Abstract
Background & Aims
Hypoxic inflammation (decreased oxygen tension at sites of inflammation) is a feature of inflammatory bowel disease (IBD). The hypoxia response is mediated by the transcription factors hypoxia-inducible factor (HIF)1α and endothelial PAS domain protein 1 (EPAS1 or HIF2α), which are induced in intestinal tissues of patients with IBD. HIF1α limits intestinal barrier dysfunction, but the role of EPAS1 has not been assessed under conditions of hypoxic inflammation or in models of IBD.
Methods
Acute colitis was induced by administration of Citrobacter rodentium ordextran sulfate sodium (DSS) to transgenic hypoxia reporter mice (ODD-Luc), mice with conditional overexpression of Epas1 (Epas1LSL/LSL), mice with intestinal epithelium-specific deletion of Epas1 (Epas1ΔIE), or wild-type littermates (controls). Colon tissues from these mice and from patients with ulcerative colitis (UC) or Crohn's disease (CD) were assessed by histologic and immunoblot analyses, immunohistochemistry, and quantitative PCR.
Results
Levels of hypoxia and EPAS1 were increased in colon tissues of mice following induction of colitis and patients with UC or CD, compared with controls. Epas1ΔIE mice had attenuated colonic inflammation and were protected from DSS-induced colitis. Intestine-specific overexpression of EPAS1, but not HIF-1α, led to spontaneous colitis, increased susceptibility to induction of colitis by C rodentium or DSS, and reduced survival times compared with controls. Disruption of intestinal epithelial EPAS1 attenuated the inflammatory response following administration of DSS or C rodentium, whereas intestine-specific overexpression of EPAS1 increased this response. We found EPAS1 to be a positive regulator of tumor necrosis factor (TNF)α production by the intestinal epithelium. Blocking TNFα completely reduced hypoxia-induced intestinal inflammation. We found EPAS1 to be a positive regulator of tumor necrosis factor (TNF)α production by the intestinal epithelium. Blocking TNFα completely reduced hypoxia-induced intestinal inflammation.
Conclusions
EPAS1 is a transcription factor that activates mediators of inflammation, such as TNFα, in the intestinal epithelium and promotes development of colitis in mice.
Keywords: mouse model, CD, UC, oxygen
Introduction
Hypoxia is a well-conserved transcriptional stimuli leading to an adaptive increase in the cellular response to limited oxygen availability. Inflammatory foci are hypoxic, and this focal hypoxia can drive the inflammatory cascade1, 2. The significance of this bidirectional crosstalk and the mechanistic role of hypoxia in inflammation are currently unclear. Hypoxia-induced signal pathway is transcriptionally mediated by hypoxiainducible factor (HIF)3, 4. In oxygen rich conditions, prolyl hydroxylases (PHD) hydroxylate HIF leading to its degradation by the von Hippel-Lindau tumor suppressor protein (VHL) coupled to the E3 ubiquitin ligase complex. Conversely, in conditions of low cellular O2 HIF is not hydroxylated and stabilized. Two transcriptionally active HIFs have been identified, HIF-1α and endothelial PAS domain protein 1 (EPAS1, also referred to as HIF-2α). They share a 48% sequence identity and regulate overlapping and distinct sets of genes critical in the adaptation to hypoxic environments.
A robust activation of HIF-1α and EPAS1 is observed in the intestinal epithelium from inflammatory bowel disease (IBD) patients5. IBD is an inflammatory disease of the intestine, which is grouped into two major types, Crohn's disease (CD) and ulcerative colitis (UC). While the etiology of CD and UC are not known, the dysregulation in intestinal epithelial barrier function and the mucosal immune response are critical in the pathogenesis of IBD. Recent studies demonstrate that HIF-1α in intestinal epithelial cells is critical in maintaining the intestinal epithelial barrier following inflammation6-10. Moreover, pharmacological activation of HIF signaling using PHD inhibitors is protective in mouse models of IBD11, 12. Therefore, it is still unclear the mechanism by which hypoxia exacerbate the inflammatory cascade2, 13. In this manuscript, using novel mouse models of intestinal HIF signaling, we demonstrate that EPAS1 is highly activated in epithelial cells from IBD patients and mouse models of colitis. Genetic activation of EPAS1 in epithelial cells leads to an increase in epithelial-derived pro-inflammatory mediators, which are critical in the initiation and progression of IBD.
Results
EPAS1 is activated in mouse models of colitis and human IBD
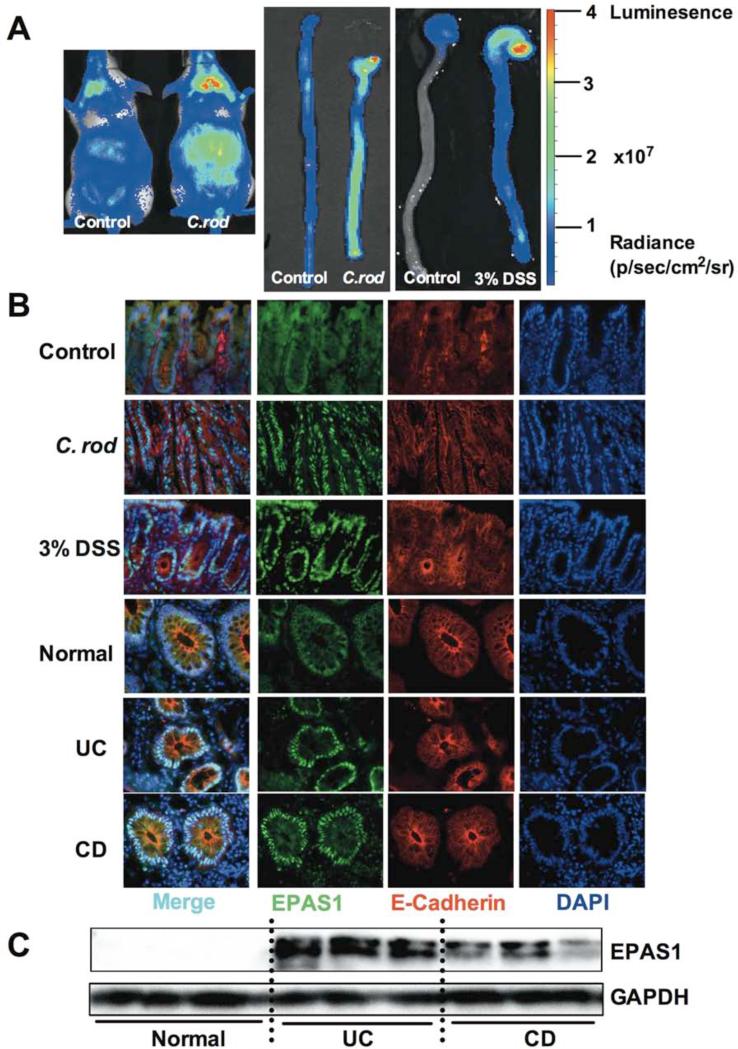
In order to test the presence of hypoxia in IBD, the oxygen dependent degradation (ODD)-luciferase hypoxia reporter mouse model was assessed following induction of acute colitis14. The ODD-luciferase mice were treated with Citrobacter rodentium (C. rod) a model of enteric infection-induced colitis or dextran sulfate sodium (DSS) an injury-induced colitis model15, 16. In vivo imaging demonstrated that the luciferase signal is increased in the abdomen of C. rod or 3% DSS treated mice (Figure 1A). This increase was consistent with a robust activation of luciferase signal in excised colons from the ODD-luciferase mice following C. rod or DSS treatment. EPAS1 expression was further assessed in colon samples from both murine experimental colitis and IBD patients by immunohistochemical staining (Figure 1B). In untreated mice and normal control human intestines there was a diffuse background staining by the EPAS1 antibody. However, it is clear that in inflamed mouse colon following C. rod or 3% DSS treatment or in UC or CD samples there was a robust increase in nuclear EPAS1 staining, which co-localizes to an epithelial specific marker, E-Cadherin. Western blot analysis further confirmed an increase in EPAS1 expression in individual UC and CD patients (Figure 1C). These results indicate that EPAS1 may play a critical role in the progression of IBD.
