Abstract
Cyclization techniques are used often to impart higher in vivo stability and binding affinity to peptide targeting vectors for molecular imaging and therapy. The two most often used techniques to impart these qualities are lactam bridge construction and disufide bond formation. While these techniques have been demonstrated to be effective, orthogonal protection/deprotection steps can limit achievable product yields. In this chapter, new α-melanocyte stimulating hormone (α-MSH) peptide analogs were synthesized and cyclized by copper-catalyzed terminal azide-alkyne cycloaddition “click” chemistry techniques. The α-MSH peptide and its cognate receptor (melanocortin receptor subtype 1, MC1R) represents a well-characterized model system to examine the effect of the triazole linkage for peptide cyclization on receptor binding in vitro and in vivo. Four new DOTA-conjugated α-MSH analogs were cyclized and evaluated by in vitro competitive binding assays, serum stability testing, and in vivo imaging by positron emission tomography (PET) of tumor bearing mice. These new DOTA-conjugated click-cyclized analogs exhibited selective high binding affinity (<2 nM) MC1R to melanoma cells in vitro, high stability in human serum, and produced high contrast PET/CT images of tumor xenografts. Gallium-68 labeled DOTA bioconjugates displayed rapid pharmacokinetics with receptor mediated tumor accumulation of up to 16±5 %ID/g. The results indicate that the triazole ring is an effective bioisosteric replacement for the standard lactam bridge assemblage for peptide cyclization. Radiolabeling results confirm that Cu catalyst is sufficiently removed prior to DOTA chelator addition to enable insertion of radiometals or stable metals for molecular imaging and therapy. Thus, these click-chemistry-cyclized variants show promise as agents for melanocortin receptor-targeted imaging and radionuclide therapy.
1 Introduction
1.1 Targeted Peptides for Molecular Imaging and Therapy
Peptides are emerging as potent and selective ligands that can be designed to bind with high affinity and specificity to cell surface receptors for a wide range of in vitro and in vivo biomedical applications. One area of research that has seen considerable proliferation is the use of peptide bioconjugates for targeted molecular imaging and therapy of cancer. For this application, peptide analogs are developed to bind selectively to specific cell surface receptors that are upregulated on malignant tumors. The peptide sequences employed are either based on the native cognate binding ligand or can be selected using peptide library approaches.(Heppeler, et al. 2000, Zwanziger and Beck-Sickinger 2008) To enable the use of the identified sequences for molecular imaging and therapy of cancer, molecular modifications are made to improve binding to cognate receptors, stabilize the bioconjugates to in vivo degradation, and install chelator moieties for radiolabeling. In this way, these same peptide ligands can be used to exploit receptor over-expression in tumor tissues for diagnosis, staging, targeted radionuclide therapy, and measurement of therapeutic response.(Heppeler, et al. 2000, Eberle and Froidevaux 2003)
While the potential of radiolabeled peptides for molecular targeting of cell surface receptors has been demonstrated, identified native peptide sequences often are degraded rapidly in vivo. Thus, investigators have devised numerous structural modifications that impart improved pharmacodynamic properties over the native peptide sequences, while preserving (and even enhancing) the molecular targeting characteristics of the peptide analogs (Vlieghe, et al. 2010, Nestor Jr. 2009). For example, N-terminal acylation and C-terminal amidation help to prevent degradation by exoproteases. Substitution of D-amino acids at positions susceptible to endoproteases can also add stability to peptide-based diagnostics and therapeutics (Vlieghe, et al. 2010). One particularly powerful approach to inhibit proteolysis of peptides is cyclization. Cyclization can not only provide added in vivo stability to peptide-based targeting vectors, it can also enhance receptor affinity and biological potency by restricting the peptide to an active conformation (Okarvi 2004, Turner, et al. 2007, Nestor Jr. 2009). Several approaches to cyclization have been advanced, including disulfide coupling, lactam bridge assembly, and metal coordination strategies (Raposinho, et al.). The receptor affinity and in vivo stability advantages of the cyclized variants of peptides prepared using these techniques have been demonstrated (Fani, et al., Froidevaux, et al. 2004, Miao, et al. 2008, Cantorias, et al. 2009, Guo, et al. 2010). On the other hand, potential drawbacks of these approaches may include reversibility (disulfide bridges), multiple protection and deprotection steps (lactam assembly), and restriction in radionuclides that can be employed for imaging and therapy (technetium/rhenium cyclizations). Here, cyclization of peptides by copper-catalyzed “click” chemistry for molecular imaging is presented. While click chemistry has been used previously for a number of peptide cyclization bioconjugate chemistry applications, few investigators have implemented this strategy for targeted molecular imaging and therapy. To highlight the potential advantages of click cyclization for radiopharmaceutical synthesis applications, a well characterized peptide/receptor model was chosen to facilitate comparison of standard lactam bridge cyclized variants to click cyclized variants. Thus, DOTA-modified α-melanocyte stimulating hormone (α-MSH) derivatives were cyclized using the lactam bridge and click triazole formation techniques. The resulting bioconjugates were evaluated in vitro by receptor binding assays to cells expressing cognate receptor melanocortin receptor subtype 1 (MC1R) and serum stability tests. Select click-cyclized derivatives were radiolabeled with positron emitting radionuclide gallium-68 (68Ga) for in vivo evaluation of pharmacodynamics and PET/CT imaging characteristics in tumor bearing mice.
1.2 Cyclized Melanocyte Stimulating Hormone
α-MSH is a peptide hormone that is the endogenous ligand for melanocortin receptor subtype 1 (MC1R), which is upregulated in malignant melanoma, along with other receptors in the MC family (Ghanem, et al. 1988, Siegrist, et al. 1988, Tatro, et al. 1990, Eberle, et al. 1991, Lunec, et al. 1992). The native sequence is a linear 13 amino acid peptide (Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2) (Harris and Lerner 1957). α-MSH binds the MCR1 with high affinity, but it has a biological half life of less than 3 minutes in vivo (Cowell, et al. 2002). Thus, for targeted molecular imaging and therapy, modifications to the native peptide sequence are required to impart sufficient in vivo stability to promote accumulation of the radiolabeled tracer in malignant tissue of interest. To enable the use of α-MSH for this application, several modified analogs have been synthesized and tested in an effort to add biological stability and improve targeting (Hruby, et al. 2003). One particularly promising conformation that has proven effective in protecting the peptide from enzymatic degradation has replaced the Met at position 4 with Nle, while L-Phe has been exchanged with D-Phe. The resulting analog, [Nle4, D-Phe7]-α-MSH (NDP-α-MSH), is a more potent peptide ligand with extended biological activity resulting from resistance to degradation by serum proteases (Sawyer, et al. 1980).
Further developments in α-MSH analogs included structure-function experiments that have helped to elucidate the amino acid residues necessary for receptor binding affinity of α-MSH for melanocortin receptors. These experiments have identified the “essential core” of native α-MSH peptide as His6-Phe7-Arg8-Trp9 (Hruby, et al. 1987). From the identification of this four amino acid core, a highly potent cyclic analog, MTII, was constructed from only 7 residues of the native α-MSH sequence with cyclization to restrict the conformation — adding further stability to the structure — while maintaining targeting affinity (Al-Obeidi, et al. 1989, Al-Obeidi, et al. 1989, Bednarek, et al. 1999). Structurally, the sequence of MTII, Ac-Nle-cyclo(Asp-His-D-Phe-Arg-Trp-Lys)-NH2, retains the core residues, while forming a lactam bridge through cyclization of the aspartic acid and lysine side chains. This cyclic analog is over 100-fold more potent than the linear α-MSH peptide in lizard skin assays (Sugg, et al. 1988, Al-Obeidi, et al. 1989). Several analogs of MTII have been prepared in an effort to increase biological activity and impart receptor subtype specificity (Bednarek, et al. 1999, Bednarek, et al. 2000, Cai, et al. 2004, Mayorov, et al. 2006, Mayorov, et al. 2006, Ying, et al. 2006, Bednarek, et al. 2007, Bednarek, et al. 2007, Grieco, et al. 2008).
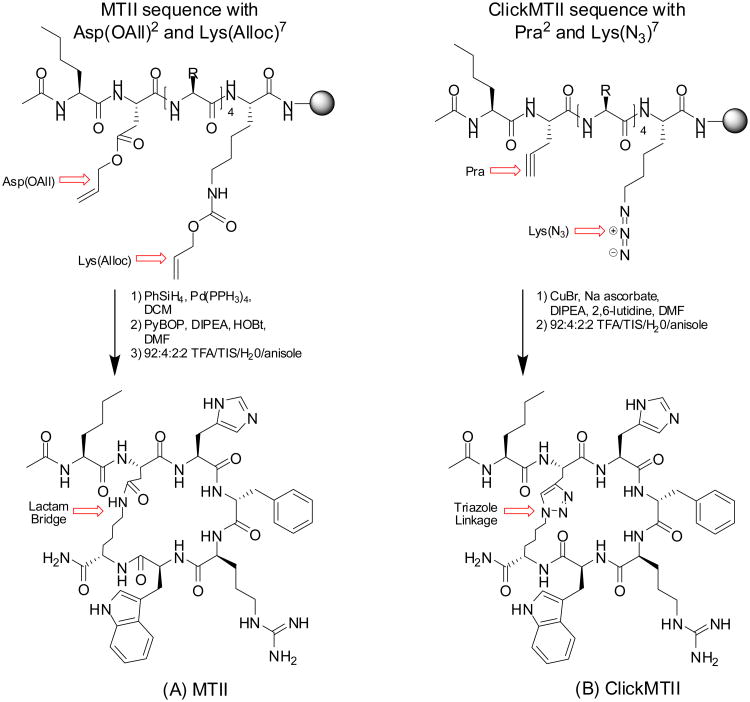
Although the reported efficacy of the lactam-bridge-cyclized variants sparked our interest in extending the use of these derivatives for our investigations, the synthetic strategy proved somewhat problematic. Cyclization by lactam bridge required orthogonal protecting strategies to keep the N-terminus protected while cyclization occurred through the side chains of aspartic acid and lysine. In our hands, deprotection of the allyl/alloc groups also partially removed the N-terminal Fmoc leaving the N-terminus unprotected and available for reaction (see Figure 1; analytical data not shown). These observations prompted us to examine alternative strategies to cyclization that could reduce the use of protective and deprotective strategies — and perhaps improve overall synthetic yields. Our assessment of the optimum characteristics of a bioconjugate strategy for the cyclization reaction included rapid kinetics, reactive selectivity that could minimize (or simplify) protection/deprotection steps, and mild reaction conditions that would be amenable to our standard automated peptide synthesis. Investigations along these lines led us to examine the use of copper-catalyzed “click” chemistry as an approach that appears to comprise these optimum reaction characteristics. In the present study, new cyclic analogs of MTII have been designed and constructed utilizing cyclization via copper-catalyzed “click” chemistry. The MTII sequence has been modified to contain propargylglycine and terminal-azide-modified Lys [Lys(N3)] to afford efficient cyclization of the peptide within the framework of standard automated peptide synthesis — through a triazole ring formation between the alkyne-modified Gly and Lys(N3) in high yield. In addition, the approach enabled facile manipulation of the synthetic positioning of the metal chelator DOTA insertion to determine its effect on receptor affinity and peptide stability. The resulting compounds were labeled with positron-emitting radionuclide copper-64 (64Cu) to perform in vitro stability testing in human serum and gallium-68 (68Ga) to enable the bioconjugates for targeted molecular imaging by positron emission tomography (PET). Thus, in this paper we present synthesis of several derivatives prepared in this manner, in vitro competitive cell binding assays, stability evaluations in serum, and preliminary evaluations of in vivo behavior by biodistribution studies and PET imaging.
