Abstract
Background
Epigenetic programming, dynamically regulated by histone acetylation, is a key mechanism regulating cell proliferation and survival. Little is known about the contribution of histone deacetylase (HDAC) activity to the development of pulmonary arterial hypertension (PAH), a condition characterised by profound structural remodelling of pulmonary arteries and arterioles.
Methods and results
HDAC1 and HDAC5 protein levels were elevated in lungs from human idiopathic PAH and in lungs and right ventricles from rats exposed to hypoxia. Immunohistochemistry localised increased expression to remodelled vessels in the lung. Both valproic acid (VPA), a class I HDAC inhibitor, and suberoylanilide hydroxamic acid (SAHA), an inhibitor of class I, II and IV HDACs, mitigated the development and reduced established hypoxia-induced pulmonary hypertension in the rat. Both VPA and SAHA inhibited the “imprinted” highly proliferative phenotype of fibroblasts and R-cells from pulmonary hypertensive bovine vessels and PDGF-stimulated growth of human vascular smooth muscle cells in culture. Exposure to VPA and SAHA was associated with increased levels of p21and FOXO3 and reduced expression of survivin. The significantly higher level of expression of cKIT, MCP-1, IL-6, SDF-1, PDGFb and S100A4 in the R-cells were down regulated by VPA and SAHA treatment.
Conclusions
Increased HDAC activity contributes to the vascular pathology of pulmonary hypertension. The effectiveness of HDAC inhibitors VPA and SAHA, in models of PAH, support a therapeutic strategy based on HDAC inhibition in PAH.
Introduction
The lungs of patients with advanced pulmonary arterial hypertension (PAH) exhibit a vascular remodelling involving all cellular elements of the vessel wall, caused by dysregulated cell proliferation and survival, inflammation and in-situ thrombosis1, 2. Current approved treatments target primarily an imbalance of vasoactive factors in PAH3 and at best retard the course of the disease. There is an urgent need for therapies that target directly the structural vascular pathology.
Aberrant epigenetic changes, such as histone acetylation state, influence gene expression and play a role in regulating cell proliferation, migration and survival and inflammation in several diseases, including cancer4, 5 Histone acetylation/deacetylation balance, dynamically maintained by two important families of enzymes, histone acetyltransferases (HAT) and histone deacetylases (HDAC)6, controls the higher-order structure of chromatin and the resultant accessibility of transcriptional factors to their target genes7. HATs catalyze the acetylation of lysine residues, neutralizing positive charges, relaxing chromatin structure and increasing accessibility to transcription machinery. HDACs remove acetyl groups from histones (and other nuclear proteins), inducing chromatin condensation and transcriptional repression8, 9. HDACs have emerged as key targets to reverse aberrant epigenetic changes associated with cancer and autoimmune disease and HDAC inhibitors show promise as anti-cancer and anti-inflammatory agents9, 10
The present understanding of epigenetic modifications through histone acetylation in PAH is very limited. Li et al (2011) have described pulmonary adventitial fibroblasts from chronically hypoxic calves that expressed an epigenetically altered proinflammatory phenotype; the phenotype was reversed by HDAC inhibition11. Recently, selective class I HDAC inhibition has been reported to attenuate the development hypoxia-induced pulmonary hypertension in the rat through an anti-proliferative mechanism12. Moreover, right ventricular (RV) function was preserved, in contrast to experience with the pan-HDAC inhibitor trichostatin A (TSA) in rat PAB models13. However, the involvement of HDACs in human PH has not been explored nor has the ability of HDAC inhibitors to reverse existing PAH. Here we show increased expression of HDACs 1, 4 and 5 in human idiopathic PAH (IPAH) lung and the association of HDAC1 and HDAC5 with remodelled vessels. Valproic acid (VPA), a class I HDAC inhibitor, and suberoylanilide hydroxamic acid (SAHA), an inhibitor of classes I, II and IV, are effective in reversing pulmonary hypertension in the hypoxic rat and exert anti-proliferative and anti-inflammatory effects in human and animal vascular cells in culture. The data provide further compelling evidence that HDACs should be explored as therapeutic targets in pulmonary vascular disease.
Methods
Human Tissues
Human lung samples (lobectomy and IPAH) were obtained from the Imperial College Pulmonary Hypertension biorepository (ethics reference numbers: 01-210 & 2001/6003). The patients’ characteristics have been described previously14.
Animals and experimental design
Adult male Sprague-Dawley (SD) rats (body weight 200-250g) (Charles River, UK) were used. All experiments were conducted in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 (London, UK). Consecutive in vivo experiments were designed as follows: a) Chronic hypoxia - time course; rats were divided into 4 groups (n=3) and exposed either to normoxia or hypoxia (normobaric, FIO2 = 10%) for 2 days, one week and two weeks15. Haemodynamic parameters were measured and tissues were collected for biochemical and histological examination. b) Chronic hypoxia - prevention study with valproic acid (VPA); rats were divided into 4 groups (n=6) and exposed to (i) normoxia (NC), (ii) hypoxia for two weeks (HC), (iii) hypoxia plus VPA (sodium salt, Sigma-Aldrich) 100mg/kg/day in drinking water (low VPA) and (iv) hypoxia plus treatment with VPA 300mg/kg/day (high VPA)16. Drug treatment was started 3 days prior to hypoxia and continued for the 2 weeks duration of exposure. c) Chronic hypoxia - treatment study with VPA and SAHA; rats were divided into five groups (n=9-12) and exposed to (i) normoxia (NC), (ii) hypoxia for 2 weeks (2WH), (iii) hypoxia for 4 weeks (4WH), (iv) hypoxia and VPA (300mg/kg/day) and (v) hypoxia and SAHA (Chemos GmbH) (50mg/kg/day)17. Drug treatment was given in drinking water and initiated after two weeks hypoxia exposure and continued for the remaining 2 weeks of exposure. VPA was dissolved in distilled water and SAHA in 5 molar equivalents 2-hydroxypropyl-β-cyclodextrin (HOP-β-CD, Sigma-Aldrich) as previously described18. The 4WH group was given HOP-β-CD solution (1.38g/kg/day) as vehicle control. Animals were weighed every other day and treatment doses were calculated accordingly.
Haemodynamic measurements, tissue collection and histology examination
Rats were anaesthetised (Hypnorm 1ml/kg i.m; Hypnovel 0.8ml/kg i.p). Right ventricular systolic pressure (RVSP) and pulmonary arterial pressure (PAP) were measured via a pre-curved catheter inserted through the right jugular vein and systemic blood pressure (SBP) recorded in the carotid artery cannulation using a PowerLab Data Acquisition system (ADInstruments Ltd).
The animals were then killed, tissues were collected, snap frozen, and stored at −80°C for biochemical measurements. Hearts were dissected and weighed, and the ratio of RV to left ventricle plus the septum mass was used as an index of RVH (RV/LV+sep). The left lung was fixed with 10% formalin in phosphate-buffered saline and processed for elastic Van Gieson (EVG) and H&E staining. Vessels less than 100 μm in peripheral lung were counted blindly under microscope (40×) and pulmonary vascular remodelling was expressed as the proportion of vessels with double elastic lamina to total vessels counted.
