Abstract
Traumatic brain injury (TBI) causes disruption of the blood brain barrier (BBB) leading to hemorrhage which can complicate an already catastrophic illness. Matrix metalloproteinases (MMPs) involved in the breakdown of the extracellular matrix may lead to brain hemorrhage. We explore the contribution of the 70 kD heat shock protein (Hsp70) to outcome and brain hemorrhage in a model of TBI. Male, wildtype (Wt), Hsp70 knockout (Ko) and transgenic (Tg) mice were subjected to TBI using controlled cortical impact (CCI). Motor function, brain hemorrhage and lesion size were assessed at 3, 7 and 14 d. Brains were evaluated for the effects of Hsp70 on MMPs.
In Hsp70 Tg mice, CCI led to smaller brain lesions, decreased hemorrhage and reduced expression and activation of MMPs compared to Wt. CCI also significantly decreased right-biased swings and corner turns in the Hsp70 Tg mice. Conversely, Hsp70 Ko mice had significantly increased lesion size, worsened brain hemorrhage and increased expression and activation of MMPs with worsened behavioral outcomes compared to Wt. Hsp70 is protective in experimental TBI. To our knowledge, this is the direct demonstration of brain protection by Hsp70 in a TBI model. Our data demonstrate a new mechanism linking TBI-induced hemorrhage and neuronal injury to the suppression of MMPs by Hsp70, and support the development of Hsp70 enhancing strategies for the treatment of TBI.
Keywords: brain injury, heat shock protein, cerebral hemorrhage
INTRODUCTION
Traumatic brain injury (TBI) remains a significant cause of lifelong cognitive, physical, behavioral and emotional impairments globally (Langlois et al., 2006 and Kiraly and Kiraly 2007). TBI frequently leads to brain edema and hemorrhage due to disruption of the blood brain barrier (BBB). Furthermore, hemorrhage can complicate brain tissue damage by the release of excitotoxic substances, free radical damage from blood breakdown products, and tissue ischemia due to loss of microvessels (Kurland et al., 2012). It is one cause of the inflammatory response, involving microglial activation, leukocyte recruitment, and upregulation of cytokine secretion after TBI (Oehmiche et al., 2003, Namas et al., 2009 and Atkins et al., 2012).
Recent work in the field has implicated matrix metalloproteinases (MMPs), a family of Zndependent endopeptidases, in the breakdown of the extracellular matrix and BBB leading to brain edema and hemorrhage (Suehiro et al., 2004 and Lo et al., 2002). MMPs are proteases normally found in the cytosol in an inactivated form, but in pathologic states, are cleaved to an active form. This activated form contributes to BBB disruption by disrupting tight junction and basal lamina proteins, and may facilitate the immune response after brain injury (Gurney et al., 2006 and Wang et al., 2007). MMP-2 and -9 are two isoforms which are increased in the brain and spinal cord following ischemia and trauma (Rosenberg, 1995 and Noble et al., 2002).
Heat shock proteins (Hsps) are induced by many stressful stimuli, including a variety of central nervous system insults, such as cerebral ischemia, neurotoxin exposure and other metabolic stresses (Kelley et al., 2002 and Yenari et al., 2005). The 70 kD inducible Hsp (Hsp70) functions as a molecular chaperone, thus preventing abnormal protein folding and facilitating protein translocation. Prior work has shown that Hsp70 is increased in brain vessels following experimental TBI (DeGracia et al., 2007). Our group and others have shown the salutary effects of Hsp70 overexpression in brain ischemia models, and that Hsp70 may have multiple mechanisms of protection (Giffard and Yenari, 2004). Overexpression of Hsp70 or its induction by heat stress reduced expression of MMPs in cultured astrocytes at the transcriptional and translational level (Lee et al., 2001). Here we propose to further explore whether Hsp70 has the potential to protect the brain against TBI.
MATERIALS AND METHODS
Three month old male Hsp70 transgenic (Hsp70 Tg) and Hsp70 deficient (Hsp70 Ko) mice (25–30g) were established from breeder mice originally generated by the Dillmann (UCSD) and Pandita (Southwestern University) labs (Zheng et al., 2008 and Hunt et al., 2004). The Hsp70 Tg and Ko mice were made a C57/B6 background, and have been backcrossed for over 10 generations in order to generate hemizygotic (Hsp70 Tg) or homozygotic (Hsp70 Ko) and wildtype (Wt) littermates. All animal housing and procedures were carried out according to a protocol approved by the local Institutional Animal Care and Use Committee (IACUC) in accordance with NIH guidelines.
Controlled cortical impact (CCI)
The CCI model of TBI was carried out according to a previously established protocol (Chang et al., 2003 and Potts et al., 2009). Briefly, mice were anesthetized with isoflurane (5% for induction and 2% for maintenance via a nosecone) in a mixture of medical air:oxygen (3:1). Rectal temperature was monitored throughout the procedure and maintained in the normal range. For induction of CCI, anesthetized mice were fixed in a stereotaxic frame and a midline scalp incision was made, followed by a circular craniotomy (5 mm in diameter) in the left parietal plate immediately posterior to the bregma. The dura was not disrupted. CCI was performed with an automated impactor (Pinpoint Precision Cortical impactor, Hatteras Instruments, Cary, NC) with a tip size of 3 mm (in diameter) at 1.5 m/s velocity to generate 2 mm penetration with a 100 ms dwell time. The excised cranial bone was replaced immediately and the incision was then closed with suture. At the end of the experiments, animals were euthanized with an overdose of isoflurane followed by decapitation.
Analysis of brain samples
Brains were collected 14d after CCI and sectioned (50 µm in thickness) on a cryostat. Brain sections taken within the lesion were stained with hematoxylin and eoson (H & E, Sigma, MO). Brain sections were photographed using a Zeiss Axio Imager, amd the lesion size was quantified using NIH Image J 1.45s computer software from 9 sequential levels (every third section) and multiplied by distance between sections (150 µm) to estimate the lesion and/or cavity volume.