Figure 1.
Cyclized α-MSH analogs. (A) MTII, a 7 amino acid derivative of a-MSH cyclized through the lactamization of Asp2 and Lys7. (B) ClickMTII, where the aspartic acid has been replaced by propargylglycine and the side chain moiety of lysine has been changed to an azide, has been cyclized utilizing click chemistry between the terminal alkyne and azide to form a triazole.
2 Experimental
2.1 Chemicals and Reagents
Fmoc-protected amino acids, 2-(7-Aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate, 1-hydroxybenzotriazole, benzotriazole-1-ly-oxy-tris-pyrrolidinophosphonium hexafluorophosphate, and Rink amide resin for peptide synthesis were obtained from Advanced ChemTech (Louisville, KY). DOTA-tris (t-Bu ester) was purchased from Macrocyclics (Dallas, TX). N,N-dimethylformamide, N,N-diisopropylethylamine, methanol, trifluoroacetic acid, anisole, acetonitrile, PhSiH3, Pd(PPH3)4, and 2,6- lutidine were purchased from Thermo Fisher Scientific (Waltham, MA). Dichloromethane, triisopropylsilane, sodium ascorbate, copper(I) bromide, BSA, 1,10-phenanthroline, HEPES, human serum, and citric acid were from Sigma Aldrich (St. Louis, MO). DMEM, MEM, FBS, and penicillin/streptomycin were from Invitrogen Life Technologies (Carlsbad, CA). B16/F10 murine melanoma cells were a gift from Dr. Michael Anderson at the University of Iowa (Iowa City, IA).
2.2 Peptide Synthesis
A total of ten linear and cyclized α-MSH peptide analogs were prepared, characterized, and tested in this study (Table 1). Four linear peptides, including α-MSH (Ac-SYSMEHFRWGKPV-NH2) and NDP-α-MSH (Ac-SYS-Nle-EHfRWGKPV-NH2), and their DOTA-conjugated variants, were synthesized on Rink amide resin at a 0.1 mmol scale following standard Fmoc procedures on an AAPPTEC Apex 396 automated multiple peptide synthesizer. DOTA-tris (t-Bu ester) was coupled to the N-terminus of the protected linear peptides on-resin. Each peptide-resin (1 equiv peptide) was suspended in N,N-dimethylformamide (DMF); and DOTA-tris (t-Bu ester) (5 equiv), 2-(7-Aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU; 5 equiv), 1-hydroxybenzotriazole (HOBt; 5 equiv), and N,N-diisopropylethylamine (DIPEA; 10 equiv) were added and the reaction was mixed at 37°C overnight. Completeness of coupling was checked by the Kaiser test for free amines (Kaiser, et al. 1970). The peptide-resin was filtered, washed with DMF, dichloromethane (DCM), and methanol, and allowed to dry. Peptides were cleaved and deprotected with 95:2.5:2.5 trifluoroacetic acid (TFA)/triisopropylsilane (TIS)/water for 2 h at room temperature, then precipitated with ice-cold ether. The peptides were purified on RP-HPLC by injection onto a Vydac C18 semipreparative column (10 × 250 mm, 10 μm, 300 Å; Grace, Deerfield, IL) eluted at 5 mL/min with 0.1% TFA and a 20-30% gradient of acetonitrile over 30 minutes while monitoring absorbance at 280 nm. The peak of interest was collected, concentrated by rotary evaporation, and lyophilized. Purified peptides were characterized on an Agilent 1100 LC-ESI-ion trap mass spectrometer.
Table 1.
α-MSH Peptide Analogs with Competitive Binding Assay and Stability Data.
| Peptide | Sequence | Mass (obs/calc) | IC50 (nM) | 24 hour stability (% remaining) |
|---|---|---|---|---|
| a-MSH | Ac-SYSMEHFRWGKPV-NH2 | 1664.7/1664.9 | 2.1 ±0.4 | N.D. |
| DOTA-a-MSH | DOTA-SYSMEHFRWGKPV-NH2 | 2009.0/2009.3 | 1.8 ±0.3 | 7.4 |
| NDP-a-MSH | Ac-SYS-Nle-EHfRWGKPV-NH2 | 1646.6/1646.9 | 0.21 ±0.03 | N.D. |
| DOTA-NDP-a-MSH | DOTA-SYS-Nle-EHfRWGKPV-NH2 | 1990.6/1991.2 | 0.59 ±0.11 | 41 |
| MTII | Ac-Nle-cyclo[DHfRWK]-NH2 | 1024.6/1024.2 | 0.26 ±0.08 | N.D. |
| ClickMTII | Ac-Nle-cyclo[Pra-HfRW-Lys(N3)]-NH2 | 1048.3/1048.2 | 0.39 ±0.06 | N.D. |
| DOTA-ClickMTII | DOTA-Nle-cyclo[Pra-HfRW-Lys(N3)]-NH2 | 1392.3/1392.6 | 25 ±1 | 100 |
| DOTA-GG-ClickMTII | DOTA-GG-Nle-cyclo[Pra-HfRW-Lys(N3)]-NH2 | 1506.1/1506.7 | 2.7 ±0.8 | 99 |
| ClickMTII-K-DOTA | Ac-Nle-cyclo[Pra-HfRW-Lys(N3)]-K(DOTA)-NH2 | 1562.4/1562.8 | 1.4 ±0.2 | 99 |
| ClickMTII-GGK-DOTA | Ac-Nle-cyclo[Pra-HfRW-Lys(N3)]-GGK(DOTA)-NH2 | 1676.6/1676.9 | 1.1 ±0.2 | 100 |
A single lactam bridge cyclized peptide (MTII, Ac-Nle-cyclo[DHfRWK]-NH2) was synthesized as described above, with the exception that Fmoc-Asp(Allyl)-OH and Fmoc-Lys(Alloc)-OH residues were inserted for selective deprotection to facilitate cyclization of the Fmoc protected peptide on-resin (Figure 1). Following synthesis, according to published procedures, the Allyl and Alloc groups were to be removed selectively by adding PhSiH3 (24 equiv) and Pd(PPH3)4 (0.10 equiv) in DCM and mixing for 10 minutes at room temperature (Thieriet, et al. 1997). The peptide-resin was filtered and washed with DCM and the deprotection was repeated. Complete removal of the Alloc group was tested by running the Kaiser test to detect the free amine of the lysine side chain. Peptide cyclization between the acid group of the aspartic acid and the primary amine of the lysine was achieved by the addition of benzotriazole-1-ly-oxy-tris-pyrrolidinophosphonium hexafluorphosphate (PyBOP; 1.1 equiv), DIPEA (2.2 equiv), and HOBt (0.5 equiv) in DMF. The reaction was mixed overnight at room temperature, and the resin was filtered and washed with DMF, DCM, and methanol. The cyclized peptide was cleaved and deprotected with 92:4:2:2 TFA/TIS/water/anisole for 2 h at room temperature, then precipitated with ice-cold ether. The cyclic MTII peptide was purified by semipreparative HPLC as described above using a 20-35% gradient of acetonitrile and the purified peptide was characterized on an Agilent 1100 LC-ESI-ion trap mass spectrometer.
Five click cyclized MTII analogs were synthesized by similar methods (Table 1; Figure 1). In this case, the internal amino acid sequences for these five MTII analogs differed with the insertion of of Pra and Lys(N3) residues to facilitate cyclization through triazole ring formation via “click” chemistry. In addition, an Fmoc-Lys(Dde)-OH was added for peptides with a C-terminal lysine residue. Following synthesis, peptides containing a Lys(Dde) were selectively deprotected by treatment with 2% hydrazine for 15 min. Peptides were cyclized by modification of methods by Turner, et al (Turner, et al. 2007). Briefly, the peptide-resin was swelled in DMF and DIPEA prior to the addition of 2,6-lutidine (10 equiv each). Sodium ascorbate was added as a 1% solution in 1:4 water/DMF (v/v; 3 equiv) followed by copper(I) bromide as a 1% solution in acetonitrile (1 equiv). Sodium ascorbate was added to maintain copper as the monovalent catalytically-active cationic form. The reaction mixture was stirred at 37°C overnight and the peptide-resin was filtered and washed with DMF, DCM, and methanol. DOTA-tris (t-Bu ester) was coupled to the primary amine (either N-terminus or Lysine side chain) and peptides were cleaved/deprotected as described above. To examine the effect of DOTA conjugation on binding affinity and stability, four ClickMTII variants were constructed with DOTA conjugated to either the N- or C-terminus of the peptide: (1) DOTA coupled directly to the free amine of the N-terminus; or (2) DOTA coupled through a glycine-glycine (GG) spacer added to the N-terminus of the peptide sequence; (3) DOTA attached to the C-terminus via the ε-amine side chain of a lysine residue added to the C-terminal end of the peptide; or (4) through the lysine side chain of a glycine-glycine-lysine spacer. The Click cyclized peptides were purified by semipreparative HPLC using a 20-30% or 20-35% gradient of acetonitrile and the purified peptides were characterized on an Agilent 1100 LC-ESI-ion trap mass spectrometer (Table 1).