Cell culture
Adventitial fibroblasts (PH-fibs) and morphologically distinct cells potentially of hematopoetic origin with high growth potential (rhomboidal or “R”-cells) were isolated from the adventitial and the medial layer of distal pulmonary arteries of chronically hypoxic calves, using explant techniques as described previously11, 19. Experiments were performed on cells at passage 4-10 and compared to fibroblasts and smooth muscle cells isolated from control animals (CO-Fibs, CO-SMC) and studied at similar passage number. For proliferation assays, cells were serum deprived and cultured for 3 days. Cell numbers were counted every day. The effects of varying concentrations of VPA (1mM, 2.5mM, and 5mM) and SAHA (10μM) on cell proliferation were also examined under serum deprived conditions and cell numbers were counted at day 3. For biochemical studies, serum starved cells were treated with VPA (5mM) or SAHA (10μM) for 24hrs and RNA were extracted for RT-PCR analysis. Primers are listed in Supplementary data Table 1.
Human pulmonary smooth muscle cells (passages 5 to 9) were assessed for proliferation by hemocytometer or BrdU incorporation assay (Millipore). Cells were serum deprived and stimulated with 50ng/ml platelet-derived growth factor (PDGF, Ebioscience) in 10% FBS with/without HDAC inhibitors treatment (VPA 1-2mM and SAHA 2.5-10 μM)20 for 72 hours. Individual experiments were repeated at least 3 times. In addition, harvested cells were stained with 50μg/ml propidium iodide (PI, Sigma) to access the cell cycle distribution and viability by using Beckton-Dickinson Fluorescent-activated cell sorting (FACS) Calibur flow cytometer system with Cell Quest software (Beckton-Dickison, Cyflogic version 1.2.1).
Western Blotting
Rat (lungs, RV and kidneys) and human (lobectomy and IPAH lung) samples were homogenized in phosphate buffer (100mM DO4 [K2HPO4:KH2PO4 = 3:1], 1mM EDTA, 1mM DTT supplemented with protease inhibitor, Roche). Proteins from cell pellets were extracted using TGN buffer (1M Tris, 2.5M NaCl, glycerol, 0.5M β-glycerophosphate, Tween 20, Nonidet P40 and protease inhibitor). Western blotting was performed as per manufactures’ suggestions (rabbit polyclonal antibodies against human HDACs, 1:1000, Cell signalling; mouse polyclonal antibodies against human Bcl-2, 1:1000, BD Biosciences). Proteins were detected by Novex® ECL chemiluminescent kit (Invitrogen). Optical densities of individual bands were measured and protein expressions were standardised with α-actin for the RV and β-actin for the other tissues.
Immunohistochemistry
Tissue sections were processed as previously described21. Sections were incubated with normal goat serum (DAKO, UK; 1:5 dilution in PBS), followed by purified mouse antibody against HDAC1 (1:50, Cell Signalling), or rabbit antibody against Ki67 (1:50, Thermo Scientific), or HDAC5 (1:50, Cell Signalling Technology), and then secondary antibody against mouse or rabbit (SignalStain® Boost IHC Detection Reagent HRP; Cell Signalling). Peroxidase activity was visualized with diaminobenzidine tetrahydrochloride (DAB 0.5 mg/ml; Sigma, UK), and sections were counterstained with Mayer’s haematoxylin.
Histone extraction and acetylation assessment
Cell pellets or tissues were washed with ice-cold PBS containing 5mM sodium butyrate. Lysis buffer (10 mM Tris, 50mM sodium bisulfate, 10 mM MgCl2, Sacarose 8.6%, 1% Triton X-100) was added on ice for 15 minutes. After homogenization, the pellets were washed with lysis buffer and then Tris-EDTA (10mM Tris, 13mM EDTA). Precipitated nuclei were suspended in ice-cold dH2O and 0.4M sulfuric acid (1:1) and kept on ice for one hour. Following centrifugation at 15000 rpm, histone was precipitated with 1ml acetone at −20°C overnight. Acetylation was detected on Western blotting using anti-acetylated H3 and H4 antibodies (Millipore). Parallel gels were executed with anti-whole H3 and H4 antibodies (Millipore) as loading control for quantification.
