Abstract
Peptide vaccination against tumor associated antigens (TAA) remains one of the most common methods of immunization in cancer vaccine clinical trials. While peptide vaccination has been reported to increase circulating antigen-specific T-cells, they have had limited clinical efficacy and there is a necessity to increase their capacity to generate strong anti-tumor responses. We sought to improve the clinical efficacy of peptide-based vaccines in cancer immunotherapy of metastatic melanoma using a LHRH-agonist (Leuprolide) as adjuvant. Seventy HLA-A*0201+ Stage IIb-IV melanoma patients were vaccinated with class I HLA-A*0201-restricted gp100209-2M peptide and stratified for HLA-DP4 restriction. HLA-DP4+ patients were also vaccinated with class II HLA-DP4-restricted MAGE-3243-258 peptide. Patients from both groups were randomized to receive 2 doses of Leuprolide or not. Here we report the increase in PBMC TREC levels at week 24 after peptide vaccination which was independent of the Leuprolide treatment. This change was mirrored by a small increase in the TREC-enriched CD8+CD45RA+RO−CD27+CD103+, but not the TREC-enriched CD4+CD45RA+RO−CD31+ T cell population. Serum concentration of two important factors for thymopoiesis was measured: IGF-1 levels were not changed, while a moderate increase in IL-7 levels was noted in the sera of all patients 6 weeks after vaccination. Increased expression of CD127 (IL-7 receptor alpha) at week 24, compared to baseline, was only seen in the CD8+CD45RA+RO−CD27+CD103+ T cell population. Our results suggest that Leuprolide has no effect on thymic output when used as peptide vaccine adjuvant, but IFA-based peptide vaccination may unexpectedly affect the thymus by increasing thymic output of new T cells.
Keywords: LHRH-agonist, melanoma, peptide-vaccination, TREC, IL-7
INTRODUCTION
It is now clear that the immune system can play an important role in antitumor response. Therapies which broadly stimulate the immune system, such as interleukin-2 and anti-CTLA-4, can result in long term complete response in some patients with advanced melanoma.1–3 Although many human tumor-associated antigens (TAA) have been identified in melanoma and other types of cancer, peptide-based vaccines have had limited clinical efficacy, with partial or complete tumor regression being observed in less than 5% of patients, despite inducing immunization in up to 50% of them.4,5 Peptide vaccines have been administered with cytokines (IFN-α, IL-2, IL-12 or GM-CSF) or nonspecific immune adjuvants (e.g. incomplete Freud’s adjuvant like Montanide ISA-51) with the aim of augmenting their immunogenicity, but with limited success.4,5 Our group has experience with a modified synthetic HLA-A*0201-restricted peptide of a melanocytic differentiation antigen (gp100209-2M), that appeared to be more immunogenic when given with incomplete Freund’s Adjuvant (IFA) and IL-2 than when given with IFA alone.6,7 Multipeptide-based anti-melanoma vaccines have also been used to overcome the possible selection and escape of antigen-negative clones, and to elicit both CD4- and CD8-mediated immune recognition.5 A class II HLA-DP4-restricted tumor-specific shared antigen peptide, encoded by the cancer-germline gene MAGE-3 (MAGE-3243-258), has been shown to be immunogenic and elicit a peptide-specific immune response.8 Despite these efforts, multi-peptide vaccination has also shown low clinical efficacy.5 Moreover, the possible effects of peptide vaccination on thymic activity have not been described.
One potential cause for the relative lack of efficacy for peptide vaccines may be the increasingly diminished activity of the thymus with age. The thymus is the immune organ where immature thymocytes derived from bone marrow stem cells undergo a series of thymic-dependent maturation steps to become antigen-specific naïve T cells. The progressive decline in thymus structure and function with age coincide with the increase of sex steroid production around puberty.9 The consequence of this atrophy is the decline of thymic input to the peripheral T cell pool,10 a limited TCR repertoire,11 altered cytokine profile12 and a bias towards memory as opposed to naïve T cells.13 Despite the atrophy of the thymus that comes with age, it still contains all the essential stromal elements to allow thymocytes to differentiate normally, although with a reduced efficiency. Proof of this comes from studies showing that the adult thymus contributes to immune reconstitution in patients after autologous or allogeneic hematopoietic stem cell transplantation14,15 and HIV infection.16
It has been shown that ablation of sex hormones by a LHRH (luteinizing hormone-releasing hormone) agonist treatment can restore age-related decline of the number of developing thymocytes in the thymus of mice17 and humans17,18 by desensitization of the pituitary gland and gonadal atrophy.19
In this study, we utilized an LHRH-agonist treatment to enhance thymic melanoma antigen-specific T cell output in combination with multi-peptide vaccination with peptides gp100209-2M and MAGE-3243-258 to try to induce melanoma specific CD8+ and CD4+ T cell activity. Our results showed that there is no effect of the LHRH agonist on vaccination rates, but suggest a possible effect of peptide vaccination on thymic activity.
MATERIAL AND METHODS
Patients
The 70 patients enrolled in this study had to provide a written informed consent and meet the following criteria: HLA-A*0201 positive; age ≥ 18 years with histologically documented diagnosis of stage IIb-IV melanomas and rendered clinically free of disease after surgery; White Blood Cell Count (WBC) ≥ 3000/mm3; Platelet count ≥ 90,000mm3; Serum creatinine ≤ 2.0mg/dl; Serum Alanine Aminotransferase (ALT) ≤ 3 X upper limit of normal (ULN); to be seronegative for HIV antibody; total bilirubin ≤ 2X ULN, except for patient with Gilbert’s syndrome who had to have a total bilirubin less than 3.0mg/dl. Women who have menstruation in the past 12 months and without sterilization surgery had to have a negative pregnancy test.
Patients were ineligible if they had prior systemic therapy (including immunomodulatory agents), radiation or surgery requiring general anesthesia for melanoma within 28 days of starting study treatment, or had autoimmune diseases, concurrent systemic or inhaled steroid therapy, any form of active primary or secondary immunodeficiency, history of immunization with gp100 or MAGE-3, had received a LHRH-agonist within the past 5 years, used oral contraceptive, hormone replacement therapy or androgen preparations, had shown hypersensitivity to gonadotropin-releasing hormone analogues or had active systemic infections requiring intravenous antibiotics.
Patients were also excluded if they had a prior malignancy, with exception of patients that has been disease-free for 5 years.
Study Design and treatment
We assessed the effects of Leuprolide on thymic activity in melanoma patients, and the corresponding effects on the ability to immunize against peptides from the melanoma antigen gp100 (class I HLA-A*0201-restricted peptide gp100209-2M) and class II DP4-restricted peptide MAGE-3243-258, derived from the MAGE-3 cancer-testis antigen.