2.3 In Vitro Competitive Binding Assay
B16/F10 murine melanoma cells were cultured in high glucose DMEM supplemented with 10% (v/v) FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin in 150 cm2 culture flasks at 37°C in a humidified 5% CO2 incubator; cells were grown to 70% confluency and used in competitive binding assays similar to the methods developed by Siegrist, et al. 1988. Cells were scraped from the culture flasks, counted on a Beckman Coulter cell counter, and resuspended in binding media (MEM containing 25 mM HEPES, 0.2% BSA, and 0.3 mM 1,10-phenanthroline). For binding assays, a total volume of 500 μL was used in 1.5 mL Eppendorf tubes. The cell suspension (50 μL containing approximately 4 million cells) was incubated with approximately 30,000 cpm of [125I]-[Nle4,D-Phe7]-α-MSH and increasing concentrations of α-MSH or peptide analog ranging from 10-6 to 10-11M. The tubes were mixed at 25°C, 600 rpm on an Eppendorf Thermomixer for 1.5 hours. Following incubation, cells were pelleted by centrifugation, the media was aspirated, pellets were transferred to 12 × 75 mm glass tubes, and the radioactivity of the cell pellet was determined using a PerkinElmer Cobra II gamma counter (PerkinElmer, Freemont, CA). All handling of radioactive materials was conducted using procedures and protocols approved through the University of Iowa Environmental Health and Safety Committee and Health Protection Office, adhering to ALARA principles. Each experiment was performed in quadruplicate and binding curves were plotted; IC50 values and their associated standard errors were calculated with GraphPad Prism 5 curve-fitting software (GraphPad Prism version 5.01 for Windows, GraphPad Software, San Diego, CA).
2.5 Radiochemistry
Copper-64 (64Cu) was obtained as 64CuCl2 solution from the College of Medicine, Washington University in Saint Louis (WashU, St. Louis, MO USA). Radiolabeling of DOTA-peptides with 64Cu was carried out as described previously (Anderson, et al. 2008, Boswell, et al. 2008, Brechbiel 2008, Hausner, et al. 2009). Briefly, 64Cu was received from WashU in approximately 10-40 μL of mildly acidic solution in a screw-top plastic “v-vial”, enclosed within appropriate lead shielding. To this vial was added ultra-pure water or pH 6 sodium acetate buffer solution to a final volume of 200 μL and the solution was mixed. The 200 μL solution was withdrawn and transferred to a 1.5 mL plastic v-vial (SealRite, natural microcentrifuge tube, Cat# 1615-5500, USA Scientific) and a measurement of the total activity was determined by dose calibrator (CRC-25R, Capintech, Ramsey, NJ). Aliquots of appropriate radioactivity amounts could then be transferred volumetrically to appropriate radiolabeling solutions. Generally, 10 nmoles of peptide was suspended in 50 μL of pH 6 acetate buffer. To this solution was added an appropriate aliquot of 64Cu master solution and the solution was incubated for 45 minutes at 70°C. This solution was used without further purification for in vitro stability testing. The identity of radiolabeled species was confirmed by radioHPLC retention time comparison to UV traces of the unlabeled species at 280 nm.
Radiolabeling with gallium-68 (68Ga) was carried out through the use of a 68Ga/68Ge generator system IGG100 (Eckert Ziegler, GmBH, Berlin, De) with a total 68Ge activity of approximately 600 MBq at the time of experiments presented here. The system was aged between 5-10 months for experiments conducted for this investigation. The methodology employed involves a single step purification of 68Ga eluted from the generator with 10 mL of 0.1 M HCl with a flow rate of approximately 2 mL per minute directly to a cation exchange (Strata™-XC, 33μm, Strong Cation, 30 mg/mL #8B-S029-TAK, Phenomenex) column mounted by supports fabricated in house. Following generator elution, the generator outflow line is disconnected and the Strata-XC column is air dried using a 20 mL plastic syringe (2×). Once dry, pure 68Ga is eluted from the Strata-XC column with 400 μL of 98% acetone/0.05 M HCl (prepared weekly and stored at 4°C) directly to a glass vial containing 5-10 nmoles DOTA-peptide conjugate; dissolved in 5 mL of pure water that had been preheated to approximately 80°C. This solution was then heated (open vessel) to 100°C and 68Ga (with the DOTA-peptide) are incubated for 15 m. Acetone is evaporated to less than 300 ppm in the final purified drug product by this method. Following the radiolabeling incubation period, the reaction mixture containing the [68Ga]-peptide and a small amount of free 68Ga was drawn up through a Strata™-X cartridge (33μm Polymeric Reversed Phase C-18, 30 mg/1mL, #8B-S100-TAK, Phenomenex® Inc., Torrance, CA USA) that had been preconditioned by passing 1 mL of 95% ethanol (USP for Injection) and 2.5 mL of pure water. The cartridge was then rinsed with 2 mL of water to remove any residual free 68Ga and finally the purified 68Ga-peptide conjugate was eluted in 500 μL of 1:1 ethanol (95%):isotonic saline solution. Radiochemical purity was determined by radioHPLC as described for 64Cu.
2.6 Serum Stability Assay
Stability of chelator-modified peptide bioconjugates was determined by radioTLC (64Cu labeled) using iTLC-SG glass microfiber chromatography paper impregnated with silica gel (Varian, Lake Forest, CA USA) as described in detail previously.(Rockey et al., 2011) Briefly, iTLC paper was cut into 5 cm × 25 cm strips and heated at 100°C for at least 30 minutes prior to measurements. Radiolabeled peptides were added to human serum and allowed to incubate at 37°C. At specified time points, 2 μl of the labeled peptide/serum mixture was spotted at a predetermined origin on the strips and the iTLC strips were developed for about 3 minutes with a mobile phase of 0.1 M citric acid. Under these conditions, it was determined in advance that stable (intact) peptide remains at the origin, while free 64Cu migrates with the mobile phase and peptide fragmentation can be observed by intermediately dispersed retention times. The strips were then dried with a common hairdryer. Possible fragmentation of the radiolabeled chelator-modified bioconjugates was determined through exposure of the iTLC strips to a storage phosphor screen (Fujifilm, Minamiashigara, Kanagawa, Japan) and subsequent screen imaging with a Typhoon™ FLA 7000 phosphorimager (GE Healthcare Bio-sciences AB, Uppsala, Sweden). Data analysis of the phosphorimages was performed using ImageQuant™ Analysis Toolbox software (TL 7.0, GE Healthcare Bio-sciences AB, Uppsala, Sweden). The percent intact radiolabeled chelator-modified peptides was calculated by dividing the integrated signal intensity over the bottom half of the TLC strips by the total integrated signal intensity over the entire length of the TLC strips. The technique was confirmed by radioHPLC. The pH of solutions was confirmed by use of an Horiba Compact pH meter (model B-213; Horiba Scientific Instruments, Ann Arbor, MI USA), calibrated daily at pH 4 and pH 7 using standard buffers or colorpHast pH paper (0-14 range; Merck KGaA, Darmstadt, De).
2.7 In Vivo PET Imaging and Biodistribution Studies
All animal experiments were performed according to approved protocols that were compliant to all rules and regulations of Federal regulatory bodies and the University of Iowa Animal Care and Use Committee.
Female SCID hairless mice (6-8 weeks old) were implanted subcutaneously with 3 million MC1R-expressing cells (B16-F10 murine melanoma). Five to seven days after inoculation, tumors had grown to approximately 100-200 mg and the tumor bearing mice were subjected to in vivo biodistribution and imaging studies using [68Ga] labeled MTII variants. For biodistribution studies, approximately 7.5 MBq (200 μCi) of [68Ga]DOTA-MTII variants (specific activity 30-50 MBq nmole-1 and radiochemical purity > 98%) was injected intravenously into the lateral tail vein using tuberculin insulin-type syringes (Becton Dickenson; 8 mm (5/16″), 500 μL, 31G Ultra-Fine™ needle).(Guo et al., 2010) Following injection of the radiolabeled tracer, animals were placed back in cages that were lined with absorbent material to sequester excretion for measurement at the conclusion of the uptake period (1.5 h). The uptake period for the comparison was determined by consideration of the radionuclide half-life of 68Ga, as well as preliminary pharmacokinetic studies using [68Ga]DOTA-Amide-NDP-α-MSH and literature reviews of other cyclized α-MSH analogs. At the conclusion of the uptake period, animals were sacrificed by approved procedures. Blood, tissues, and organs of interest (heart, liver, kidneys, lung, brain, muscle, and tumors) were resected, dabbed on blotter paper to remove excess blood, transferred to pre-weighed snapcap v-vials, weighed, and assayed for radioactivity by scintillation counting. The radioactivity in excretion (urine and feces) was determined by the total activity that could be wiped from the floor of wire-bottom cages using the inserted absorbent pads and urine extracted from the bladder. Following resections and removal of urine from the bladder, the carcass was also counted by dose calibrator measurement to complete a measure of radioactivity balance. The radioactivity measurements were normalized to percent injected dose per gram (%ID/g) of tissue for each tissue sample with appropriate decay corrections applied. An evaluation of the biodistribution of “free” 68Ga3+ was conducted to determine the possible contribution of free radiometal to tumor accumulation of injected dose. Finally, receptor mediated tumor accumulation to xenograft tumors was confirmed by co-injection of 50-750 μg unlabeled NDP-α-MSH together with 68Ga DOTA-peptide conjugates.
In vivo imaging studies were conducted to complete the preliminary evaluation of the click-cyclized peptides. For imaging, animals were anesthetized and injected with approximately 25 MBq [68Ga]-peptide (specific activity 30 to 50 MBq nmole-1) suspended in approximately 100 μL of saline solution. Animals were allowed access to food and water and all times. Imaging of the biodistribution of the radiolabeled peptide was conducted at 1.5 h post injection (p.i.) using an Inveon™ small animal PET/CT (Siemens, Inc., Knoxville, TN USA). A 15 min. static scan was employed for imaging. Immediately prior to scanning, the bladder contents were carefully expunged and collected in an absorbent pad for quantification of urine radioactivity and to prevent urinary contamination of the imaging bed.
