Abstract
Deregulated expression of zinc transporters was linked to several cancers. However, the detailed expression profile of all human zinc transporters in normal human organs and in human cancer, especially in pancreatic cancer is not available. The objectives of this study are to investigate the complete expression patterns of 14 ZIP and 10 ZnT transporters in a large number of normal human organs and in human pancreatic cancer tissues and cell lines. We examined the expression patterns of ZIP and ZnT transporters in 22 different human organs and tissues, 11 pairs of clinical human pancreatic cancer specimens and surrounding normal/benign tissues, as well as 10 established human pancreatic cancer cell lines plus normal human pancreatic ductal epithelium (HPDE) cells, using real time RT-PCR and immunohistochemistry. The results indicate that human zinc transporters have tissue specific expression patterns, and may play different roles in different organs or tissues. Almost all the ZIPs except for ZIP4, and most ZnTs were down-regulated in human pancreatic cancer tissues compared to the surrounding benign tissues. The expression patterns of individual ZIPs and ZnTs are similar among different pancreatic cancer lines. Those results and our previous studies suggest that ZIP4 is the only zinc transporter that is significantly up-regulated in human pancreatic cancer and might be the major zinc transporter that plays an important role in pancreatic cancer growth. ZIP4 might serve as a novel molecular target for pancreatic cancer diagnosis and therapy.
Keywords: Pancreatic cancer, profile, zinc transporter, ZIP4
Introduction
Zinc is an essential trace element and catalytic cofactor involved in normal cell growth and development [1]. Alterations in intracellular zinc concentration impact many cellular processes. Zinc deficiency in animals leads to impaired DNA synthesis, decreased food intake, and stunted growth [2]. Cells have evolved a complex system to maintain the balance of zinc influx and efflux. In mammalian cells, there are two zinc transporter protein families, ZIPs and ZnT [3], which have opposite roles in maintaining cellular zinc homeostasis: ZIP transporters are responsible for dietary zinc uptake, while ZnTs prompt zinc efflux [4]. There are at least 14 ZIP and 10 ZnT proteins identified in human [5], which exhibit unique tissue specific expression and differential responsiveness to dietary zinc deficiency and excess [3]. However, a comprehensive gene profile of the 24 human zinc transporters in a large amount of normal human organs and diseased tissues such as cancers are not available.
Deregulated zinc transporters have been linked with several cancers. Low level of ZnT1 was found in mammary gland tumor cells, which leads to a higher concentration of zinc than normal cells, suggesting a misregulation of zinc transport in these proliferating tumor cells [3, 6]. ZIP6, 7, and 10 have been associated with breast cancer metastasis [4, 7-10]. Our previous studies indicated that ZIP4 is up-regulated in human pancreatic cancer, and aberrant expression of ZIP4 contributes to pancreatic cancer pathogenesis [11, 12]. Those reports suggest a positive correlation between zinc importers and cancer progression.
Pancreatic cancer has the lowest overall survival rate in all cancers. The 5-year survival rate for all stages is less than 5% [13]. The standard therapy for patients with advanced pancreatic cancer is gemcitabine-based chemotherapy, which only provides modest improvement of overall survival [14, 15]. Very little is known about the molecular mechanism of pancreatic cancer, therefore, new markers and novel therapeutic targets are urgently needed for this terrible disease. The deregulated zinc transport pathway (such as ZIP4) in pancreatic cancer might provide new insights on cancer growth and metabolism. Over-expression of zinc transporters in cancers may regulate the expression of many other genes or transcription factors by providing sufficient zinc ions to the fast growing tumor cells. However, the complete gene profile of zinc transporters in human pancreatic cancer is not available. In this study, we examined the expression profile of all 14 ZIP transporters and 10 ZnT transporters in a large number of normal human organs, clinical human pancreatic cancer specimens and cell lines, to further characterize the involvement of zinc transporters in human cancers such as pancreatic cancer, and identify key zinc transporters which play important roles in pancreatic cancer pathogenesis.
Materials and Methods
Chemicals and Reagents
The real time PCR reagents SYBR Green supermix and iScript cDNA synthesis kits were purchased from Bio-Rad (Hercules, CA). The Ambion “RNAqueous-4PCR” kit and DNA removing kits were obtained from Ambion (Austin, Texas). Other chemicals were obtained from Sigma (St. Louis, MO).