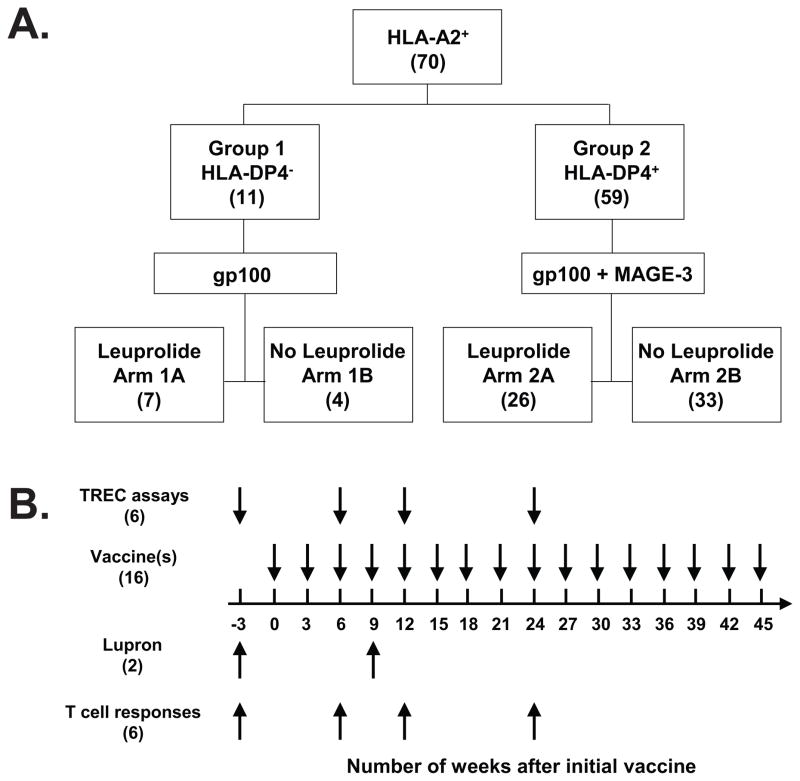
This is a phase II randomized study in patients with stage IIb-III melanoma who have undergone surgical resection with curative intent, and stage IV patients whose metastatic lesion(s) has been removed surgically. Clinically all patients were rendered free of disease (NED) at the study entry. Patients were screened for their HLA types and only those with an HLA-A*0201 positive allele were eligible. All HLA-A*0201 positive patients were assigned into 2 groups based on their DP4 status: Patients in Group 1 (DP4 negative) received the gp100 vaccine. Patients in Group 2 (DP4 positive) received both the gp100 and MAGE-3 vaccines. Within each group, patients were randomized to either receive Leuprolide (Arm A) or not (Arm B) in addition to the vaccine (FIG. 1A).
FIGURE 1. Randomization of patients, treatment schema and time points for laboratory evaluations.
All patients entering the trial were HLA-A*0201+. Patients were stratified by whether they were HLA-DP4+ or HLA-DP4−; Group 1 consisted of patients who were HLA-DP4−, while Group 2 consisted of patients who were HLA-DP4+. Within these groups, patients were randomized in a 1:1 ratio to receive Leuprolide or not (A). A schematic summary of treatments and time points for laboratory evaluations is also shown (B). The number in parenthesis is the total numbers of treatments and immunological evaluations for each patient.
For patients receiving Leuprolide in Arm A, a 3-month 11.25 mg sustained-release formulation of Leuprolide (Lupron Depot®) was administrated intramuscularly approximately every 12 weeks for a total of 2 injections. Patients received the first injection of Leuprolide (Lupron Depot®) at −3 weeks (FIG. 1B).
gp100 and MAGE-3 vaccines were administrated subcutaneously in extremities. For patients in Group 2, gp100 and MAGE-3 peptide vaccines were administrated subcutaneously in separate extremities, each peptide consists of two separate injections, with each injection being 1.0 ml in volume. All vaccines were given at an interval of 3 weeks (FIG. 1B). For those patients receiving Leuprolide, the first vaccination(s) were given 3 weeks following the first Leuprolide injection at time 0 (FIG. 1B). In order to boost reactivity in the same lymph node, the same peptide was injected into the same extremity with subsequent cycles. Because T-cells are stimulated in lymph nodes, extremities that had not undergone lymph node dissection were used for vaccine injections. All patients received peptide vaccines for a total of 48 weeks (32 injections for each vaccine).
Flow Cytometry Analysis
Multiparameter flow cytometric analysis of different cell subsets was performed using Frozen/thawed PBMC from patients at different time points. For the analysis of antigen-specific T cells (gp100/MAGE-3), cells were stained with class I HLA-A*0201-restricted gp100209-2M APC-labeled tetramer (Beckman Coulter, CA), and class II HLA-DP4-restricted MAGE-3243-258 PE-labeled multimer.8 The net tetramer/multimer increase was calculated by subtracting the net baseline frequency (baseline frequency – Control tetramer frequency) from the net frequency at any given time point (time point frequency – Control tetramer frequency at that time point). The control for the multimer assay was a staining without the multimer. Cells were also stained with anti-CD11c/CD14/CD15/CD16/CD19 FITC (dump channel), CD4 PerCP-Cy5.5 and CD8-APC/Cy7.
For the analysis of TREC-enriched T cell subsets (CD4+CD45RA+RO−CD31+ and CD8+CD45RA+RO−CD27+CD103+), cells were stained with anti-CD4-AmCyan, CD8-Pacific Blue, CD45RA-FITC, CD103-PE, CD127-PerCP-Cy5.5, CD45RO-PE-Cy7, CD27-APC-H7 and CD31-Alexa Fluor 647.
For the analysis of T regulatory cells (CD4+CD25highFoxp3+), cells were stained with anti-CD4-PerCP-Cy5.5, Foxp3-Pacific Blue, CD25-FITC, CD3-PE and Aqua (Live/dead staining, Life Technologies, NY). All antibodies, except for FoxP3-Pacific Blue (eBioscience, CA), are from BD Biosciences. The acquisition was carried out on a FACS Canto II (BD Biosciences, CA). All analysis was done with the software FlowJo (Tree Star, OR).
Quantitative real-time PCR for TREC
Quantitative real-time PCR for TREC was performed following a protocol previously described.20 In brief, One million snap-frozen PBMC were lysed overnight (up to 18 hours) at 56°C in lysis buffer (LB)20 and heat inactivated 10 minutes at 95°C. DNA was purified by phenol-chloroform extraction. The DNA was precipitated using a 0.2x volume of 7.5 M ammonium acetate and a 2x volume of cold 100% ethanol. The DNA pellets were dried down and reconstituted in 35 μL of Tris-EDTA (TE) buffer and stored at −20°C until use in the real-time PCR TREC assay.
Thymic function was assessed using the method of Harris et al.21 Delta-deletion TRECs were amplified and quantified in a Biorad iCycler iQ Real-Time Detection System (BioRad Laboratories, Hercules, CA) using fluorescently labeled oligonucleotides as reporter probes in a 50 μL PCR reaction using 2X iQ Supermix (with additional MgCl2 to a final 3.5 mM concentration) (Biorad). Primers for the TREC sequence were 5′-CCC TTT CAA CCA TGC TGA CAC-3′ (forward) and 5′-GGG TGC AGG TGC CTA TGC-3′ (reverse), and the probe was 5′-FAM-TCT GGT TTT TGT AAA GGT GCC CAC TCC TG-BHQ-1-3′. TREC abundance was normalized to input cell number by a parallel amplification for the β-globin gene. Human β-globin primers were 5′-GAA GAG CCA AGG ACA GGT ACG-3′ (forward) and 5′-CCT GGG AGT AGA TTG GCC AA-3′ (reverse), with the probe 5′-FAM-CTG TCA TCA CTT AGA CCT CAC CCT GTG-BHQ1-3′. Primers (all from Sigma-Genosys, St Louis, MO) were used at 10 pmol per reaction well, and probes (both from Biosearch Technologies, Novato, CA) were used at 5 pmol per reaction well. All standards were run in duplicate, and all samples were run in triplicate.