3 Results
3.1 Peptide Synthesis
The lactam-bridge cyclized α-MSH peptide analog MTII and click-cyclized variant ClickMTII were synthesized utilizing similar Fmoc procedures (Rink amide resin) and final Fmoc removal and N-terminal acylation. Thus, the molecular compositions were identical, with the exception of the linkage used for cyclization (Figure 1). For lactam bridge formation of MTII, removal of allyl/alloc protecting groups was required to prepare the peptide for cyclization by amide bond formation via the acid moiety of the aspartic acid and the primary amine of lysine (lactam bridge formation). While much of the synthetic strategy for preparation of the ClickMTII derivative was the same, the cyclization strategy in this case was achieved through cycloaddition of the alkyne group of propargylglycine and the azide of an azido-modified lysine residue to form the triazole ring (Figure 1). Following cyclization, peptides were deprotected and cleaved from the resin, purified by semipreparative HPLC, and characterized by LC-MS (data not shown).
To facilitate radiolabeling with 64Cu and 68Ga, a DOTA chelator was conjugated to the MTII and ClickMTII variants by standard methods. To conjugate DOTA to the N-terminus of MTII, the N-terminal Fmoc was left in place at the end of the peptide synthesis. The peptide was then treated with palladium catalyst and phenyltrihydrosilane for removal of the allyl and alloc protecting groups and cyclized as previously described (see section 2.2 Peptide Synthesis). However, HPLC analysis of the N-terminal Fmoc-protected MTII peptide before cyclization by HPLC showed the presence of two major peaks, corresponding to the peptide with and without the N-terminal Fmoc (data not shown). Thus, difficulty in synthesizing an N-terminal Fmoc-protected MTII prevented smooth attachment of DOTA to this peptide and alternative click chemistry strategies were pursued.
On the other hand, ClickMTII was synthesized smoothly without N-terminal acylation, facilitating DOTA addition to the free amine of the N-terminus. In this case, the N-terminal Fmoc-protection was not necessary as the cyclization was carried out by click chemistry, which does not involve a primary amine. DOTA was then coupled to the peptide by formation of an amide bond between a carboxylic acid on DOTA and the primary amine of the N-terminus. DOTA-ClickMTII was finally deprotected and cleaved from the resin, purified by semipreparative HPLC, and characterized by LC-MS (Table 1).
In addition to the MTII and ClickMTII peptides, native α-MSH, NDP-α-MSH, and their N-terminal DOTA derivatives were prepared for use in in vitro experiments for structure-activity comparisons (Table 1). Preparative HPLC-purified peptides were re-chromatographed on analytical RP-HPLC to establish structure on the basis of retention time and mass determined by LC-MS. For each purified peptide, a single major peak demonstrated >95% purity that also produced a singly charged positive ion in the ESI-MS corresponding to the calculated mass of the desired peptide (Table 1).
3.2 Radiolabeling
DOTA-GG-ClickMTII and ClickMTII-GGK-DOTA were radiolabeled with 64Cu in acetate buffer (pH 6) for 45 m at 70 °C. For 64Cu, no further purification was required to obtain radiochemical purity of >98% with specific activity of >35 MBq nmole-1 peptide conjugate (FIG). Similar results were obtained for radiolabeling with 68Ga. In this case, radiolabeling was conducted by elution of 68Ga from a manual generator system. Following a radiolabeling step (5 mL water, pH 3.5, 15 min., 100°C), final purification of the 68Ga labeled peptides was performed using a disposable C18 reverse phase cartridge. Radiochemical purity was routinely >98% with specific activities up to 45 MBq nmole-1 of peptide conjugate.
3.3 In Vitro Competitive Binding Assays
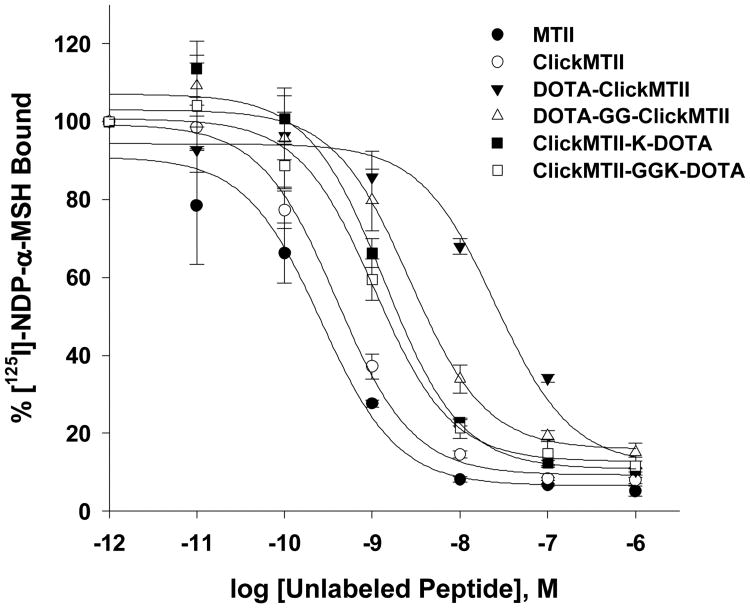
The ClickMTII peptide analogs, without and with DOTA, were evaluated for their binding affinity to melanocortin receptors in B16/F10 murine melanoma cells. Competitive binding assays were carried out using the radiolabeled ligand [125I]-NDP-α-MSH and increasing amounts of α-MSH analog. Experiments were carried out in quadruplicate, and binding curves were plotted as the mean and standard error of the four sets of data (Figure 2, Table 1). The uncertainties of these results are based on the standard deviation of the regression-curve fit-derived affinity results of the quadruplicate measurements. MTII (closed circle) and ClickMTII (open circle) give similar curves, with IC50 values of 0.26 and 0.39 nM, respectively. Addition of DOTA to the N-terminus of ClickMTII (DOTA-ClickMTII; closed triangle) results in a 64-fold decrease in binding affinity and an IC50 of 25 nM. Due to the large increase in the IC50 with the addition of DOTA, three other analogs were synthesized to optimize the placement of DOTA. DOTA was conjugated to the C-terminus through the side chain of an added lysine residue (ClickMTII-K-DOTA; IC50 = 1.4 nM). Two peptides were also synthesized with glycine spacers to see if the added distance between the DOTA and the peptide would increase binding affinity (DOTA-GG-ClickMTII and ClickMTII-GGK-DOTA; IC50 = 2.7 and 1.1 nM, respectively). Binding affinity data is summarized in Table 1.
Figure 2.
Competitive inhibition of 125I-NDP-α-MSH binding to B16/F10 murine melanocytes by α-MSH analogs. Competitive binding curves are the mean and standard error of quadruplicate experiments.
3.4 Serum Stability
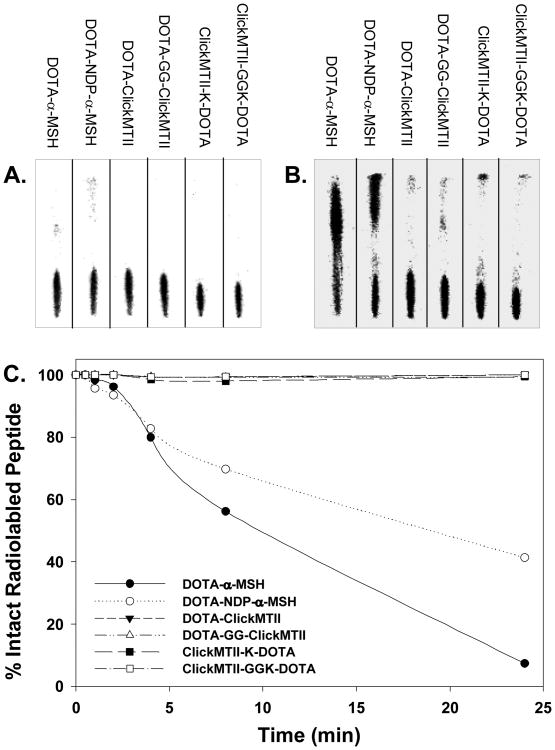
DOTA-conjugated peptides were radiolabeled with 64Cu in pH 6.0 acetate buffer and used without further purification. Labeled peptides were added to human serum and incubated at 37°C to determine both the stability of the radionuclide within the DOTA complex and the stability of the peptide to serum proteases. At specified time points, samples were removed and analyzed by radioTLC as described in the experimental procedures. When spotted on the iTLC strip, intact [64Cu]-DOTA-peptide remained where initially spotted, while free 64Cu and peptide fragments migrated with variable retention characteristics with the solvent. Immediately upon addition of the peptides to the serum, a reference sample was taken of each peptide. At this zero time point, all [64Cu]-DOTA-peptides remained intact and did not migrate with the solvent (Figure 3). Analysis of the iTLC strips at 24 hours showed that a significant amount of both linear test peptides (DOTA-α-MSH and DOTA-NDP-α-MSH) were degraded by the serum, while the cyclized DOTA-ClickMTII analogs were intact (Figure 3). The four DOTA-ClickMTII peptides remained stable throughout the entire 24 hour experiment, while the linear DOTA-α-MSH and DOTA-NDP-α-MSH begin to degrade after 1 hour in serum. By 24 hours, only 7.4% of DOTA-α-MSH and 41% of NDP-α-MSH remained, while >99% of all four DOTA-conjugated cyclized ClickMTII analogs remained stable (Figure 3, Table 1).
Figure 3.
Stability of [64Cu]-DOTA-peptide analogs in human serum. Radiolabeled peptides were incubated in human serum at 37°C, and at specified time points samples were spotted on iTLC paper and developed with 0.1 M citric acid. Panel A shows the peptides in serum at t = 0 min, and all of the radiolabeled peptide remains at the bottom of the plate. By 24 h, the linear peptides α-MSH and NDP-α-MSH are degraded by the serum while the cyclic ClickMTII analogs remain intact (Panel B). Panel C graphically represents the degradation data over the 24 h time period for all peptides.