Cell Culture and Tissue Sample Collection
Human pancreatic cancer cell lines Panc-1, MIA PaCa-2, BxPC-3, Hs766T, ASPC-1, Capan-1, Capan-2, HPAFII, Panc03.27, PL45 were purchased from the American Type Culture Collection (ATCC, Rockville, MD). The human pancreatic ductal epithelium (HPDE) cells were provided as a generous gift from Dr. Ming-Sound Tsao [16, 17]. All cells were cultured as previously described [18]. Human pancreatic cancer tissue specimens were collected from patients who underwent surgery according to an approved human protocol at the University of Texas Health Science Center at Houston (UTHealth), Medical School. The RNA samples from 22 normal human organs and tissues were purchased from Clontech (Mountain View, CA).
RNA Extraction and Real Time RT PCR
Total RNA was extracted from the pancreatic cancer cell lines and homogenized pancreatic tumor tissues using an Ambion “RNAqueous-4PCR” kit following the manufacturer's instructions. Briefly, cells or tissue samples were lysed by Ambion lysis buffer for 20 min and the cell lysates were mixed with equal volume of 64% ethanol. The lysates were transferred to an Ambion mini-column in a 2 ml collection tube, and centrifuged at 10,000 ×g for 1 min. The column was washed once with 700 μl of wash buffer 1, and twice with 500 μl of wash buffer 2/3. After incubation with 50 μl of pre-warmed elution buffer, the eluted fluid was collected using a new tube, and another 50 μl of elution buffer was added followed by a second centrifugation. The RNA solution was treated with DNase I to remove the trace amount of genomic DNA contamination by using an Ambion DNA removing kit (Austin, Texas). The mRNA levels for 14 ZIPs and 10 ZnTs were analyzed by real time RT PCR using iCycler system (Bio-Rad, Hercules, CA) as described previously [18]. PCR reaction included the following components: 100 nM each primer, diluted cDNA templates and iQ SYBR Green supermix, and running for 40 cycles at 95°C for 20 sec and 60°C for 1 min. PCR efficiency was examined by serially diluting the template cDNA and the melting curve data were collected to check PCR specificity. Each cDNA sample was run as triplicates and the corresponding no-reverse transcriptase (RT) mRNA sample was included as a negative control. The primer sequences for the zinc transporters are listed in Table 1. The β-actin primer was included in every plate to avoid sample variations. The mRNA level of each sample for each gene was normalized to that of the β-actin mRNA. The relative mRNA level was presented as 2̂[(Ct/β-actin - Ct/gene of interest)].
Table 1. Sequences of the Primers Used for Real Time PCR Analysis of ZIP and ZnT Genes.
| Gene Name | Primer Name | Sequence (5′ -> 3′) | Length (nt) |
|---|---|---|---|
| ZnT1 | FhZnT1 | CCTCGCGTTAAGAGCACCC | 19 |
| RhZnT1 | CAATTTCAGCCCGTTGGAGTT | 21 | |
| ZnT2 | FhZnT2 | TGATCGGAGAAGTCGTTGAGA | 21 |
| RhZnT2 | CCGTCAATTTCATAGTCCCCAGA | 23 | |