ELISA
ELISA kit for human IGF-1 was obtained from R&D Systems (Minneapolis, MN). Multiplex cytokine kits for human IL-7, IL-2 (for serum) and Th1/Th2 cytokines (IFN-γ, IL-2, IL-4, IL-5, IL-10, IL-12p70 and IL-13; for tissue culture) were obtained from Meso Scale Discovery (MSD, MD). Each kit was used according to the manufacturer’s instructions.
Statistical methods
To study the Leuprolide effect on the generation of tumor-specific T cells and antigen-specific cytokine secretion, we used a 2-tailed Mann-Whitney test. To compare different time points within patients, a 2-tailed Nonparametric Wilcoxon signed rank test was used. Statistical tests were performed with Prism 4 software (San Diego, Calif), and P < 0.05 was considered significant.
RESULTS
Patient Demographics and Vaccination Schedule
Between November 2005 and December 2008, 70 eligible patients underwent class I HLA-A*0201-restricted gp100209-2M and class II HLA-DP4-restricted MAGE-3243-258 peptide (for DP4 positive patients) vaccinations together with or without sex steroid ablation treatment with luteinizing hormone-releasing hormone (LHRH) agonist Leuprolide. All patients were HLA-A*0201, with fifty nine of them being HLA-DP4 as well (FIG. 1A). No patient had prior chemotherapy. All patients received the gp100 peptide vaccine, whereas only patients that were HLA-DP4+ received the MAGE-3 peptide vaccine (FIG. 1A). The schedule of peptide vaccination and Leuprolide treatment is shown in FIG. 1B. The primary end point of this clinical study was the comparison of tumor-specific immune responses to melanoma-specific peptide vaccines, gp100 and MAGE-3 in the presence or absence of Leuprolide. Secondary endpoints were the evaluation of the enhanced thymic activity measured by T-cell receptor excision circle (TREC) analysis and flow cytometric analysis following peptide vaccination and Leuprolide treatments. The median (range) age of the patients was 52 (23 to 85) years. Patients recruited had histologically documented diagnosis of stage IIb-IV melanomas and were clinically rendered free of disease after surgery. Forty seven patients were male, and 23 were female. Eight patients had American Joint Committee on Cancer (AJCC) stage IIB disease, five patients had stage IIC, four patients had stage IIIA, ten patients had stage IIIB, thirty patients had stage IIIC and thirteen patients had stage IV disease.
Peptide vaccines were administered at an interval of 3 weeks for a total of 48 weeks (FIG. 1B). For those patients receiving Leuprolide, it was administrated approximately every 12 weeks for a total of 2 injections starting at week −3, and the first vaccination(s) was given following the first Leuprolide injection at time 0 (FIG. 1B).
Most of the treatment-related Adverse Events (AE; National Cancer Institute Common Toxicity Criteria, version 3) were grade 1. Only a few AEs were grade 2: Hot flashes (3/70; 4.3 %), sweating (2/70; 2.9 %), mood alterations (1/70; 1.40 %) and Fatigue (2/70; 2.9 %) in the whole population; libido (1/47; 2.1 %) and gynecomastia (4/47; 8.5 %) in males. No treatment-related grade 4 or 5 AEs occurred.
Leuprolide treatment does not increase peptide-specific T cell frequency after vaccination
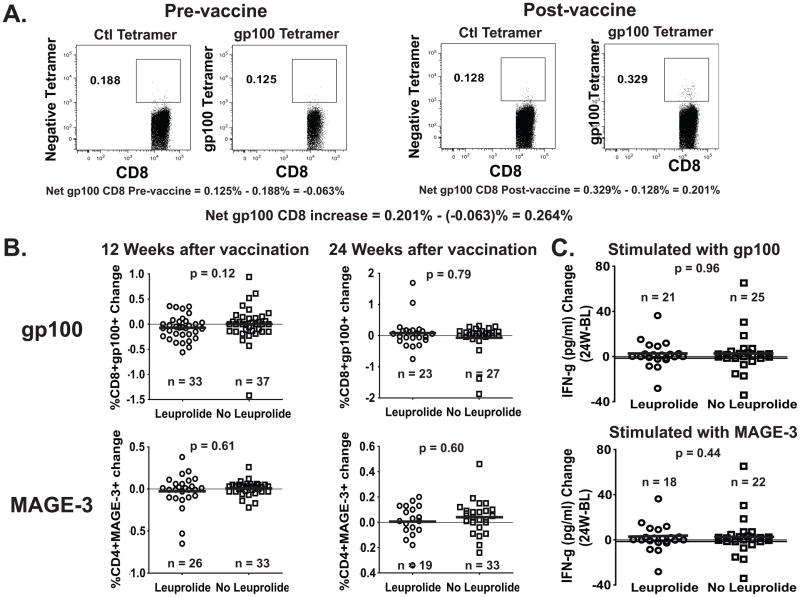
Paired samples from cryopreserved patient peripheral blood, with a viability of at least 80% were obtained at different time points before and after Leuprolide and vaccine treatments (FIG. 1B). Using tetramer staining, we analyzed the samples from treated patients before therapy (Week −3; Baseline) and on weeks 12 (70 patients) and 24 (50 patients) after treatment for percentages of CD8+ cells capable of recognizing gp100 peptide compared to an HIV control peptide. Similarly, using a class II multimer, we evaluated CD4+ T-cells capable of recognizing MAGE-3 peptide.8 As shown in FIG. 2A, a series of calculations were made to determine the specificity of the response, whether a relevant antigen-specific response was taking place at each time point, and whether there was a significant change at each particular time point relative to the baseline sample at Week −3. FIG. 2B shows there was no significant difference in the percentage change of CD8+ gp100 tetramer+, or CD4+ MAGE-3 multimer+ T cells at 12 or 24 weeks after vaccination when compared to the baseline, between patients treated with Leuprolide and patients who received the vaccine only (means of tetramer/multimer percentage change between Leuprolide/No Leuprolide are: gp100 week 12: −0.072/0.003; gp100 week 24: 0.080/−0.069; MAGE-3 week 12: −0.027/0.007 and MAGE-3 week 24: 0.005/0.040). Additionally, mean fluorescence intensity for the gp100+CD8+ and the MAGE-3+CD4+ T cells was not different between the two groups (data not shown). Moreover, separation of patients by gender did not reveal any gender-specific difference in the percentage change of CD8+ gp100 tetramer+ or CD4+ MAGE-3 multimer+ T cells after Leuprolide treatment (Supplemental Digital Content 1). Functionally, FIG. 2C shows that overnight in vitro stimulation of PBMCs with the same gp100 or MAGE-3 peptides used during vaccination did not yield any significant difference in the percentage change of IFN-γ secretion at 24 weeks after vaccination when compared to the baseline, as measured by multiplex cytokine assay. This assay did not show any significant difference in the percentage change of secretion of the other cytokines tested (IL-2, IL-4, IL-5, IL-10, IL-12p70 and IL-13; data not shown).