Figure 1. Activation of EPAS1 in mouse models of colitis and IBD.
(A) Whole animal or excised colon luminescent imaging of ODD-luciferase mice following C. rod infection for 11-days or 3% DSS treatment for 3-days or vehicle treatment (control). Luminescence intensity is scaled for radiance according to scale bars with gradient color peaks. (B) Immunohistochemical staining for the expression of EPAS1 and E-Cadherin in colons from mouse models of colitis (C. rod and DSS) or control mice and ulcerative colitis (UC), Crohn's Disease (CD) or normal colon tissues. (C) Western blotting analysis for the expression of EPAS1 in UC, CD or normal colon tissues.
Disruption of EPAS1 protects mice from DSS-induced colonic damage
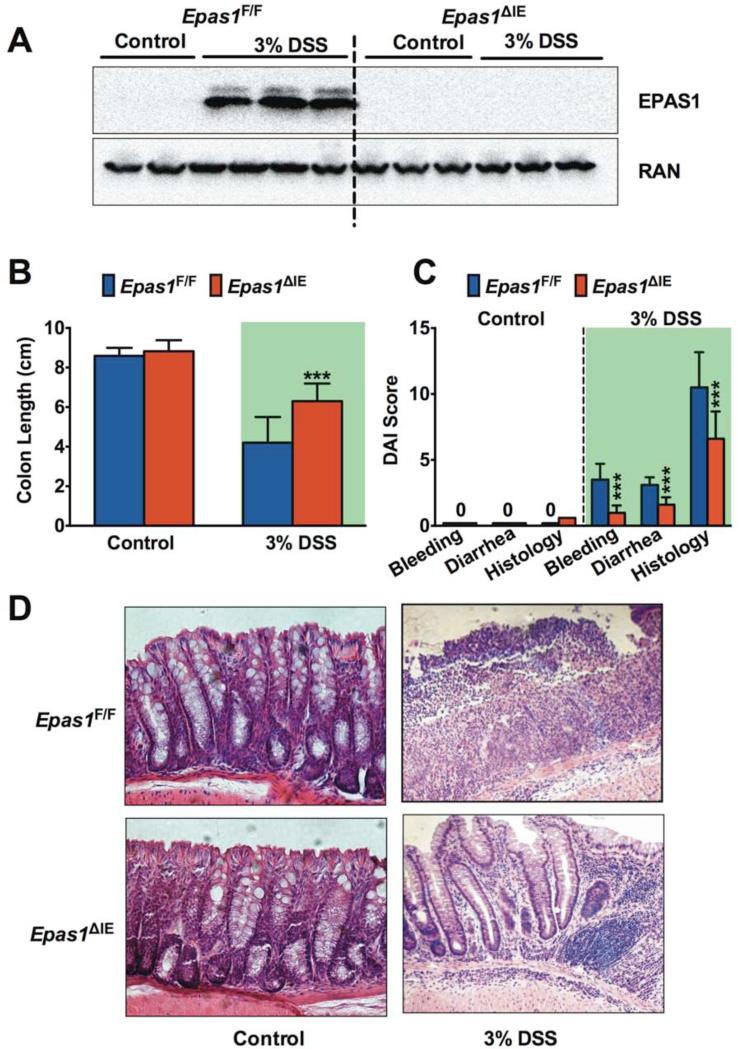
Since EPAS1 expression was rapidly activated in epithelial cells in acute models of colitis and predominantly observed in epithelium in UC and CD, mice with an intestinal epithelial-specific deletion of Epas1 (Epas1ΔIE) were assessed following DSS treatment (Figure 2, Supplemental Figure 1). Following 7-days of 3% or 5% DSS treatment no difference in body weight was observed between Epas1ΔIE and Epas1F/F mice (Supplemental Figure 1, A-C). Western blot analysis from scraped mucosal samples demonstrated that the expression of EPAS1 in the colon was induced in wild-type littermate mice (Epas1F/F) with 3% or 5% DSS treatment for 3 days, while this induction was inhibited in Epas1ΔIE mice (Figure 2A, Supplemental Figure 1D). This data provides further evidence that an increase of EPAS1 following acute insult is primarily due to EPAS1 activation in intestinal epithelial cells. A treatment of 5% DSS for 7-days did not lead to a difference in the severity of colitis between Epas1ΔIE and Epas1F/F mice (Supplemental Figure 1, E and F). However, in a moderate model of colitis induced by 3% DSS the Epas1ΔIE mice were protected from DSS-induced injury as assessed by colon length (Figure 2B). The disease activity index (DAI) based on rectal bleeding, diarrhea and histology were significantly improved in the Epas1ΔIE mice compared to Epas1F/F mice (Figure 2C). Colons from the Epas1ΔIE mice had a decrease in inflammatory infiltrates, and damage to crypts and surface epithelium compared to Epas1F/F mice treated with 3% DSS for 7-days (Figure 2D). Together this data suggest that intestinal epithelial EPAS1 is an important transcription factor in the pathogenesis of colitis.
Figure 2. Disruption of intestinal EPAS1 protects mice from DSS-induced colitis.
(A) Western blot analysis of EPAS1 in colons from Epas1F/F and Epas1ΔIE mice treated with 3% DSS or regular water (Control) for 3-days. (B) Colon lengths, (C) disease activity index (DAI) and (D) H&E staining of colon tissue after 3% DSS or Control treatment for 7 days in Epas1F/F (n=15) and Epas1ΔIE (n=16) mice. Shaded areas highlight the 3% DSS treatment. Each bar represents the mean value ± S.D.***p<0.001, compared to Epas1F/F mice.
