with an overall survival end point is awaiting interim analysis (NCT01515189). The benefit of maintenance ipilimumab administered every 3 months is unknown; however, some limited data suggest that reinduction therapy with four additional doses of ipilimumab administered every 3 weeks is active in patients who progressed after initial response or initial disease stability. In the registration trial, six of 31 reinduced patients (19%) achieved an objective response, with an additional 15 patients (48%) achieving stable disease [3, 16].
With the approval of both ipilimumab and vemurafenib and no head-to-head randomized comparisons of the two drugs, the optimal sequencing is still an unanswered question. One retrospective analysis suggested inferior overall survival when vemurafenib was administered prior to immunotherapy [17]. However, it is our practice to treat BRAF-mutant patients with vemurafenib first when they are symptomatic or have high tumor burden at the baseline, given the greater likelihood of a rapid response with vemurafenib [18]. Additionally, a vemurafenib “induction” strategy is being evaluated for safety in a single-arm phase II trial: patients will receive vemurafenib (960mg per os twice daily) for 6 weeks followed by ipilimumab (four doses of 10 mg/kg), followed by vemurafenib at first progression (NCT01673854).
Ipilimumab and Targeted Therapy: Bevacizumab and Vemurafenib
Preclinical studies indicate that B-Raf inhibitors can increase melanoma antigen expression, decrease secretion of immunosuppressive cytokines, and induce T-cell infiltration of tumor sites while preserving T-cell function [19–21]. Additionally, the vascular endothelial growth factor inhibitor bevacizumab increases dendritic cell maturation, primes T cells, and inhibits maturation of myeloid-derived suppressor cells (MDSCs) [22, 23]. These agents, in combination with checkpoint agents, could enhance immune clearance of tumor. Bevacizumab combined with ipilimumab is currently being evaluated in a phase I trial (NCT00790010). Unfortunately the vemurafenib combined with ipilimumab phase I/II study was stopped early secondary to hepatic toxicity. [24]. Skin reactions were additionally seen in this concurrent regimen which resembled skin toxicity seen when patients were treated with vemurafenib soon after completing ipilimumab therapy [25]. It is likely that scheduling of vemurafenib and ipilimumab will be important to their overlapping toxicity profile. A strategy of vemurafenib “induction” followed by ipilimumab is being prospectively evaluated (NCT001673854).
Ipilimumab and Central Nervous System Metastases
Central nervous system (CNS) metastases occur in more than 50% of patients with advanced metastatic melanoma, and the median overall survival has been reported as 4.4 months [26, 27]. A phase II open-label trial of ipilimumab at a dose of 10 mg/kg in patients with CNS metastases served to determine whether the drug would be effective in this scenario [28]. Two parallel cohorts—neurologically asymptomatic patients not requiring corticosteroids and neurologically symptomatic patients requiring steroids—received induction therapy followed by maintenance infusions every 3 months if clinical benefit was achieved. CNS and non-CNS responses were evaluated by both modified World Health Organization (mWHO) criteria and immune-related response criteria (irRC). The primary end point was disease control at week 12 determined by mWHO criteria (partial response, complete response, and stable disease). The study reported 18% control in asymptomatic patients versus 5% control in patients requiring steroids. Disease control rates within the CNS and outside the CNS were concordant. It is most likely that the reduced response rate and overall survival of the corticosteroid cohort was due to the overall poorer health of this study cohort. A possible detrimental effect of steroids blunting the immune response to ipilimumab, however, could also be involved. On the basis of these data, ipilimumab is a reasonable consideration for treatment of asymptomatic CNS lesions; however, more data are required to validate this approach for treatment of symptomatic lesions.
Ipilimumab and Cytotoxic Chemotherapy
Although cytotoxic chemotherapy may induce lymphopenia and immune suppression, it may also stimulate the immune system by a variety of mechanisms, such as depleting the levels of MDSCs, inducing tumor antigen release, and enhancing T-cell function [29–31]. Therefore, combination regimens with chemotherapy are actively being investigated (Table 1). The initial combination trials included a phase II study showing a trend of increased disease control with ipilimumab plus dacarbazine versus ipilimumab alone [32], and a phase III study demonstrating improved survival of patients receiving ipilimumab plus dacarbazine compared with patients receiving dacarbazine alone [4]. More recently, the Italian phase II NIBIT-M1 trial was conducted that combined ipilimumab at a dose of 10 mg/kg with fotemustine, an alkylating agent that crosses the blood–brain barrier and may prolong the time to progression of CNS metastases [33, 34]. The results were encouraging, with a favorable objective response rate compared with historical rates from the ipilimumab registration trials (29% determined by irRC, compared with 11% [3] and 15% [4] determined by mWHO criteria). Twenty patients with asymptomatic brain metastases were treated, with nine of the 20 patients (45%) experiencing an objective response and ten of the 20 patients (50%) achieving disease control. Similarly, temozolomide was tested in combination with ipilimumab at a dose of 10 mg/kg. An interim analysis demonstrated a best ORR of 28.1% as determined by irRC, with tolerable toxicity and evidence of activity in CNS metastases [35, 36].