FIGURE 2. Change in Frequency of gp100209-2M –specific CD8+ and MAGE-3243-258 –specific CD4+ T cells between baseline and weeks 12 or 24 after treatment, measured by tetramer (CD8) and multimer (CD4) staining assay.
Frozen/thawed PBMC from patients at different time points were stained with class I HLA-A*0201-restricted gp100209-2M tetramer (A) and class II HLA-DP4-restricted MAGE-3243-258 multimer (not shown). How the net tetramer/multimer calculation is carried out is represented (A). Cells were stained with anti-CD11c/CD14/CD15/CD16/CD19 FITC (dump channel), MAGE3 Multimer PE, CD4 PerCP-Cy5.5, gp100 Tetramer-APC and CD8-APC/Cy7. The numbers in (B) represent the net change of gp100209-2M –specific CD8+ and MAGE-3243-258 –specific CD4+ T cell subsets within the CD8 and CD4 T cell population, respectively, at 12 and 24 weeks after vaccination. The numbers in (C) represent the net change of IFN-γ secretion after O/N stimulation of patients’ PBMC with peptides gp100209-2M or MAGE-3243-258 at 24 weeks after vaccination when compared to the Baseline (W24-BL).
TREC levels were increased in all patients, independent of Leuprolide treatment
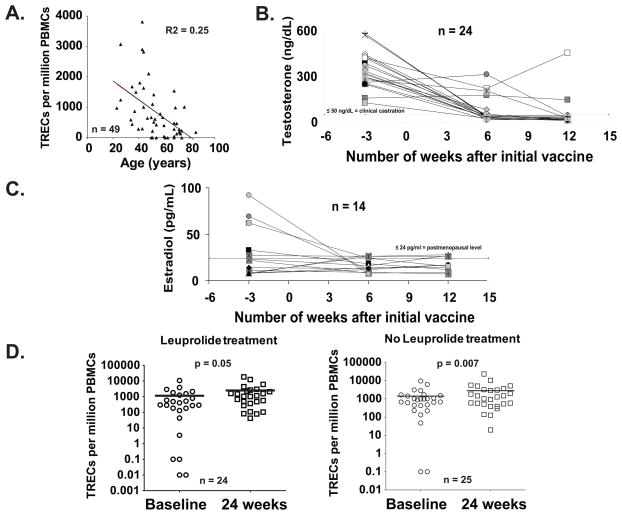
Forty nine of seventy patients were analyzed for T-cell receptor excision circle (TREC) content in their peripheral blood. It has been previously reported that Leuprolide treatment increases thymic output of naïve T cells in hemopoietic stem cell transplant recipients18 and in prostate cancer patients,17 as evidenced by the increase of TREC levels in the blood. The analysis of the samples before therapy showed the expected decrease of TREC levels in the blood with age (FIG. 3A).10 The Leuprolide treatment was effective in inhibiting the signaling of LHRH, reducing the production of testosterone in men to clinical castration levels (50 ng/dL, FIG. 3B) and estradiol in women to postmenopausal levels (24 pg/ml, FIG. 3C) in sera.
FIGURE 3. Number of molecules of TRECs per million of PBMC detected in the blood of patients increase during the course of the intervention independently of Leuprolide treatment.
Molecules of TREC in patients from age 23 through 85 yr (median = 52; n = 50) were analyzed by QC-PCR. The number of molecules of TRECs detected per million of PBMC in the blood of patients at baseline decreases with age (A). Analysis of blood testosterone levels in men (B) and estradiol levels in women (C) after Leuprolide treatment. Most men had testosterone levels compatible with clinical castration (50 ng/dL), whereas almost all women had postmenopausal estradiol levels (24 pg/ml), after 2 doses of Leuprolide. At 24 weeks post-vaccine, most patients showed an increase in total TREC cells/million PBMC compared with pretreatment, independently of Leuprolide treatment (D). In 18 of 24 patients for the Leuprolide-treated, and 15 of 25 for the non-Leuprolide group, changes were deemed substantial (>25% increase).
A comparison between TREC levels at baseline and 24 weeks after initial vaccine showed there was a Leuprolide-independent increase in TREC levels in the blood of both Leuprolide treated and untreated groups at week 24 (FIG. 3D; means of TREC/million PBMCs between Baseline/Week 24 are: Leuprolide treatment: 1,145/2,482; No Leuprolide treatment: 1,421/2,815). This result suggests that the vaccination, and not Leuprolide treatment, may be stimulating thymic activity, possibly masking the effect of the Leuprolide treatment during peptide vaccination.
The Frequency of T regulatory cells does not change after Leuprolide treatment and vaccination
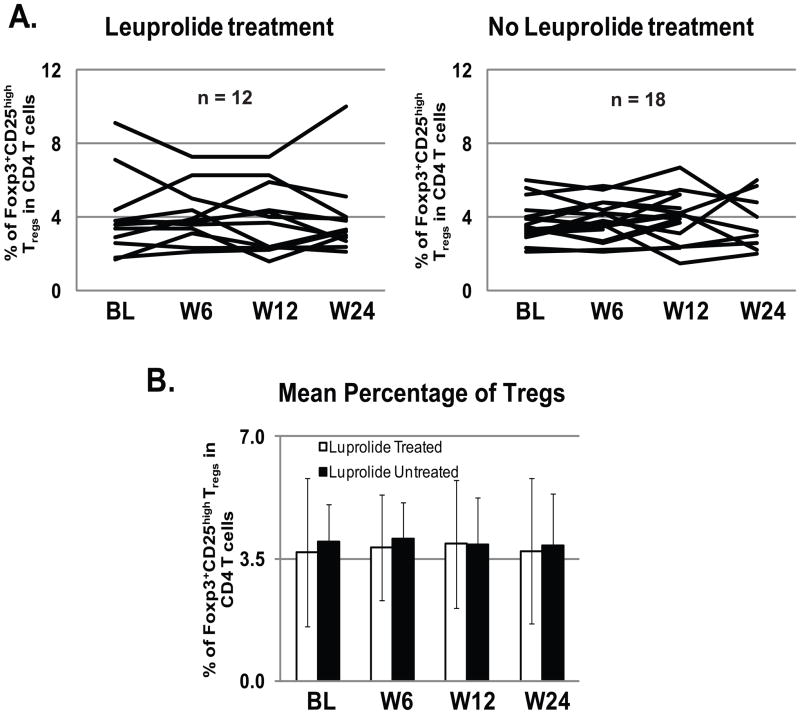
It has been shown that castration in C57BL/6 mice increased splenic Tregs after immunization with a murine sarcoma cell line, causing a decline in frequency and function of tumor-specific CD8 T cells.22 To study the role of Tregs in the lack of effect of Leuprolide on peptide-specific T cell frequency after vaccination, thirty patients were analyzed for T regulatory cell frequency in the blood at baseline and at weeks 6, 12 and 24 after vaccination, with (12 patients) or without (18 patients) Leuprolide treatment (FIG 4). The data shows that Treg frequency in the CD4 T cell compartment does not significantly change between Baseline (before Leuprolide treatment and vaccination) and weeks 6, 12 and 24, independently of whether the patients received Leuprolide or not (FIG. 4A). This is more evident when comparing the average of Treg frequency per time point between Leuprolide treated and not treated patients: the mean Treg frequency is very similar (around 4% CD4+CD25highFoxp3+ of the total CD4 T cells) between the two groups (FIG. 4B). IL-2 has been shown to be the most important cytokine for Treg expansion and survival.23,24 We were unable to detect any major change in the levels of IL-2 in the serum of 50 patients after 24 weeks after peptide vaccination (25 Leuprolide treated and 25 non treated; with a detection limit = 0.31 pg/mL; data not shown).