RT-PCR
Total RNA isolation from cultured cells, first-strand cDNA synthesis, and real-time RT-PCR were performed as described previously22. mRNAs were quantified by TaqMan® real-time RT-PCR using the commercially available specific primer-probes(Supplementary data Table1). Real-time PCR reactions were set following the manufacture’s conditions. Threshold cycle (Ct) values obtained for each gene were converted to the linear form using the term 2−ΔΔCt as a value directly proportional to the copy number of mRNA23, where ΔΔCt was the Ct value normalized by β-Actin and referenced to the values obtained for each gene under normoxic conditions.
Statistics
One-way ANOVA was applied for data analysis, followed by Mann-Whitney t-test between two groups. Data are presented as the mean ± SEM. Statistical significance was set at p value<0.05.
Results
HDAC1 and HDAC5 expression is increased in lungs from IPAH patients and lungs and RV from the chronically hypoxic rat
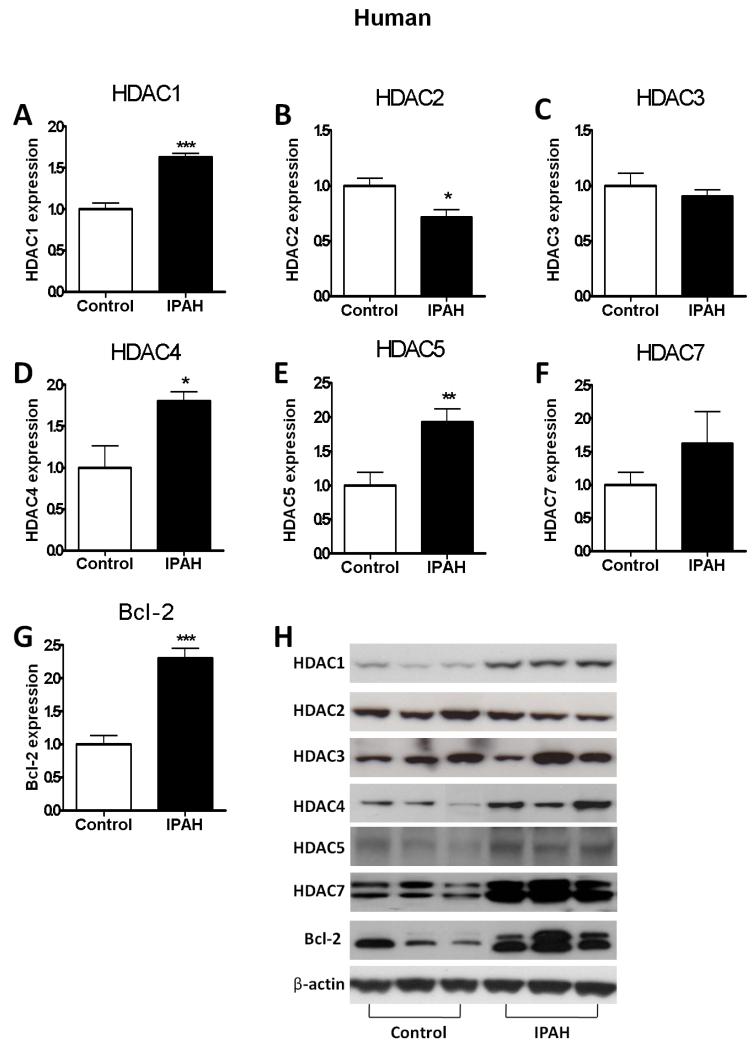
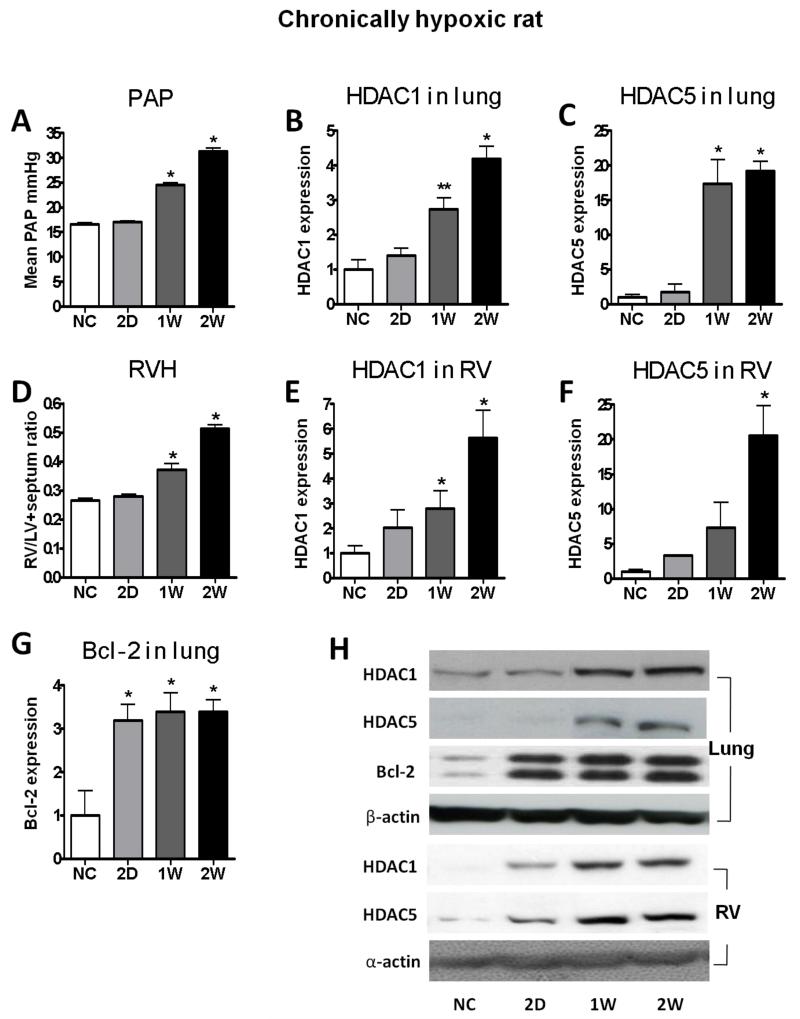
HDAC (Class I: HDAC1, 2, 3; Class II: HDAC 4, 5, 7) proteins levels were measured in human and rat lung samples. Human IPAH lung exhibited increased expression of HDAC1, HDAC4 and HDAC5 and decreased expression of HDAC2 and HDAC 3 compared with control lung tissue, along with a two-fold increase in the anti-apoptotic regulator, Bcl-2 (Figure 1). Hypoxic rats with an elevated mean PAP and RVH (Figure 2A, 2D) exhibited a striking increase in HDAC1 (Figure 2B, 2E) and HDAC5 (Figure 2C, 2F) expression in both lung and RV, again accompanied by elevation of Bcl-2 in the lung (Figure 2G and Supplementary data).
Figure 1.
HDAC protein expression levels in human lung extracts from IPAH patients (n=12) and lobectomy (control, n=21). (A) HDAC1, (B) HDAC2, (C) HDAC3, (D) HDAC4, (E) HDAC5, (F) HDAC7 (G) Bcl-2, and (H) representative bands. The data are generated from optical density measurements of individual bands from Western blots and normalised to β-actin. The ratios are presented as mean ± SEM of fold change relative to control. * p<0.05, ** p<0.005, *** p<0.0001 compared with control group.
Figure 2.
Time course of development of pulmonary hypertension phenotype and HDAC expression in chronically hypoxic rat. (A) PAP, mean pulmonary arterial pressure, (B) HDAC1 in lung, (C) HDAC5 in lung, (D) RVH, RV hypertrophy, (E) HDAC1 in RV, (F) HDAC5 in RV, (G) Bcl-2 in lung, and (H) representative bands. Rats were exposed to normal air (NC) or hypoxia for 2 days (2D), 1 week (1W) and 2 weeks (2W). The data are generated from optical density measurements of individual bands from Western blots and normalised to β-actin. The ratios are presented as mean ± SEM of fold change relative to control (normal air). n≥3 for each group. * p<0.05, ** p<0.005 compared with NC group.
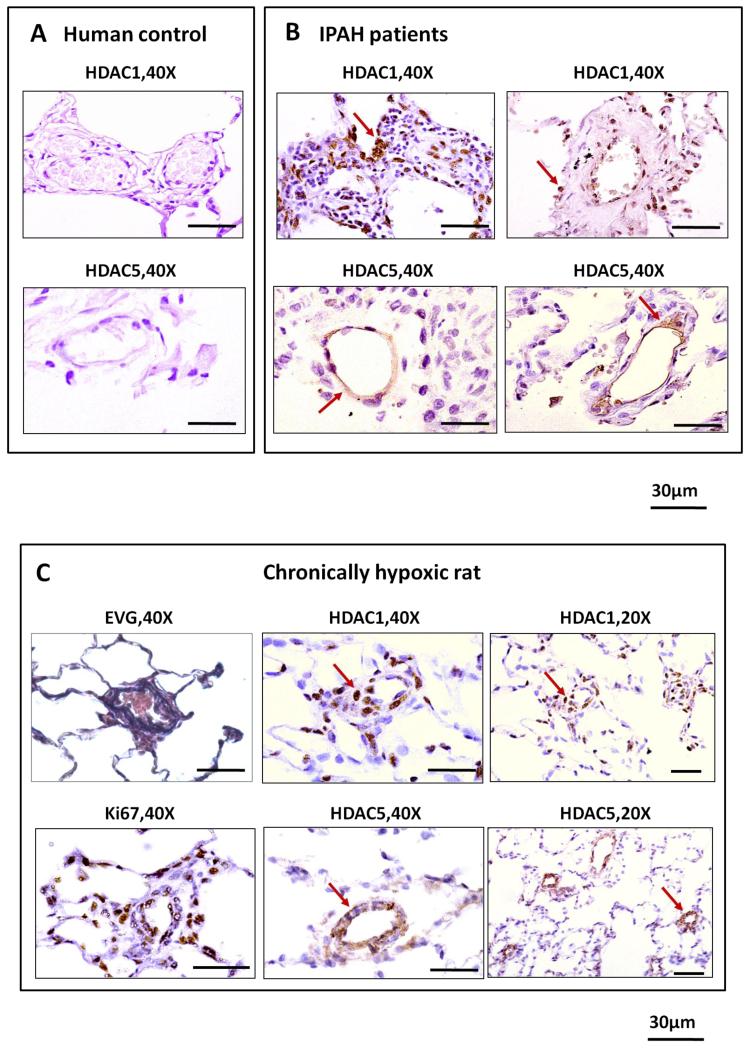
Consistent with a role in the pathology of pulmonary hypertension, HDAC1 and HDAC5 immunostaining was observed in remodeled pulmonary vessels of IPAH and hypoxic rat lungs, in contrast to control tissues (Figure 3 and Supplementary data). In keeping with their known function, HDAC1 showed a predominantly nuclear distribution while HDAC5 was cytoplasmic. The vascular distribution of HDAC1 nuclear immunostaining overlapped with Ki67 expression, a marker of proliferation, in the rat lung (Figure 3C).
Figure 3.
Immunohistochemistry of HDAC1 and HDAC5 in human and rat lungs. (A) Both HDAC1 and HDAC5 exhibit relatively weak expression in control lobectomy lung sections. (B) HDAC1 exhibits predominantly nuclear distribution in perivascular and vascular cells of remodelled vessels while HDAC5 is seen mainly in cytoplasm in IPAH lung sections. (C) Prominent HDAC1 and Ki67 expression in nuclei of perivascular and vascular cells while HDAC5 has a cytoplasmic distribution in vascular cells of remodelled vessels in lung sections from rats exposed to hypoxia for 4 weeks.