In a separate cohort of animals, brains were harvested 3d post-CCI and gross sections measuring 2 mm thick were prepared and photographed to measure hemorrhage volume using a previously published Image J based technique (Tang et al., 2010). The volume was quantified by digitally measuring the volume of hemorrhage (in µl) and using the “Integrated Density” measurement function in ImageJ to account for variation in hemorrhage intensity.
Expression of Hsp70, MMP-2 and -9 proteins were determined from brains 3 d post injury (Kim et al., 2011). Briefly, mice were anesthetized and transcardially perfused with saline and then paraformaldehyde (PFA, 4%). Brains were dissected, post-fixed in 4% PFA, incubated in 20% sucrose at 4°C for 24 hrs, frozen, and cryosectioned. Brain sections (10 µm in thickness) from mice 3 days after CCI (n=3/group) were subjected to immunostaining. Brain sections were incubated sequentially with 0.1% hydrogen peroxidase (3 min), a blocking buffer (0.5% Triton X-100, 0.1% BSA, 1.5% normal horse serum in PBS) for 30 min, and mouse anti-Hsp70 (1:100; Stressgen, NY) or rabbit anti-MMP-2 and -9 (1:100; abcam, MA) antibodies overnight at 4°C. Immunoreactivity was amplified and detected with biotinylated anti-mouse IgG and atni-rabbit IgG (1:200; Vector Laboratories, CA), peroxidase-conjugated avidin (ABC Elite, Vector Laboratories, CA) and DAB substrate. Sections were counterstained with hematoxylin (Sigma, MO). For immunoblots, ipsilateral hemispheres were homogenized and solubilized in Laemmli’s lysis buffer containing the following protease inhibitors: Leupeptin 1 µg/ml, Aprotinin 5 µg/ml, Pepstatin 1 µg/ml, phenylmethylsulfonylfluoride 1 mM. Aliquots containing 30–60 µg of protein were subjected to 10% SDS-polyacrylamide gel electrophoresis, then transferred to polyvinylidinene fluoride (PVDF) membranes (Millipore, MA), and probed for the protein of interest by incubating in the mouse anti-Hsp70 (1:500; Stressgen, NY) or rabbit anti-MMP-2 and -9 (1:500; Abcam, MA) antibody of interest followed by a horseradish peroxidase (HRP) conjugated secondary antibody. Blots were visualized using the ECL system (Amersham, NJ) according to the manufacturer’s directions, and exposed to X-ray film for 5 min. Equal protein loading was confirmed by stripping the membrane, then re-probing for mouse anti-β-actin (Sigma, MO). Densitometric measurements were made from the film using a BioRad Multianalyst scanner and densitometer (Hercules, CA). To detect of MMP-2 and -9 activation, similarly prepared protein samples as for western blot analysis were subjected to gelatin zymography with Gelatin-zymography Kit (Cosmo Bio Co., Japan) according to the manufacturer’s instructions. In briefly, each sample was mixed with sample buffer and incubated for 15 min at RT. The samples and markers were loaded to gelatin-gel plate for electrophoresis. The gel was washed and incubated 40h in reaction buffer at 37°C. After the enzymatic reaction, the gel was stained with coomassie blue and incubated for 30 min at RT.
Behavior tests
A panel of behavioral assays was carried out to assess neurological function as previously described (Wang et al., 2009 and Tang et al., 2008). For the swing test, mice were suspended vertically by the tail with their heads lifted 3 inches above the test bench. A lateral swing was counted if the animal moved its head >10 degrees away from the vertical axis (Borlongan, 1995). A total of 20 swings were counted in each trial and frequency of right-biased swing was calculated for each animal. The corner test was performed by placing two sheets of cardboard in the animal’s home cage and positioned to create a 30° corner with a 0.5–1 cm gap, which permitted light to shine through. Mice were placed between the boards and encouraged to enter the corner. Once the animal reached to corner, vibrissae were stimulated by the cardboard, causing the animal to rear and turn away from the corner. The direction of turns was recorded. Each animal was tested 20 times, and the number of turns toward the ipsilateral injured hemisphere (right) was counted.
Statistical analysis
All data analysis was carried out by investigators blinded to experimental conditions. Experiments were carried out in a randomized fashion to minimize bias. Standard statistical tests were applied depending on the condition (ANOVA or T-test for continuous data, and non-parametric tests for non-continuous data, repeated measures where appropriate) and analyzed (Systat Software, Inc., CA). P-values <0.05 were considered significant. Data are presented x±SE.