| ZnT3 | FhZnT3 | CACCCGCACCATGACCTTT | 19 |
| RhZnT3 | CCCCCTCGATGTGGTAGTC | 19 | |
| ZnT4 | FhZnT4 | GACCTAAGCGCCATCATACTC | 21 |
| RhZnT4 | AGCTGACAAAACCTCTAAGCG | 21 | |
| ZnT5 | FhZnT5 | ACCAAACACCAGTGGATCAAAA | 22 |
| RhZnT5 | CAGCAAAGTCCTTAGTGGTCC | 21 | |
| ZnT6 | FhZnT6 | ATCTTCCTTCCCCGAATGAATCC | 23 |
| RhZnT6 | CCAAATGTCATCAAGGCAATAGC | 23 | |
| ZnT7 | FhZnT7 | GCTTAGGCTTGATTTCCGACT | 21 |
| RhZnT7 | CCAGAACTTCCGCTCTAACATAC | 23 | |
| ZnT8 | FhZnT8 | GTACGCCTATGCCAAGTGGAA | 21 |
| RhZnT8 | CAGCAAGACTCCCAGCAATGT | 21 | |
| ZnT9 | FhZnT9 | ACTGGAGTCAGAGCGATAAATGA | 23 |
| RhZnT9 | GCTTCCCCAAACTTCCAAAGAT | 22 | |
| ZnT10 | FhZnT10 | GTCCCAAAAGGAGTCAACAT | 20 |
| RhZnT10 | TTTCCACTTACAAGTTCCC | 19 | |
| ZIP1 | FhZIP1 | ACTACCTGGCTGCCATAGATG | 21 |
| RhZIP1 | GCCCTGACTGCTCCTTGTAAG | 21 | |
| ZIP2 | FhZIP2 | CTGCTGGAGGATCGACAGTG | 20 |
| RhZIP2 | CACAAGCCCCTTATGAGCCA | 20 | |
| ZIP3 | FhZIP3 | TGGCCGAAACCATCCTCCT | 19 |
| RhZIP3 | GATCCGGCGTTGAAGGTCTC | 20 | |
| ZIP5 | FhZIP5 | GGGGCTGTCAGTGCTCGGAG | 20 |
| RhZIP5 | TCCGGATCCAAGTTGCGTGTT | 21 | |
| ZIP6 | FhZIP6 | TCTCTGTCACAAATCCCCTTCA | 22 |
| RhZIP6 | GATATTGCCGTGTGGAAATTGC | 22 | |
| ZIP7 | FhZIP7 | CCACAGGCACTCACATGAAGA | 21 |
| RhZIP7 | CGTGATCGTGGGTATGTCCAT | 21 | |
| ZIP8 | FhZIP8 | TGCTACCCAAATAACCAGCTCC | 22 |
| RhZIP8 | ACAGGAATCCATATCCCCAAACT | 23 | |
| ZIP9 | FhZIP9 | TCTCTGGCTATGTTGGTGGGA | 21 |
| RhZIP9 | CCAGCACCCAAAACAGTCAC | 20 | |
| ZIP10 | FhZIP10 | ACACCAGATTCTGACTGGCTT | 21 |
| RhZIP10 | TAGGAGGGGATTCTTGTTGGC | 21 | |
| ZIP11 | FhZIP11 | TGCTGGGGACCTTCTTCAC | 19 |
| RhZIP11 | GCCAAGACTTCCATCTAAGATCC | 23 | |
| ZIP12 | FhZIP12 | TTTCCTGGGATCAGACCTGCT | 21 |
| RhZIP12 | GTTGGTCCTTGGGTAAGTGGC | 21 | |
| ZIP13 | FhZIP13 | TCCTGGGTTCCCTCATGGT | 19 |
| RhZIP13 | AGATGCAGAAACACATTGCCC | 21 | |
| ZIP14 | FhZIP14 | CCTGAGGCTCACGCTTCATC | 20 |
| RhZIP14 | TCACCCTCGCCATACCGAT | 19 |
F, forward; R, reverse; h, human.
Immunohistochemical Staining
Human pancreatic adenocarcinoma and surrounding benign tissues were processed into 5 μm slices and incubated in blocking buffer for 30 min at room temperature before adding anti-hZIP4 antibody and incubated for 60 min at room temperature. The rabbit polyclonal anti-hZIP4 antibody was prepared as previously described [19]. After washing with PBS, the section was incubated with biotinylated secondary antibody for 30 min. An avidin-biotin reaction using peroxidase enzyme was used for protein detection (ABC kit; Vector Laboratories, Burlingham, CA). Immune complexes were detected with diaminobenzidine (DAB) under a phase contrast microscope.
Statistical Analysis
Quantitative results are shown as means ± standard deviations. The statistical analysis was performed by the Student's t test between normal and tumor tissues. All data shown are the mean±SD of three separate experiments. A p-value of < 0.05 was considered statistically significant.