VPA and SAHA ameliorate established pulmonary arterial hypertension in chronic hypoxia
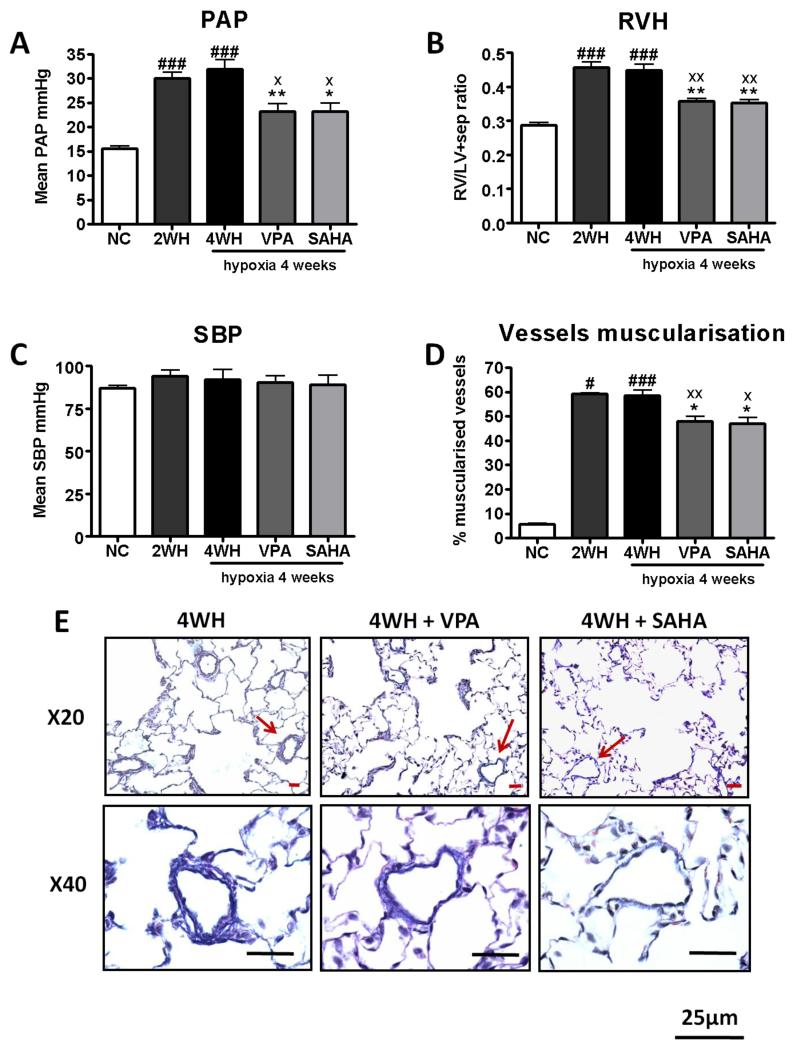
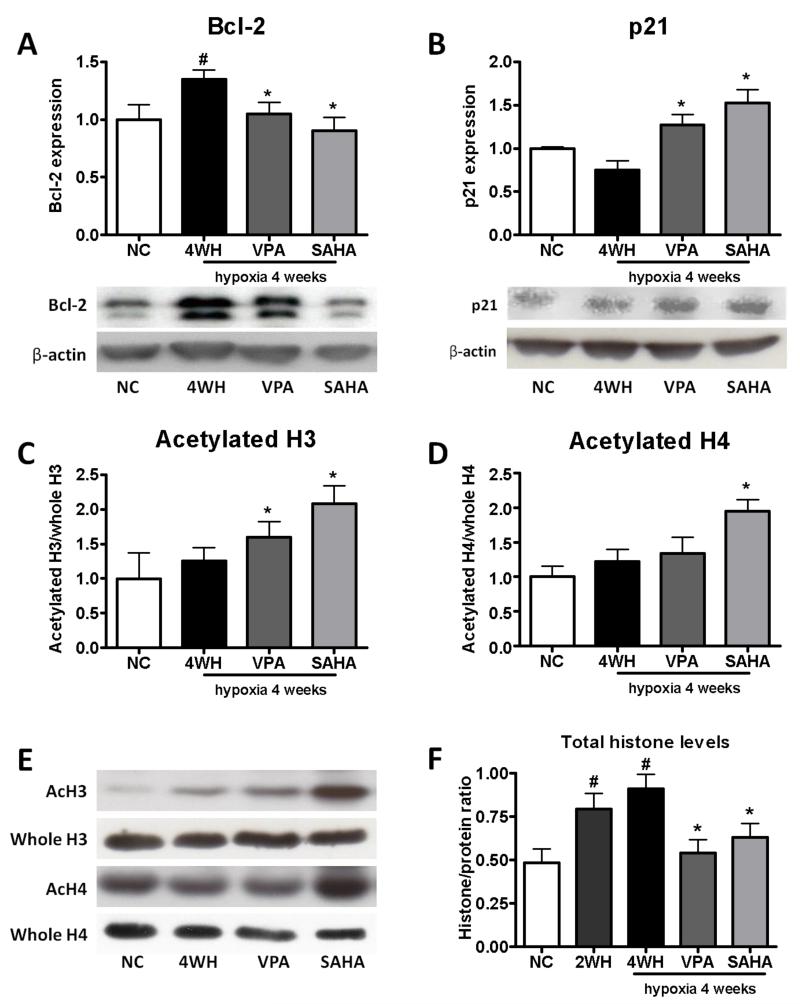
To address the role of increased HDAC1 and HDAC5 levels, we investigated the effects of VPA (Class I inhibitor) and SAHA (Class I, II and IV inhibitor) in the hypoxic rat. Supportive of a previous study, treatment with VPA at the start of hypoxia attenuated the development of pulmonary hypertension (Supplementary data). More important, treatment with VPA or SAHA after pulmonary hypertension was established reduced mean PAP (Figure 4A) and RV hypertrophy (Figure 4B) with no significant changes in heart rate or systemic blood pressure (Figure 4C). Consistent with an effect on vascular remodelling, pulmonary arteriolar muscularisation was significantly reduced (Figure 4D, 5E), along with Bcl-2 (Figure 5A), while lung p21 levels were increased by VPA and SAHA treatment (Figure 5B).
Figure 4.
Treatment of established hypoxia-induced pulmonary hypertension. Valproic acid or SAHA administered during the last 2 weeks of 4 week hypoxia exposure. (n≥6 for each group). (A) Mean pulmonary artery pressure, PAP, (B) right ventricular hypertrophy, RV/LV+sep, (C) systolic blood pressure, SBP, (D) percentage of muscularised vessels, and (E) Elastic van Gieson. NC: normoxia; 2WH: hypoxia for 2 weeks; 4WH: hypoxia for 4 weeks; VPA: hypoxia with VPA 300 mg/kg/day, and SAHA: hypoxia with SAHA 50 mg/kg/day. The data are presented as mean± SEM. n≥6 for each group. * p<0.05, ** p<0.005 compared with 4WH. x p<0.05, xx p<0.005 compared with 2WH, # p<0.05, ## p<0.05, ### p<0.0005 compared with NC.
Figure 5.