RESULTS
Hsp 70 reduced lesion size and brain hemorrhage following TBI
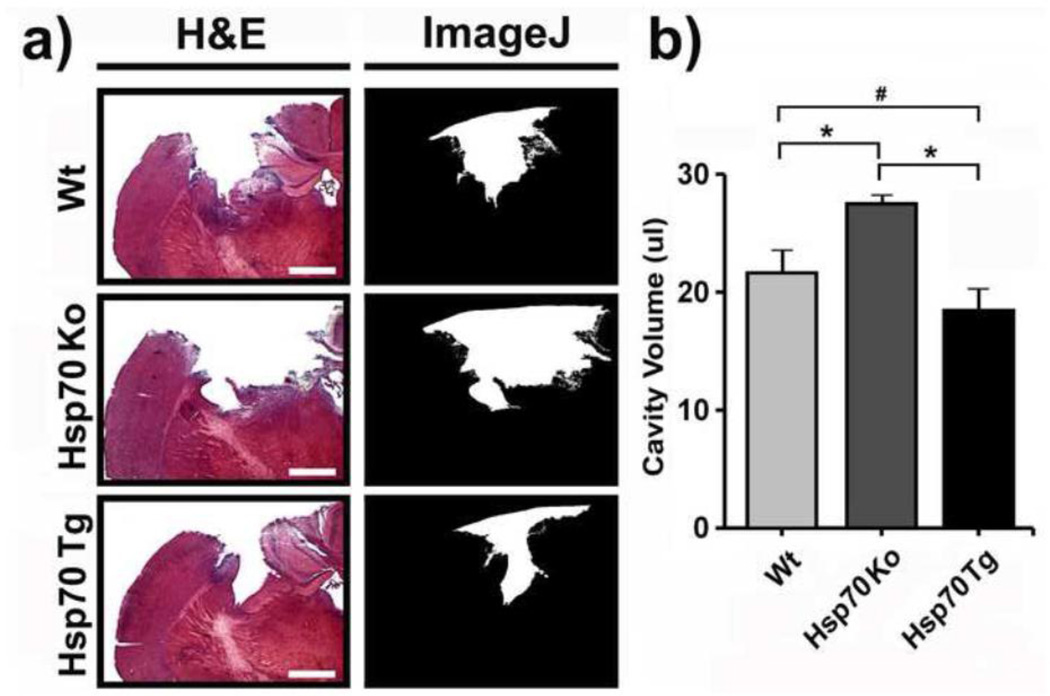
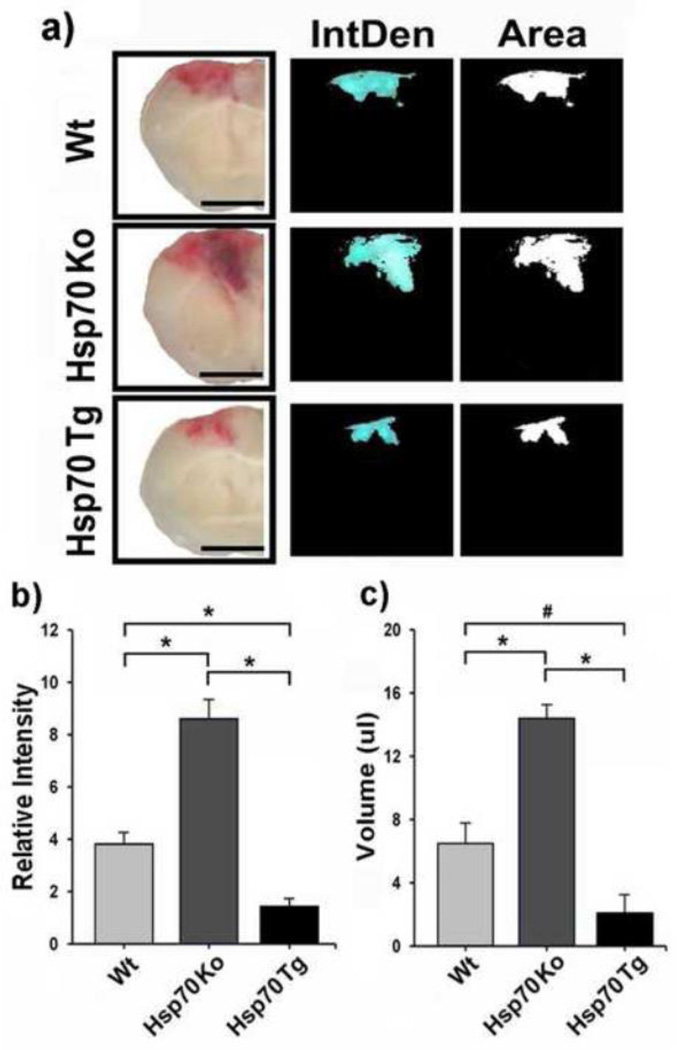
To study the neuroprotective effect of Hsp70 against TBI, we compared the degree of injury in mouse brains subjected to CCI in Wt, Hsp70 Ko and Hsp70 Tg mice. After a 14-day recovery period, the size of cortical brain lesion from H&E-stained sections was quantified as described above. Hsp70 Tg mice had significantly decreased brain lesion size compared to Wt, whereas Hsp70 Ko mice had increased lesion size (Fig. 1). The extent of brain hemorrhage was similarly less amongst Hsp70 Tg mice, and significantly greater in Hsp70 Ko mice brains 3d post CCI (Fig. 2).
Figure 1. Hsp70 reduces cortical lesion size following CCI in mice.
a) Left panels show hematoxylin & eosin (H&E) stained brain sections from wildtype (Wt), Hsp70 knockout (Ko) and Hsp70 Transgenic (Tg) mice 14 d after CCI. Right panels show delineation of cortical lesion area (white area) from the corresponding images on the left by using the NIH Image J software. Bar = 100 µm. b) Cortical cavity volumes were quantified using H&E stained sections as described in the methods. Lesion volumes were smallest in Hsp70 Tg mice, while lesions were the largest amongst Hsp70 Ko mice. *p<0.01, #p<0.05; n=6~8 mice/group.
Figure 2. Hsp70 reduces brain hemorrhage after CCI in mice.
a) Each column of images shows coronal brain sections. The left hand columns are gross brain sections, while the center and right hand columns are NIH ImageJ derived maps of intensity (IntDen) and area (Area) of Wt, Hsp70 Ko and Hsp70 Tg mice 3 d after CCI. Bar = 25 mm. b) Average relative intensity and c) volume of hemorrhage is quantified from the brain sections. Statistical analysis showed a significant reduction in the intensity and volume of hemorrhage in Hsp70 Tg mice vs. Wt and Hsp70 Ko mice groups. *p<0.01, #p<0.05; n=6~8 mice/group.