Table 1.
Examples of active clinical trials evaluating combination strategies with checkpoint modulation
| Combination | Trial identifier | Phase | Regimen |
|---|---|---|---|
| Checkpoint agents | NCT01714739 | I | Anti-PD-1 + anti-KIR |
| NCT01024231 | I | Dose-escalation ipilimumab + nivolumab | |
| NCT01750580 | I | Ipilimumab + anti-KIR | |
| NCT01844505 | III | Ipilimumab vs nivolumab vs ipilimumab + nivolumab | |
| Chemotherapy | NCT01590082 | I/II | Ipilimumab + doxycycline + temozolomide |
| NCT01676649 | II | Ipilimumab + carboplatin/taxol in melanoma | |
| NCT01323517 | II | Ipilimumab + ILI melphalan/dactinomycin | |
| NCT01740401 | II | Ipilimumab + low-dose cyclophosphamide | |
| Immunotherapy | NCT01701674 | 0 | Ipilimumab + adoptive T-cell therapy + lymphodepletion |
| NCT01629758 | I | Anti-PD-1 and IL-21 | |
| NCT01176461 | I | Anti-PD1 + vaccine + montanide | |
| NCT01489059 | I | Ipilimumab + IL-21 | |
| NCT01838200 | I | Ipilimumab + intralesionally administered BCG | |
| NCT01672450 | I | Ipilimumab + intralesionally administered IL-2 | |
| NCT01750983 | I | Ipilimumab + lenalidomide | |
| NCT01810016 | I | Ipilimumab + NY-ESO-1 vaccine | |
| NCT01103635 | I | Tremelimumab + anti-CD40 (CP-870,893) | |
| NCT01740297 | I/II | Ipilimumab +/− talimogene laherparepvec | |
| NCT01689870 | I/II | Ipilimumab + anti-OX40 | |
| NCT00871481 | I/II | Ipilimumab + antigen-specific T cells | |
| NCT01409174 | I/II | Ipilimumab + biochemotherapy | |
| NCT01743157 | I/II | Ipilimumab + biochemotherapy + bevacizumab | |
| NCT01363206 | II | Ipilimumab + GM-CSF | |
| NCT01708941 | II | Ipilimumab +/ IFNα2b | |
| NCT01134614 | II | Ipilimumab +/− sargramostim | |
| NCT01302496 | II | Ipilimumab + TriMix-DC | |
| Radiotherapy | NCT01703507 | I | Ipilimumab + whole-brain radiotherapy or SRS in brain mets |
| NCT01497808 | I/II | Ipilimumab + stereotactic body radiotherapy | |
| NCT01565837 | II | Ipilimumab + stereotactic ablation in oligometastases | |
| NCT01689974 | II | Ipilimumab +/− radiotherapy | |
| Targeted therapy | NCT01767454 | I | Ipilimumab + dabrafenib +/− trametenib in V600E/K+ |
| NCT00790010 | I | Ipilimumab + bevacizumab | |
| NCT01738139 | I | Ipilimumab + imatinib in c-KIT mutation | |
| NCT01633970 | I | MPDL3280A + bevacizumab +/− chemotherapy | |
| NCT01656642 | I | MPDL3280A + vemurafenib | |
| NCT01604889 | I/II | Ipilimumab +/− INCB024360 |
GM-CSF granulocyte–macrophage colony stimulating factor, IFN interferon, ILI isolated limb infusion, KIR killer inhibitory receptor, PD-1 programmed cell death 1, SRS stereotactic radiosurgery
Ipilimumab and Radiation: The Abscopal Effect
The abscopal effect describes the phenomenon of tumor regression at sites distant from the primary site of radiotherapy. This concept was demonstrated in a melanoma case report published in 1975 [37] and rests on the theory that radiation induces antigen and cytokine release, which subsequently potentiates a systemic immune response to the tumor. In the past year, two case studies have provided additional anecdotal evidence of the abscopal effect, along with associated immunologic correlates. In the first case, a patient with unresectable scalp melanoma and in-transit lesions developed complete response of both irradiated and nonirradiated lesions following external beam radiotherapy [38]. Later, after developing metastases to the skin and brain, he received concurrent ipilimumab and stereotactic brain radiotherapy, which resulted in a complete resolution of skin lesions. In the second case, a female with metastatic disease initially progressed while receiving ipilimumab with worsening disease in the spleen, soft tissue, and lymph nodes. After palliative radiotherapy for the soft tissue lesion, she later experienced disease regression within the irradiated field but more impressively also in areas outside the irradiated field, including the spleen and lymph nodes [39].