Expression of Bcl-2, p21 and acetylated histone levels in rat lung extracts from chronic hypoxia treatment study. (A) Bcl-2, (B) p21, (C) acetylated H3, (D) acetylated H4, (E) representative blots, (F) total histone levels. The data are generated from optical density measurements of individual bands from Western blots. Bcl-2 and p21 protein expression is normalised to β-actin, acetlyated histone to total hisotne, and total histone levels to total protein level. Data are presented as mean ± SEM of fold change relative to NC. n=6. NC: normoxia; 4WH: hypoxia for 4 weeks; VPA: hypoxia with VPA 300 mg/kg/day; SAHA: hypoxia with SAHA 50 mg/kg/day. * p<0.05 compared with 4WH, # p<0.05 compared with NC.
Histone acetylation levels were augmented by SAHA and VPA treatments
As evidence of HDAC inhibition, VPA increased acetylated histone H3 levels while both H3 and H4 acetylation levels were increased > 60% in SAHA treated lungs (Figure 5C-E). Interestingly, total histone levels were elevated in the rat lung after hypoxia exposure, a measure of protein synthesis, and this was reduced by VPA and SAHA treatment (Figure 5F).
HDAC inhibitors prevent constitutive growth of PH-Fibs and ‘R’-cells
Previous studies have shown that cells with high proliferative potential and stably increased HDAC activity can be isolated and perpetuated in culture from both the adventitia (PH-Fibs) and media (R-cells) of the hypertensive bovine pulmonary artery11, 24. It has been speculated that these cells with high proliferative capacity contribute selectively to the remodeling process in pulmonary hypertension3. Significantly increased class I HDAC catalytic activity has been shown in PH-Fibs compared to CO-Fibs11. In this study, class I HDAC mRNA levels were also significantly increased in R-cells compared to CO-SMC; no differences of HDAC-2 and HDAC-3 levels were detected (Supplementary data).
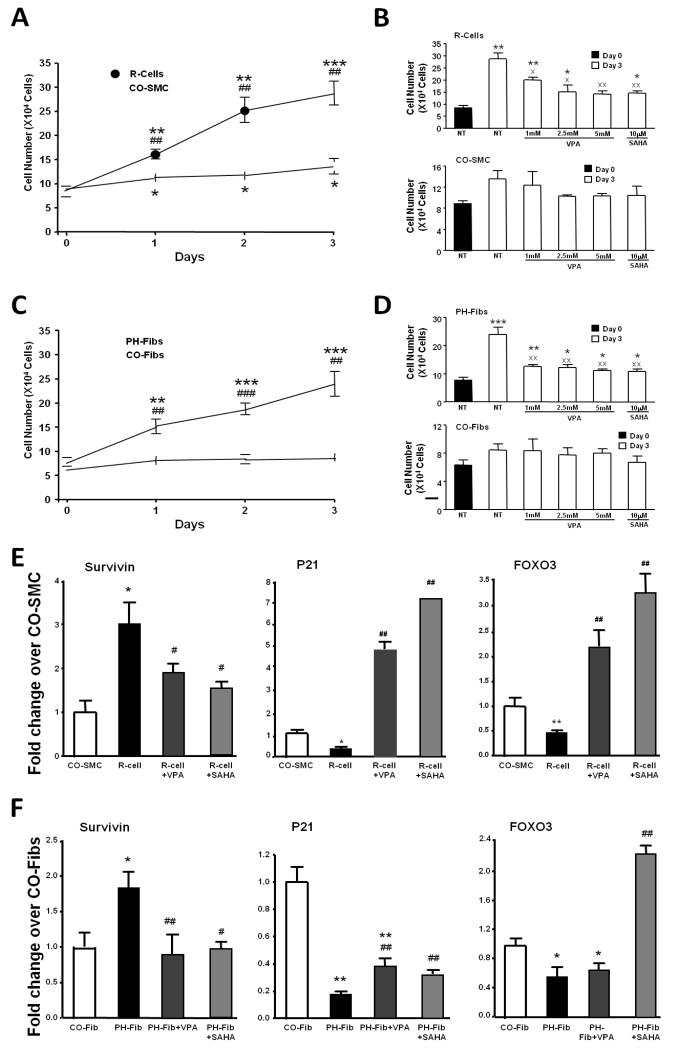
Under serum deprived conditions, R-cells and PH-Fibs exhibited greater proliferation than CO-SMC and CO-Fibs (Figure 6A, 6C). The HDAC inhibitor VPA, at concentrations of 2.5mM and 5mM, significantly inhibited the growth of “R”-cells but had no effect on CO-SMC growth or viability (Fig. 6B). A similar response was observed in PH-Fibs with again no effect seen on CO-Fibs (Fig. 6D). SAHA (10μM) also inhibited cell growth, with the greatest sensitivity exhibited by “R”-cells and PH-Fibs (Figure 6B, 6D). At the concentrations tested, no significant effects on cell viability were found (Supplementary data).
Figure 6.
Effects of histone deacetylase inhibition on bovine R-cells and fibroblasts PH-Fibs in culture. Effects on proliferation are assessed by counting: (A) Daily cell counts of serum deprived R-cells and control smooth muscle cells (CO-SMC) in 3 days, (B) Cell counts of R-cells and CO-SMC, untreated and treated with increasing concentrations of VPA (1mM, 2.5mM, and 5mM), SAHA (10μM) for 3 days, (C) Daily cell counts of serum deprived PH-Fibs and control fibroblasts (CO-Fibs) in 3 days, (D) Cell counts of PH-Fibs and CO-Fibs, untreated and treated with increasing concentrations of VPA (1mM, 2.5mM, and 5mM), SAHA (10μM) for 3 days. The data are presented as mean ± SEM of cell numbers. NT: untreated. * p<0.05, ** p<0.01, *** p<0.001 compared with day 0 within the same cell type, ## p<0.01, ### p<0.001 compared with CO-SMC or CO-Fibs on the same day, x p<0.05, xx p<0.01 compared with untreated on day 3. Effects of VPA (5mM) and SAHA (10μM) for 24 hours on gene expression are assessed by RT-PCR: (E) mRNA expression of genes involved in the cell cycle and apoptosis, including survivin, p21 and FOXO3. The data are presented as mean ± SEM of fold change to either CO-SMC or CO-Fibs. * p<0.05, ** p<0.01 compared with CO-SMC or CO-Fibs, #, p<0.05, ## p<0.01 compared with untreated R-cells or PH-Fibs.
We sought to determine mechanisms for VPA and SAHA induced growth inhibition. Constitutively activated “R”-cells and PH-Fibs expressed significantly higher levels of survivin and lower levels of p21 and FOXO3 under basal, serum-free conditions than their respective control cells, CO-SMC and CO-Fibs (Figure 6E and F). Treatment with VPA and SAHA led to significant increases in p21 and FOXO3 mRNA levels in both “R”-cells and PH-Fibs and a significant decrease in survivin in both cell types (Figure 6E, 6F).