3.5 In Vivo Biodistribution and Imaging
The tumor targeting properties of the click cyclized peptides for targeted molecular imaging, were evaluated in tumor-bearing female scid hairless mice. The in vivo biodistribution of DOTA-GG-ClickMTII and ClickMTII-GGK-DOTA were examined at 90 minutes post injection of 25-33 MBq 68Ga labeled DOTA-peptide conjugate. Receptor blocking studies were conducted for the DOTA-GG-ClickMTII derivative by co-injection of unlabeled NDP-α-MSH peptide to confirm receptor mediated tumor accumulation of the tracers in vivo. The tumor accumulation was 16.0±5.7 %ID/g for DOTA-GG-ClickMTII and 10.2±1.3 %ID/g for ClickMTII-GGK-DOTA at 90 minutes post injection. Urinary excretion of the radiolabeled peptides was assessed by collection of urine in an absorbent pad during the uptake period. These results demonstrated fast pharmacokinetics with 78.8±8.6% of the injected dose voided within the 90 minute uptake period. Blocking studies reduced tumor uptake to 5±0.9 %ID/g of tumor tissue. Normal organ uptake of the tracers was low with the highest values observed in kidney (5 %ID/g), lung (5 %ID/g), and heart (5 %ID/g) with a tumor:kidney ratio >3 for each DOTA-peptide conjugate at 90 minutes post injection. The tumor:blood ratios were 5.0±3.4 for the DOTA-GG-MTII variant and 3.2±1.1 for the MTII-GGK-DOTA derivative at 90 minutes post injection. Low liver retention was observed for both variants (1.1±0.7 %ID/g and 1.3±0.1 %ID/g respectively). Injection of 68Ga3+ in saline produced a completely different biodistribution pattern indicative of a relatively stable radiometal-DOTA coupling.
4 Discussion
4.1 Click Cyclizations and Peptide Synthesis
We have prepared five cyclic α-MSH analogs with high affinity for MC1R and prolonged stability in human serum using copper-catalyzed click chemistry. Upregulation of cell surface receptor expression, such as melanocortin receptors, in cancer cells has been identified as a promising target for the development of targeted molecular imaging agents and tumor-targeting drugs. Many of the endogenous ligands of these receptors are peptides, such as α-MSH, which is the native peptide ligand for the melanocortin receptors. In its native form, a linear 13 amino acid peptide, α-MSH is rapidly degraded and not very useful in the clinical realm. Alanine replacement scans led to the discovery of the “essential core” needed for receptor binding and bioactivity, along with replacements of Nle4 and D-Phe7 to give NDP-α-MSH with very high bioactivity combined with increased stability (Sawyer, et al. 1980, Sahm, et al. 1994, Cone 2000). The design of cyclic α-MSH analogs has further increased receptor binding affinities and biological stability (Al-Obeidi, et al. 1989, Al-Obeidi, et al. 1989). A variety of techniques have been utilized for cyclization of α-MSH peptides, the most common strategies being the lactam bridge, disulfide coupling, and metal coordination (Miao et al., 2008; Cantorius et al., 2009; Guo et al., 2010; Guo et al., 2011). Cyclization restricts the peptide to a secondary structure designed to better fit the binding pocket of the receptor in hope of enhancing binding affinity, stability, and potentially even receptor selectivity (Raposinho, et al.).
In the present study, a novel class of cyclic α-MSH analogs has been synthesized utilizing cyclization by copper-catalyzed click chemistry. The aspartic acid has been substituted with propargylglycine, while the terminal-primary amine on the side chain of the internal lysine has been replaced by an azide function. The Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of the alkyne and azide is a mild and selective bioconjugation reaction that forms a 1,4-disubstituted-1,2,3-triazole (Huisgen 1963). The triazole moiety has been described as an isosteric replacement for the amide bond (Brik, et al. 2005, Bock, et al. 2007). This study not only describes the potential advantageous of the click chemistry approach for the cyclization reaction of a specific peptide molecular targeting vector, but also examines the effect of the triazole ring on binding of the small peptide to the melanocortin receptors as an alternative to lactam, disulfide, and other compositional cyclization constructs for molecular imaging and radionuclide therapy.
Based on the sequence of the cyclic peptide MTII, five ClickMTII analogs were prepared and tested for their binding affinity and serum stabililty as compared to the original MTII peptide (Table 1). MTII, a 7 amino acid peptide containing the core residues His-D-Phe-Arg-Trp for receptor binding, was cyclized by a lactam bridge through the side chains of aspartic acid and lysine. This required orthogonal protection of the peptide so that the Asp and Lys could be deprotected and cyclized while the rest of the peptide (side chains and N-terminus) remained protected on the resin (Figure 1). Following synthesis, the peptide was acylated on the N-terminus, the allyl/alloc groups were deprotected, the lactam bridge was formed on-resin, and the peptide was deprotected and cleaved from the resin. For cyclization by the click reaction, propargylglycine and azido-modified lysine were substituted for aspartic acid and lysine, respectively. As expected, the click chemistry approach did not require protection and subsequent deprotection at this step, greatly simplifying our synthetic strategy and potentially increasing our overall synthetic yields.
After solid phase synthesis of the peptide, the N-terminus was acylated, the peptide was directly cyclized by addition of Cu(I) bromide (with ascorbate), and then deprotected and cleaved from the resin by standard techniques. In our hands, the alkyne and azide moieties are stable (unreactive) to all steps of the peptide synthesis and cyclization may be performed at any point in the reaction scheme without involving protecting group chemistry. For lactam bridge formation, cyclization was only possible on-resin prior to deprotection and cleavage so that cyclization occurred between the selected acid and amine groups. Cyclization of the N-terminal acylated MTII peptide by click reaction also proved more efficient than lactamization (Figure 1). This means that the peptide could also be synthesized and then deprotected and cleaved from the resin with cyclization performed as a final step under mild aqueous conditions. It is expected that dilution could be used at this step to minimize dimerization if required.
In order to be useful as an in vivo imaging agent, a metal chelator was incorporated into the peptide sequence. DOTA is a 12-membered macrocyclic compound that is a prototype chelation moiety used for coupling of a variety of di- and trivalent metals (including 64Cu) for both imaging and therapy. Numerous bifunctional derivatives are available for facile conjugation to peptides through the formation of an amide bond (as well as other potential bioconjugate strategies). DOTA positioning has been described previously to have a profound effect on the tumor targeting and kidney uptake characteristics of linear and cyclized α-MSH analogs (Froidevaux et al., 2004, Froidevaux et al., 2005, Guo et al., 2009). For our investigation, we initially utilized the free amine of the N-terminus as an efficient connection point for MTII and ClickMTII analogs. Thus, both the lactam-bridge cyclized MTII and click-cyclized ClickMTII peptides were synthesized without acylation of the N-terminus so that the free amine could serve as the point of attachment for DOTA. In the case of MTII, to enable efficient cyclization, the N-terminus should remain Fmoc-protected during the allyl/alloc deprotection and subsequent lactam bridge cyclization. Based on previous studies, we expected that these orthogonal protecting groups would allow for the selective deprotection and cyclization to occur — through the side chains of the aspartic acid and lysine — while preserving the N-terminal amine Fmoc protection (Bloomberg, et al. 1993, Dessolin, et al. 1995, Thieriet, et al. 1997). However, in our hands, during the allyl/alloc deprotection step, a fraction of the Fmoc was removed from the N-terminus of peptide, exposing the terminal amine for potential reaction with the aspartic acid side chain at the cyclization step (data not shown). This observation indicated the potential for reduced selectivity of the technique. This behavior was observed despite numerous minor modifications to our synthetic strategy and the results prompted us to examine alternative strategies.
With this knowledge, our efforts turned solely to the more selective click chemistry approach to cyclization of DOTA-conjugated ClickMTII peptides. As mentioned above, the ClickMTII peptide sequence was prepared without N-terminal acylation. In addition, the N-terminus was left as a free amine as protection was not needed for cyclization by the click reaction. Following peptide synthesis, the ClickMTII was cyclized by introduction of CuBr and sodium ascorbate (to preserve the catalytic monovalent Cu+ state), which is selective for the cycloaddition reaction of terminal alkynes with azide moieties. DOTA was then coupled to the N-terminus and the peptide was purified by semipreparative RP-HPLC, followed by characterization by LC-MS. When analyzed by RP-HPLC, the crude peptide (data not shown) had a major peak corresponding to the DOTA-conjugated cyclized peptide, with a much simplified synthetic process. The propargyl and azido amino acids required for cyclization of the peptide were stable to the solid phase peptide synthesis reagents and are selective for the click cyclization and did not require orthogonal protecting groups. In addition, because the click reaction utilizes an internal azide (rather than an amine), the N-terminus could be Fmoc-deprotected following peptide synthesis without interfering with subsequent steps. Following synthesis, the crude peptide was purified by standard semipreparative RP-HPLC techniques in high purity (>95%). Overall, the synthesis of DOTA-ClickMTII required fewer steps and provided a more efficient pathway to formation of the cyclized peptide. These characteristics opened efficient avenues for synthesis of three other novel peptides based on the click cyclization (Table 1). Studies to optimize this process for the highest possible chemical yields are underway in our laboratories.
Previous reports demonstrate not only the profound effect of DOTA positioning on molecular targeting, but also the significant effect of the composition of chelator-amino-acid linkers on the binding affinity and pharmacodynamic properties of peptide targeting vectors for molecular imaging (Garrison et al., 2008, Fragogeorgi et al., 2009, Guo et al., 2011). Three unique DOTA conjugates were prepared based on these findings. In an effort to increase the spacing between the DOTA and the peptide, a glycine spacer was added to afford N-terminal conjugated DOTA-GG-ClickMTII. A C-terminal end DOTA conjugate of the click cyclized peptide was achieved by attachment to the ε-amine of a lysine residue placed at the C-terminus, ClickMTII-K-DOTA. Finally, a glycine spacer was added to the C-terminal DOTA derivative resulting in the conjugate ClickMTII-GGK-DOTA (Table 1). These conjugates were evaluated by in vitro cell binding assays and the most promising candidates were further evaluated by in vivo biodistribution studies and finally by PET/CT imaging.