In both of these cases, antibody responses to melanoma antigens were evaluated by enzyme-linked immunosorbant assay. The scalp melanoma patient exhibited preexisting antibodies against melanoma antigen A3 (MAGEA3), which increased in titer following ipilimumab therapy with radiotherapy. Following ipilimumab therapy, the patient mounted a new response to PAS domain containing 1 (PASD1), another cancer antigen [38]. In the second patient, a serologic analysis of over 9,000 human antigens revealed ten antigenic targets that exhibited greater than fivefold increase in reactivity following radiotherapy. Additionally, the immunosuppressive MDSC population decreased after radiotherapy.
Although these cases are anecdotally interesting, prospective evaluation is required to fully evaluate the possibility of synergy between radiotherapy and immunotherapy as suggested by these case reports, preclinical models, and the results of a phase I study [15, 40, 41]. Since the dose of radiotherapy likely has immunologic consequences that may be important to consider in combination with immunotherapy, we will soon initiate a randomized, phase II study to evaluate whether high dose per fraction radiotherapy is more effective than conventionally fractionated radiotherapy. Additional prospective trials of the combination of ipilimumab and radiotherapy are ongoing (Table 1).
Ipilimumab and Uveal Melanoma
Ipilimumab has recently been evaluated as a treatment for uveal melanoma, a rare melanoma subtype with a distinct genetic profile and no known systemic therapy conferring survival benefit [42, 43]. A recent single-institution review of patients receiving ipilimumab for treatment of uveal melanoma demonstrated similar response rates and adverse effects compared with those for cutaneous melanoma. At 24 weeks, the response rate determined by irRC was 5% (one of 20 patients with a partial response), with 25% of patients achieving either a response or stable disease. One patient achieved a partial response later, consistent with an immune-related response, making the ORR 10%. These responses were ongoing at the time of publication, and median overall survival was 8.6 months [44]. These findings were confirmed by several smaller series, one of which reported two of five patients with durable stable disease (15 months, more than 12 months) but no objective responses, and another which reported three of 13 patients with durable stable disease (71 weeks, 75 weeks, more than 172 weeks), but no objective responses [45, 46]. In all of the above-mentioned studies, adverse events were comparable to those of patients treated for cutaneous melanoma. These findings provide a rationale for currently accruing prospective studies, including a phase I/II trial investigating ipilimumab in both the adjuvant and the metastatic setting (NCT01585194), as well as a pilot study evaluating sequential radioembolization followed by ipilimumab therapy for treatment of liver metastases (NCT01730157).
Ipilimumab and Mucosal Melanoma
Like uveal melanoma, mucosal melanoma is a rare subtype of melanoma with genetically distinct features [47]. The efficacy of ipilimumab in mucosal melanoma is largely unknown. Recently, an experience of 70 mucosal melanoma patients treated with 3 mg/kg ipilimumab induction under a European expanded access program was reported. The response rate was 6%, with one complete response; however, 23.1% of patients achieved disease control [48]. Our experience with mucosal melanoma in patients treated at Memorial Sloan-Kettering Cancer Center, Dana-Farber Cancer Institute, and Massachusetts General Hospital shows similar response rates, with an irRC ORR of 6% and a disease control rate of 26.7% across 30 patients (unpublished data). This indicates that ipilimumab is a reasonable choice to consider in patients with mucosal melanoma, especially in patients for whom a targetable mutation such as a c-KIT mutation cannot be identified. A prospective trial of ipilimumab for treatment of mucosal melanoma is ongoing (NCT01355120).