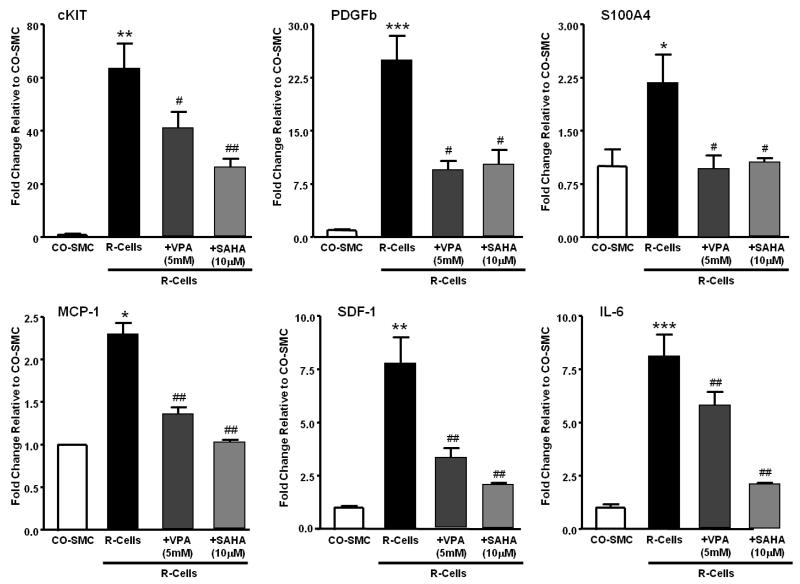
A previous study showed that R-cells expressed significantly higher levels of progenitor cell markers (cKIT), pro-inflammatory factors (MCP-1, IL-6, SDF-1) and growth factors (PDGFb and S100A4)19. In this study, treatment with VPA and SAHA significantly decreased expression of these genes in R-cells (Figure 7).
Figure 7.
Effects of VPA (5mM) and SAHA (10μM) on gene expression of growth factor (cKIT, PDGFb, S100A4) and pro-inflammatory factor (MCP-1, SDF-1, IL-6) in serum deprived R-cells. Cells were untreated or treated with VPA (5mM) for 24 hours and mRNA expression is analysed by RT-PCT. The data are presentes as mean ± SEM of fold change to either CO-SMC. * p<0.05, ** p<0.01 *** p<0.001 compared with CO-SMC, #, p<0.05, ## p<0.01 compared with untreated R-cells.
HDAC inhibitors prevent PDGF-induced human pulmonary smooth muscle cell proliferation
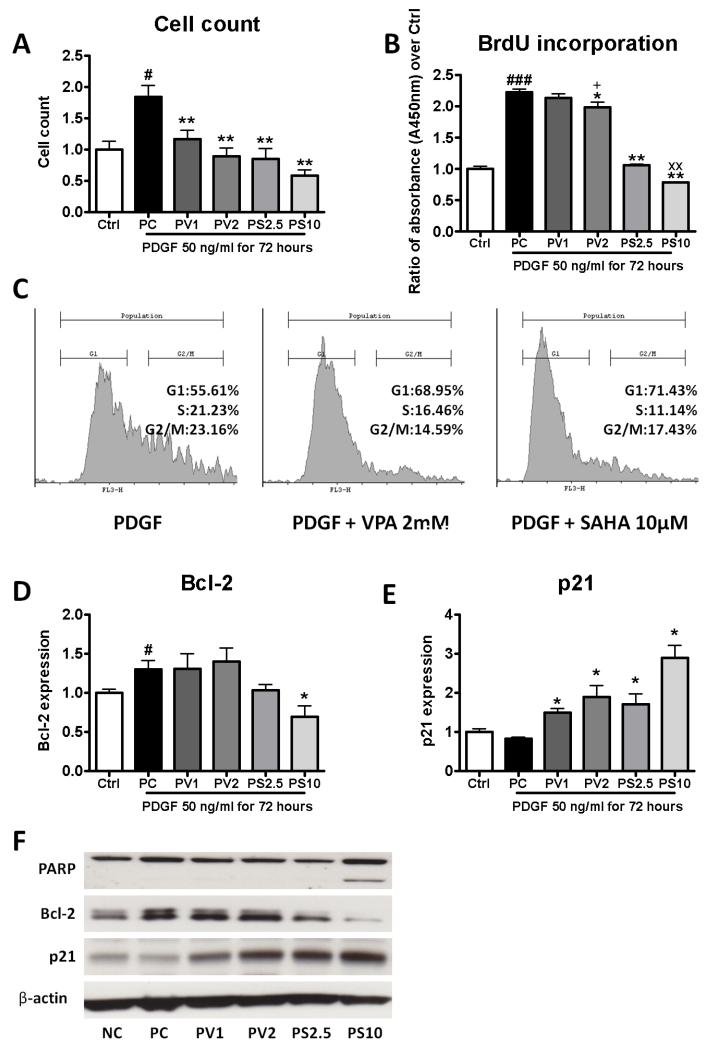
A direct inhibitory effect of VPA and SAHA on PDGF-stimulated human pulmonary smooth muscle cell proliferation was observed (Figure 8A, 8B). VPA and SAHA arrested cell growth at the G1-S phase, reversing the shift caused by PDGF stimulation (Figure 8C). The percentage of cells in G1 (PDGF: 55.6%) was higher in VPA (VPA 1mM: 63.4%; VPA 2mM: 69.0% p<0.05) and SAHA treated cells (SAHA 2.5μM: 71.5%; SAHA 10μM: 71.4%, p<0.05) (Supplementary data). Bcl-2 expression was reduced by SAHA treatment (Figure 8D). Both VPA and SAHA led to increased p21 expression in cells (Figure 8E). Furthermore, SAHA treatment at 10 μM resulted in PARP cleavage to an 85kDa fragment (Figure 8F).
Figure 8.
Effect of histone deacetylase inhibition on PDGF-stimulated human pulmonary smooth muscle cells in culture. (A) cell counts; (B) BrdU incorporation assay; (C) FACS analysis for cell cycle distribution showing both VPA and SAHA arrested cell growth at G1-S phase; (D) p21 and (E) Bcl-2 protein expression. (F) PARP cleavage. Data are presented as mean ± SEM of fold change relative to control (Ctrl). Each experiment was repeated at least 3 times with separate cell preparations. Ctrl: control, PC: PDGF, PV1: PDGF+VPA 1mM, PV2: PDGF+VPA 2mM, PS2.5: PDGF+SAHA 2.5μM, PS10: PDGF+SAHA 10μM. * p<0.05, ** p<0.005 compared with PC, xx p<0.005 compared with PS2.5, + p<0.05 compared with PV1, # p<0.05, ### p<0.0005 compared with Ctrl.
Discussion
This study demonstrates for the first time changes in the expression of HDAC proteins, specifically increased HDAC1 (class I) and HDAC5 (Class II), in human IPAH lung. These data were replicated in lungs and RV from rats with hypoxia-induced pulmonary hypertension. The lack of change in HDAC expression in the kidneys from these animals links the observed changes in HDAC expression to the pathology/vascular remodelling of pulmonary hypertension rather than the hypoxic stimulus per se. Immunohistochemical assessment of human IPAH and chronic hypoxic rat lungs confirmed increased nuclear expression of HDAC1 and cytoplasmic expression of HDAC5 in remodelled vessels, which also express the proliferative marker Ki67. Consistent with a functional role for HDACs in pulmonary hypertension, chronic administration of the HDAC class I inhibitor, VPA, not only prevented hypoxia-induced pulmonary hypertension but attenuated the phenotype in the rat when administered after pulmonary hypertension had become established. A similar effect was produced by the broad spectrum HDAC inhibitor, SAHA. VPA and SAHA were also effective in cell culture models. Both inhibited PDGF-stimulated human smooth muscle cell proliferation and the hyper-proliferation of epigenetically altered bovine R- cells and fibroblasts in culture. The precise molecular mechanisms by which HDAC inhibition exerts its effects in these models remain to be elucidated, but the changes in Bcl-2 and p21 expression in vivo and p21, FOXO3, cKIT, PDGFb, S100A4 and survivin in vitro support strongly a direct effect on cell division and survival. Downregulation of pro-inflammatory factors such as MCP-1, IL-6 and SDF-1 may also be involved.