Activation of HIF signaling potentiates colitis in an EPAS1-dependent manner
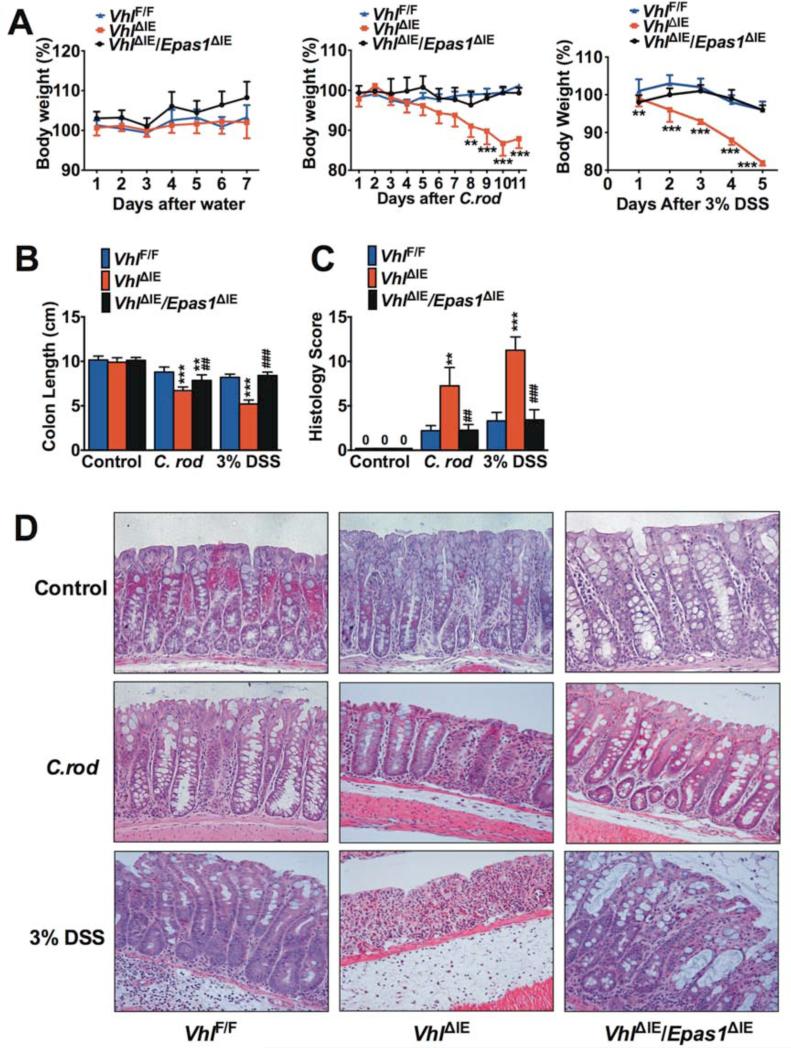
To assess if activation of EPAS1 potentiates colitis, mice with an intestinal epithelium-specific disruption of Vhl or double disruption of Vhl and Epas1 were assessed. Disruption of Vhl activates HIF signaling by stabilizing both HIF-1α and EPAS1, whereas the double disruption of Vhl and Epas1 prevents the formation of functional EPAS1 with a slight compensatory upregulation of HIF-1α (Supplemental Figure 2)13, 17-19. Intestinal epithelium-specific deletion of Vhl (VhlΔIE) increased the susceptibility of mice to C. rod- and 3% DSS-induced colitis (Figure 3). The VhlΔIE mice demonstrated significant decrease in body weight and the experiment was stopped following 11 days of C. rod infection and 5 days of 3% DSS treatment (Figure 3A). A decrease in the colon length in the VhlΔIE mice compared to wild-type littermates (VhlF/F) was observed (Figure 3B). Intestinal epithelial-specific deletion of both Vhl and Epas1 (VhlΔIE/Epas1ΔIE) did not demonstrate any overt intestinal phenotype, and completely ameliorated the decrease in body weight and colon length in acute models of colitis (Figure 3, A and B). Accordingly, histological assessment confirmed severe inflammation in VhlΔIE, while only very limited inflammation could be observed in VhlΔIE/Epas1ΔIE mice (Figure 3, C and D). VhlΔIE/Hif-1αΔIE mice were indistinguishable from VhlΔIE mice following DSS treatment (Supplemental Figure 3). These results provide evidence that activation of EPAS1 potentiates inflammation and suggest the increase in EPAS1 expression that is observed in UC and CD exacerbates disease progression.
Figure 3. EPAS1 is critical for the development of experimental colitis.
(A) Body weight, (B) colon lengths, (C) histological scoring, and (D) H&E staining of colon tissue after C. rod, 3% DSS or regular water (Control) treatment in VhlF/F (n=20),VhlΔIE (n=16) and VhlΔIE/Epas1ΔIE (n=17) mice. Each bar or time point represents the mean value ± S.D.*p<0.05, **p<0.01 and ***p<0.001, compared to VhlF/F mice. ##p<0.01 and ###p<0.001, compared to VhlΔIE mice.
Overexpression of EPAS1 leads to spontaneous colitis
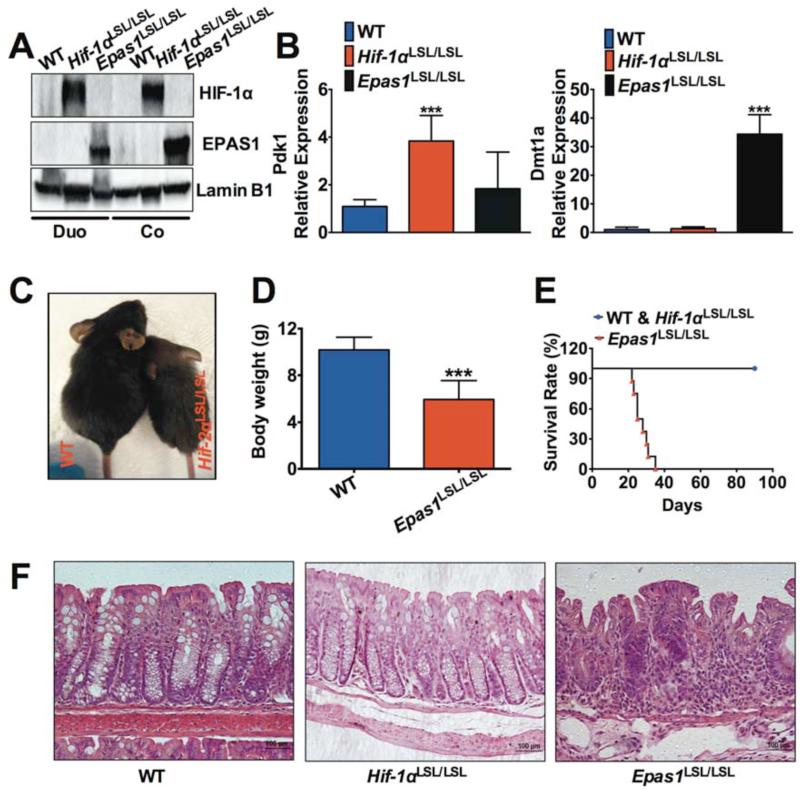
VHL disruption is an indirect mouse model to assess HIF signaling. To directly understand the role of HIF in the intestinal epithelium, mouse models with intestinal epithelial-specific overexpression of HIF-1α or EPAS1 were generated. An oxygen stable Hif-1α and Epas1 cDNA was expressed downstream of a loxP-stop-loxP cassette20. These mice were crossed to villin-Cre transgenic mice21 to overexpress HIF-1α (Hif-1αLSL/LSL) or EPAS1 (Epas1LSL/LSL). Western blot analysis from scraped intestinal epithelial cells confirmed specific overexpression of HIF-1α (not EPAS1) in the Hif-1αLSL/LSL or EPAS1 (not HIF-1α) in the Epas1LSL/LSL mice (Figure 4A). The expression of HIF-1α target gene Pdk1 22 was only induced in intestinal tissues from Hif-1αLSL/LSL mice, whereas the expression of EPAS1 target gene Dmt1a 17 was only induced in Epas1LSL/LSL mice (Figure 4B). The Epas1LSL/LSL mice were smaller and their body weights were significantly lower compared to wild-type littermates (WT) (Figure 4, C and D). No differences were observed in Hif-1αLSL/LSL mice compared to their littermate controls (data not shown). In addition, Epas1LSL/LSL mice did not survive past 40 days after birth, whereas the WT and Hif-1αLSL/LSL mice were healthy at the observation time of 3 months after birth (Figure 4E). H & E staining showed no histological signs of inflammation in control littermates and Hif-1αLSL/LSL mice in the colon and small intestine, but severe spontaneous inflammation was found in Epas1LSL/LSL mice in both the small intestine and colon (Figure 4F and data not shown). Bromodeoxyuridine (BrdU) incorporation and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay revealed no significant difference in cell proliferation and cell apoptosis between the colon tissues of WT and Epas1LSL/LSL mice (Supplemental Figure 4). However, immunohistochemical staining revealed an increase of the macrophage marker F4/80 in the colon tissues of Epas1LSL/LSL mice compared to WT mice. These data demonstrate that epithelial activation of EPAS1 induces overt inflammation in the intestine.