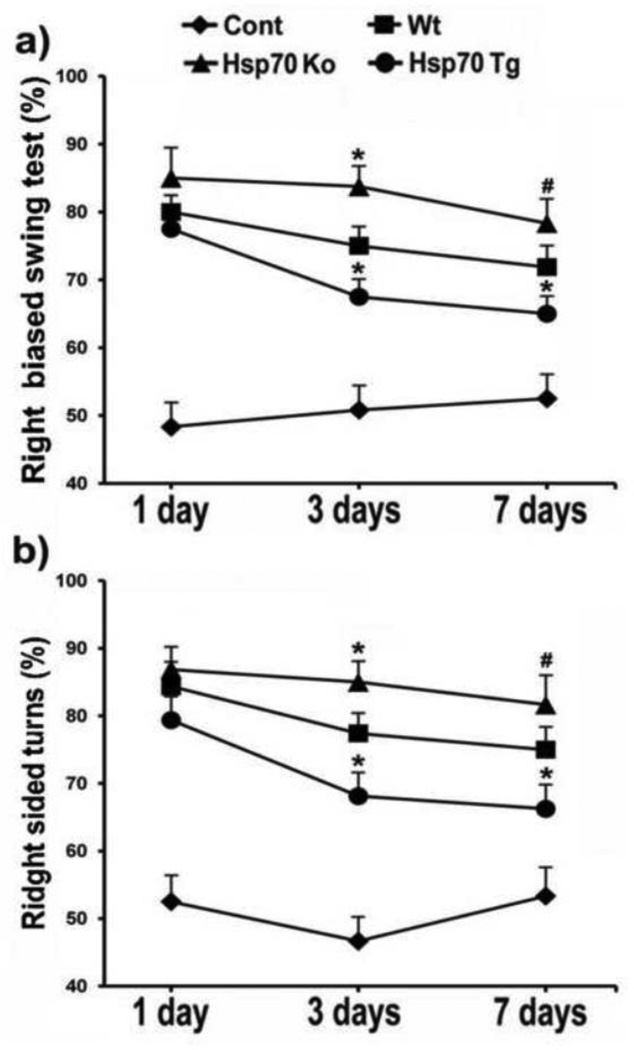
Hsp70 improves motor function following experimental TBI
Motor function following CCI was also improved amongst Hsp70 Tg mice, and worsened in Hsp70 Ko mice. Both elevated body swing and corner tests showed less asymmetry amongst Hsp70 Tg mice, and worsened asymmetry amongst Hsp70 Ko mice following CCI (Fig 3), observations that indicate better neurological function amongst Hsp70 Tg mice. Behavioral indices amongst sham controls did not reveal and baseline differences due to gene manipulation.
Figure 3. Hsp70 overexpression improves motor function after CCI.
a) Elevated body swing and b) corner tests were performed following CCI. The frequency of right-biased swing and corner turn was calculated after 20 trials for each test on each animal. Hsp70 Tg mice performed better than wildtype (Wt) and Hsp70 Ko mice, whereas Hsp70 Ko mice performed worse than Wt and Hsp70 Tg. *p<0.05 (Wt mice vs. Hsp70 Ko and Hsp70 Tg mice), #p<0.1 (Wt mice vs. Hsp70 Ko mice); n=6~8 mice/group.
Hsp70 blunts TBI-induced MMP-2 and MMP-9 expression and activation
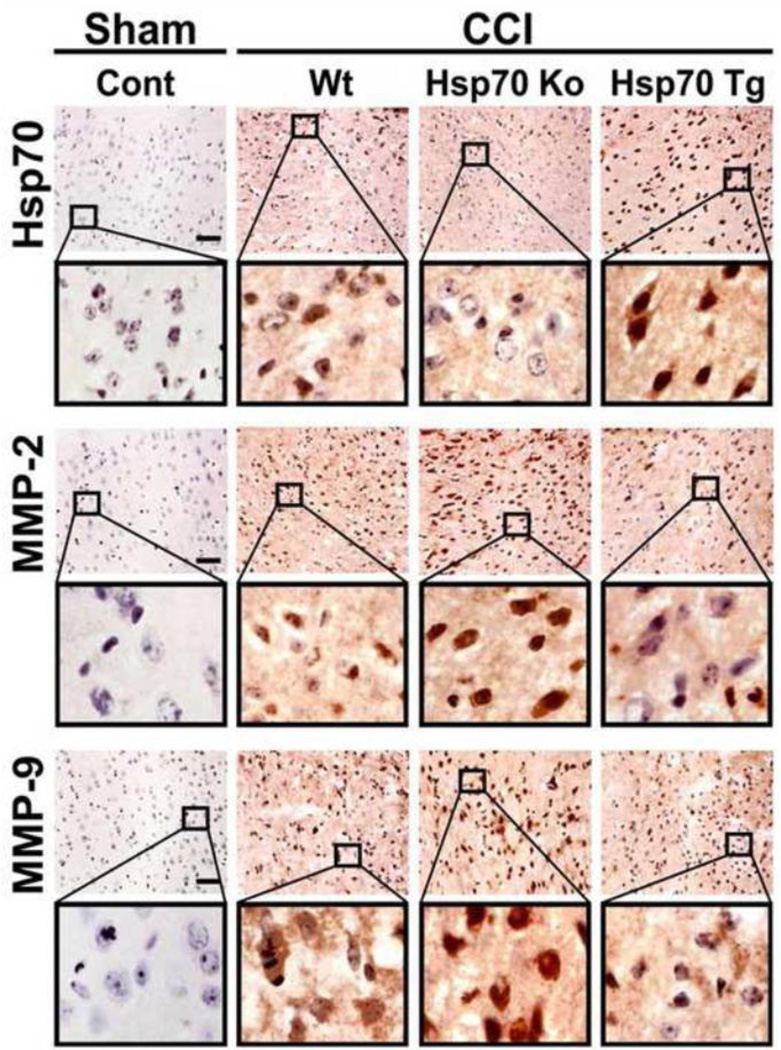
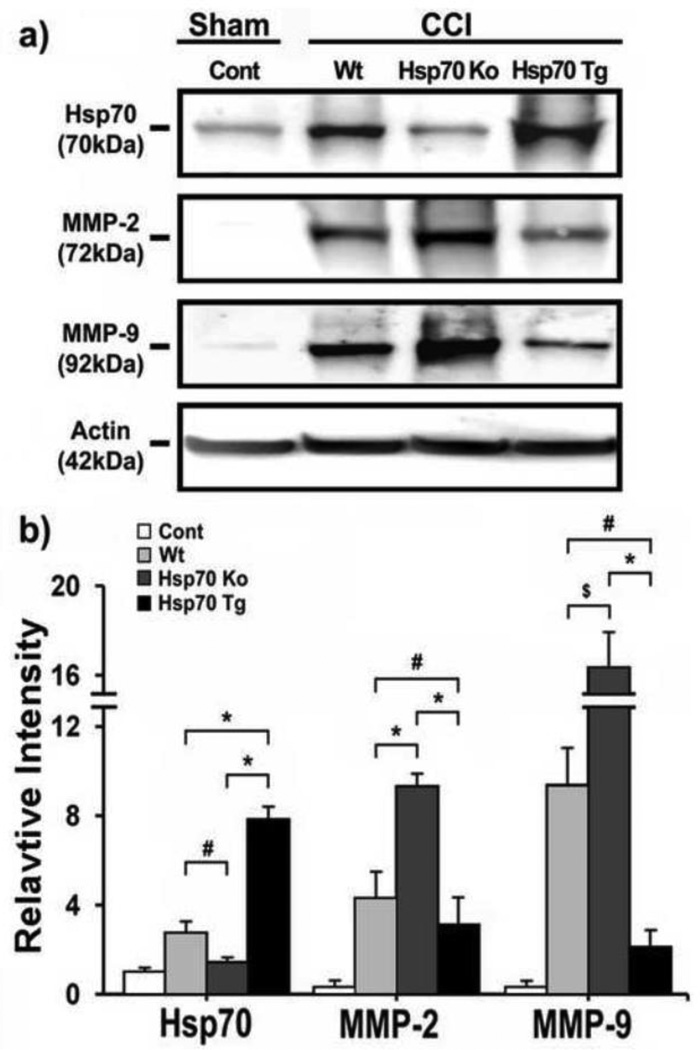
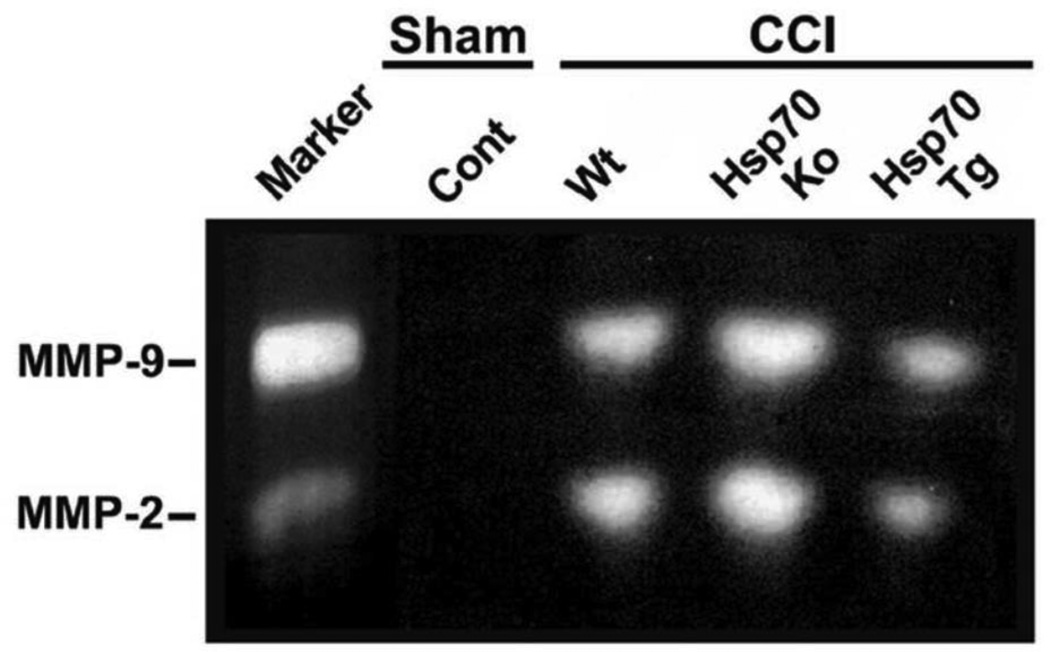
We previously showed in an in vitro stroke model that Hsp70 can suppress expression and activation of MMPs (Lee et al., 2004). We next explored whether similar patterns might be observed in our TBI model. Immunostains of mice subjected CCI are