Abstract
Williams Syndrome (WS) is a neurogenetic developmental disorder characterized by peaks and valleys of cognitive abilities. One peak that has been understudied is the affinity that many individuals with WS have toward music. It remains unknown whether their high levels of musical interest, skill and expressivity are related to their sociable personality or their verbal intelligence. We examined the relationships between musicality (musical interest, creativity and expressivity), sociability (social-emotionality, social approach) and language comprehension in WS and typically developing (TD) controls. Findings suggest that emotion-expressivity through music in WS may be linked to their sensitivity and responsivity to emotions of others, whereas general interest in music may be related to greater linguistic capacity in TD individuals. Musicality and sociability may be more closely related in WS relative than in typical development; implications for future interventions for this neurodevelopmental condition will be discussed.
Keywords: Social-Emotionality, Musicality, Williams Syndrome
“Music is like soup for the soul…”
Williams syndrome (WS) is a neurodevelopmental disorder that stems from a deletion of an estimated 25 genes normally linked to the elastin protein on chromosome 7q11.23 (Korenberg et al., 2000). Those with this condition typically demonstrate striking peaks and valleys of cognitive abilities (i.e., impaired visual-spatial skills and a relative strength in language), and an overtly social phenotype complemented by increased anxiety. Hypersocial behaviors in WS include an increased propensity to approach strangers (Doyle, Bellugi, Korenberg, & Graham, 2004; Järvinen-Pasley et al., 2008), increased attention to faces (Jones et al., 2000), social closeness (Klein-Tasman & Mervis, 2003), and greater use of social language during ordinary discourse (Losh, Bellugi, Reilly, & Anderson, 2000; Reilly, Klima, & Bellugi, 1990). Moreover, individuals with WS appear to be more sensitive to others’ emotions and display greater signs of emotional empathy (Tager-Flusberg & Sullivan, 2000).
While the social phenotype of WS has been given considerable research attention, a characteristic less explored is their musical behaviors. Music in WS has been described as a relative strength, with reported equivalent interests and engagement compared to typically developing (TD) individuals and greater musical affinity than others with contrasting neurodevelopmental disorders including Down syndrome (DS) and autism-spectrum disorders (ASD; Lai, 2009; Lenhoff, Wang, Greenberg, & Bellugi, 1997; Levitin & Bellugi, 2006; Levitin et al., 2004; Levitin, Cole, Lincoln, & Bellugi, 2006). Individuals with WS are demonstrably strong and unusual in their musical affinity and interest, as evidenced in our ongoing studies, in the public media (“A Very Special Brain”, 1997; “Embraceable”, 2011; “Growing Up Different”, 2001), and in the number of music camps and academies that have developed within the past decade.
Evidence suggests that this musical phenotype may be linked to their social behaviors. For example, Bhatara et al. (2010) administered an emotion-perception task with musical stimuli to individuals with ASD and those with WS; WS individuals understood and evaluated emotionality in music better than those with ASD. Järvinen-Pasley et al. (2010) found that music facilitated social-emotional processes in WS, further supporting the relationship between music and affective processing ability in this population.
Although research in music abilities in WS is scarce, some studies suggest that atypical neural organization may underpin WS musical abilities compared to TD individuals (Galaburda, Wang, Bellugi, & Rossen, 1994). An initial functional resonance magnetic imaging study (Levitin et al., 2003) found more amygdala activation to music and pleasing noise in WS than TD. Moreover, Thornton-Wells et al. (2010) noted that musical stimuli also activated visual cortical areas suggesting that these individuals may process auditory stimuli similarly to those with synesthesia. The neural networks underlying music in WS may be atypically organized and functionally different than normal development, however, more research in this field is required to tease apart neural and functional differences in this population.