Figure 4. Overexpression of EPAS1 in intestinal epithelial cells leads to spontaneous colitis.
(A) Western blotting analysis for HIF-1α and EPAS1 in the duodenum (Duo) or colon (Co) from Hif-1αLSL/LSL, Epas1LSL/LSL mice and their wild-type littermate controls (WT). (B) qPCR analysis for the expression of Dmt1a and Pdk1 in intestinal tissues from Hif-1αLSL/LSL, Epas1LSL/LSL mice and WT. (C) Representative image and (D) body weights for WT (n=15) and Epas1LSL/LSL mice (n=5) at the age of 25 days. (E) Survival rate for WT and Hif-1αLSL/LSL (n=15) and Epas1LSL/LSL (n=8) mice followed for 3 months. (F) H&E staining of colon tissues from WT, Hif-1αLSL/LSL and Epas1LSL/LSL mice. Each bar represents the mean value ± S.D. ***p<0.001, compared to WT mice.
Intestinal EPAS1 specific overexpression predisposes mice to experimental colitis
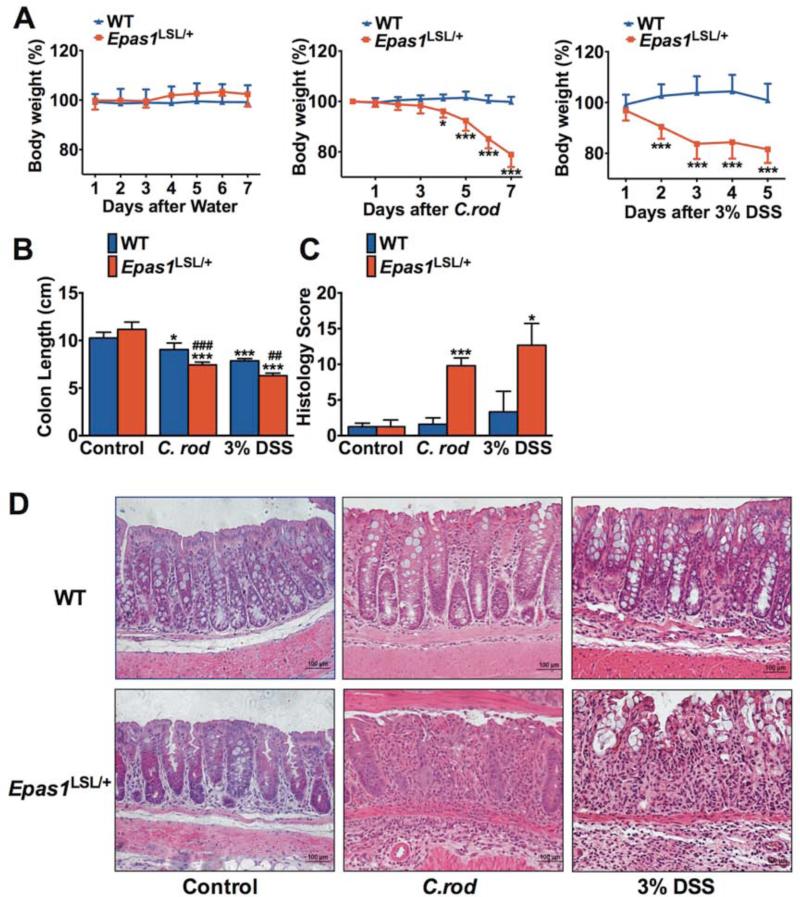
The Epas1LSL/LSL mice demonstrated severe overt inflammation and decreased survival. However Epas1LSL/+ mice overexpressing EPAS1 from one allele, did not display any overt phenotypes as assessed up to 3 months of age (data not shown). Therefore, these mice were further assessed following C. rod and DSS treatment (Figure 5). The Epas1LSL/+ mice demonstrated significant decrease in body weight and the experiment was stopped following 7 days of C. rod infection and 5 days following 3% DSS treatment (Figure 5A). A decrease in the colon length in the Epas1LSL/+ mice compared to WT was observed (Figure 5B). Histological scoring confirmed severe inflammatory cells infiltration and tissue damage in Epas1LSL/+ mice, while only moderate inflammation could be observed in WT (Figure 5, C and D). These data demonstrate that EPAS1augments the development of colitis. Interestingly, double overexpression of HIF-1α and EPAS1 (Hif-1α LSL/+/Epas1LSL/+) lead to a phenotype very similar to the Epas1LSL/+ mice (Supplemental Figure 5). The Hif-1α LSL/+/Epas1LSL/+ mice had no overt phenotype at the age assessed. However, the Hif-1α LSL/+/Epas1LSL/+ mice had an increased susceptibility to an acute colitis model, similar to that observed in the Epas1LSL/+ mice. This data suggests that when HIF-1α and EPAS1 are expressed in the same cell the pro-inflammatory role of EPAS1 is the dominant response in the intestinal epithelial cells.
Figure 5. EPAS1 potentiates acute experimental colitis.
(A) Body weight, (B) colon length, (C) histological scoring, and (D) H&E staining of colon tissue after C. rod treatment for 7 days, 3% DSS for 5 days or regular water (Control) in Epas1LSL/+ (n=8) or littermate control mice (WT) (n=9) mice. Each bar or time point represents the mean value ± S.D.*p<0.05, **p<0.01 and ***p<0.001, compared to WT mice.