FIGURE 4. The Frequency of T regulatory cells does not change in patients after concomitant Leuprolide treatment and peptide vaccination.
Multiparameter flow cytometric analysis of CD4+CD25highFoxp3+ Tregs was performed using Frozen/thawed PBMC from 30 patients during peptide vaccination (12 treated with Leuprolide and 18 non treated) at Baseline, week 6, 12 and 24. Cells were stained with anti-CD4-PerCP-Cy5.5, Foxp3-Pacific Blue, CD25-FITC, CD3-PE and Aqua. Treg levels did not significantly change throughout the time course of the vaccination +/− Leuprolide (A). The mean frequency of CD4+CD25highFoxp3+ Tregs was around 4% of the total CD4 T cells, independent from the Leuprolide treatment (B).
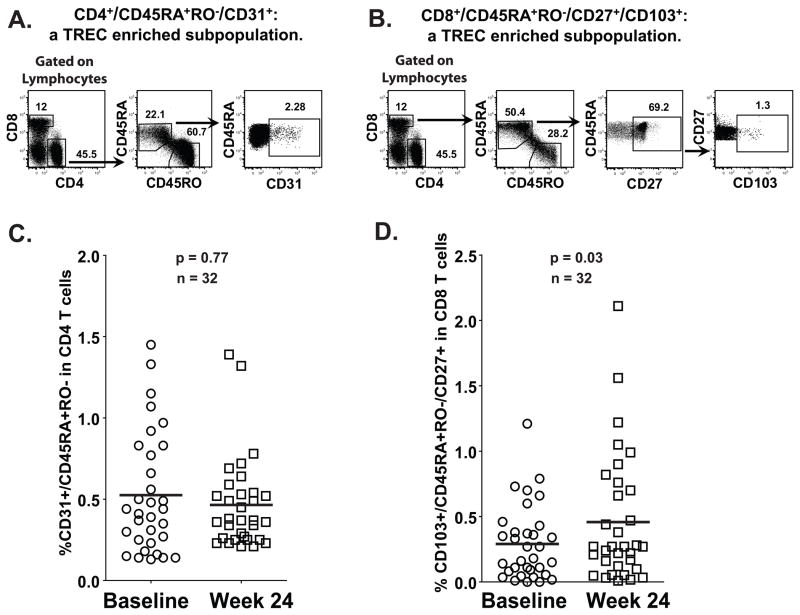
TREC level increase at week 24 after treatment correlates with the increase of a TREC-enriched CD8+ T cell population, but not the equivalent CD4+ T cell population
It has been reported that specific CD8+ and CD4+ T cells subpopulations are enriched for TREC content. In the case of CD4+ T cells, TRECs are almost exclusively confined to CD31+ cells, more specifically, to the naïve CD4+CD45RA+RO−CD31+ T cells.25 Therefore, this subpopulation depicts the CD4+ Recent Thymic Emigrants (RTE) (gating strategy is shown in FIG. 5A). Likewise, in the CD8+ T cells, CD103+ is expressed in a subpopulation with relatively high TREC levels,26 and is differentiated from circulating CD103+ mucosa-associated memory T cells by its naive T cell phenotype: CD8+CD45RA+RO−CD27+CD103+. This CD8+CD103+ naive subset constitutes a major population of RTE (gating strategy is shown in FIG. 5B). In order to determine the relative contribution of CD4+ and CD8+ T cells to the increase in TREC levels observed at week 24 after treatment, these subpopulations were analyzed by flow cytometry. Thirty two patients for which TREC data was available and had enough cells were used to perform the staining and analysis. As shown in FIG. 5C, the increase on TREC levels seen on week 24 after treatment was not mirrored by an increase in naïve CD4+CD45RA+RO−CD31+ T cells, but rather by an increase of naive CD8+CD45RA+RO−CD27+CD103+ T cells (FIG. 5D; mean of percentage of CD4+CD45RA+RO−CD31+ in CD4 T cells or CD8+CD45RA+RO−CD27+CD103+ in CD8 T cells between Baseline/Week 24 is: 0.53/0.47 and 0.29/0.46, respectively). Therefore, it appears that CD8+ recent thymic emigrants were the major population responsible for the increase in TREC levels following immunization.
FIGURE 5. The TREC-enriched CD8+CD103+ naive CD8+ T cell subset, but not the TREC-enriched CD4+CD31+ naive CD4+ T cell subset, increased at week 24 after treatment in both Leuprolide treated and untreated patients.
Multiparameter flow cytometric analysis of CD4+CD45RA+RO−CD31+ and CD8+CD45RA+RO−CD27+CD103+ subsets was performed using Frozen/thawed PBMC from 32 patients at different time points. Cells were stained with anti-CD4-AmCyan, CD8-Pacific Blue, CD45RA-FITC, CD103-PE, CD127-PerCP-Cy5.5, CD45RO-PE-Cy7, CD27-APC-H7 and CD31-Alexa Fluor 647. The gating strategy to study CD4+CD45RA+RO−CD31+ (A) and CD8+CD45RA+RO−CD27+CD103+ (B) subsets is shown. A comparison of CD4+CD45RA+RO−CD31+ (C) or CD8+CD45RA+RO−CD27+CD103+ (D) subpopulations between baseline and week 24 post-treatment of all patients shows that only an increase in the CD8+CD45RA+RO−CD27+CD103+ subpopulation correlates with the increase of TREC levels in PBMC.