Results
Gene Profiling Identifies Tissue Specific Expression Patterns of Human Zinc Transporters
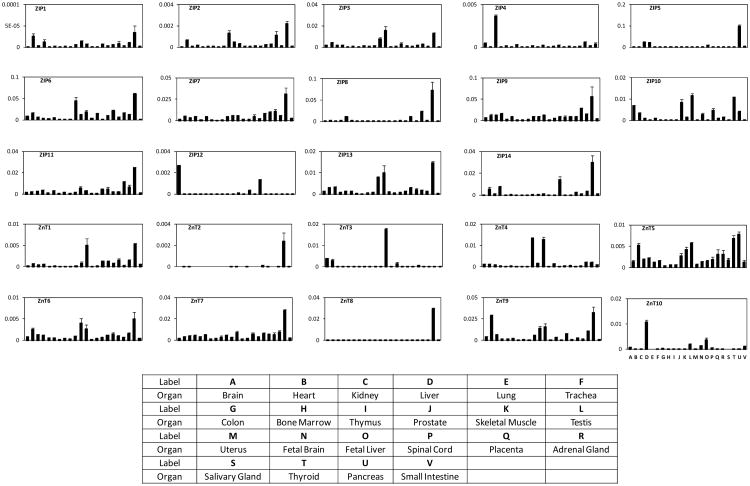
We have previously shown that ZIP4 is aberrantly overexpressed in human pancreatic cancer [11, 12], however, little is known about the distribution of ZIP4 in a large number of normal human organs and tissues, and there are limited studies on the expression patterns of all ZIP and ZnT proteins in normal human organs and diseased tissues such as cancers. To determine the tissue specificity of the human zinc transporters, we examined the expression of 14 ZIPs and 10 ZnTs in 22 normal human organs and tissues by using quantitative real time PCR. As shown in Figs. (1, 2), ZIP6 and ZIP9 are the most abundant ZIPs that are highly expressed in many human organs, especially in heart, testis, salivary gland, thyroid, and pancreas. ZIP3, ZIP7, ZIP8, ZIP10, ZIP11, ZIP13 and ZIP14 were also detected in most of the organs at medium to high levels, but with distinct expression patterns. ZIP3 and ZIP13 had very similar expressions in most of the organs, and they were both highly expressed in testis and pancreas. ZIP7 and ZIP11 seemed to be in the same cluster and were both highly expressed in salivary gland and pancreas. ZIP8 was highly expressed in lung, placenta, salivary gland and pancreas; ZIP10 was most abundant in testis, thyroid, and prostate; while ZIP14 was mainly seen in fetal liver and pancreas.
Fig. (1).
Expression patterns of ZIPs and ZnTs in normal human organs and tissues by real time RT PCR. The relative mRNA of target gene was calculated and normalized to that of β-actin. All data shown are the mean ± SD (n=3) of three separate experiments.
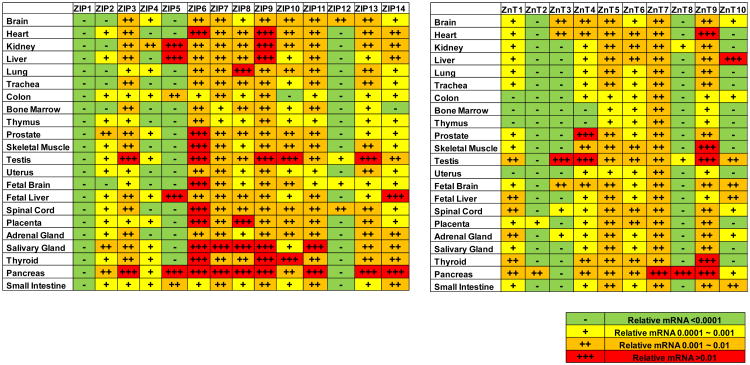
Fig. (2).
Summaries of the ZIPs and ZnTs expression profiles in different human organs and tissues. The relative mRNA per β-actin has been converted into “-”, “+”, “++” and “+++” according to the following standard: -, relative mRNA level <0.0001; +, relative mRNA level 0.0001 ∼ 0.001; ++, relative mRNA level 0.001 ∼ 0.01; +++, relative mRNA level >0.01.
ZIP5 was found to be highly expressed in kidney, liver and pancreas, and was also found in colon and small intestine at medium levels, but was not detected in any other organs. ZIP2 and ZIP4 were found with relatively low expression levels in many organs: ZIP2 was mostly seen in prostate, salivary gland and pancreas; while kidney is the major organ for ZIP4 expression. ZIP12 was expressed in brain and spinal cord at medium level, and in testis and fatal brain at low level, but was undetectable in all other organs. The ZIP1 expression level was the lowest in all ZIP4 proteins, and was below the threshold in the organs and tissues that we have examined.
Among the ZnT family, ZnT7 is the most abundant zinc exporter that was expressed in all the organs tested at medium to high level, especially in pancreas. ZnT5, ZnT6 and ZnT9 were also universally expressed in all the organs, and most organs showed medium to high levels of expression, especially ZnT9 was found to be highly expressed in heart, skeletal muscle, testis, thyroid and pancreas. ZnT1 and ZnT4 were found to be present in the majority of the organs: both of them were found in thyroid and pancreas at medium levels, and ZnT1 expression in testis, fatal liver, spinal cord and adrenal gland were also at medium level. The expression of ZnT4 in prostate and testis were high compared with that in other organs.
ZnT3, ZnT8 and ZnT10 were expressed at very high levels only in certain organs, such as testis (ZnT3), pancreas (ZnT8) and liver (ZnT10), but at low to undetectable levels in most other organs. ZnT2 was not detected in most of the organs except for pancreas and placenta, in which the expression was medium to low. Those results are consistent with previous reports and confirmed that human zinc transporters have tissue specific expression patterns, and may play different roles in different organs or tissues.