The epithelial inflammatory response is dependent on intestinal EPAS1
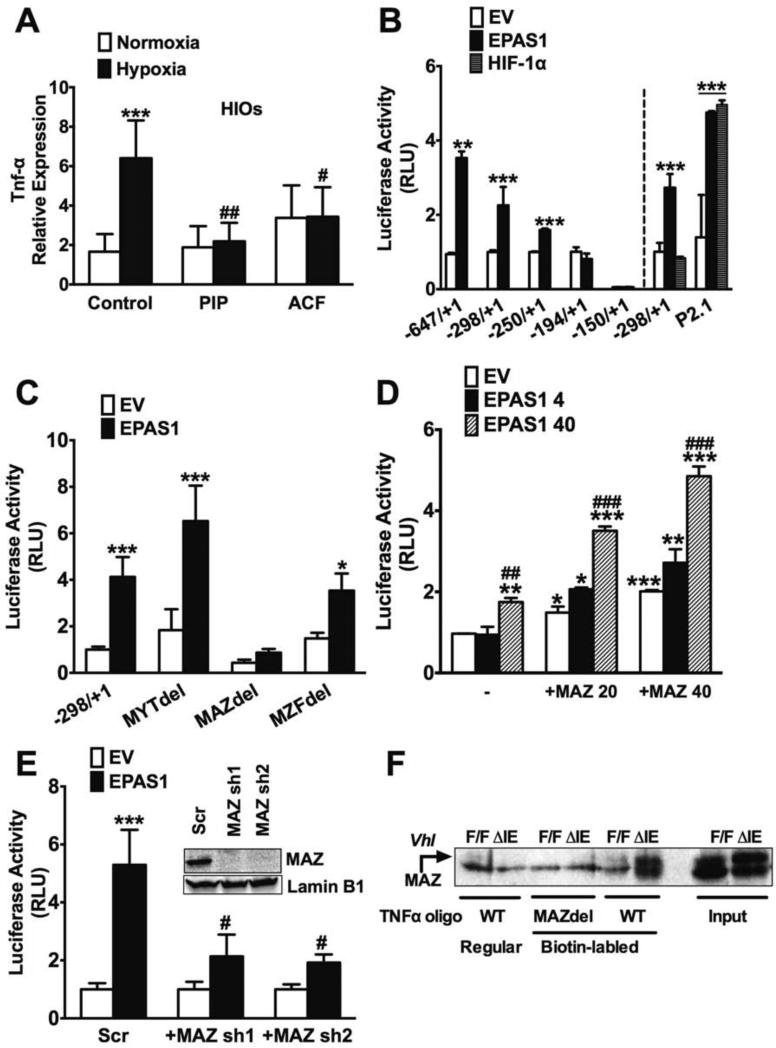
To investigate the detailed molecular mechanisms for the regulatory role of EPAS1 in experimental colitis, gene expression profiles following DSS treatment between Epas1ΔIE and Epas1F/F mice were compared by microarray (Gene Expression Omnibus database accession number: GSE43416 http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE43416). In contrast to what has been shown with HIF-1α6-8, 23, EPAS1 did not regulate genes important in the intestinal epithelial-barrier as demonstrated by the microarray and qPCR analysis (data not shown). Functional annotation analysis suggested a significant decrease in the pro-inflammatory response in Epas1ΔIE mice compared to Epas1F/F mice following 3% DSS treatment for 3 days. qPCR analysis confirmed marked reductions of mRNAs encoding IL-6, IL1RL1, IL-1β, TNF-α and MIF in Epas1ΔIE mice (Supplemental Figure 6A). Whereas overexpression of EPAS1 increased expression of pro-inflammatory meditators, which was further potentiated in acute colitis models (Supplemental Figure 6 and 7). The gene expression analysis demonstrates an increase in TNF-α specifically by EPAS1 (but not HIF-1α) in epithelial cells, which precedes overt colonic inflammation (Supplemental Figure 8A and B). Activation of Tnf-α expression by EPAS1 is similar to that of direct targets such as Ptgs2 and Ptges24. To assess direct regulation of TNF-α by hypoxic signaling, human embryonic stem cell (hESC)-derived intestinal organoids (HIOs) were assessed25-28. The intestinal organoid cultures provide an ideal model system to assess hypoxic activation of TNF-α in the context of normal human intestinal epithelial cells. HIOs were incubated in 1% O2 (hypoxia) or 21% O2 (normoxia) with or without EPAS1 inhibitor piplartine (PIP) or acriflavine (ACF) for 24-hours29, 30. qPCR analysis demonstrated that the expression of DMT-1a and TNF-α was significantly induced by hypoxia and inhibited by PIP and ACF (Figure 6A and Supplemental Figure 8C). Furthermore, EPAS1 but not HIF-1α activated the proximal promoter of Tnf-α in HCT116 cells, whereas both EPASI and HIF-1α activated the canonical hypoxia response element (HRE)-luciferase construct p2.1 (Figure 6B). These data demonstrate the specificity of HIF-2α for the regulation of TNFα maintained in an in vitro promoter assay, and in vivo in intestinal epithelial cells. The nuclear factor-kappaB (NF-κB) p65 transcription factor is a master regulator of Tnf-α expression31. Repressing the nuclear translocation of NF-κB with a super-repressor form of IκBα (SR-IκBα) could suppress TNF-α mediated activation of the NF-κB promoter but did not reverse EPAS1-mediated activation of the Tnf-α promoter 32(Supplemental Figure 9, A and B). Moreover, the NF-κB pathway was not activated in mice overexpressing intestinal EPAS1 (Supplemental Figure 9C). Taken together, these results provide evidence that EPAS1 is a critical transcription factor regulating intestinal epithelial inflammatory response independent of the NF-κB pathway. EPAS1 responsive element was narrowed down to 50 bp region by 5’ promoter deletion constructs (Figure 6B). This region however contains no HREs, suggesting a novel EPAS1-dependent mechanism. By analyzing the sequence of this Tnf-α promoter region with MatInspector (www.genomatix.de), several putative transcription factor-binding sites were identified. Deletion of the myc-associated zinc finger protein (MAZ) binding site, but not myelin transcription factor (MYT) or myeloid zinc finger protein (MZF) sites abolished EPAS1-mediated activation of TNF-α (Figure 6C). Overexpression of MAZ together with EPAS1 potentiated the transcriptional activity of TNF-α in a dose dependent manner (Figure 6D). Moreover, in cells in which MAZ was reduced by shRNAs, the EPAS1-induced TNF-α promoter activity was attenuated (Figure 6E). To assess MAZ DNA-binding activity, we performed DNA affinity precipitation assays (DAPA) using nuclear extracts from the colons of VhlΔIE and VhlF/F mice and biotinylated TNF-α oligos containing the putative MAZ binding site to precipitate proteins that bind. Immunoblotting of the precipitates confirmed that HIF-2α strongly induced MAZ binding which was absent when using a TNF-α oligo in which the MAZ site was mutated (Figure 6F). Together, our data suggest that EPAS1 induces the promoter activity of TNF-α through MAZ binding.
Figure 6. EPAS1 activates the TNF-α promoter through MAZ.