HDACs are expressed in all eukaryotic cells and regulate many genes engaged in controlling cell proliferation, differentiation and survival. Eighteen HDACs have been identified in humans. Eleven contain highly conserved deacetylase domains and are zinc-dependent: class-I (HDAC1, 2, 3 and 8, nuclear localization); class-IIa (HDACs 4, 5, 7 and 9)/ class-IIb (HDACs 6 and 10) (cytoplasm and nuclear localization) and class-IV (HDAC11). Another seven HDACs, known as class III or sirtuins, require NAD for their enzymatic activity. The precise balance between the acetylated and deacetylated states of histones is an important feature of gene regulation. HDAC expression is increased in a number of human tumours and cancer cell lines25. In general, increased HDAC activity results in histone hypoacetylation, and this has been implicated in the initiation and progression of various tumours26-28. For example, HDAC1 is overexpressed in gastric, pancreatic, colorectal, prostate and hepatocellular cancers, and correlates with poor prognosis26, 29-31. The increase in total histone levels and HDAC1 and HDAC5 in human IPAH and lungs from pulmonary hypertensive rats, together with the increase in Bcl-2 expression, are consistent with the proliferative, apoptosis-resistant vascular pathology that characterises pulmonary hypertension2. The reduction in HDAC2 and HDAC3 in lungs from IPAH patients and hypoxic rats is consistent with a compensatory reaction to HDAC1 overexpression27.
We selected two HDAC inhibitors on the basis of (i) the pattern of HDAC expression in human lung and (ii) the potential to translate findings into clinical studies. VPA is used clinically as an antiepileptic drug and for some painful neuropathies, and has a low toxicity profile32. It has a complex pharmacology, which includes sodium channel blockade and class I HDAC inhibition (IC50 0.4mM)33. The doses chosen for our experiments were based on published studies in the range reported to inhibit HDAC116. Recent studies have reported that VPA has antitumor effects in various cancers through HDAC inhibition and clinical studies are ongoing34, 35. SAHA is a relatively broad spectrum HDAC inhibitor (class I, II and IV) that was approved by the FDA as a therapy for cutaneous T cell lymphoma in 2006 under the generic name Vorinostat36. In keeping with inhibition of HDAC activity, both VPA and SAHA increased lung histone H3 acetylation levels and SAHA increased H4 histone acetylation.
Both agents reversed the increase in total histone levels in the chronic hypoxic rat lung, an indirect measure of hyper-proliferation37. HDAC inhibitors exert their antineoplastic effects through multiple interacting processes38. Both VPA and SAHA have been reported to downregulate the antiapoptotic factor, Bcl-2, and activate proapoptotic factors, such as Bid and Bim39, 40, thereby increasing the ratio of pro- to anti-apoptotic proteins. The cyclin-dependent kinase (CDK) inhibitor, p21, is directly transcriptionally upregulated within hours of HDAC inhibitor treatment, leading to cell cycle arrest at G1/S phase via the tumour suppressor p5341, 42. Upregulation of p21 and downregulation of Bcl-2 expression in hypoxic rat lungs by VPA and SAHA is consistent with the notion that HDAC inhibition may reverse vascular remodelling by inhibiting proliferation and promoting apoptosis.
Further support for this comes from the measurements made on cells in vitro. Human pulmonary vascular smooth muscle cells exposed to VPA and SAHA showed increased p21 levels, and FACS analysis confirmed cell cycle arrest at the G1-S phase. We observed a significant attenuation of PDGF stimulated Bcl-2 expression with SAHA, but not VPA. PARP cleavage, another signature of apoptosis, was observed in cells exposed to SAHA (10μM), indicating a pro-apoptosis action.
The fibroblasts and R-cells (potentially of haematopoetic origin) derived from the distal pulmonary arteries of chronically hypoxic calves display a stable hyperproliferative / apoptosis resistant phenotype, even under serum deprived ex-vivo conditions11, 19, and thus offer a cell model to study the effects of HDAC inhibition in cells displaying an epigenetically altered and stable ex-vivo phenotype. The phenotypic changes displayed by the PH-Fibs are strikingly similar to those described in rheumatoid arthritis fibroblasts where changes in HDAC activity have been shown to contribute to their inflammatory/destructive phenotype43. HDAC1 catalytic activity is significantly elevated in PH-Fibs and R-cells compared to control cells11. The greater growth inhibitory effect of VPA and SAHA on PH-Fibs and R-cells compared to control cells might reflect specific modulation of epigenetically altered signalling pathways in these cells. Similar results have been observed in studies of cancer, where transformed cells are highly sensitive to the apoptosis-inducing effects of HDAC inhibitors compared to normal controls44. Here, we show that PH-Fibs and “R” cells express significantly lower levels of p21 and FOXO3 and higher levels of survivin compared to cells from control animals (CO-fibs and CO-SMC) under serum-starved conditions. The increase in p21 and FOXO3 when exposed to VPA and SAHA is compatible with the inhibition of constitutive cell growth. Survivin, a member of the ‘inhibitor of apoptosis’ family, is associated with the development of PH etiologically45. Interestingly, both VPA and SAHA reduced survivin levels in PH-Fibs and R-cells, arguing that VPA and SAHA target the apoptosis-resistant remodelled cells in PAH. The significantly higher levels of pro-inflammatory factors, including MCP-1, SDF-1 and IL-6 in the R-cell was downregulated by VPA treatment, confirming the anti-inflammatory mechanism of HDAC inhibition as described previously19.
The data in this study come from end-stage human PAH, a rodent model and cells in culture. Each has their limitations as a readout for the human condition but collectively support the argument that changes in HDAC activity may participate in the vascular pathology of pulmonary hypertension, both IPAH and secondary to hypoxia. RV function was not examined in the rat model but no adverse effects on animal survival were observed. The data to date in the literature on RV function post pulmonary artery banding13, 46 are conflicting and indicate caution. However, recent data on RV function and gene expression with selective class I HDAC inhibition are more encouraging12. There is extensive human experience with VPA and it is generally well tolerated. SAHA is active against HDACs in the low nanomolar concentration range and does not accumulate in cardiac tissue47, 48. As the safety profile of both drugs in humans is well established, HDAC inhibition is an accessible therapeutic strategy to examine in patients with PAH.