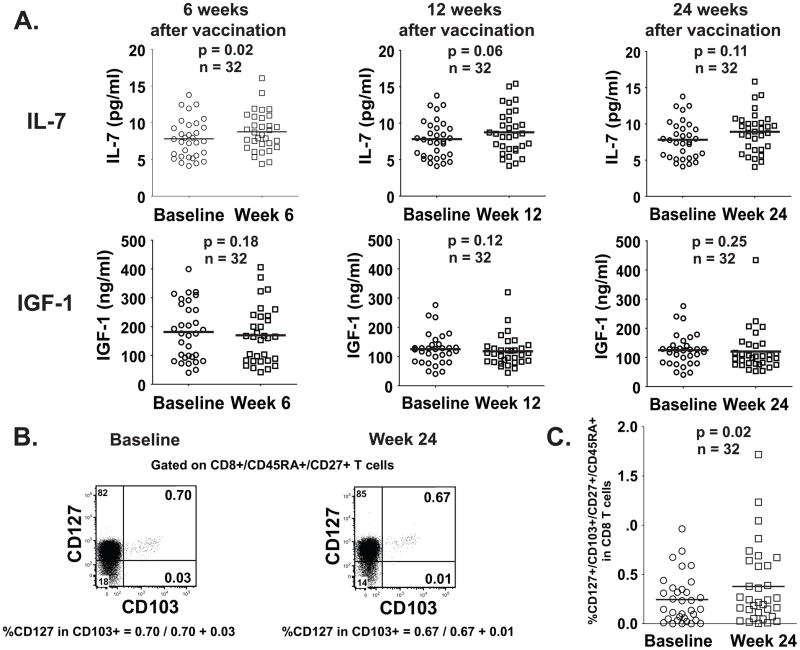
IL-7 levels in patient’s sera increase at week 6 after treatment
Several research groups have focused on the factors that influence thymic activity, especially on factors which increase thymic output. Special attention has been placed on cytokines such as IL-7,27 and growth factors such as Growth Hormone (GH),28 Keratinocyte Growth Factor 1 (KGF-1)29 and Insulin Growth Factor 1 (IGF-1).30 IL-7 in particular, has been shown to increase thymocyte proliferation and directly enhance TREC generation.31
We therefore set to analyze the sera from the same thirty two patients we used to identify the increase of CD8+CD103+ naive subset after treatment, and measured the levels of IL-7 and IGF-1 at weeks 6, 12 and 24 after vaccine (FIG. 6). We found that IL-7 serum levels showed a statistically significant increase from baseline to week 6 (mean pg/ml of IL-7 in sera for Baseline/Week 6 = 7.81/8.76; p=0.015), and marginally significant to week 12 (mean pg/ml of IL-7 in sera for Baseline/Week 12 = 7.81/8.75; p=0.058), but did not reach statistical significance at week 24 after treatment, although it showed the same trend (mean pg/ml of IL-7 in sera for Baseline/Week 24 = 7.81/8.90; p=0.1141) (FIG. 6A). Serum levels of IGF-1 showed no difference with baseline throughout the same period of time (FIG. 6A). Both T cell subsets, CD8+CD45RA+RO−CD27+CD103+ (FIG. 6B and C) and CD4+CD45RA+RO−CD31+ (not shown) expressed CD127 (IL-7 receptor alpha), rendering them potentially susceptible to slightly higher IL-7 levels in serum. Increased expression of CD127 at week 24, compared to baseline, was only seen in CD8+CD45RA+RO−CD27+CD103+ T cells. (FIG. 6C).
FIGURE 6. Serum levels of IL-7, but not those of IGF-1, increase at week 6 after treatment.
The levels of IL-7 and IGF-1 in the sera of the same thirty two patients used to identify the increase of the CD8+CD103+ naive subset were determined using ELISA (A). The percentage of expression of CD127 was determined by flow cytometry with the same gate strategy used in FIGURE 4B and using the quadrants depicted (B). The percentage of CD127+CD45RA+RO−CD27+CD103+ in CD8 T cells was calculated by multiplying the percentage of CD127+ cells in CD103+ (calculated as shown in B) by the percentage of CD8+CD45RA+CD27+ T cells. The graphic shows the result from these calculations for all 32 patients at baseline and at week 24 after treatment (C).
DISCUSSION
The ability to mount an immune response against TAAs is paramount to achieve an immune-mediated antitumor response. Traditionally, one of the most popular methods to induce TAA-specific T cell responses is peptide immunization, due to the number of different TAAs that have been identified and the lack of toxicity associated with this method of immunization.6 Melanocytic differentiation antigens such as tyrosinase, MART-1 (Melan-A) and gp100 have been identified as being over-expressed in cutaneous melanoma. Other TAAs, including MAGE-3 (Melanoma AntiGEn), are not only expressed in melanoma, but also in a number of other cancers. While relatively good immunization rates with peptides derived from these antigens have been achieved, clinical efficacy has been limited at best.4,5 Clinical trials carried out transferring melanoma specific T infiltrating lymphocytes into melanoma patients have shown the importance of melanoma-specific T cell frequency and cell numbers to achieve positive clinical response.32 Since the thymus diminishes its output to the periphery with age, we aimed to restore its production and increase melanoma-specific T cell frequency by ablating sex hormones with a LHRH agonist (Leuprolide; LHRH-A) treatment together with melanoma-specific multipeptide vaccination in a randomized clinical trial. Sex hormones ablation can restore age-related decline of the number of developing thymocytes in the thymus.17,18
We have shown that concomitant blockade of sex steroids and multipeptide vaccination (gp100209-2M and MAGE-3243-258) results in no significant increase in antigen-specific T cell frequency at weeks 12 and 24 after vaccination. A blood sample for TREC analysis between the first dose of Leuprolide (week −3) and the first peptide vaccination dose (Week 0) was not included in the design of this clinical trial. We cannot rule out the possibility that Leuprolide had an effect on thymic output in those three weeks, and that peptide vaccination may have reversed that effect.
Although differences in thymic output between males and females have been demonstrated33,34, gender does not seem to affect the outcome after vaccination and LHRH-agonist treatment. Moreover, the functionality of vaccine-generated antigen-specific T cells was unchanged, as IFN-γ secretion after peptide stimulation, both by Multiplex cytokine assay and Intracellular Staining (data not shown) was not perturbed by Leuprolide treatment.
A mouse model of immunization and castration suggested that after an initial increase of frequency and effector function of antigen-specific CD8 T cell response, a decline in this response was due to a concomitant increase in T regulatory cell frequency 22. Our results indicate that Treg levels remain constant from Baseline (week −3; before vaccination and chemical castration) through week 24 after vaccination. Moreover, IL-2 serum concentration was undetectable throughout the same period of time (data not shown).
Determination of thymic output change (measured as TREC change between baseline and week 24 after start of the treatment) hinted at the reason for the failure of LHRH-agonist treatment to increase tumor-specific T cell numbers: Leuprolide treatment, when used as an adjuvant for the vaccination of melanoma patients with gp100209-2M and MAGE-3243-258 peptides in Montanide ISA-51, did not induce the expected chemical castration-dependent increase on TREC level, even though chemical castration was achieved. For most of the patients analyzed, we could see a significant increase on TREC levels at week 24, independent of whether patients were treated with Leuprolide or not.
The unexpected increase in TREC content in the blood between baseline and week 24 of most patients, would suggest that something else, perhaps previous treatment, the gp100/MAGE-3 peptide vaccination or the Montanide ISA-51 used as adjuvant for vaccination, may be involved. It has been reported that thymic damage induced by cytoablative chemotherapy or radiotherapy causes acute involution followed by eventual regeneration.35 Patients on this clinical trial did not have prior chemotherapy/radiotherapy, ruling out this prospect. Another possibility could be an unforeseen effect of vaccination/Montanide ISA-51 in all patients.
IFA alone without antigen has been shown to have immunological response modifying activity. For example, it has been indicated that it can reduce diabetes incidence in the NOD mouse36 and induce a myeloid cell population capable of suppressing tumor immunity in BALB/c mice.37 Moreover, it has been reported that intraperitoneal injection of IFA induces lupus-related autoantibodies to nRNP/Sm in non-autoimmune BALB/c mice,38 can reduce autoimmune encephalomyelitis (EAE), inhibit both mitogen and antigen-induced T cell proliferation and increase secretion of IFN-gamma and IL-10 by neuroantigen specific T cells in mice. 39 It is therefore plausible that IFA has a direct effect (on thymic epithelial cells -TEC- or thymocytes) or indirect effect (induction of cytokine production by accessory cells) on thymopoiesis. To our knowledge, there are no reports on the effect of peptide vaccination and/or IFA on thymopoiesis.