4.2 In Vitro Evaluation
Binding Affinity Assays
The lactam cyclized MTII peptide and ClickMTII α-MSH analogs, along with DOTA-conjugated ClickMTII variants described above were evaluated for selective binding to MC1R on B16/F10 murine melanoma cells in competitive binding assays (Figure 2). While the affinity figure of merit IC50 was observed to be slightly elevated (lower affinity) for the click cyclized ClickMTII peptide in the comparison to the lactam-bridge cyclized MTII (ClickMTII IC50 = 0.39 nM; MTII IC50 = 0.26 nM), the results were not statistically significant based on the uncertainty derived from n=4 experimental runs for each peptide. These data suggest that replacement of the lactam bridge by a triazole ring does not significantly affect binding of the peptide to the receptors in B16/F10 cells. On the other hand, conjugation of DOTA directly to the N-terminus, DOTA-ClickMTII, resulted in a 64-fold increase in the IC50 over the ClickMTII peptide without DOTA (25 nM). This decrease in binding affinity indicated that DOTA was interfering with binding, possibly as a result of being too close to the core amino acids that interact with the binding pocket of the receptors, thereby impeding the docking reaction by steric hindrance. To test this hypothesis, a glycine spacer was added to increase the distance between the binding domain of the peptide and the DOTA moiety. The effect on binding for the resulting derivative (DOTA-GG-ClickMTII) was a reduction in the observed IC50 by a factor of 9 to a value of 2.7 nM, which is comparable to previously reported values for lactam-bridge cyclized DOTA-conjugated α-MSH analogs (1.77 nM; Guo et al., 2010; 2.1 nM; Guo et al., 2011). Attachment at the C-terminus, ClickMTII-K-DOTA, gave an IC50 of 1.4 nM, which was lowered to 1.1 nM in ClickMTII-GGK-DOTA with the addition of a glycine spacer. These binding measurements compare favorably with high affinity linear peptide derivatives DOTA-α-MSH and DOTA-NDP-α-MSH (IC50 2.1 and 0.59 nM respectively) and are similar to binding affinities of linear variants reported by Froidevaux et al. (1.37 nM; Froidevaux et al., 2004; see Table 1). Thus, the three novel analogs of DOTA-GG-ClickMTII, ClickMTII-K-DOTA, and ClickMTII-GGK-DOTA show promise as potential imaging agents with comparable binding affinities based on in vitro competitive receptor affinity assessment.
Serum Stability Comparisons
DOTA-conjugated peptides were also tested for their stability in human serum (Figure 3). A method was developed that enabled efficient qualitative examination of the stability of the peptide constructs in serum — with operationally-defined quantitative information about the stability of the 64Cu-DOTA complexation and fragmentation of the peptide obtained by examining the migration of radioactivity signal on iTLC strips. Further experimental details of the method development can be obtained in Rockey et al., 2011. Peptides were radiolabeled with 64Cu and incubated with human serum to test for stability of the radionuclide within the DOTA chelator and the stability of the peptide to serum proteases. As expected and based on the migration of apparent peptide fragments, the linear-peptide forms DOTA-α-MSH and DOTA-NDP-α-MSH were relatively unstable in serum, with only 7.4% and 41% of the radioactivity signal remaining at iTLC strip origin at the 24 hour sampling timepoint, respectively. α-MSH is known to have a half-life of less than 3 minutes in vivo, so while the DOTA may stabilize it from some proteases, relatively rapid degradation in serum is expected. DOTA-NDP-α-MSH, while more biologically stabile due to the amino acid substitutions and DOTA conjugation, as a linear peptide is expected to be susceptible to serum proteases and with enough time is expected to be degraded, albeit at a slower rate. In contrast, but in agreement with previously reported lactam-bridge cyclized α-MSH analogs (Guo et al., 2010), all four click-cyclized DOTA-conjugated (ClickMTII) analogs appeared to remain stable for at least 24 hours in human serum (>99% intact radiolabeled peptides) and the 64Cu-DOTA complex remained stable as well. Thus, from analysis of the iTLC strips, the DOTA-conjugated ClickMTII peptides were not significantly degraded by serum proteases within 24 hours, and they were also able to retain the 64Cu within the DOTA chelator. This is in contrast to linear DOTA-α-MSH and DOTA-NDP-α-MSH, which appear as a degraded “smear” on the iTLC strip at 24 hours (Figure 3).
4.3 In Vivo Biodistributions and PET Imaging
The tumor targeting properties of the click cyclized peptides for targeted molecular imaging were evaluated in tumor-bearing female SCID mice. The in vivo biodistribution of DOTA-GG-ClickMTII and ClickMTII-GGK-DOTA were examined at 90 minutes post injection of 25-33 MBq 68Ga labeled DOTA-peptide conjugate. Receptor blocking studies were conducted for the DOTA-GG-ClickMTII derivative by co-injection of unlabeled NDP-α-MSH peptide to confirm receptor mediated tumor accumulation of the tracers in vivo. The results are comparable to previous investigations of similar lactam-bridge and stable-metal cyclized α-MSH analogs (Miao et al., 2008, Guo et al., 2009, Cantorias et al., 2009, Guo et al., 2010, Guo et al., 2011), indicating that the triazole linkage is an effective replacement for the lactam bridge for peptide cyclization. The tumor accumulation was 16.0±5.7 %ID/g for N-terminal DOTA-conjugate DOTA-GG-ClickMTII and 10.2±1.3 %ID/g for C-terminal DOTA-modified ClickMTII-GGK-DOTA at 90 minutes post injection. A reduced ring-size lactam-bridge variant presented by Guo et al., 2010 appears to have advantages over all other such cyclized derivatives, with tumor accumulation of greater than 24%ID/g of tumor tissue reported. These results and our findings suggest that further modifications to the click cyclization approach could result in improved tumor targeting characteristics, which is the subject of continued investigation in our laboratories. Urinary excretion of the radiolabeled peptides was assessed by collection of urine during the uptake period. These results demonstrated fast pharmacokinetics, with 78.8±8.6% of the injected dose voided by urinary excretion within the 90 minute uptake period, which is comparable to previously reported pharmacokinetic behavior of cyclized α-MSH analogs (Miao et al., 2008, Guo et al., 2009, Cantorias et al., 2009, Guo et al., 2010, Guo et al., 2011). Blocking studies reduced tumor uptake to 5±0.9 %ID/g, demonstrating that tumor accumulation of the examined peptide derivatives is receptor mediated. Interestingly, blocking to 5%ID/g required co-injection of nearly 500 μg of cold NDP-α-MSH. Based on a comparison of our blocking results to other studies reported, it appears that the B16/F10 cell line cultured in our laboratory possesses higher receptor density that the B16/F1 cell line used for studies presented by others, which is the subject of further investigation. Normal organ uptake of the tracers was low and similar to recent reports of other DOTA-modified cyclized α-MSH analogs, with the highest values observed in kidney (5 %ID/g), lung (5 %ID/g), and heart (5 %ID/g) with a tumor:kidney ratio >3 for each DOTA-peptide conjugate at 90 minutes post injection. The tumor:blood ratios were 5.0±3.4 for the DOTA-GG-MTII variant and 3.2±1.1 for the MTII-GGK-DOTA derivative at 90 minutes post injection. Low liver retention was observed for both variants (1.1±0.7 %ID/g and 1.3±0.1 %ID/g respectively) labeled with 68Ga. Injection of 68Ga3+ in saline produced a completely different biodistribution pattern, indicative of a relatively stable radiometal-DOTA coupling. The two most similar lactam-bridge cyclized DOTA-conjugates examined for comparison (Guo et al., 2010, Guo et al., 2011) were evaluated at 30 minutes and 120 minutes post injection, while our results were evaluated in this preliminary investigation at 90 minutes post injection. Interestingly, the tumor:normal ratios reported here are roughly intermediate to the time points presented elsewhere, suggesting that the click-cyclized variants are displaying similar behavior to lactam-bridge cyclized derivatives of α-MSH.
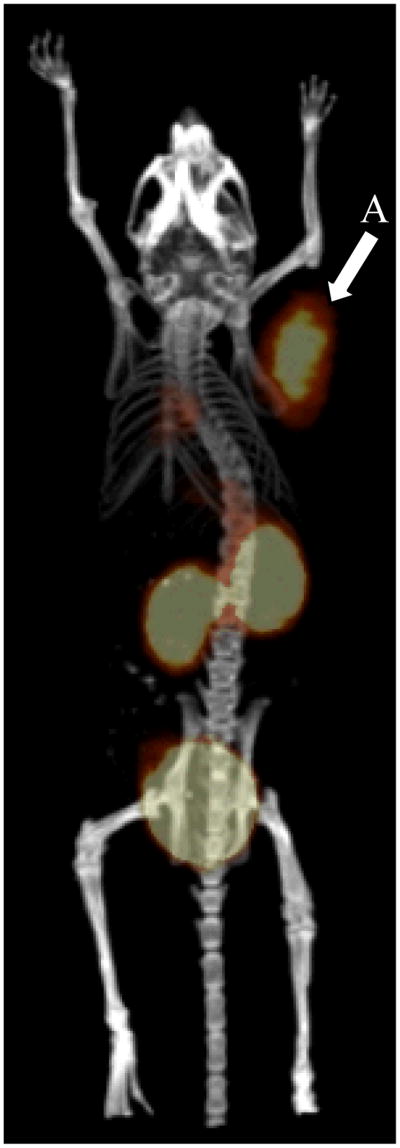
Good tumor accumulation properties observed for the DOTA-GG-ClickMTII variant prompted us to examine the potential of this α-MSH analog for in vivo PET imaging. These studies were carried out by tail vein injection of approximately 18.5 MBq of 68Ga labeled DOTA-GG-ClickMTII and imaging at 90 minutes post injection. Excellent tumor contrast was observed with clear tumor visualization of MC1R positive B16/F10 tumor xenograft (Figure 4). The PET/CT image reveals rapid tumor accumulation and renal excretion of the click cyclized peptide with only tumor, kidneys, and bladder apparent in the whole-body field of view of the PET/CT image (Figure 4). Tumor contrast observed for this investigation is excellent and comparable to previously reported findings for lactam bridge cyclized α-MSH (Miao et al., 2008, Guo et al., 2009, Cantorias et al., 2009, Guo et al., 2010, Guo et al., 2011).
Figure 4.
PET/CT image of the biodistribution of the biodistribution of [68Ga]DOTA-GG-MTII; 35 MBq radiolabeled peptide; tail vein injection; 90 minute accumulation period; 15 minute static acquisition: (A) xenograft B16-F10 mouse melanoma tumor; (B) kidney accumulation and retention; (C) bladder accumulation and retention. The average and standard deviation of the biodistribution of [68Ga]DOTA-GG-MTII is given in Table 2.