Supplementary Material
Acknowledgments
SOURCE OF FUNDING
This research was supported by a project grant (PG/11/28/28844) from British Heart Foundation
Footnotes
Author Disclosures
Lan Zhao:
Research Grant: British Heart Fundation PG/11/28/28844, Amount: >= $10,000
Chien-Nien Chen: No disclosures
Nabil Hajji: No disclosures
Eduardo Oliver: No disclosures
Emanuele Cotroneo: No disclosures
John Wharton: No disclosures
Daren Wang: No disclosures
Min Li: No disclosures
Timothy A. McKinsey: No disclosures
Kurt R. Stenmark: No disclosures
Martin R. Wilkins: No disclosures
DISCLOSURES
None
Reference List
- 1.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, Haworth SG. Development and pathology of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S3–S9. doi: 10.1016/j.jacc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberharter A, Becker PB. Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002;3:224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards EJ, Elgin SC. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell. 2002;108:489–500. doi: 10.1016/s0092-8674(02)00644-x. [DOI] [PubMed] [Google Scholar]
- 6.Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006;23:289–296. doi: 10.1016/j.molcel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 8.Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, McKeon BA, Lemon DD, Horn TR, Anwar A, Barajas C, Stenmark KR. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol. 2011;187:2711–2722. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavasin MA, Demos-Davies K, Horn TR, Walker LA, Lemon DD, Birdsey N, Weiser-Evans MC, Harral J, Irwin DC, Anwar A, Yeager ME, Li M, Watson PA, Nemenoff RA, Buttrick PM, Stenmark KR, McKinsey TA. Selective Class I Histone Deacetylase Inhibition Suppresses Hypoxia-Induced Cardiopulmonary Remodeling Through an Antiproliferative Mechanism. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.111.258426. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogaard HJ, Mizuno S, Hussaini AA, Toldo S, Abbate A, Kraskauskas D, Kasper M, Natarajan R, Voelkel NF. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care Med. 2011;183:1402–10. doi: 10.1164/rccm.201007-1106OC. [DOI] [PubMed] [Google Scholar]
- 14.Abdul-Salam VB, Wharton J, Cupitt J, Berryman M, Edwards RJ, Wilkins MR. Proteomic analysis of lung tissues from patients with pulmonary arterial hypertension. Circulation. 2010;122:2058–2067. doi: 10.1161/CIRCULATIONAHA.110.972745. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L, Mason NA, Morrell NW, Kojonazarov B, Sadykov A, Maripov A, Mirrakhimov MM, Aldashev A, Wilkins MR. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;104:424–428. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Menendez V, Gilardini A, Bossi M, Canta A, Oggioni N, Carozzi V, Tremolizzo L, Cavaletti G. Valproate protective effects on cisplatin-induced peripheral neuropathy: an in vitro and in vivo study. Anticancer Res. 2008;28:335–342. [PubMed] [Google Scholar]
- 17.Wise LD, Turner KJ, Kerr JS. Assessment of developmental toxicity of vorinostat, a histone deacetylase inhibitor, in Sprague-Dawley rats and Dutch Belted rabbits. Birth Defects Res B Dev Reprod Toxicol. 2007;80:57–68. doi: 10.1002/bdrb.20104. [DOI] [PubMed] [Google Scholar]
- 18.Hockly E, Richon VM, Woodman B, Smith DL, Zhou X, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PA, Steffan JS, Marsh JL, Thompson LM, Lewis CM, Marks PA, Bates GP. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2003;100:2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, Sylman JL, Stenmark KR. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1059–L1072. doi: 10.1152/ajplung.90611.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedier A, Dedes KJ, Imesch P, Von Bueren AO, Fink D. The histone deacetylase inhibitors suberoylanilide hydroxamic (Vorinostat) and valproic acid induce irreversible and MDR1-independent resistance in human colon cancer cells. Int J Oncol. 2007;31 [PubMed] [Google Scholar]
- 21.Jin JS, Tsao TY, Sun PC, Yu CP, Tzao C. SAHA Inhibits the Growth of Colon Tumors by Decreasing Histone Deacetylase and the Expression of Cyclin D1 and Survivin. Pathol Oncol Res. 2012 doi: 10.1007/s12253-012-9499-7. In press. [DOI] [PubMed] [Google Scholar]
- 22.Oliver E, Rovira E, Monto F, Valldecabres C, Julve R, Muedra V, Ruiz N, Barettino D, D’Ocon P. beta-Adrenoceptor and GRK3 expression in human lymphocytes is related to blood pressure and urinary albumin excretion. J Hypertens. 2010;28:1281–1289. doi: 10.1097/HJH.0b013e3283383564. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Das M, Burns N, Wilson SJ, Zawada WM, Stenmark KR. Hypoxia exposure induces the emergence of fibroblasts lacking replication repressor signals of PKCzeta in the pulmonary artery adventitia. Cardiovasc Res. 2008;78:440–448. doi: 10.1093/cvr/cvn014. [DOI] [PubMed] [Google Scholar]
- 25.Marks PA. The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Exper Opin Investig Drugs. 2010;19:1049–1066. doi: 10.1517/13543784.2010.510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, Kristiansen G. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98:604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagger G, O’Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 2002;21:2672–81. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jurkin J, Zupkovitz G, Lagger S, Grausenburger R, Hagelkruys A, Kenner L, Seiser C. Distinct and redundant functions of histone deacetylases HDAC1 and HDAC2 in proliferation and tumorigenesis. Cell Cycle. 2011;10:406–12. doi: 10.4161/cc.10.3.14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JH, Kwon HJ, Yoon BI, Kim JH, Han SU, Joo HJ, Kim DY. Expression profile of histone deacetylase 1 in gastric cancer tissues. Jpn J Cancer Res. 2001;92:1300–1304. doi: 10.1111/j.1349-7006.2001.tb02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyake K, Yoshizumi T, Imura S, Sugimoto K, Batmunkh E, Kanemura H, Morine Y, Shimada M. Expression of hypoxia-inducible factor-1alpha, histone deacetylase 1, and metastasis-associated protein 1 in pancreatic carcinoma: correlation with poor prognosis with possible regulation. Pancreas. 2008;36:e1–e9. doi: 10.1097/MPA.0b013e31815f2c2a. [DOI] [PubMed] [Google Scholar]
- 31.Rikimaru T, Taketomi A, Yamashita Y, Shirabe K, Hamatsu T, Shimada M, Maehara Y. Clinical significance of histone deacetylase 1 expression in patients with hepatocellular carcinoma. Oncology. 2007;72:69–74. doi: 10.1159/000111106. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg G. The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? Cell Mol Life Sci. 2007;64:2090–2103. doi: 10.1007/s00018-007-7079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 34.Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo CF, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de lC-H, Herrera LA. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev. 2008;34:206–222. doi: 10.1016/j.ctrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z, Edwards RJ, Boobis AR. Increased expression of histone proteins during estrogen-mediated cell proliferation. Environ Health Perspect. 2009;117:928–934. doi: 10.1289/ehp.0800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci U S A. 2005;102:16090–16095. doi: 10.1073/pnas.0505585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu W, Ngo L, Perez G, Dokmanovic M, Marks PA. Intrinsic apoptotic and thioredoxin pathways in human prostate cancer cell response to histone deacetylase inhibitor. Proc Natl Acad Sci U S A. 2006;103:15540–15545. doi: 10.1073/pnas.0607518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zupkovitz G, Grausenburger R, Brunmeir R, Senese S, Tischler J, Jurkin J, Rembold M, Meunier D, Egger G, Lagger S, Chiocca S, Propst F, Weitzer G, Seiser C. The cyclin-dependent kinase inhibitor p21 is a crucial target for histone deacetylase 1 as a regulator of cellular proliferation. Mol Cell Biol. 2010;30:1171–1181. doi: 10.1128/MCB.01500-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawabata T, Nishida K, Takasugi K, Ogawa H, Sada K, Kadota Y, Inagaki J, Hirohata S, Ninomiya Y, Makino H. Increased activity and expression of histone deacetylase 1 in relation to tumor necrosis factor-alpha in synovial tissue of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R133. doi: 10.1186/ar3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A, Altucci L, Nervi C, Minucci S, Pelicci PG. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med. 2005;11:71–6. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- 45.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho YK, Eom GH, Kee HJ, Kim HS, Choi WY, Nam KI, Ma JS, Kook H. Sodium valproate, a histone deacetylase inhibitor, but not captopril, prevents right ventricular hypertrophy in rats. Circ J. 2010;74:760–770. doi: 10.1253/circj.cj-09-0580. [DOI] [PubMed] [Google Scholar]
- 47.Munster PN, Troso-Sandoval T, Rosen N, Rifkind R, Marks PA, Richon VM. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res. 2001;61:8492–8497. [PubMed] [Google Scholar]
- 48.Hendricks JA, Keliher EJ, Marinelli B, Reiner T, Weissleder R, Mazitschek R. In vivo PET imaging of histone deacetylases by 18F-suberoylanilide hydroxamic acid (18F-SAHA) J Med Chem. 2011;54:5576–5582. doi: 10.1021/jm200620f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.