Other CTLA-4 Agents: Tremelimumab
Another CTLA-4 antibody, tremelimumab, continues to be investigated in clinical trials. A phase II trial of tremelimumab monotherapy in 251 melanoma patients demonstrated an ORR of 6.6%, with prolonged duration of response among responders ranging from 8.9 to 29.8 months [49]. This prompted a phase III randomized trial of tremelimumab (15 mg/kg) versus dacarbazine or temozolomide [50]. Six hundred and fifty-five patients were enrolled, but after the second interim analysis, the trial was stopped for futility. Final analysis demonstrated a nonsignificant overall survival benefit of 12.6 months versus 10.7 months (hazard ratio 0.88, p = 0.127). Objective responses were equal (10.7% versus 9.8%); however, the median duration of response was longer in patients responding to tremelimumab (35.8 months versus 13.7 months, p = 0.0011). Toxicity was similar to that of ipilimumab, and seven patients (2%) died from treatment-related causes. It is possible that the lack of an overall survival benefit was due to exclusion of patients with elevated LDH levels and the fact that a number of patients in the control arm subsequently received ipilimumab.
Despite the trial’s failure to demonstrate survival benefit, tremelimumab may still hold promise, particularly in combination with other therapeutics. For example, a phase II trial combining tremelimumab with interferon alfa-2b demonstrated a best ORR of 24%, with an additional 38% of subjects experiencing stable disease [51]. Overall survival was 21 months, significantly longer than reported with ipilimumab or tremelimumab monotherapy, with grade III/IV toxicities of neutropenia (17%), diarrhea/colitis (11%), liver abnormalities (11%), rash (11%), fatigue (40%), and anxiety/depression (14%). Moving forward, tremelimumab will be evaluated in combination with the anti-CD40 antibody CD-870,893, as well as in combination with other immunomodulatory therapies (NCT01103635) and as monotherapy in other malignancies.
Biomarkers for Ipilimumab
Despite the improvement in overall survival with ipilimumab, only a minority of patients experience long-term overall survival. Therefore, considerable efforts are ongoing to discover biomarkers that may predict response to ipilimumab and other immunomodulatory agents. One of the first and most thoroughly described potential biomarkers is the absolute lymphocyte count (ALC). Patients with an ALC of more than 1,000 cells per microliter 7 weeks after starting therapy exhibited increased overall survival in a single-institution cohort of patients receiving ipilimumab at a dose of 10 mg/kg [52]. Recently, the ALC was evaluated in 137 patients receiving ipilimumab at the commercial dose (3 mg/kg) and similar findings were obtained. The association between ALC and overall survival 7 weeks into ipilimumab therapy retained significance in a multivariate analysis accounting for LDH, M stage, and number of prior therapies [53]. Further work must be performed to prospectively evaluate this biomarker and determine if it could be appropriately used clinically. One foreseeable hypothesis is that this ALC cutoff could be used to determine whether a patient should continue with the commercial dose of ipilimumab or receive additional therapy or perhaps higher doses of ipilimumab.
Various correlates of cellular and humoral response have been previously examined as possible biomarkers in patients treated with ipilimumab, including antibodies and CD8+ antigen-specific responses to NY-ESO-1, the percentage of CD4+ICOShi cells following treatment, expression of genes involved in immune response, and posttreatment increases the levels of in tumor infiltrating lymphocytes [54–58]. At the annual meeting of American Society of Clinical Oncology in 2012, an additional cellular marker, the percentage of MDSCs (determined as the percentage of cells that are CD14+, HLA-DR−/low among peripheral blood mononuclear cells), was reported as being associated with overall survival in melanoma patients treated with ipilimumab. Low MDSC quantity was associated with improved overall survival (p = 0.002), an effect which was associated with overall survival in a multivariate analysis when accounting for the baseline LDH level [59]. It is quite possible that a high quantity of MDSCs may be a poor prognostic factor in melanoma, regardless of therapy. Most of these biomarker analyses have been retrospective and included only small numbers of patients. Nonetheless, they highlight the potential of immunologic monitoring in patients treated with immunotherapy.
In efforts to produce a mainstream assay to predict response to therapy, an assay that evaluates antibody response to a proprietary panel of melanoma antigens such as BRAF and NY-ESO-1 has been developed. Among 34 patients receiving ipilimumab who were tested with the assay, most of the patients exhibited antibodies to at least one of the antigens tested (22 of 34 patients, 65%). Patients who produced antibodies to at least two antigens exhibited increased overall survival (39.4 weeks versus 16.4 weeks, p = 0.02) [60]. Similarly, expression array analyses of patients receiving ipilimumab were evaluated for potential use as an assay to predict immune-mediated gastrointestinal events. Twenty-seven genes were identified as differentially expressed among patients developing immune-related gastrointestinal toxicities. Expression of two neutrophil activation markers, CD177 and CEACAM1, was associated strongly with gastrointestinal events, as well as several immunoglobulin genes. These findings were confirmed in a validation cohort, indicating that expression of these genes might serve to predict gastrointestinal toxicity [61].