(A) qPCR analysis for Tnf-α expression in HIOs with or without EPAS1 inhibitor 10 μM piplartine (PIP) or 5 μM acriflavine (ACF) for 24-hours. Luciferase-reporter assays with (B) TNF-α promoter using 5’ truncated constructs (−647/+1, −298/+1, −250/+1, −194/+1 or −150/+1) or HRE reporter P2.1 activity following co-transfection with empty vector (EV), EPAS1 or HIF-1α. (C) transcription factor binding site deleted constructs, (D) following co-transfection with MAZ (20 and 40ng of plasmid) and HIF-2α (4 and 40ng of plasmid) in the 298/+1 promoter construct and (E) in HCT116 cells with a stable shRNA knockdown of MAZ (MAZ sh1 and sh2) or scrambled shRNA (Scr) using the −298/+1 construct. Western blot analysis of MAZ from scrambled and MAZ knockdown HCT116 cells (inset). Expression was normalized to β-actin. (F) DNA affinity precipitation analysis for MAZ in colonic nuclear extracts from VhlF/F or VhlΔIE mice. Each bar represents the mean value ± S.D. *P < 0.05, **P < 0.01 and ***P < 0.001, compared with empty transfection. ##p<0.05, ###p<0.001, compared to without EPAS1 inhibitor treatment, EPAS1 40ng or Scr.
Blocking TNF-α antagonizes EPAS1-mediated colitis
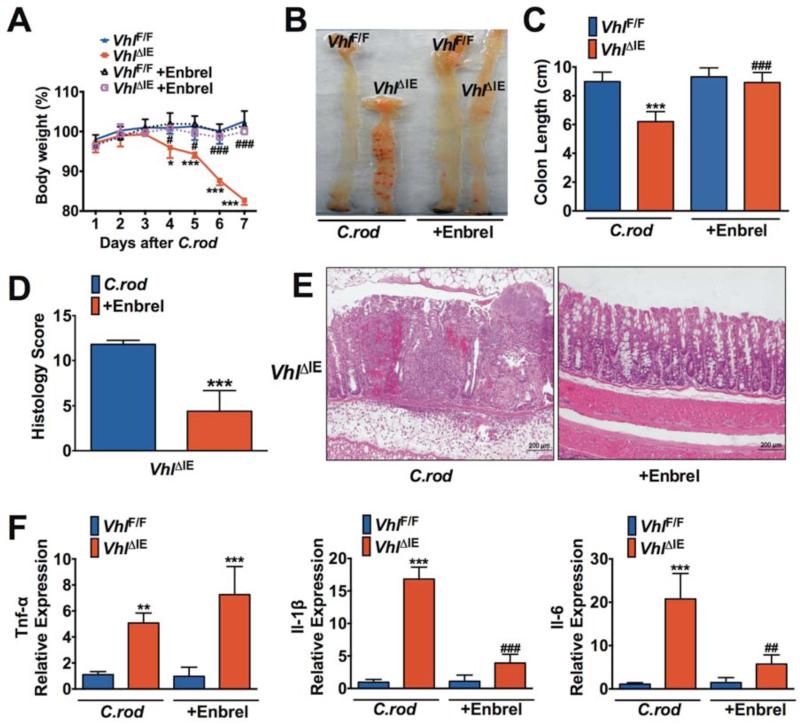
TNF-α antagonist treatment is an approved therapy for UC patients33. To test whether TNF-α is a critical downstream mediator for EPAS1-mediated colitis, VhlΔIE mice were treated with TNF-α antagonists Enbrel (soluble TNF-α decoy receptor) or Remicade (monoclonal neutralizing antibody) in C. rod-induced colitis model. As expected, following C. rod treatment the VhlΔIE mice demonstrated a significant decrease in body weight compared to VhlF/F mice (Figure 7A, Supplemental Figure 10A). The TNF-α inhibitors Enbrel or Remicade, completely abolished the decrease in body weight following C.rod treatment in VhlΔIE mice (Figure 7A, Supplemental Figure 10A). The VhlΔIE mice had more severe colonic hemorrhage and decreased colon length compared to VhlF/F mice, which were attenuated by Enbrel or Remicade treatment (Figure 7, B and C; Supplemental Figure 10, B and C). Histological scoring confirmed severe tissue damage and massive inflammatory cells infiltration in VhlΔIE mice following C.rod treatment, while Enbrel or Remicade treatment ameliorated these histological changes (Figure 7, D and E; Supplemental Figure 10D). Moreover, the expressions of Tnf-α, Il-6 and Il-1β were significantly increased in the colons of VhlΔIE mice compared with VhlF/F mice following C.rod treatment (Figure 7F, Supplemental Figure 10E). Enbrel or Remicade treatment did not reduce the expression of Tnf-α in VhlΔIE mice, however it significantly reduced the expressions of Il-6 and Il-1β (Figure 7F, Supplemental Figure 10E). This is consistent with the data that TNF-α is directly downstream of EPAS1, whereas activation of other pro-inflammatory cytokines by EPAS1 is dependent on TNF-α expression. In addition, Remicade also protected Epas1LSL/+ mice from C. rod or DSS-induced colitis (Supplemental Figure 11). These data demonstrate that EPAS1 regulates the epithelial inflammatory response through TNF-α.
Figure 7. Enbrel ameliorates EPAS1 potentiated pathogen-induced colitis in mice.
(A) Body weight, (B) representative gross image, (C) colon lengths, (D) histological scoring, (E) H&E staining and (F) qPCR analysis for the expressions of pro-inflammatory cytokines in colon tissues from VhlF/F (n=11) and VhlΔIE (n=10) mice following C. rod treatment with or without Enbrel for 7 days. Expression was normalized to β-actin. Each bar represents the mean value ± S.D. *p<0.05, **p<0.01 and ***p<0.001, compared to VhlF/F mice without Enbrel treatment. #p<0.05, ##p<0.01 and ###p<0.001, compared to VhlΔIE mice without Enbrel treatment.
Discussion
Intestinal epithelial cells play a major role in IBD as a barrier to the luminal mircroflora. However, recent data demonstrates that epithelial cells are also critical in regulating the mucosal immune response34-38. Through studying the role of EPAS1 in IBD the present manuscript demonstrates that EPAS1 is a critical transcription factor regulating the epithelial inflammatory response. It is difficult to discern the relative importance of the epithelial inflammatory response compared to the innate and adaptive immune cells. However, this work clearly demonstrates that attenuation of the epithelial elicited inflammation through disruption of EPAS1 limits colonic inflammation. Moreover, overexpression of epithelial EPAS1 leads to increased expression of pro-inflammatory cytokines and potentiates colitis. In addition to activating the epithelial-elicited inflammatory response EPAS1 activates iron transporters17, 18, 39, which may play a role in epithelial inflammation and contribute to dysregulation of iron transporters in IBD40. Although TNF-α neutralization reverses EPAS1-induced inflammation it will be interesting to understand if EPAS1-medited intestinal iron homeostasis plays any contributory role in epithelial inflammation or changes in the gut microflora that are associated with IBD.