The Expression of Zinc Transporters in Human Pancreatic Cancer Tissues
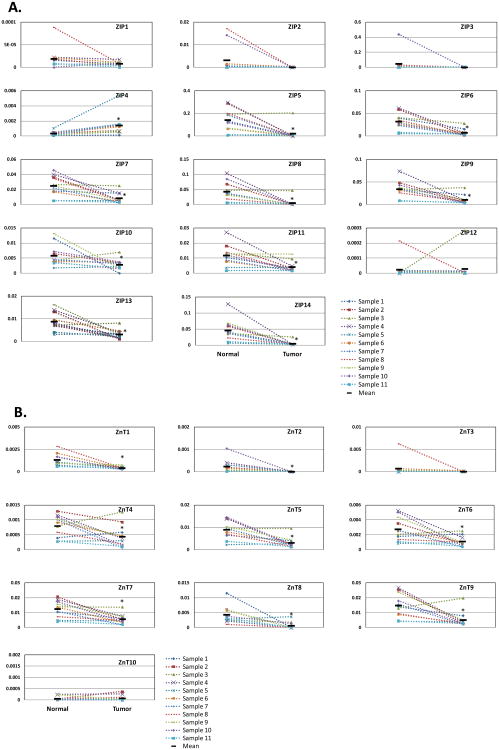
Our previous results have shown that ZIP4 is overexpressed in human pancreatic cancer. To expand our knowledge on the expression profile of zinc transporters in human pancreatic cancer, we examined the mRNA levels of ZIPs and ZnTs in paired human pancreatic cancer tissues and surrounding benign tissues. We found that all 13 ZIPs other than ZIP4 were down-regulated in the pancreatic tumors compared with the surrounding benign tissues (Fig. 3A). The relatively high expressing zinc transporters such as ZIP5, ZIP8 and ZIP14 and the low expressing zinc transporters such as ZIP7, ZIP9, ZIP11, and ZIP13 all showed a substantial decrease in tumor tissues for at least 2-fold (p<0.05). Almost all the paired tissues showed similar trend of down-regulation for the 13 ZIPs. Similar expression patterns were found in ZnT family. Nine out of ten ZnTs showed significant decrease at the mRNA level in tumor tissues compared to the surrounding benign tissues, except for ZnT 10 (Fig. 3B). Almost all the paired tissues showed similar trend of down-regulation for the nine ZnTs as well.
Fig. (3).
Expression of ZIP and ZnT mRNA levels in human pancreatic adenocarcinoma and surrounding normal tissues by real time RT PCR. (A) ZIP expression in human pancreatic cancer tissues. (B) ZnT expression in human pancreatic cancer tissues. The relative mRNA of target gene was calculated and normalized to that of β-actin. All data shown are the mean±SD of three separate experiments. *p < 0.05.
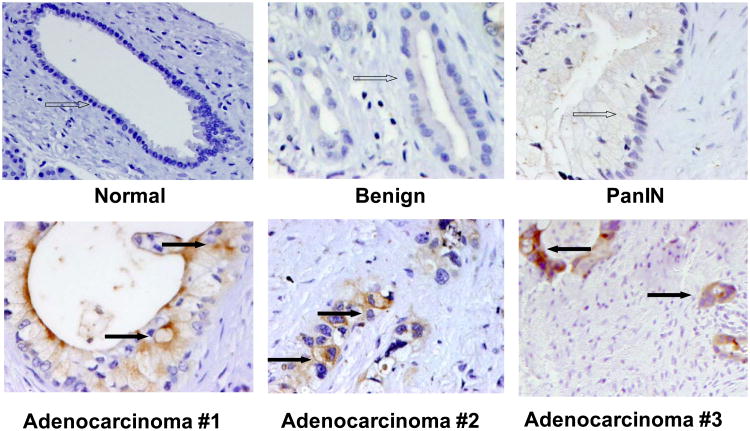
Those results and our previous studies have indicated that ZIP4 is the only zinc transporter that is significantly up-regulated in pancreatic cancer and might be the major zinc transporter that plays an important role in pancreatic cancer growth. To further confirm the expression of ZIP4 in human pancreatic cancer, we also examined the ZIP4 protein level in a larger cohort of human pancreatic tumor samples. Representative higher power pictures from the normal, benign, pancreatic intraepithelial neoplasia (PanIN), and three adenocarcinoma tissues are shown in Fig. (4). The tumors showed specific positive staining of ZIP4 on the membrane of cancer cells in the pancreatic adenocarcinoma, while the surrounding benign or PanIN lesions next to the duct adenocarcinoma showed negative or weak positive staining of ZIP4. Thus, the overexpression of ZIP4 in the majority of pancreatic cancer tissues suggests that this zinc transporter may contribute to pancreatic cancer growth.