shown in Fig. 4. We first demonstrated that Hsp70 is increased following CCI in Wt and Hsp70 Tg brains, but is absent in brains from Hsp70 Ko mice. Both MMP-2 and MMP-9 increased in brains from Wt and Hsp70 Ko mice, but was notably decreased in brains of Hsp70 Tg mice (Fig. 4). Similar patterns were observed on Western blots (Fig. 5). While MMP expression was decreased in the brains of Hsp70 Tg mice, the Ko mice had 3-fold higher MMP-2 expression, and 10-fold higher MMP-9 expression (Fig. 5). To estimate Hsp’s influence on the proteolytic properties of the MMPs, gelatin zymograhy showed that MMP-2 and MMP-9 activity was decreased by Hsp70 overexpression, but increased when Hsp70 was deficient (Fig. 6).
Figure 4. Hsp70, MMP-2 and MMP-9 expression.
Immunohistological detection of Hsp70, MMP-2 and MMP-9 in cortical neurons in Wt, Hsp70 Ko and Hsp70 Tg mice subjected to CCI vs. sham control, followed by 3 day recovery. Black boxes contain images of higher-power view of the corresponding brain regions. Brown DAB stain depicts immunoreactivity in the brain sections counterstained with hematoxylin. Hsp70 expression was highest amongst Hsp70 Tg mice, and nearly absent in the Hsp70 Ko mice. Some immunoreactivity in brains of Hsp70 Ko likely represent cross reactivity with other Hsp70 isoforms. Immunostains of MMP-2 and -9 show upregulation by CCI in Wt, but less reactivity amongst Hsp70 Tg and more reactivity amongst Hsp70 Ko brains. Bar=25 µm.