The data in the VhlΔIE and Epas1LSL/+ mice clearly demonstrates that activation of epithelial EPAS1 signaling potentiates inflammation. This data is contrary to the well-characterized function of PHD inhibitors or genetic disruption of PHDs that mimic hypoxic stimulation, and protects mice in acute colitis models11, 12, 41. The PHD inhibitor dimethyloxaloylglycine (DMOG) at a dose that was protective in DSS-induced colitis was assessed for EPAS1 activation. DMOG stimulated colonic hypoxic signaling was confirmed in vivo and ex vivo with the ODD-luciferase mice (Supplemental Figure 12A). The expression of HIF-1α specific target gene pdk1 was increased but no increase in the expression of EPAS1 or its specific target genes such as Dmt1a and Dcytb was observed (Supplemental Figure 12, B-D)17, 39, suggesting the DMOG treatment maybe a more potent activator of HIF-1α in the intestine for protection in acute colitis models. It is also possible that activation of EPAS1 by PHD inhibitors may require significantly longer treatment times. This is consistent with the data demonstrating a rapid activation of HIF-1α compared to EPAS1 in several cell lines 42.
Hypoxic activation of pro-inflammatory mediators has been described in innate immune cells43-45. However, the present study revealed that hypoxia is rapidly increased in epithelial cells of the inflammatory foci and regulates expression levels of a number of inflammatory mediators in an EPAS1-dependent manner. Our previous work had demonstrated the importance of macrophage migration inhibitory factor signaling to hypoxic inflammation13. However, the relative importance of these and likely other gene products to the increased colon inflammation has not been precisely defined. In mice overexpressing EPAS1 but not HIF-1α a significant increase in Tnf-α gene expression is observed. Moreover, wild-type mice demonstrated a robust increase in the expression of Tnf-α following DSS treatment, which was significantly attenuated in Epas1ΔIE mice. To identify putative functional EPAS1 binding sites, promoter analysis of Tnf-α was performed. Interestingly, EPAS1 (but not HIF-1α) regulated the promoter activity of Tnf-α through a NF-κB independent mechanism. The data suggests that HIF-2α activates Tnf-α through a MAZ-dependent manner. Currently studies are being performed to assess if EPAS1 is in a complex with MAZ on the TNF-α promoter. A similar mechanism has been demonstrated for EPAS1 interaction with c-myc, which is critical in the regulation of cell proliferation46. The present study demonstrates that TNF-α is the major pro-inflammatory mediator required for induction of hypoxic inflammation. As anti-TNF-α therapy is expensive and has become the main costs driver for IBD patients47, alternative cost-effective medication is needed. Moreover, anti-TNF-α therapies also lead to several deleterious side effects48-52. Therefore, EPAS1 may represent a potential target for treatment of IBD since its expression is nearly undetectable in most normal tissues and is present in inflammatory foci.
In conclusion we demonstrate that hypoxia signaling is robustly induced in intestinal epithelial cells. Using a powerful collection of mouse models it was demonstrated that hypoxia and EPAS1 is essential for the proper activation of the epithelial inflammatory response. Together with previous studies assessing the role of HIF-1α in the intestine8, 10, the present study establishes a working model where HIF-1α tightens the epithelial barrier limiting luminal bacteria antigen, and EPAS1 activates the epithelial inflammatory response to eliminate bacteria. This study clearly shows that the EPAS1/TNF-α axis is a critical signaling pathway in activating hypoxic inflammation. Since the dominant role of HIF activation in the intestine is a pro-inflammatory response mediated by EPAS1, more work is required to determine the temporal regulation of HIF-1α and EPAS1 in colitis. It is possible that HIF-1α and EPAS1 are activated in colitis, but HIF-1α response persists for proper barrier repair and resolution of inflammation.
Methods
Animals and treatment
VhlF/F, VhlΔIE, Epas1F/F, Epas1ΔIE, VhlF/F/Hif-1αF/F, VhlΔIE/Hif-1αΔIE, VhlF/F/Epas1F/F, VhlΔIE/Epas1ΔIE and ODD-luciferase mice were described previously13, 14, 18, 19, 53. LSL-HIF1 dPA and LSL-HIF2 dPA mice are previously described20. The LSL-HIF1 dPA and LSL-HIF2 dPA mice were crossed with murine villin promoter driven cre mice 21 to generate intestinal epithelial specific HIF-1α and EPAS1 overexpressing mice (Hif-1αLSL/LSL and Epas1LSL/LSL), respectively. Hif-1αLSL/LSL and Epas1LSL/LSL mice were crossed to each other to generate intestinal epithelial specific HIF-1α and EPAS1 double overexpressing mice (Hif-1αLSL/+/Epas1LSL/+). For DMOG study, 6- to 8-week-old ODD-luciferase mice (n=3) were intraperitoneally (i.p.) injected with 400 mg/kg DMOG daily or saline for two days, a regimen previously shown to stabilize HIF-1α in vivo11. For TNF-α inhibition study, 6- to 8-week-old VhlF/F and VhlΔIE mice were subcutaneously injected with 5 mg/kg Enbrel (Immunex Corporation, Thousand Oaks, CA) 4 hours prior to C. rod infection, and at day 1 and day 4 following C. rod infection or i.p. injected with 10 mg/kg Remicade (Janssen Biotech, Inc., Horsham, PA) one day and 4 hours prior to and every other day following C. rod infection or DSS treatment. All mice were maintained in standard cages in a light and temperature-controlled room and were given standard chow and water ad libitum. All animal studies were carried out in accordance with Institute of Laboratory Animal Resources guidelines and approved by the University Committee on the Use and Care of Animals at the University of Michigan (UCUCA approval number: 10299).
Supplementary Material
Acknowledgments
The authors thank Dr. Gabriel Nunez and Dr. Nobuhiko Kamada for providing Citrobacter rodentium and The University of Michigan Cancer Center Microarray Core for Affymetrix gene array services and analysis.
Funding: National Institutes of Health (grant CA148828 to Y.M.S.; KO1DK091415 to J.R.S); The University of Michigan Gastrointestinal Peptide Center; Jeffrey A.Colby Colon Cancer Research and the Tom Liu Memorial Funds of the University of Michigan Comprehensive Cancer Center.
Abbreviations
- ACF
acriflavine
- CD
Crohn's disease
- C.rod
Citrobacter rodentium
- DMOG
dimethyloxaloylglycine
- DSS
dextran sulfate sodium
- EPAS1
Endothelial PAS domain protein 1
- HIF
Hypoxia-Inducible Factor
- HRE
hypoxia response elements
- IBD
inflammatory bowel disease
- PHD
prolyl hydroxylase
- PIP
piplartine
- TNF-α
tumor necrosis factor-α
- UC
ulcerative colitis
- VHL
von Hippel-Lindau tumor suppressor protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conception and design: X Xue; Y.M. Shah.
Development of methodology: X. Xue, E. Anderson, S. Ramakrishnan, S. Huang,Y.M. Shah
Acquisition of data: X. Xue, M. Taylor, S. Ramakrishnan, Y.M. Shah
Analysis and interpretation of data: X. Xue, J.K. Greenson, Y.M. Shah
Writing, review, and/or revision of the manuscript: X. Xue, E. Anderson, J M. Taylor, S. Ramakrishnan, J.R. Spence, E.M. Zimmermann, Y.M. Shah
Study supervision: Y.M. Shah
Conflicts of Interest: none declared.
Author names in bold designate shared co-first authorship.