Targeting the PD-1 Axis
PD-1 Blockade
In 2012, preliminary investigations of PD-1 inhibitors came to fruition, demonstrating a strong signal of efficacy and safety. PD-1 signaling serves to regulate T-cell activation in peripheral tissues, limiting autoimmunity and sequelae of chronic inflammation. Inhibition of these interactions can enhance T-cell response in vitro and stimulate antitumor activity in preclinical models [62, 63]. The sentinel anti-PD-1 phase I trial evaluated the safety of nivolumab (BMS-936558), a fully human immunoglobulin G4 (IgG4) blocking monoclonal antibody, against PD-1 [6, 64]. In the most recent analysis, 106 melanoma patients were accrued, receiving doses ranging from 0.1 to 10.0 mg/kg every 2 weeks, with options for maintenance and reinduction dosing in select clinical scenarios. Unlike for ipilimumab, a dose–response correlation was not observed. All doses had acceptable safety, and a maximum tolerated dose was not defined. The objective response rate was 31% (33 of 106 patients), with an additional 6% of patients (six of 106) achieving stable disease lasting 24 weeks or more [64]. Most of the responses were durable for more than 1 year (13 of 18 patients). As with ipilimumab, some patients experienced progression or stable disease before ultimately responding to therapy. Additionally, responses to anti-PD-1 reinduction have been reported [65].
Grade III/IV drug-related toxicities occurred in 14% of patients in the trial, some with potential immune-mediated mechanisms (pneumonitis 1%, diarrhea 1%, and increased alanine aminotransferase/aspartate aminotransferase levels 2%). Frequent grade I/II adverse events included fatigue, anorexia, diarrhea, pruritus, rash, and nausea. Three treatment-related deaths occurred secondary to pneumonitis, which contrasts with ipilimumab’s most frequent life-threatening toxicity of colitis. With additional experience, algorithms to address pneumonitis may mitigate progression to life-threatening pneumonitis, as has been achieved with ipilimumab and colitis [66].
In an unplanned analysis, 42 patients with pretreatment biopsies were evaluated for PD-L1 expression by immunohistochemistry. Patients with tumors expressing PD-L1 had an ORR of 36%, versus 0% among PD-L1-negative patients (p = 0.006) [6]. Despite these data, we emphasize that the currently available assays have not yet been validated and prospectively evaluated. Additionally, PD-L1 and PD-1 expression is dynamic and heterogeneous, and baseline PD-L1 expression might by modified by clinical factors. Further, durable stable disease is felt to be a benefit of therapy, and whether patients with PD-L1-negative tumors achieved durable stable disease has not yet been reported.
An additional anti-PD-1 antibody, lambrolizumab (MK-3475), is currently being investigated. Interim analysis of a phase I trial investigating three dosing regimens revealed a 51% ORR by the irRC among 85 evaluable melanoma subjects. Of these, 9% experienced complete response, and 41% of patients pretreated with ipilimumab achieved an immune-related response (with no complete responses) [67]. Seven grade III/IV adverse events were reported. This trial was followed by an actively accruing phase II trial (NCT01704287) comparing lambrolizumab with the investigator’s choice chemotherapy. Additional agents directed towards PD-1 are actively being investigated, including AMP-224 and CT-011 (NCT01352884, NCT01435369).
PD-L1 Blockade
PD-L1 antibodies are being developed in tandem with PD-1 antibodies. The rationale is similar: antibodies against PD-L1 impede the inhibitory interactions of PD-1 with PD-L1. Notably, PD-1 ligand 2/PD-1 interactions are spared, and PD-L1/CD80 are additionally inhibited [68]. The therapeutic significance of these differences remains to be determined.
A multicenter phase I dose-escalation trial of the PD-L1 antibody BMS-936559 provides the first compelling clinical evidence of efficacy. Two hundred and seven patients were enrolled, of whom 55 were melanoma patients. Objective responses were observed in 17% of patients(nine of 52) with melanoma, including three complete responses. Many responses were durable, with five responses lasting more than 1 year, and an additional 27% of patients achieving stable disease lasting more than 24 weeks [7]. Four trial patients experienced a response as determined by irRC that would be classified as progression by standard RECIST [69]. Benefit was observed across all doses, and a maximum tolerated dose was not achieved up to 10 mg/kg administered every 2 weeks. Grade III/IV adverse effects were observed in 9% of patients treated with the drug, with common toxicities including fatigue, emesis, infusion reaction, and lymphopenia. No treatment-related deaths were reported.