5 Summary and Conclusions
In summary, a novel class of cyclized MTII-type α-MSH peptide analogs have been synthesized and cyclized by Cu(I) catalyzed terminal azide-alkyne cycloaddition “click” chemistry techniques. Three new DOTA-conjugated analogs – DOTA-GG-ClickMTII, ClickMTII-K-DOTA, and ClickMTII-GGK-DOTA – have exhibited <2 nM affinity for MC1R receptor positive malignant cells in vitro and high stability in human serum. The DOTA-GG-ClickMTII variant was further evaluated by PET imaging in a xenograft mouse model of melanoma. Thus, these click-chemistry-cyclized variants show promise as agents for melanocortin receptor-targeted imaging and radionuclide therapy. These results indicate that the triazole ring is an effective bioisosteric replacement for the lactam bridge in cyclization of the MTII peptide as the ClickMTII has comparable affinity in receptor positive cells compared to MTII (cyclized by lactamization). Radiolabeling results confirm that Cu catalyst is sufficiently removed prior to DOTA chelator addition to enable preparation with sufficient radiochemical purity (>98%) and high specific activity (>35 MBq nmole-1) for molecular imaging applications. Cyclization by this click chemistry strategy is advantageous for this application because it obviates the need for protecting groups required for standard lactam bridge formation used in previous approaches to cyclizing α-MSH analogs. Both the propargylglycine and azido-lysine residues are stabile to all steps of the peptide synthesis and selective for the click cyclization, so side-chain protection/deprotection is not necessary. The click cyclization approach is a valuable tool for preparation of DOTA-conjugated peptides for molecular imaging and radionuclide therapy.
Table 2.
In vivo biodistribution summary of 68Ga labeled DOTA conjugated click cyclized peptides.§ Animals were injected via tail vein with approximately 25 MBq of 68Ga labeled compound with specific activity >35 MBq nmole-1 and >98% radiochemical purity. Animals were sacrificed at 90 min post injection.
| DOTA-GG-ClickMTII | DOTA-GG-ClickMTII (blocked) | ClickMTII-GGK-DOTA | Free 68Ga3+ | |
|---|---|---|---|---|
| Tissue | %ID/gδ | %ID/g | %ID/g | %ID/g |
| Blood | 3.2±1.0 | 3.6±0.6 | 3.2±1.0 | 12.5±3.2 |
| Heart | 4.6±2.5 | 1.8±0.3 | 5.0±0.2 | 5.4±0.5 |
| Liver | 1.1±0.7 | 2.5±1.2 | 1.3±0.1 | 2.0±0.8 |
| Kidney | 4.7±0.9 | 11.7±2.1 | 5.4±0.1 | 5.0±0.6 |
| Brain | 2.3±1.1 | 0.4±0.1 | 1.3±0.0 | 0.6±0.0 |
| Muscle | 3.1±1.8 | 1.7±0.4 | 2.9±0.4 | 2.6±0.9 |
| Lung | 4.9±1.3 | 3.8±0.9 | 4.2±0.5 | 6.9±4.4 |
| Tumor | 16.0±5.7 | 5.0±0.9 | 10.2±1.3 | 11.0±2.2 |
| Tumor:Blood | 5.0±3.4 | 1.4±0.7 | 3.2±1.1 | 0.9±2.7 |
| Tumor:Kidney | 3.4±3.3 | 0.4±1.5 | 1.9±0.7 | 2.2±1.4 |
| Tumor:Liver | 14.0±3.2 | 2.0±1.0 | 7.9±0.7 | 5.4±1.5 |
| %ID | %ID | %ID | %ID | |
| Excretion | 78.8±8.6 | 61.7±9.0 | NDζ | 12.9±1.7 |
mean ± std. dev. (n = 3);
%ID/g = percent injected dose per gram.
not determined.
Acknowledgments
Support for this work was provided by American Cancer Society (IRG-77004-31; MKS), the Holden Comprehensive Cancer Center (MEM, MKS), Neuroendocrine Tumor Fund (MSO), and University of Iowa Dance Marathon (MEM). MEM is supported by T32 University of Iowa Institutional Training Grant in Hematologic and Oncologic Childhood Diseases (HL080070). The authors thank Dr. Kevin Rice, Dr. Lynn Teesch and Vic Parcell for spirited assistance with mass spectral analyses.
References
- Al-Obeidi F, Castrucci AMdL, Hadley ME, Hruby VJ. Potent and prolonged-acting cyclic lactam analogs of α-melanotropin: design based on molecular dynamics. Journal of Medicinal Chemistry. 1989;32(12):2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- Al-Obeidi F, Hadley ME, Pettitt BM, Hruby VJ. Design of a new class of superpotent cyclic α-melanotropins based on quenched dynamic simulations. Journal of the American Chemical Society. 1989;111(9):3413–3416. doi: 10.1021/ja00191a044. [DOI] [Google Scholar]
- Anderson CJ, Wadas TJ, Wong EH, Weisman GR. Cross-bridged macrocyclic chelators for stable complexation of copper radionuclides for PET imaging. Q J Nucl Med Mol Imaging. 2008;52(2):185–192. R39072063 [pii] [PMC free article] [PubMed] [Google Scholar]
- Bednarek MA, Macneil T, Kalyani RN, Tang R, Van der Ploeg LHT, Weinberg DH. Analogs of MTII, Lactam Derivatives of α-Melanotropin, Modified at the N-Terminus, and Their Selectivity at Human Melanocortin Receptors 3, 4, and 5. Biochemical and Biophysical Research Communications. 1999;261(1):209–213. doi: 10.1006/bbrc.1999.0981. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, MacNeil T, Kalyani RN, Tang R, Van der Ploeg LHT, Weinberg DH. Analogs of Lactam Derivatives of α-Melanotropin with Basic and Acidic Residues. Biochemical and Biophysical Research Communications. 2000;272(1):23–28. doi: 10.1006/bbrc.2000.2589. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, MacNeil T, Tang R, Fong TM, Angeles Cabello M, Maroto M, Teran A. Potent and Selective Peptide Agonists of α-Melanocyte Stimulating Hormone (αMSH) Action at Human Melanocortin Receptor 5; their Synthesis and Biological Evaluation in vitro. Chemical Biology & Drug Design. 2007;69(5):350–355. doi: 10.1111/j.1747-0285.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, MacNeil T, Tang R, Fong TM, Cabello MA, Maroto M, Teran A. Potent and selective agonists of human melanocortin receptor 5: cyclic analogues of α-melanocyte-stimulating hormone. J Med Chem. 2007;50(10):2520–2526. doi: 10.1021/jm0614275. 10.1021/jm0614275 [doi] [DOI] [PubMed] [Google Scholar]
- Bednarek MA, Silva MV, Arison B, MacNeil T, Kalyani RN, Huang RR, Weinberg DH. Structure-function studies on the cyclic peptide MT-II, lactam derivative of alpha-melanotropin. Peptides. 1999;20(3):401–409. doi: 10.1016/s0196-9781(99)00048-0. S0196-9781(99)00048-0 [pii] [DOI] [PubMed] [Google Scholar]
- Bloomberg GB, Askin D, Gargaro AR, Tanner MJA. Synthesis of a branched cyclic peptide using a strategy employing Fmoc chemistry and two additional orthogonal protecting groups. Tetrahedron Letters. 1993;34(29):4709–4712. [Google Scholar]
- Bock VD, Speijer D, Hiemstra H, van Maarseveen JH. 1,2,3-Triazoles as peptide bond isosteres: synthesis and biological evaluation of cyclotetrapeptide mimics. Organic & Biomolecular Chemistry. 2007;5(6):971–975. doi: 10.1039/b616751a. [DOI] [PubMed] [Google Scholar]
- Boswell CA, Regino CA, Baidoo KE, Wong KJ, Bumb A, Xu H, Milenic DE, Kelley JA, Lai CC, Brechbiel MW. Synthesis of a cross-bridged cyclam derivative for peptide conjugation and 64Cu radiolabeling. Bioconjug Chem. 2008;19(7):1476–1484. doi: 10.1021/bc800039e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechbiel MW. Bifunctional chelates for metal nuclides. Q J Nucl Med Mol Imaging. 2008;52(2):166–173. R39072061 [pii] [PMC free article] [PubMed] [Google Scholar]
- Brik A, Alexandratos J, Lin YC, Elder JH, Olson AJ, Wlodawer A, Goodsell DS, Wong CH. 1,2,3-Triazole as a Peptide Surrogate in the Rapid Synthesis of HIV-1 Protease Inhibitors. ChemBioChem. 2005;6(7):1167–1169. doi: 10.1002/cbic.200500101. [DOI] [PubMed] [Google Scholar]
- Cai M, Cai C, Mayorov AV, Xiong C, Cabello CM, Soloshonok VA, Swift JR, Trivedi D, Hruby VJ. Biological and conformational study of β-substituted prolines in MT-II template: steric effects leading to human MC5 receptor selectivity*. The Journal of Peptide Research. 2004;63(2):116–131. doi: 10.1111/j.1399-3011.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- Cantorias MV, Figueroa SD, Quinn TP, Lever JR, Hoffman TJ, Watkinson LD, Carmack TL, Cutler CS. Development of high-specific-activity 68Ga-labeled DOTA-rhenium-cyclized α-MSH peptide analog to target MC1 receptors overexpressed by melanoma tumors. Nuclear Medicine and Biology. 2009;36(5):505–513. doi: 10.1016/j.nucmedbio.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Cone RD, editor. The Melanocortin Receptors. Humana Press; Totowa, New Jersey: 2000. [Google Scholar]
- Cowell SM, Balse-Srinivasan PM, Ahn JM, Hruby VJ. Design and synthesis of peptide antagonists and inverse agonists for G protein-coupled receptors. Methods Enzymol. 2002;343:49–72. doi: 10.1016/s0076-6879(02)43127-8. [DOI] [PubMed] [Google Scholar]
- Dessolin M, Guillerez MG, Thieriet N, Guibé F, Loffet A. New allyl group acceptors for palladium catalyzed removal of allylic protections and transacylation of allyl carbamates. Tetrahedron Letters. 1995;36(32):5741–5744. [Google Scholar]
- Eberle AN, Froidevaux S. Radiolabeled α-melanocyte-stimulating hormone analogs for receptor-mediated targeting of melanoma: from tritium to indium. Journal of Molecular Recognition. 2003;16(5):248–254. doi: 10.1002/jmr.633. [DOI] [PubMed] [Google Scholar]
- Eberle AN, Verin VJ, Solca F, Siegrist W, Kuenlin C, Bagutti C, Stutz S, Girard J. Biologically Active Monoiodinated α-MSH Derivatives for Receptor Binding Studies Using Human Melanoma Cells. Journal of Receptor Research. 1991;11(1-4):311–322. doi: 10.3109/10799899109066410. [DOI] [PubMed] [Google Scholar]