Figure 5. Western blots of Hsp70, MMP-2 and MMP-9 after CCI.
a) Immunoblots show expression of Hsp70, MMP-2 and MMP-9 proteins from Wt, Hsp70 Ko and Hsp70 Tg mouse brains 1 d after CCI. β-actin is shown as a housekeeping control. b) Relative intensities of Hsp70, MMP-2 and MMP-9 protein were quantified by NIH Image J software, and normalized to the intensity of β-actin. MMP-2 and -9 were decreased in Hsp70 Tg brains compared to Wt and Hsp70 Ko, whereas MMP-2 and -9 were increased in Hsp70 Ko brains compared to Wt and Hsp70 Tg. *p<0.01,s #p<0.05, $p<0.1; n=3 mice/group
Figure 6. Gelatin zymograms show relative activities of MMP-2 and MMP-9 after CCI.
MMP-2 and MMP-9 gelatinolytic activity 1 d post CCI are shown for injured brains of Wt, Hsp70 Ko and Hsp70 Tg mice. Similar to MMP expression patterns observed on immunostains and immunoblots, zymography shows that MMP-2 and MMP-9 activity are upregulated in Hsp70 Ko mice relative to that of Wt and Hsp70 Tg mice, whereas gelatinolytic activity is decreased in Hsp70 Tg mice.
DISCUSSION
Studies of the events involved in brain injury are essential to developing effective therapies for TBI. We and others previously showed that Hsp70 overexpression was protective in stroke models (Kelly et al., 2002, Lee et al., 2001, Zheng et al., 2008, Hoehn et al., 2001, Yenari et al., 1998 and Rajdev et al., 2000). We now show that overexpression of Hsp70 is protective in a model of TBI which, to our knowledge, is the first demonstration in experimental brain trauma. This protection also led to decreased brain hemorrhage and improvement in neurological function. We further showed that MMP expression and its proteolytic activity were decreased in Hsp70 Tg mice. Hsp70 deficiency resulted in the converse, or increased injury, worsened neurological outcome and more brain hemorrhage and MMP expression and activity. These data suggest that the Hsp70 may be an appropriate therapeutic target to limit damage due to TBI.
Brain injury due to TBI, especially when complicated by disruption of the BBB leading to hemorrhage can lead to permanent neurological deficits or death (Vajtr et al., 2009). Recent work in TBI and related brain injuries has implicated, among other processes, matrix metalloproteinases (MMPs) in the breakdown of the extracellular matrix (ECM) and BBB, leading to brain edema and hemorrhage (Lescot et al., 2010 and Higashida et al., 2011). MMPs are first expressed as pro-proteins, which are cleaved by other activated MMPs and proteases in the extracellular space (Cunningham et al., 2005). MMP are activated plasmin as well as other MMPs. Processed, or active MMPs then cleave a variety of extracellular substrates including the basal lamina and lead to disruption of the BBB. MMP-2, MMP-9 and MMP-3 have been shown to increase in the central nervous system following TBI in experimental models (Truettner et al., 2005) (Grossetete and Rosenberg, 2008 and Jia et al., 2010) and in patients (Grossetete et al., 2009, Vilalta et al., 2008a and Vilalta et al., 2008b). Several studies have shown that at least during the acute phases of TBI, MMPs exacerbate damage. For instance, the elevation of MMP-9 is associated with inflammatory events following TBI, in part, by degrading components of the basal lamina disrupting the BBB (Suehiro et al., 2004). Other studies have shown that MMP-9 deletion (Wang et al., 2000) or pharmacologic inhibition of MMPs is protective (Khan et al., 2009 and Homsi et al., 2009). These findings are also in line with a study from our labs which showed that therapeutic hypothermia, a robust neuroprotectant, is associated with decreased expression of both MMP-2 and MMP-9 in a model of stroke (Lee et al., 2005). MMPs may also play beneficial roles in brain injury, as they are important in several processes involved in recovery and repair (Cunningham et al., 2005), which occur during the more chronic phases of stroke. ECM disruption may pave the road to angiogenesis, for instance (Lo et al., 2002). Thus, MMPs play different roles during TBI, although the specific role may depend on the timing. In this study, we focused on the acute stages where we previously established protection by Hsp70 (Zheng et al., 2008 and Yenari et al., 1998)(Yenari et al., 1998; Zheng et al., 2008), expression of MMPs (Lee et al., 2005) and disruption of the BBB in a stroke model.
Hsp70 is one of several stress proteins upregulated following brain injury. It is expressed in a variety of cells in the brain in a time and spatially dependent pattern. Neurons are among the first cells to express Hsp70, followed by astrocytes and finally blood vessels and microglia (Sharp et al., 1999). While it is known that Hsp70 is protective, the mechanism of protection is only partially understood. Work by our lab and others’ have shown its beneficial effects against experimental brain injury (Kelly et al., 2002, Rajdev et al., 2000, Truettner et al., 2007 and Yi et al., 2008). Hsp70’s chaperone functions relate to its ability to bind to and assist in the conformational folding of nascent proteins, and its protective properties have been attributed to its chaperone functions, presumably leading to enhancement of nascent protein folding and preventing protein aggregation (Giffard et al., 2004). We and other groups have shown that Hsp70 may have multiple mechanisms of protection (Yenari et al., 2005 and Giffard and Yenari, 2004). Hsp70 can prevent ischemic apoptotic cell death at multiple levels (Giffard et al., 2004 and Matsumori et al., 2005), and Hsp70 is capably of interacting with and inhibiting the inflammatory transcription factor, nuclear factor kappa B (NFkB) (Zheng et al., 2008 and Ran et al., 2004). There is very little published regarding the link between Hsp70 and MMPs, but we previously showed that overexpression of Hsp70 in cultured astrocytes inhibited MMP-2 and -9, and MMP-9 is one of many genes regulated by NFkB.