Future Perspectives: Novel Targets and Combinations
Preclinical studies suggest that concurrent blockade of immunologic checkpoints with multiple inhibitory molecules may enhance efficacy [70, 71]. The sentinel clinical trial investigating this strategy is a phase I trial combining ipilimumab with nivolumab, with clinical arms comparing various dose combinations (NCT01024231). Another trial will compare nivolumab and the combination of nivolumab with ipilimumab versus ipilimumab monotherapy (NCT01844505). Sequential monotherapy is also being investigated in a phase II trial, since clinical responses to anti-PD-1 have been observed in patients previously receiving ipilimumab (NCT01783938).
In addition to CTLA-4 and PD-1, numerous other immunologic inhibitory and activating targets have been identified preclinically, many with corresponding therapeutic antibodies that are being investigated in phase I clinical trials (Fig. 1). In murine models, these next-generation checkpoint antibodies appear to work synergistically when delivered in combination [72, 73]. Additionally, multiple other viable immune strategies could be combined with checkpoint modulators, for example, tumor vaccines, cytokine therapy, adoptive T-cell therapy, and biochemotherapy [74–76]. A promising next step is to combine immunomodulatory agents with such strategies (Table 1).
Fig. 1.
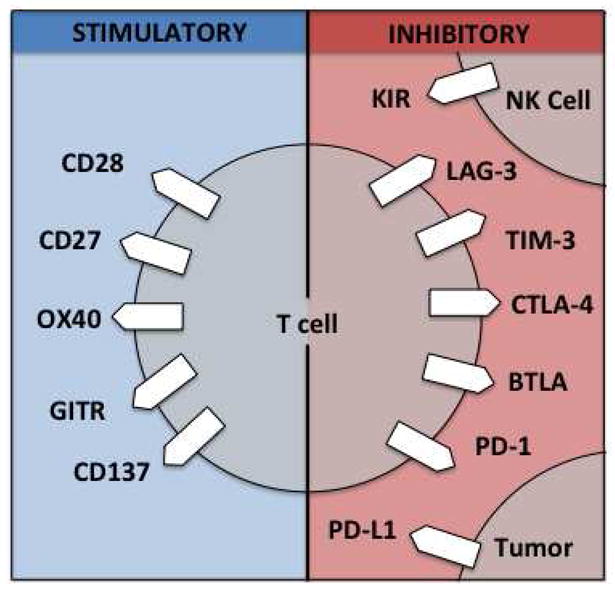
Therapeutic targets for immunoregulatory antibodies. GITR glucocorticoid-induced tumor necrosis factor receptor related protein, NK cell natural killer cell, KIR killer inhibitory receptor, LAG-3 lymphocyte activation gene 3, TIM-3 T-cell immunoglobulin- and mucin-domain containing 3, CTLA-4 cytotoxic T lymphocyte antigen 4, BTLA B- and T-cell attenuator; PD-1 programmed cell death 1, PD-L1 programmed cell death 1 ligand 1
Conclusion
Ipilimumab has paved the way for a host of next-generation immunomodulatory agents. Preliminary data on anti-PD-1 and anti-PD-L1 antibodies demonstrate response rates that exceed those of ipilimumab, with an acceptable toxicity profile. Despite decades of skepticism of immunotherapy, these agents have proven immunotherapy is a viable therapeutic strategy, both in melanoma and in other malignancies. We must continue to investigate this new class of therapeutic agents, and incorporate them into clinical practice with previously existing and novel therapies. We anticipate that work performed in 2013 will reaffirm the clinical utility of anti-PD-1 and anti-PD-L1 therapy, setting the stage for ultimate FDA approval. Additionally, clinical trials combining multiple checkpoint agents will come to fruition, allowing even more novel subsequent combination approaches.
Footnotes
Conflict of Interest
Margaret K. Callahan has received a research grant from Bristol-Myers Squibb.
Michael A. Postow has served on a nonpaid advisory board for Bristol-Myers Squibb and has received a research grant and travel reimbursement from Bristol-Myers Squibb.
Jedd D. Wolchok has been a consultant for Bristol-Myers Squibb and Merck, has received grants from Bristol-Myers Squibb, Merck, and AstraZeneca, and has received travel accommodation from Bristol-Myers Squibb.
David B. Page declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
David B. Page, Email: paged@mskcc.org.
Michael A. Postow, Email: postowm@mskcc.org.
Margaret K. Callahan, Email: callaham@mskcc.org.
Jedd D. Wolchok, Email: wolchokj@mskcc.org.