- Fani M, Mueller A, Tamma ML, Nicolas G, Rink HR, Cescato R, Reubi JC, Maecke HR. Radiolabeled Bicyclic Somatostatin-Based Analogs: A Novel Class of Potential Radiotracers for SPECT/PET of Neuroendocrine Tumors. J Nucl Med. 51(11):1771–1779. doi: 10.2967/jnumed.110.076695. [DOI] [PubMed] [Google Scholar]
- Fragogeorgi EA, Zikos C, Gourni E, Bouziotis P, Paravatou-Petsotas M, Loudos G, Mitsokapas N, Xanthopoulos S, Mavri-Vavayanni M, Livaniou E, Varvarigou AD, Archimandritis SC. Spacer Site Modifications for the Improvement of the in Vitro and in Vivo Binding Properties of 99mTc-N3S-X-Bombesin[2–14] Derivatives. Bioconj Chem. 2009;20:856–867. doi: 10.1021/bc800475k. [DOI] [PubMed] [Google Scholar]
- Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. A Gallium-Labeled DOTA-α-Melanocyte- Stimulating Hormone Analog for PET Imaging of Melanoma Metastases. J Nucl Med. 2004;45(1):116–123. [PubMed] [Google Scholar]
- Garrison JC, Rold TL, Sieckman GL, Naz F, Sublett SV, Figueroa AD, Volkert WA, Hoffman TJ. Evaluation of the Pharmacokinetic Effects of Various Linking Groups Using the 111In-DOTA-X-BBN(7-14)NH2 Structural Paradigm in Prostate Cancer Model. Bioconj Chem. 2008;19:1803–1812. doi: 10.1021/bc8001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem GE, Comunale G, Libert A, Vercammen-Grandjean A, Lejeune FJ. Evidence for alpha-melanocyte-stimulating hormone (α-MSH) receptors on human malignant melanoma cells. International Journal of Cancer. 1988;41(2):248–255. doi: 10.1002/ijc.2910410216. [DOI] [PubMed] [Google Scholar]
- Grieco P, Cai M, Liu L, Mayorov A, Chandler K, Trivedi D, Lin G, Campiglia P, Novellino E, Hruby VJ. Design and Microwave-Assisted Synthesis of Novel Macrocyclic Peptides Active at Melanocortin Receptors: Discovery of Potent and Selective hMC5R Receptor Antagonists. Journal of Medicinal Chemistry. 2008;51(9):2701–2707. doi: 10.1021/jm701181n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Yang J, Gallazzi F, Prosnitz ER, Sklar LA, Miao Y. Effect of DOTA Position on Melanoma Targeting and Pharmacokinetic Properties of 111In-Labeled Lactam Bridge-Cyclized α-MSH Peptide. Bioconj Chem. 2009;20:2162–2168. doi: 10.1021/bc9003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Yang J, Gallazzi F, Miao Y. Reduction of the Ring Size of Radiolabeled Lactam Bridge-Cyclized α-MSH Peptide Resulting in Enhanced Melanoma Uptake. J Nucl Med. 2010;51(3):418–426. doi: 10.2967/jnumed.109.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Yang J, Gallazzi F, Miao Y. Effects of the Amino Acid Linkers on the Melanoma-Targeting and Pharmacokinetic Properties of 111In-Labeled Lactam Bridge-Cyclized α-MSH Peptide. J Nucl Med. 2011;52:608–616. doi: 10.2967/jnumed.110.086009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JI, Lerner AB. Amino-Acid Sequence of the [alpha]-Melanocyte-Stimulating Hormone. Nature. 1957;179(4574):1346–1347. doi: 10.1038/1791346a0. [DOI] [PubMed] [Google Scholar]
- Hausner SH, Kukis DL, Gagnon MK, Stanecki CE, Ferdani R, Marshall JF, Anderson CJ, Sutcliffe JL. Evaluation of [64Cu]Cu-DOTA and [64Cu]Cu-CB-TE2A chelates for targeted positron emission tomography with an alphavbeta6-specific peptide. Mol Imaging. 2009;8(2):111–121. [PMC free article] [PubMed] [Google Scholar]
- Heppeler A, Froidevaux S, Eberle AN, Maecke HR. Receptor Targeting for Tumor Localisation and Therapy with Radiopeptides. Current Medicinal Chemistry. 2000;7:971–994. doi: 10.2174/0929867003374516. [DOI] [PubMed] [Google Scholar]
- Hruby VJ, Cai M, Grieco P, Han G, Kavarana M, Trivedi DEV. Exploring the Stereostructural Requirements of Peptide Ligands for the Melanocortin Receptors. Annals of the New York Academy of Sciences. 2003;994(1):12–20. doi: 10.1111/j.1749-6632.2003.tb03157.x. [DOI] [PubMed] [Google Scholar]
- Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, DeVaux AE, Dym O, Castrucci AMdL. α-Melanotropin: the minimal active sequence in the frog skin bioassay. Journal of Medicinal Chemistry. 1987;30(11):2126–2130. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- Huisgen R. 1,3-Dipolar Cycloadditions. Past and Future. Angewandte Chemie International Edition in English. 1963;2(10):565–598. doi: 10.1002/anie.196305651. [DOI] [Google Scholar]
- Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Analytical Biochemistry. 1970;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Lunec J, Pieron C, Thody AJ. MSH receptor expression and the relationship to melanogenesis and metastatic activity in B16 melanoma. Melanoma Research May. 1992;2(1):5–12. doi: 10.1097/00008390-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Mayorov AV, Cai M, Chandler KB, Petrov RR, Van Scoy AR, Yu Z, Tanaka DK, Trivedi D, Hruby VJ. Development of Cyclic Î3-MSH Analogues with Selective hMC3R Agonist and hMC3R/hMC5R Antagonist Activities. Journal of Medicinal Chemistry. 2006;49(6):1946–1952. doi: 10.1021/jm0510326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorov AV, Han SY, Cai M, Hammer MR, Trivedi D, Hruby VJ. Effects of Macrocycle Size and Rigidity on Melanocortin Receptor-1 and -5 Selectivity in Cyclic Lactam α-Melanocyte-Stimulating Hormone Analogs. Chemical Biology & Drug Design. 2006;67(5):329–335. doi: 10.1111/j.1747-0285.2006.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Gallazzi F, Guo H, Quinn TP. 111In-Labeled Lactam Bridge-Cyclized α-Melanocyte Stimulating Hormone Peptide Analogues for Melanoma Imaging. Bioconjugate Chemistry. 2008;19(2):539–547. doi: 10.1021/bc700317w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor JJ., Jr The Medicinal Chemistry of Peptides. Current Medicinal Chemistry. 2009;16:4399–4418. doi: 10.2174/092986709789712907. [DOI] [PubMed] [Google Scholar]
- Okarvi SM. Synthesis, radiolabeling and in vitro and in vivo characterization of a technetium-99m-labeled alpha-M2 peptide as a tumor imaging agent. J Pept Res. 2004;63(6):460–468. doi: 10.1111/j.1399-3011.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- Raposinho PD, Correia JDG, Oliveira MC, Santos I. Melanocortin-1 receptor-targeting with radiolabeled cyclic α-melanocyte-stimulating hormone analogs for melanoma imaging. Peptide Science. 2010;94(6):820–829. doi: 10.1002/bip.21490. [DOI] [PubMed] [Google Scholar]
- Rockey WM, Huang L, Kloepping KC, Baumhover NJ, Giangrande PH, Schultz MK. Synthesis and radiolabeling of chelator-RNA aptamer bioconjugates with copper-64 for targeted molecular imaging. Bioorg Med Chem. 2011 Jul 1;19(13):4080–90. doi: 10.1016/j.bmc.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm UG, Olivier GWJ, Branch SK, Moss SH, Pouton CW. Synthesis and biological evaluation of [alpha]-MSH analogues substituted with alanine. Peptides. 1994;15(7):1297–1302. doi: 10.1016/0196-9781(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, Hadley ME. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(10):5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist W, Oestreicher M, Stutz S, Girard J, Eberle AN. Radioreceptor Assay for α-MSH Using Mouse B16 Melanoma Cells. Journal of Receptor Research. 1988;8(1-4):323–343. doi: 10.3109/10799898809048996. [DOI] [PubMed] [Google Scholar]
- Sugg EE, De L, Castrucci AM, Hadley ME, Van Binst G, Hruby VJ. Cyclic lactam analogs of Ac-[Nle4].alpha.-MSH4-11-NH2. Biochemistry. 1988;27(21):8181–8188. doi: 10.1021/bi00421a029. [DOI] [PubMed] [Google Scholar]
- Tatro JB, Entwistle ML, Lester BR, Reichlin S. Melanotropin Receptors of Murine Melanoma Characterized in Cultured Cells and Demonstrated in Experimental Tumors in Situ. Cancer Research. 1990;50(4):1237–1242. [PubMed] [Google Scholar]
- Thieriet N, Alsina J, Giralt E, Guibé F, Albericio F. Use of Alloc-amino acids in solid-phase peptide synthesis. Tandem deprotection-coupling reactions using neutral conditions. Tetrahedron Letters. 1997;38(41):7275–7278. [Google Scholar]
- Turner RA, Oliver AG, Lokey RS. Click Chemistry as a Macrocyclization Tool in the Solid-Phase Synthesis of Small Cyclic Peptides. Organic Letters. 2007;9(24):5011–5014. doi: 10.1021/ol702228u. [DOI] [PubMed] [Google Scholar]
- Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discovery Today. 2010;15(1-2):40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Ying J, Gu X, Cai M, Dedek M, Vagner J, Trivedi DB, Hruby VJ. Design, Synthesis, and Biological Evaluation of New Cyclic Melanotropin Peptide Analogues Selective for the Human Melanocortin-4 Receptor. Journal of Medicinal Chemistry. 2006;49(23):6888–6896. doi: 10.1021/jm060768f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanziger D, Beck-Sickinger AG. Radiometal Targeted Tumor Diagnosis and Therapy with Peptide Hormones. Current Pharmaceutical Design. 2008;14:2385–2400. doi: 10.2174/138161208785777397. [DOI] [PubMed] [Google Scholar]