To date, there are no satisfactory treatments for TBI or brain hemorrhage complicating TBI. Our data establish a protective role of Hsp70 overexpression which leads to improved neurological function and decreased brain hemorrhage. However, it should be noted that Hsp70 overexpression was already occurring prior to the injury, and this has obvious translational limitations. Our lab has previously reported that Hsp70 overexpression using gene transfer is also protective when given 2 hours following middle cerebral artery occlusion (Hoehn et al., 2001). Since these particular viral vectors do not begin to express transgene until 4 hours after the vector is administered, this suggests that Hsp70 overexpression may be protective with a temporal therapeutic window of at least 6 hours. Of late, it is now possible to pharmacologically induce Hsp70 in the brain (Kim et al., 2012), and pharmacologic induction of Hsp70 by geldanamycin was found to be protective against experimental stroke and brain hemorrhage (Lu et al., 2002, Manaenko et al., 2011, Kwon et al., 2008, Ouyang et al., 2005 and Xu et al., 2003). Thus, the possibility of acute treatment of traumatic brain injury by Hsp70 induction should be explored, especially to establish whether its induction can be delayed. Other questions such as whether this effect is durable and positively influences long term outcome should also be addressed.
HIGHLIGHTS.
Hsp70 improves neurological & histological outcome in experimental TBI.
Hsp70 decreases brain hemorrhage following experimental TBI.
Hsp70 decreases TBI-induced MMP expression and activity.
This is the first report of the neuroprotection by Hsp70 in experimental TBI.
Acknowledgements
This work was supported by grants from the National Institutes of Health (NS40516) and the Veteran’s Merit Award to MY, an American Heart Association Western States Affiliate Postdoctoral Fellowship (13POST14810019) to JYK and Basic Science Research Program through the NRF of KOREA funded by the ministry of Education, Science and Technology (2012-0005827) to JEL. Grants to MY and JYK were administered by the Northern California Institute for Research and Education, and supported by resources of the Veterans Affairs Medical Center, San Francisco, California. The authors with to thank Dr. Linda Noble-Haeusslein for helpful discussion and advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Atkins CM, Kang Y, Furones C, Truettner JS, Alonso OF, Dietrich WD. Postinjury treatment with rolipram increases hemorrhage after traumatic brain injury. J Neurosci Res. 2012 Sep;90(9):1861–1871. doi: 10.1002/jnr.23069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Sanberg PR. Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995 Jul;15(7 Pt 2):5372–5378. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Wong RJ, Vreman HJ, et al. Heme oxygenase-2 protects against lipid peroxidation-mediated cell loss and impaired motor recovery after traumatic brain injury. J Neurosci. 2003 May 1;23(9):3689–3696. doi: 10.1523/JNEUROSCI.23-09-03689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005 Jun;50(4):329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- DeGracia DJ, Kreipke CW, Kayali FM, Rafols JA. Brain endothelial HSP-70 stress response coincides with endothelial and pericyte death after brain trauma. Neurol Res. 2007 Jun;29(4):356–361. doi: 10.1179/016164107X204666. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Xu L, Zhao H, et al. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J Exp Biol. 2004 Aug;207(Pt 18):3213–3220. doi: 10.1242/jeb.01034. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004 Jan;16(1):53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009 Oct;65(4):702–708. doi: 10.1227/01.NEU.0000351768.11363.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossetete M, Rosenberg GA. Matrix metalloproteinase inhibition facilitates cell death in intracerebral hemorrhage in mouse. J Cereb Blood Flow Metab. 2008 Apr;28(4):752–763. doi: 10.1038/sj.jcbfm.9600572. [DOI] [PubMed] [Google Scholar]
- Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006 Jul;23(1):87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Higashida T, Kreipke CW, Rafols JA, et al. The role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg. 2011 Jan;114(1):92–101. doi: 10.3171/2010.6.JNS10207. [DOI] [PubMed] [Google Scholar]
- Hoehn B, Ringer TM, Xu L, et al. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J Cereb Blood Flow Metab. 2001 Nov;21(11):1303–1309. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Homsi S, Federico F, Croci N, et al. Minocycline effects on cerebral edema: relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res. 2009 Sep 29;1291:122–132. doi: 10.1016/j.brainres.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Hunt CR, Dix DJ, Sharma GG, et al. Genomic instability and enhanced radiosensitivity in Hsp70.1-and Hsp70.3-deficient mice. Mol Cell Biol. 2004 Jan;24(2):899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pan YH, Mao Q, Liang YM, Jiang JY. Matrix metalloproteinase-9 expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. J Neurotrauma. 2010 Jun;27(6):1059–1068. doi: 10.1089/neu.2009.1067. [DOI] [PubMed] [Google Scholar]
- Kelly S, Zhang ZJ, Zhao H, et al. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann Neurol. 2002 Aug;52(2):160–167. doi: 10.1002/ana.10264. [DOI] [PubMed] [Google Scholar]
- Khan M, Im YB, Shunmugavel A, et al. Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact. J Neuroinflammation. 2009;6:32. doi: 10.1186/1742-2094-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Kim N, Yenari MA, Chang W. Mild Hypothermia Suppresses Calcium-Sensing Receptor (CaSR) Induction Following Forebrain Ischemia While Increasing GABA-B Receptor 1 (GABA-B-R1) Expression. Transl Stroke Res. 2011 Jun 1;2(2):195–201. doi: 10.1007/s12975-011-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Kim JY, Yenari MA. Anti-inflammatory properties and pharmacological induction of Hsp70 after brain injury. Inflammopharmacology. 2012 Jun;20(3):177–185. doi: 10.1007/s10787-011-0115-3. [DOI] [PubMed] [Google Scholar]