Fig. (4).
IHC staining of ZIP4 in human pancreatic cancer tissues. Representative pictures from a normal, benign, PanIN, and three adenocarcinoma samples are shown in higher power field (400 ×). Open arrows, negative or weak positive staining of ZIP4 in the normal, benign, and PanIN lesions. Black arrows, positive staining of ZIP4 in pancreatic adenocarcinomas.
The Expression of Zinc Transporters in Human Pancreatic Cancer Cell Lines
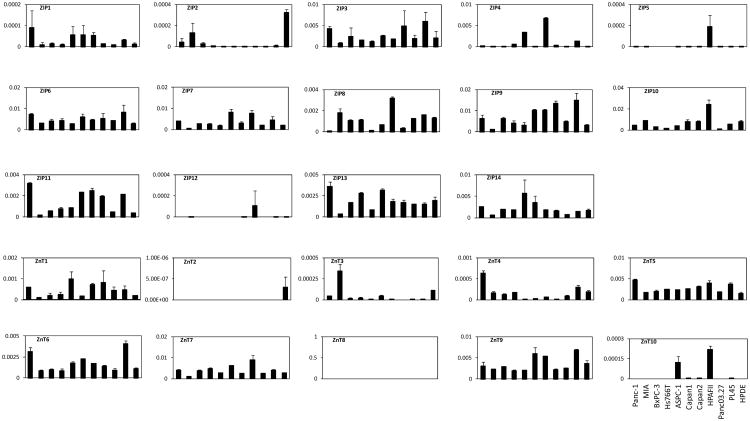
The expression patterns of individual ZIPs and ZnTs are very similar among different pancreatic cancer lines (Figs. 5, 6). The mRNA levels of ZIP3, ZIP4, ZIP6, ZIP7, ZIP8, ZIP9, ZIP10, ZIP11, ZIP13 and ZIP14 are maintained at medium to high levels in most of the pancreatic cancer cell lines, as well as in HPDE cells. ZIP9 is highly expressed in Capan1, Capan2, HPAFII and PL45 cells, while ZIP10 is highly expressed in HPAFII cells. ZIP4 expression level is relative higher in ASPC-1, Capan2 and PL45 cells. The expression levels of ZIP1, ZIP2, ZIP5 and ZIP12 are undetectable in most of the pancreatic cell lines.
Fig. (5).
Expression patterns of ZIPs and ZnTs in pancreatic cancer cell lines. The relative mRNA of target gene was calculated and normalized to that of β-actin. All data shown are the mean ± SD (n=3) of three separate experiments.
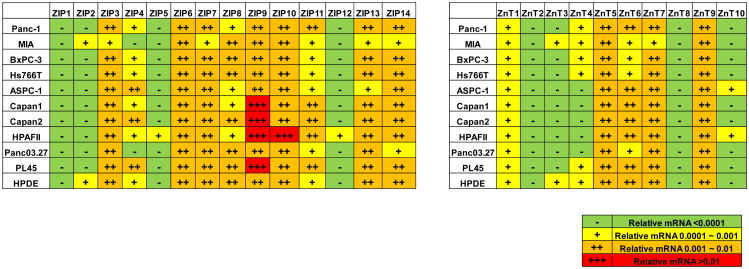
Fig. (6).
Summaries of the ZIPs and ZnTs expression profiles in different pancreatic cancer cell lines. The relative mRNA per β-actin has been converted into “-”, “+”, “++” and “+++” according to the following standard: -, relative mRNA level <0.0001; +, relative mRNA level 0.0001 ∼ 0.001; ++, relative mRNA level 0.001 ∼ 0.01; +++, relative mRNA level >0.01.
In ZnT family, ZnT5, ZnT6, ZnT7 and ZnT9 are expressed in almost all the pancreatic cell lines at medium levels. ZnT1 were also detected in all the cell lines, but at low levels; while ZnT4 was detected in half of the cells we tested at low levels. ZnT3 and ZnT10 were only detected in very few cell lines at low levels. ZnT2 and ZnT8 were undetectable in all the pancreatic cancer cell lines.