Reference
- 1.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nature reviews. Immunology. 2009;9:609–17. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. The New England journal of medicine. 2011;364:656–65. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giatromanolaki A, Sivridis E, Maltezos E, et al. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. Journal of clinical pathology. 2003;56:209–13. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuta GT, Turner JR, Taylor CT, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. The Journal of experimental medicine. 2001;193:1027–34. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comerford KM, Wallace TJ, Karhausen J, et al. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer research. 2002;62:3387–94. [PubMed] [Google Scholar]
- 8.Karhausen J, Furuta GT, Tomaszewski JE, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. The Journal of clinical investigation. 2004;114:1098–106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. The Journal of clinical investigation. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirota SA, Fines K, Ng J, et al. Hypoxia-inducible factor signaling provides protection in Clostridium difficile-induced intestinal injury. Gastroenterology. 2010;139:259–69. e3. doi: 10.1053/j.gastro.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummins EP, Seeballuck F, Keely SJ, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–65. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Robinson A, Keely S, Karhausen J, et al. Mucosal protection by hypoxiainducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–55. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah YM, Ito S, Morimura K, et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–48. 2048, e1–3. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson ER, Taylor M, Xue X, et al. The hypoxia-inducible factor-C/EBPalpha axis controls ethanol-mediated hepcidin repression. Molecular and cellular biology. 2012;32:4068–77. doi: 10.1128/MCB.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues DM, Sousa AJ, Hawley SP, et al. Matrix metalloproteinase 9 contributes to gut microbe homeostasis in a model of infectious colitis. BMC microbiology. 2012;12:105. doi: 10.1186/1471-2180-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nature protocols. 2007;2:541–6. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 17.Shah YM, Matsubara T, Ito S, et al. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell metabolism. 2009;9:152–64. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor M, Qu A, Anderson ER, et al. Hypoxia-inducible factor-2alpha mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011;140:2044–55. doi: 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue X, Taylor M, Anderson E, et al. Hypoxia-inducible factor-2alpha activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer research. 2012;72:2285–93. doi: 10.1158/0008-5472.CAN-11-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim WY, Safran M, Buckley MR, et al. Failure to prolyl hydroxylate hypoxiainducible factor alpha phenocopies VHL inactivation in vivo. The EMBO journal. 2006;25:4650–62. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 22.Kim JW, Tchernyshyov I, Semenza GL, et al. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell metabolism. 2006;3:177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Louis NA, Hamilton KE, Canny G, et al. Selective induction of mucin-3 by hypoxia in intestinal epithelia. Journal of cellular biochemistry. 2006;99:1616–27. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- 24.Xue X, Shah YM. Hypoxia-inducible factor-2alpha is essential in activating the COX2/mPGES-1/PGE2 signaling axis in colon cancer. Carcinogenesis. 2013;34:163–9. doi: 10.1093/carcin/bgs313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCracken KW, Howell JC, Wells JM, et al. Generating human intestinal tissue from pluripotent stem cells in vitro. Nature protocols. 2011;6:1920–8. doi: 10.1038/nprot.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du A, McCracken KW, Walp ER, et al. Arx is required for normal enteroendocrine cell development in mice and humans. Developmental biology. 2012;365:175–88. doi: 10.1016/j.ydbio.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkbeiner SR, Zeng XL, Utama B, et al. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio. 2012;3:e00159–12. doi: 10.1128/mBio.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K, Zhang H, Qian DZ, et al. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci U S A. 2009;106:17910–5. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Bokesch HR, Gardella RS, Rabe DC, et al. A new hypoxia inducible factor-2 inhibitory pyrrolinone alkaloid from roots and stems of Piper sarmentosum. Chem Pharm Bull (Tokyo) 2011;59:1178–9. doi: 10.1248/cpb.59.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Molecular and cellular biology. 1990;10:1498–506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang CY, Mayo MW, Baldwin AS., Jr. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–7. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 33.Valesini G, Iannuccelli C, Marocchi E, et al. Biological and clinical effects of anti-TNFalpha treatment. Autoimmunity reviews. 2007;7:35–41. doi: 10.1016/j.autrev.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Stadnyk AW. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Canadian journal of gastroenterology. 2002;16:241–6. doi: 10.1155/2002/941087. [DOI] [PubMed] [Google Scholar]
- 35.Goncalves NS, Ghaem-Maghami M, Monteleone G, et al. Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis. Infection and immunity. 2001;69:6651–9. doi: 10.1128/IAI.69.11.6651-6659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roulis M, Armaka M, Manoloukos M, et al. Intestinal epithelial cells as producers but not targets of chronic TNF suffice to cause murine Crohn-like pathology. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5396–401. doi: 10.1073/pnas.1007811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shioya M, Nishida A, Yagi Y, et al. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clinical and experimental immunology. 2007;149:480–6. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolachala V, Asamoah V, Wang L, et al. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cellular and molecular life sciences : CMLS. 2005;62:2647–57. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastrogiannaki M, Matak P, Keith B, et al. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. The Journal of clinical investigation. 2009;119:1159–66. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomollon F, Gisbert JP. Intravenous iron in inflammatory bowel diseases. Curr Opin Gastroenterol. 2013;29:201–7. doi: 10.1097/MOG.0b013e32835bdc2e. [DOI] [PubMed] [Google Scholar]
- 41.Tambuwala MM, Cummins EP, Lenihan CR, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 42.Lin Q, Cong X, Yun Z. Differential hypoxic regulation of hypoxia-inducible factors 1alpha and 2alpha. Mol Cancer Res. 2011;9:757–65. doi: 10.1158/1541-7786.MCR-11-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imtiyaz HZ, Williams EP, Hickey MM, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. The Journal of clinical investigation. 2010;120:2699–714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doedens AL, Stockmann C, Rubinstein MP, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer research. 2010;70:7465–75. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordan JD, Bertout JA, Hu CJ, et al. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Valk ME, Mangen MJ, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFalpha therapy: results from the COIN study. Gut. 2012 doi: 10.1136/gutjnl-2012-303376. [DOI] [PubMed] [Google Scholar]
- 48.Coffin CS, Fraser HF, Panaccione R, et al. Liver diseases associated with anti-tumor necrosis factor-alpha (TNF-alpha) use for inflammatory bowel disease. Inflammatory bowel diseases. 2011;17:479–84. doi: 10.1002/ibd.21336. [DOI] [PubMed] [Google Scholar]
- 49.Hoentjen F, van Bodegraven AA. Safety of anti-tumor necrosis factor therapy in inflammatory bowel disease. World journal of gastroenterology : WJG. 2009;15:2067–73. doi: 10.3748/wjg.15.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee HH, Song IH, Friedrich M, et al. Cutaneous side-effects in patients with rheumatic diseases during application of tumour necrosis factor-alpha antagonists. The British journal of dermatology. 2007;156:486–91. doi: 10.1111/j.1365-2133.2007.07682.x. [DOI] [PubMed] [Google Scholar]
- 51.Niess JH, Danese S. Anti-TNF and skin inflammation in IBD: a new paradox in gastroenterology? Gut. 2013 doi: 10.1136/gutjnl-2013-304683. [DOI] [PubMed] [Google Scholar]
- 52.Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-gamma-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2013 doi: 10.1136/gutjnl-2012-302853. [DOI] [PubMed] [Google Scholar]
- 53.Haase VH, Glickman JN, Socolovsky M, et al. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1583–8. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.