- Kiraly M, Kiraly SJ. Traumatic brain injury and delayed sequelae: a review--traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. ScientificWorldJournal. 2007;7:1768–1776. doi: 10.1100/tsw.2007.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J Neurotrauma. 2012 Jan 1;29(1):19–31. doi: 10.1089/neu.2011.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HM, Kim Y, Yang SI, Kim YJ, Lee SH, Yoon BW. Geldanamycin protects rat brain through overexpression of HSP70 and reducing brain edema after cerebral focal ischemia. Neurol Res. 2008 Sep;30(7):740–745. doi: 10.1179/174313208X289615. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006 Sep-Oct;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Lee JE, Kim YJ, Kim JY, Lee WT, Yenari MA, Giffard RG. The 70 kDa heat shock protein suppresses matrix metalloproteinases in astrocytes. Neuroreport. 2004 Mar 1;15(3):499–502. doi: 10.1097/00001756-200403010-00023. [DOI] [PubMed] [Google Scholar]
- Lee JE, Yenari MA, Sun GH, et al. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol. 2001 Jul;170(1):129–139. doi: 10.1006/exnr.2000.7614. [DOI] [PubMed] [Google Scholar]
- Lee JE, Yoon YJ, Moseley ME, Yenari MA. Reduction in levels of matrix metalloproteinases and increased expression of tissue inhibitor of metalloproteinase-2 in response to mild hypothermia therapy in experimental stroke. J Neurosurg. 2005 Aug;103(2):289–297. doi: 10.3171/jns.2005.103.2.0289. [DOI] [PubMed] [Google Scholar]
- Lescot T, Fulla-Oller L, Palmier B, et al. Effect of acute poly(ADP-ribose) polymerase inhibition by 3-AB on blood-brain barrier permeability and edema formation after focal traumatic brain injury in rats. J Neurotrauma. 2010 Jun;27(6):1069–1079. doi: 10.1089/neu.2009.1188. [DOI] [PubMed] [Google Scholar]
- Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002 Jul 1;69(1):1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- Lu A, Ran R, Parmentier-Batteur S, Nee A, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002 Apr;81(2):355–364. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- Manaenko A, Fathali N, Williams S, Lekic T, Zhang JH, Tang J. Geldanamycin reduced brain injury in mouse model of intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:161–165. doi: 10.1007/978-3-7091-0693-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumori Y, Hong SM, Aoyama K, et al. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab. 2005 Jul;25(7):899–910. doi: 10.1038/sj.jcbfm.9600080. [DOI] [PubMed] [Google Scholar]
- Namas R, Ghuma A, Hermus L, et al. The acute inflammatory response in trauma / hemorrhage and traumatic brain injury: current state and emerging prospects. Libyan J Med. 2009;4(3):97–103. doi: 10.4176/090325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002 Sep 1;22(17):7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehmichen M, Walter T, Meissner C, Friedrich HJ. Time course of cortical hemorrhages after closed traumatic brain injury: statistical analysis of posttraumatic histomorphological alterations. J Neurotrauma. 2003 Jan;20(1):87–103. doi: 10.1089/08977150360517218. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Xu L, Giffard RG. Geldanamycin treatment reduces delayed CA1 damage in mouse hippocampal organotypic cultures subjected to oxygen glucose deprivation. Neurosci Lett. 2005 Jun 3;380(3):229–233. doi: 10.1016/j.neulet.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Potts MB, Adwanikar H, Noble-Haeusslein LJ. Models of traumatic cerebellar injury. Cerebellum. 2009 Sep;8(3):211–221. doi: 10.1007/s12311-009-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000 Jun;47(6):782–791. [PubMed] [Google Scholar]
- Ran R, Lu A, Zhang L, et al. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004 Jun 15;18(12):1466–1481. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in brain injury. J Neurotrauma. 1995 Oct;12(5):833–842. doi: 10.1089/neu.1995.12.833. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Massa SM, Swanson RA. Heat-shock protein protection. Trends Neurosci. 1999 Mar;22(3):97–99. doi: 10.1016/s0166-2236(98)01392-7. [DOI] [PubMed] [Google Scholar]
- Suehiro E, Fujisawa H, Akimura T, et al. Increased matrix metalloproteinase-9 in blood in association with activation of interleukin-6 after traumatic brain injury: influence of hypothermic therapy. J Neurotrauma. 2004 Dec;21(12):1706–1711. doi: 10.1089/neu.2004.21.1706. [DOI] [PubMed] [Google Scholar]
- Tang XN, Berman AE, Swanson RA, Yenari MA. Digitally quantifying cerebral hemorrhage using Photoshop and Image J. J Neurosci Methods. 2010 Jul 15;190(2):240–243. doi: 10.1016/j.jneumeth.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008 Jun 23;154(2):556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truettner JS, Hu B, Alonso OF, Bramlett HM, Kokame K, Dietrich WD. Subcellular stress response after traumatic brain injury. J Neurotrauma. 2007 Apr;24(4):599–612. doi: 10.1089/neu.2006.0186. [DOI] [PubMed] [Google Scholar]
- Truettner JS, et al. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2005;25:1505–1516. doi: 10.1038/sj.jcbfm.9600150. [DOI] [PubMed] [Google Scholar]
- Vajtr D, Benada O, Kukacka J, et al. Correlation of ultrastructural changes of endothelial cells and astrocytes occurring during blood brain barrier damage after traumatic brain injury with biochemical markers of BBB leakage and inflammatory response. Physiol Res. 2009;58(2):263–268. doi: 10.33549/physiolres.931253. [DOI] [PubMed] [Google Scholar]
- Vilalta A, Sahuquillo J, Poca MA, et al. Brain contusions induce a strong local overexpression of MMP-9. Results of a pilot study. Acta Neurochir Suppl. 2008;102:415–419. doi: 10.1007/978-3-211-85578-2_81. [DOI] [PubMed] [Google Scholar]
- Vilalta A, Sahuquillo J, Rosell A, Poca MA, Riveiro M, Montaner J. Moderate and severe traumatic brain injury induce early overexpression of systemic and brain gelatinases. Intensive Care Med. 2008 Aug;34(8):1384–1392. doi: 10.1007/s00134-008-1056-1. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007 Mar;184(1–2):53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, van Hoecke M, Tang XN, et al. Pyruvate protects against experimental stroke via an anti-inflammatory mechanism. Neurobiol Dis. 2009 Oct;36(1):223–231. doi: 10.1016/j.nbd.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ouyang YB, Giffard RG. Geldanamycin reduces necrotic and apoptotic injury due to oxygen-glucose deprivation in astrocytes. Neurol Res. 2003 Oct;25(7):697–700. doi: 10.1179/016164103101202183. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Fink SL, Sun GH, et al. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann Neurol. 1998 Oct;44(4):584–591. doi: 10.1002/ana.410440403. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005 Aug;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008 Dec 9;1244:164–172. doi: 10.1016/j.brainres.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008 Jan;28(1):53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]