References
- 1.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 2.Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res. 2000;19(1):21–34. [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. This was the first phase III study to demonstrate an overall survival benefit with therapy in metastatic melanoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26. doi: 10.1056/NEJMoa1104621. This is a phase III study showing improved overall survival with ipilimumab plus dacarbazine compared with a previous standard of care, dacarbazine. [DOI] [PubMed] [Google Scholar]
- 5.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782. This is a phase III trial establishing vemurafenib as a standard of care for metastatic melanoma patients harboring the V600E BRAF mutation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. This is a sentinel phase I trial demonstrating safety and clinical activity for PD-1 blockade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. This is a sentinel phase I trial demonstrating safety and clinical activity for PD-L1 blockade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 9.Revicki DA, van den Eertwegh AJ, Lorigan P, et al. Health related quality of life outcomes for unresectable stage III or IV melanoma patients receiving ipilimumab treatment. Health Qual Life Outcomes. 2012;10:66. doi: 10.1186/1477-7525-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18(7):2039–47. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12(12):1005–16. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maker AV, Yang JC, Sherry RM, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29(4):455–63. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postow MA, Callahan MK, Wolchok JD. The antitumor immunity of ipilimumab: (T-cell) memories to last a lifetime? Clin Cancer Res. 2012;18(7):1821–3. doi: 10.1158/1078-0432.CCR-12-0409. [DOI] [PubMed] [Google Scholar]
- 15.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 16.Robert C, Schadendorf D, Messina M, et al. Efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin Cancer Res. 2013;19(8):2232–9. doi: 10.1158/1078-0432.CCR-12-3080. [DOI] [PubMed] [Google Scholar]
- 17.Ackerman A, McDermott D, Lawrence D, et al. Outcomes of patients with malignant melanoma treated with immunotherapy prior to or after vemurafenib. Paper presented at: 2012 ASCO Annual Meeting; 2012 Jun 1–5; Chicago. [Google Scholar]
- 18.Wolchok J. How recent advances in immunotherapy are changing the standard of care for patients with metastatic melanoma. Ann Oncol. 2012;23(Suppl 8):viii15–21. doi: 10.1093/annonc/mds258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70(13):5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 20.Comin-Anduix B, Chodon T, Sazegar H, et al. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res. 2010;16(24):6040–8. doi: 10.1158/1078-0432.CCR-10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilmott JS, Long GV, Howle JR, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18(5):1386–94. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 22.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang DH, Park JS, Jin CJ, et al. The dysfunction and abnormal signaling pathway of dendritic cells loaded by tumor antigen can be overcome by neutralizing VEGF in multiple myeloma. Leuk Res. 2009;33(5):665–70. doi: 10.1016/j.leukres.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Ribas A, Hodi FS, Callahan M, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368(14):1365–6. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 25.Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med. 2012;366(9):866–8. doi: 10.1056/NEJMc1114329. [DOI] [PubMed] [Google Scholar]
- 26.Bafaloukos D, Gogas H. The treatment of brain metastases in melanoma patients. Cancer Treat Rev. 2004;30(6):515–20. doi: 10.1016/j.ctrv.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181(3):193–201. [PubMed] [Google Scholar]
- 28.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–65. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 29.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 30.Carson WE, 3rd, Shapiro CL, Crespin TR, et al. Cellular immunity in breast cancer patients completing taxane treatment. Clin Cancer Res. 2004;10(10):3401–9. doi: 10.1158/1078-0432.CCR-1016-03. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 32.Hersh EM, O’Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs. 2011;29(3):489–98. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 33.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22(6):1118–25. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 34.Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13(9):879–86. doi: 10.1016/S1470-2045(12)70324-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Patel SG, Hwu WJ, et al. Development of brain metastases in patients with metastatic melanoma treated with ipilimumab plus temozolomide. Paper presented at: 2012 Annual ASCO Meeting; 2012 Jun 1–5; Chicago. [Google Scholar]
- 36.Patel SP, Hwu WJ, Kim KB, et al. Phase II study of the frontline combination of ipilimumab and temozolomide in patients with metastatic melanoma. Paper presented at: 2012 Annual ASCO Meeting; 2012 Jun 1–5; Chicago. [Google Scholar]
- 37.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol. 1975;48(574):863–6. doi: 10.1259/0007-1285-48-574-863. [DOI] [PubMed] [Google Scholar]