Discussion
The deregulated zinc transport pathway has been linked with several cancers. However, the complete gene profile of all zinc transporters in normal human organs and in human pancreatic cancer is not available. In this study, we examined the expression profile of all 14 ZIP transporters and 10 ZnT transporters in a large number of normal human organs and tissues. We also investigated the expression of these zinc transporters in clinical human pancreatic cancer specimens and established human pancreatic cancer cell lines. The results indicate that human zinc transporters have very specific tissue expression patterns, and may play different roles in different organs or tissues. Almost all the ZIPs except for ZIP4, and most ZnTs were down-regulated in human pancreatic cancer tissues compared to the surrounding benign tissues. Those results and our previous studies suggest that ZIP4 is the only zinc transporter that is significantly up-regulated in pancreatic cancer and might be the major zinc transporter that plays an important role in pancreatic cancer growth.
Most ZIP proteins showed specific expression in organs with high metabolism index such as liver, heart, or pancreas. This may be due to the function of ZIPs in uptaking zinc ions, which are essential cofactors for many metabolic enzymes. It seems that each organ prefers a few ZIPs as their specific zinc transporters. Testis, salivary gland and pancreas have the most ZIPs at high expression levels; Heart, kidney, liver, fetal liver, placenta, and thyroid also have a few ZIPs that are highly expressed. The expression levels of the ZIPs in other organs are generally medium to low. Previous studies have shown that ZIP1 was expressed in the small intestine and pancreas [20]. The expression of ZIP2 could be induced by the zinc depletion but the level was low in the prostate, uterus, cervical epithelium, optic nerve, and monocytes [21]. Abundant expressions of ZIP4 and ZIP5 were identified in small intestine, stomach, colon and kidney which were associated with the zinc absorption [22, 23]. ZIP6 and ZIP9 are the most abundant ZIPs that are highly expressed in many human organs, including heart, testis, salivary gland, thyroid, and pancreas. ZIP6 expression was elevated in prostate, placenta and mammary gland cells. ZIP6, ZIP7 and ZIP10 were identified as a potential marker for metastatic breast cancer [9, 10, 24]. Multiple studies showed increased expression of ZIP14 in intestine and liver, which play a role in inflammation response [25, 26]. For the ZnT transporters, most of the organs we tested only have medium levels of ZnTs except for ZnT9 which is highly expressed in heart, skeletal muscle, testis, thyroid, and pancreas. ZnT7 is the most abundant ZnT that was expressed in all the organs tested at medium to high levels. ZnT5 and ZnT6 were shown mostly in human endocrine pancreas, ovary, prostate, and testis tissues. The ZnTs also showed tissue specific expression patterns. Testis and pancreas are the two major organs that have the most ZnTs at high expressed levels, and ZnTs in other organs are generally medium to low. These results suggest that zinc transporters have distinct expression patterns among different organs, which may be attributed to the different requirement for zinc transport in different organs.
We previously reported that one of the zinc importers, ZIP4, is highly induced in pancreatic tumor compared with normal or PanIN tissues, which is responsible for the high proliferation and migration phenotypes of the tumor cells caused by the increased intracellular zinc concentration. Here we studied the expression levels of all the other ZIP family members, as well as their counterparts ZnTs. The results showed that in most of the paired tissues, the expression levels of all the other ZIP family members except ZIP4 [11] are significantly lower in tumors than that in the surrounding benign tissues, especially those that have high endogenous expression levels (ZIP5, ZIP8 and ZIP14). Similar results were also report for ZIP3 in pancreatic adenocarcinoma tissues [27]. As for the ZnT family members, only ZnT10 showed increased expression in tumors than that in benign tissues; all the other 9 ZnT members were down-regulated in tumors. This result is consistent with our previous findings that ZIP4 might be the major zinc transporter that is responsible to the increased cellular zinc concentration in pancreatic cancer cells, and promotes pancreatic cancer cell proliferation and tumor growth [11], which strongly suggests that ZIP4 plays a central and decisive role in pancreatic cancer progression, and may serve as a novel therapeutic target for pancreatic cancer treatment.
This study represents a detailed analysis of the expression profiles of two zinc transporter families, ZIP and ZnT, in a large number of normal human organs, human pancreatic cancer tissues, and established pancreatic cancer cell lines. Those results and our previous studies may provide new insights of the underlying mechanisms of pancreatic cancer pathogenesis and progression, and may suggest new direction of research on heavy metal transport and pancreatic cancer.
Acknowledgments
Supported by the National Institutes of Health (NIH) grants R21CA133604, R01CA138701 (M. Li), the MacDonald General Research Fund and the Vivian L. Smith Department of Neurosurgery at the University of Texas Health Science Center at Houston, Medical School.