Although a possible association between vaccination with IFA and increased TREC levels was found here, we must emphasize that this study could not define a cause-effect relationship between these parameters because a control arm of unvaccinated patients (without IFA) was not included. In addition, our results cannot rule out the possibility that natural oscillations in thymic output occur through time in patients that may account for the TREC level changes seen in our study here, although this is unlikely.
We also investigated whether any of the peripheral T cell sub-populations, CD4+ or CD8+, was particularly associated with the TREC level increase seen in this clinical trial. Published evidence suggested that no particular T cell sub-population had more TREC contribution after Leuprolide treatment: it has been shown that chemical castration does not change the CD4/CD8 ratio in the periphery of humans,40 although it increases thymic cellularity and T cell output.17,18 We stained patient’s PBMC before and 24 weeks after treatment for markers reported to be associated with higher content of TRECs: CD4+CD45RA+RO−CD31+ for CD4 T cells25 and CD8+CD45RA+RO−CD27+CD103+ for CD8 T cells.26 We determined that the increase on TRECs was paralleled by an increase in the CD8+CD45RA+RO−CD27+CD103+ T cell sub-population, but not by an increase in the CD4+CD45RA+RO−CD31+ sub-population. This difference in CD4/CD8 TREC distribution also suggests that the cause of the TREC level increase has no relation with the Leuprolide treatment.
It is known that thymic productivity is influenced by cytokines, growth factors, and hormones.41 Cytokines not only influence the production and turnover of peripheral T cells, but can also lead to alterations of the thymus and RTE homeostasis. IL-7 has been shown to be a very important cytokine in the early stages of thymocyte development,42 with IL-7 expression levels decreasing with age.43 IL-7 levels inside the thymus are critical for the production of T cells.44 However, this is not without controversy. Investigations in mice have demonstrated some rejuvenation of the thymus following IL-7 treatment45, but IL-7 administration into thymectomized mice resulted in patterns of increased total TREC contents similar to those in IL-7–treated thymus-intact mice46 with a dose-dependent effect on T cell development.42 Other factors, such as GH,47 IGF-130 and KGF-1,48 through different mechanisms, can enhance thymopoiesis and immune reconstitution.
We measured IL-7 and IGF-1 levels in the sera of 32 patients for which TREC TREC data was available. While IL-7 serum levels showed a statistically significant increase from baseline to week 6 and marginally significant to week 12, IGF-1 levels remained constant. The increase in serum IL-7 levels could help explain why TREC levels, and therefore the CD8+CD45RA+RO−CD27+CD103+ T cell sub-population, increased after vaccination. It is worth mentioning that both T cell sub-populations, CD8+CD45RA+RO−CD27+CD103+ and CD4+CD45RA+RO−CD31+, expressed CD127 (IL-7 receptor alpha), but they were not proliferating (they were KI67−, data not shown). It is curious that although CD8+CD103+ and CD4+CD31+ T cell subsets express CD127, only CD8+CD103+ T cells appeared to be affected by slightly higher concentrations of IL-7 in the sera. This could at least be partially explained by concomitant increase of CD127 expression at week 24 only in CD8+CD103+ T cells, but also points to a different need of cytokines or other factors for the two subsets. In particular we have not measured all reported factors and cytokines that could affect both subsets, such as GH or KGF-1.
We have shown that peptide vaccinations in IFA may be associated with increased TREC levels in melanoma patients, independent of LHRH agonist treatment. Only the increase of one sub-population of CD8 T cells, CD8+CD45RA+RO−CD27+CD103+, seemed to reflect the TREC level increase. IL-7 was identified as one of the factors that may contribute to the increase of CD8+CD45RA+RO−CD27+CD103+ T cells. Animal studies will be helpful to understand the mechanism by which TREC levels increase after peptide vaccination.
Supplementary Material
Frozen/thawed PBMC from patients at different time points were stained with class I HLA-A*0201-restricted gp100209-2M tetramer. Cells were stained with anti-CD11c/CD14/CD15/CD16/CD19 FITC (dump channel), CD4 PerCP-Cy5.5, gp100 Tetramer-APC and CD8-APC/Cy7. The numbers represent the net change of gp100209-2M –specific CD8+ T cell subset within the CD8 T cell population at 12 and 24 weeks after vaccination.
Acknowledgments
This work has been supported by the Norwood Immunology Limited grant # CS2004-00012566SP and the TAP Pharmaceutical products, INC grant # CS2006-00015509SP. The authors acknowledge the M.D. Anderson Cancer Center Immune Monitoring Core Laboratory (IMCL) for assistance with the immunological assays. The IMCL is funded by the M.D. Anderson Cancer Center Support Grant (NCI # CA16672).
Abbreviations Used
- TAA
Tumor Associated Antigens
- TREC
T-cell receptor excision circle
- IFA
incomplete Freund’s Adjuvant
- LHRH
luteinizing hormone-releasing hormone
- RTE
Recent Thymic Emigrants
- GH
Growth Hormone
- KGF-1
Keratinocyte Growth Factor 1
- IGF-1
Insulin Growth Factor 1
Footnotes
Financial Disclosure: All authors have declared there are no conflicts of interest in regards to this work.
ClinicalTrials.gov identifier: NCT00254397
Contributor Information
Chiyu Wang, Email: Chiyu.Wang@usoncology.com.
Himabindu Pappu, Email: HPappu@mdanderson.org.
Ryan Anson, Email: Ryan_E_Anson@bd.com.
Tejal A. Patel, Email: tapatel@mdanderson.org.
Priscilla Miller, Email: pmiller@mdanderson.org.
Roland Bassett, Email: rlbasset@mdanderson.org.
Gregory Lizee, Email: glizee@mdanderson.org.
Willem W. Overwijk, Email: woverwijk@mdanderson.org.
Krishna Komanduri, Email: KKomanduri@med.miami.edu.
Cara Benjamin, Email: CBenjamin@med.miami.edu.
Gladys Alvarado, Email: galvarad@mdanderson.org.
Sapna P. Patel, Email: SPPatel@mdanderson.org.
Kevin Kim, Email: kkim@mdanderson.org.
Nicholas E. Papadopoulos, Email: npapadop@mdanderson.org.
Agop Y. Bedikian, Email: abedikia@mdanderson.org.
Jade Homsi, Email: jade.homsi@bannerhealth.com.
Wen-Jen Hwu, Email: wenjhwu@mdanderson.org.
Richard Boyd, Email: Richard.Boyd@med.monash.edu.au.
Laszlo Radvanyi, Email: lradvanyi@mdanderson.org.
Patrick Hwu, Email: phwu@mdanderson.org.