- 38.Stamell EF, Wolchok JD, Gnjatic S, et al. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85(2):293–5. doi: 10.1016/j.ijrobp.2012.03.017. This is a case report demonstrating immune correlates of the abscopal effect in a patient treated with ipilimumab and external beam radiotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2—tumor and immunological responses. Sci Transl Med. 2012;4(137):137ra74. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 41.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Augsburger JJ, Correa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009;148(1):119–27. doi: 10.1016/j.ajo.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 43.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363(23):2191–9. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan S, Callahan M, Postow MA, et al. Ipilimumab in the treatment of uveal melanoma: The Memorial Sloan-Kettering Cancer Center experience. Paper presented at: 2012 ASCO Annual Meeting; 2012 Jun 1–5; Chicago. [Google Scholar]
- 45.Khattak MA, Fisher R, Hughes P, et al. Ipilimumab activity in advanced uveal melanoma. Melanoma Res. 2013;23(1):79–81. doi: 10.1097/CMR.0b013e32835b554f. [DOI] [PubMed] [Google Scholar]
- 46.Danielli R, Ridolfi R, Chiarion-Sileni V, et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol Immunother. 2012;61(1):41–8. doi: 10.1007/s00262-011-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327–34. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Vecchio M, Simeone E, Chiarion Sileni V, et al. Efficacy and safety of ipilimumab in patients with pretreated, mucosal melanoma: experience from Italian clinics participating in the European expanded access programme (EAP). Paper presented at: ESMO 2012 Congress; 2012 Sep 28–October 2; Vienna. [Google Scholar]
- 49.Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16(3):1042–8. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 50.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–22. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarhini AA, Cherian J, Moschos SJ, et al. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol. 2012;30(3):322–8. doi: 10.1200/JCO.2011.37.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Postow MA, Yuan J, Panageas KS, et al. Evaluation of the absolute lymphocyte count as a biomarker for melanoma patients treated with the commercially available dose of ipilimumab (3mg/kg). Paper presented at: 2012 ASCO Annual Meeting; 2012 Jun 1–5; Chicago. [Google Scholar]
- 54.Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16(10):2861–71. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108(40):16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan J, Ginsberg B, Page D, et al. CTLA-4 blockade increases antigen-specific CD8+ T cells in prevaccinated patients with melanoma: three cases. Cancer Immunol Immunother. 2011;60(8):1137–46. doi: 10.1007/s00262-011-1011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61(7):1019–31. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kitano S, Postow MA, Cortez C, et al. Myeloid-derived suppressor cell quantity prior to treatment with ipilimumab at 10mg/kg to predict for overall survival in patients with metastatic melanoma. Paper presented at: 2012 ASCO Annual Meeting; 2012 Jun 1–5; Chicago. [Google Scholar]
- 60.Ellis SG, Wheater M, Tier K, et al. Biomarker for benefit from ipilimumab: correlation of breadth of humor tumor-antigen-specific immunity with outcome. Paper presented at: 2012 ASCO Annual Meeting; 2012 Jun 1–5; Chicago. [Google Scholar]
- 61.Shahabi V, Berman D, Chasalow SD, et al. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-mediated gastrointestinal adverse events. Paper presented at: 2012 ASCO Annual Meeting; 2012 Jun 1–5; Chicago. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Topalian SL, Brahmer JR, Hodi FS, et al. Anti-programmed death-1 (PD-1) (BMS-936558/MDX-1106/ONO-4538) in patients with advanced solid tumors: clinical activity, safety, and molecular markers. Paper presented at: ESMO 2012 Congress; 2012 Sep 28–Oct 2; Vienna. [Google Scholar]
- 65.Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 67.Hamid O, Daud A, Robert C, et al. Preliminary clinical efficacy and safety of MK-3475 (anti-PD-1 monoclonal antibody) in patients with advanced melanoma. Pigment Cell Melanoma Res. 2012;25(6):836–903. [Google Scholar]
- 68.Park JJ, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116(8):1291–8. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 70.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19(5):997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 71.Curran MA, Kim M, Montalvo W, et al. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6(4):e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fourcade J, Sun Z, Pagliano O, et al. CD8+ T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72(4):887–96. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ngiow SF, von Scheidt B, Akiba H, et al. Anti-TIM3 antibody promotes T cell IFN-gamma- mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71(10):3540–51. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 74.Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26(35):5748–54. doi: 10.1200/JCO.2008.17.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chi M, Dudek AZ. Vaccine therapy for metastatic melanoma: systematic review and meta-analysis of clinical trials. Melanoma Res. 2011;21(3):165–74. doi: 10.1097/CMR.0b013e328346554d. [DOI] [PubMed] [Google Scholar]
- 76.Bernatchez C, Radvanyi LG, Hwu P. Advances in the treatment of metastatic melanoma: adoptive T-cell therapy. Semin Oncol. 2012;39(2):215–26. doi: 10.1053/j.seminoncol.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