Abbreviations
- HPDE
Human pancreatic ductal epithelium
- SLC
Solute-linked carrier
- TMD
Transmembrane domain
- DAB
Diaminobenzidine
- PanIN
Pancreatic intraepithelial neoplasia
Footnotes
Specific Author Contributions: Planning and/or conducting the study: Jingxuan Yang, Yuqing Zhang, Xiaobo Cui, Wantong Yao, Xianjun Yu, and Min Li. Collecting and/or interpreting data: Jingxuan Yang, Yuqing Zhang, Xiaobo Cui, Wantong Yao, Xianjun Yu, Putao Cen, Sally E. Hodges, William E. Fisher, F. Charles Brunicardi, Changyi Chen, Qizhi Yao, and Min Li. Drafting the manuscript: Jingxuan Yang, Yuqing Zhang, Xiaobo Cui, and Min Li. All the authors have approved the submission of the paper.
Conflict of Interest: We declare no financial conflict of interest.
References
- 1.Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271(5252):1081–5. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000;130(5S Suppl):1500S–8S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 3.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–72. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 4.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–76. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 5.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763(7):711–22. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Lee R, Woo W, Wu B, Kummer A, Duminy H, Xu Z. Zinc accumulation in N-methyl-N-nitrosourea-induced rat mammary tumors is accompanied by an altered expression of ZnT-1 and metallothionein. Exp Biol Med (Maywood) 2003;228(6):689–96. [PubMed] [Google Scholar]
- 7.Taylor KM, Morgan HE, Johnson A, Hadley LJ, Nicholson RI. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem J. 2003;375(Pt 1):51–9. doi: 10.1042/BJ20030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor KM, Hiscox S, Nicholson RI. Zinc transporter LIV-1: a link between cellular development and cancer progression. Trends Endocrinol Metab. 2004;15(10):461–3. doi: 10.1016/j.tem.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98(5):692–7. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer cells. Endocrinology. 2008;149(10):4912–20. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Zhang Y, Liu Z, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci USA. 2007;104(47):18636–41. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Zhang Y, Bharadwaj U, et al. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res. 2009;15(19):5993–6001. doi: 10.1158/1078-0432.CCR-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Li Y, Ahmad A, et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8(1):27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 15.Shi S, Yao W, Xu J, Long J, Liu C, Yu X. Combinational therapy: New hope for pancreatic cancer? Cancer Lett. 2012;317(2):127–35. doi: 10.1016/j.canlet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol. 1996;148(6):1763–70. [PMC free article] [PubMed] [Google Scholar]
- 17.Ouyang H, Mou L, Luk C, et al. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol. 2000;157(5):1623–31. doi: 10.1016/S0002-9440(10)64800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Yang H, Chai H, et al. Pancreatic carcinoma cells express neuropilins and vascular endothelial growth factor, but not vascular endothelial growth factor receptors. Cancer. 2004;101(10):2341–50. doi: 10.1002/cncr.20634. [DOI] [PubMed] [Google Scholar]
- 19.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinalpancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci USA. 2004;101(40):14355–60. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaither LA, Eide DJ. The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. J Biol Chem. 2001;276(25):22258–64. doi: 10.1074/jbc.M101772200. [DOI] [PubMed] [Google Scholar]
- 21.Cousins RJ, Blanchard RK, Popp MP, et al. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc Natl Acad Sci USA. 2003;100(12):6952–7. doi: 10.1073/pnas.0732111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. 2002;71(1):66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Kim BE, Dufner-Beattie J, Petris MJ, Andrews G, Eide DJ. Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter. Hum Mol Genet. 2004;13(5):563–71. doi: 10.1093/hmg/ddh049. [DOI] [PubMed] [Google Scholar]
- 24.Taylor KM, Nicholson RI. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim Biophys Acta. 2003;1611(1-2):16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 25.Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA. 2005;102(19):6843–8. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tominaga K, Kagata T, Johmura Y, Hishida T, Nishizuka M, Imagawa M. SLC39A14, a LZT protein, is induced in adipogenesis and transports zinc. FEBS J. 2005;272(7):1590–9. doi: 10.1111/j.1742-4658.2005.04580.x. [DOI] [PubMed] [Google Scholar]
- 27.Costello LC, Levy BA, Desouki MM, et al. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol Ther. 2011;12(4):297–303. doi: 10.4161/cbt.12.4.16356. [DOI] [PMC free article] [PubMed] [Google Scholar]