References
- 1.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 2.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17(22):6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrella T, Quirt I, Verma S, Haynes AE, Charette M, Bak K. Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treat Rev. 2007;33(5):484–496. doi: 10.1016/j.ctrv.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Parmiani G, Castelli C, Dalerba P, et al. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst. 2002;94(11):805–818. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- 5.Pilla L, Rivoltini L, Patuzzo R, Marrari A, Valdagni R, Parmiani G. Multipeptide vaccination in cancer patients. Expert Opin Biol Ther. 2009;9(8):1043–1055. doi: 10.1517/14712590903085109. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4(3):321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Renkvist N, Sun Z, et al. A polyclonal anti-vaccine CD4 T cell response detected with HLA-DP4 multimers in a melanoma patient vaccinated with MAGE-3.DP4-peptide-pulsed dendritic cells. Eur J Immunol. 2005;35(4):1066–1075. doi: 10.1002/eji.200425847. [DOI] [PubMed] [Google Scholar]
- 9.Hirokawa K, Utsuyama M, Kasai M, Kurashima C, Ishijima S, Zeng YX. Understanding the mechanism of the age-change of thymic function to promote T cell differentiation. Immunol Lett. 1994;40(3):269–277. doi: 10.1016/0165-2478(94)00065-4. [DOI] [PubMed] [Google Scholar]
- 10.Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunol Res. 2000;22(2–3):253–261. doi: 10.1385/IR:22:2-3:253. [DOI] [PubMed] [Google Scholar]
- 11.Mackall CL, Hakim FT, Gress RE. T-cell regeneration: all repertoires are not created equal. Immunol Today. 1997;18(5):245–251. doi: 10.1016/s0167-5699(97)81664-7. [DOI] [PubMed] [Google Scholar]
- 12.Kurashima C, Utsuyama M, Kasai M, Ishijima SA, Konno A, Hirokawa K. The role of thymus in the aging of Th cell subpopulations and age-associated alteration of cytokine production by these cells. Int Immunol. 1995;7(1):97–104. doi: 10.1093/intimm/7.1.97. [DOI] [PubMed] [Google Scholar]
- 13.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332(3):143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 14.Douek DC, Vescio RA, Betts MR, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355(9218):1875–1881. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97(5):1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 16.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland JS, Goldberg GL, Hammett MV, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175(4):2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland JS, Spyroglou L, Muirhead JL, et al. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin Cancer Res. 2008;14(4):1138–1149. doi: 10.1158/1078-0432.CCR-07-1784. [DOI] [PubMed] [Google Scholar]
- 19.Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- 20.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110(13):4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15(14):1749–1756. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 22.Tang S, Moore ML, Grayson JM, Dubey P. Increased CD8+ T-cell function following castration and immunization is countered by parallel expansion of regulatory T cells. Cancer Res. 2012;72(8):1975–1985. doi: 10.1158/0008-5472.CAN-11-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 24.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201(5):723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Junge S, Kloeckener-Gruissem B, Zufferey R, et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol. 2007;37(11):3270–3280. doi: 10.1002/eji.200636976. [DOI] [PubMed] [Google Scholar]
- 26.McFarland RD, Douek DC, Koup RA, Picker LJ. Identification of a human recent thymic emigrant phenotype. Proc Natl Acad Sci U S A. 2000;97(8):4215–4220. doi: 10.1073/pnas.070061597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolotin E, Smogorzewska M, Smith S, Widmer M, Weinberg K. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood. 1996;88(5):1887–1894. [PubMed] [Google Scholar]
- 28.Napolitano LA, Schmidt D, Gotway MB, et al. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest. 2008;118(3):1085–1098. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi SW, Jeker LT, Ueno T, et al. Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells. Blood. 2007;109(9):3803–3811. doi: 10.1182/blood-2006-10-049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu YW, Schmitz S, Choudhury B, et al. Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood. 2008;112(7):2836–2846. doi: 10.1182/blood-2008-04-149435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamoto Y, Douek DC, McFarland RD, Koup RA. Effects of exogenous interleukin-7 on human thymus function. Blood. 2002;99(8):2851–2858. doi: 10.1182/blood.v99.8.2851. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323(9):570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell WA, Lang PO, Aspinall R. Tracing thymic output in older individuals. Clin Exp Immunol. 2010;161(3):497–503. doi: 10.1111/j.1365-2249.2010.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pido-Lopez J, Imami N, Aspinall R. Both age and gender affect thymic output: more recent thymic migrants in females than males as they age. Clin Exp Immunol. 2001;125(3):409–413. doi: 10.1046/j.1365-2249.2001.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sfikakis PP, Gourgoulis GM, Moulopoulos LA, Kouvatseas G, Theofilopoulos AN, Dimopoulos MA. Age-related thymic activity in adults following chemotherapy-induced lymphopenia. Eur J Clin Invest. 2005;35(6):380–387. doi: 10.1111/j.1365-2362.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 36.Liddi R, Beales PE, Rosignoli G, Pozzilli P. Incomplete Freund’s adjuvant reduces diabetes in the non-obese diabetic mouse. Horm Metab Res. 2000;32(6):201–206. doi: 10.1055/s-2007-978622. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Jiang J, Li Z, Zhang J, Wang H, Qin Z. A myeloid cell population induced by Freund adjuvant suppresses T-cell-mediated antitumor immunity. J Immunother. 33(2):167–177. doi: 10.1097/CJI.0b013e3181bed2ba. [DOI] [PubMed] [Google Scholar]
- 38.Kuroda Y, Nacionales DC, Akaogi J, Reeves WH, Satoh M. Autoimmunity induced by adjuvant hydrocarbon oil components of vaccine. Biomed Pharmacother. 2004;58(5):325–337. doi: 10.1016/j.biopha.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Zamora A, Matejuk A, Silverman M, Vandenbark AA, Offner H. Inhibitory effects of incomplete Freund’s adjuvant on experimental autoimmune encephalomyelitis. Autoimmunity. 2002;35(1):21–28. doi: 10.1080/08916930290005873. [DOI] [PubMed] [Google Scholar]
- 40.Page ST, Plymate SR, Bremner WJ, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290(5):E856–863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- 41.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 42.El Kassar N, Lucas PJ, Klug DB, et al. A dose effect of IL-7 on thymocyte development. Blood. 2004;104(5):1419–1427. doi: 10.1182/blood-2004-01-0201. [DOI] [PubMed] [Google Scholar]
- 43.Andrew D, Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp Gerontol. 2002;37(2–3):455–463. doi: 10.1016/s0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 44.Aspinall R. T cell development, ageing and Interleukin-7. Mech Ageing Dev. 2006;127(6):572–578. doi: 10.1016/j.mad.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Pido-Lopez J, Imami N, Andrew D, Aspinall R. Molecular quantitation of thymic output in mice and the effect of IL-7. Eur J Immunol. 2002;32(10):2827–2836. doi: 10.1002/1521-4141(2002010)32:10<2827::AID-IMMU2827>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 46.Chu YW, Memon SA, Sharrow SO, et al. Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood. 2004;104(4):1110–1119. doi: 10.1182/blood-2003-10-3635. [DOI] [PubMed] [Google Scholar]
- 47.Chen BJ, Cui X, Sempowski GD, Chao NJ. Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice. Exp Hematol. 2003;31(10):953–958. doi: 10.1016/s0301-472x(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 48.Erickson M, Morkowski S, Lehar S, et al. Regulation of thymic epithelium by keratinocyte growth factor. Blood. 2002;100(9):3269–3278. doi: 10.1182/blood-2002-04-1036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Frozen/thawed PBMC from patients at different time points were stained with class I HLA-A*0201-restricted gp100209-2M tetramer. Cells were stained with anti-CD11c/CD14/CD15/CD16/CD19 FITC (dump channel), CD4 PerCP-Cy5.5, gp100 Tetramer-APC and CD8-APC/Cy7. The numbers represent the net change of gp100209-2M –specific CD8+ T cell subset within the CD8 T cell population at 12 and 24 weeks after vaccination.