The extent that the musical phenotype in WS is related to their social behaviors is not well understood. While a few experiments have indirectly suggested the music phenotype in WS may influence or correlate to their social-emotional behaviors (Bhatara et al., 2010; Järvinen-Pasley et al., 2010), to our knowledge there have been no direct studies examining the relationship between these two aspects of the WS profile. Thus, the present study sought to address this question by comparing music and social-emotional and social approach behaviors in WS and in TD individuals. A verbal measure was also included to explore whether linguistic ability plays a role in these two domains. The current study primarily focused on three domains of music: musical interest, frequency of creating musical pieces, and emotion expressivity. General social approach and social-emotional behaviors (i.e., empathy, sensitivity and responsivity to emotions) were measured in addition to verbal comprehension. Given past studies indicating social-emotional processes may be influenced by high musical interest in WS, we hypothesized that measures of musicality, in particularly musical interest and affect-expressivity, will be correlated with social-emotional behaviors in WS participants to a greater degree than TD controls. While it is unclear how language in WS may interact with music or social behaviors due to the limited literature in this realm, research in TD has implicated the associations between verbal intelligence with musical engagement (Hille, Gust, Bitz, & Kammer, 2011); consequently, it is hypothesized that more linguistic competence in TD would be associated with more musical interest and creative frequency.
Method
Participants
A total of 74 individuals participated in this study; 55 participants had WS (30F, 25M) and 19 (12F, 7M) TD, both groups matched on chronological age. Participants with WS and their caregivers were recruited through the Williams Syndrome Association National Conference and from the Laboratory for Cognitive Neuroscience (LCN) at the Salk Institute for Biological Studies. Diagnoses for WS were confirmed through either the florescent insitu hybridization test (FISH; Korenberg et al., 2000) or by the WS Diagnostic Score Sheet (American Academy of Pediatrics Committee on Genetics, 2001). The TD controls were recruited through LCN and undergraduate psychology classes at the University of California, San Diego. These participants were asked to complete the inventory while consulting with a caregiver for accuracy. A t-test found no significant intergroup age differences, WS: Mage = 29.5 years; range = 16.3–52.3 years; TD: Mage = 28.2 years; range = 18.2–41.0 years; t(72) = .51, ns.
Participation in the questionnaires was voluntary; however, for the participation in the vocabulary test, $10 per session was paid per enrolled subject.
Materials
Two questionnaires were administered to evaluate musicality and sociability respectively. First, Salk/McGill Music Inventory (SAMMI) was employed (Bhatara, et al,, 2010; Levitin, 2005; 2012; Levitin et al. 2004; Quintin, Bhatara, Fombonne & Levitin, 2011; 2012) The SAMMI was developed to characterize musicality among WS, ASD, and developmentally-delayed (DD) populations and validated for reliability and other psychometric properties (Levitin, 2005; Levitin et al., 2004). This inventory consists of 46 items, of which 33 are multiple choice and 13 are free response questions. Factor analysis revealed that the individual items cluster into six higher order factors representing demographic, musical interest, emotion responsivity to music, musical creativity, musical training, and age of onset. The Demographic construct addresses relevant physical deficits (e.g., hyperacusis, hearing loss, handedness). Musical Interest assesses level of musical involvement, interest in music relative to peers, time spent on musical activities, and types of musical activities the participant engages in. Musical creativity measures frequency, accuracy, and complexity of musical pieces produced by participants and the ability to recall musical songs. Musical training addresses whether the participant had previous formal training in musical theory, and the number and frequency of instruments played. Emotion responsivity evaluates the intensity of participant’s affective response to music, and the intensity of reactions across emotional musical pieces. Lastly, age of onset explores the age when the participant began to show interest in musical activities, including playing instruments and singing or listening to songs. Statistical properties of the inventory can be found in Levitin et al. (2004). Here, we were primarily interested in specific musical domains and their relevance to social behavior. Thus, the subscores for musical interest, emotion responsivity/expressivity with music, and musical creativity were emphasized in the current study.
The second inventory used was the Salk Institute Sociability Questionnaire (SISQ). This inventory has been used in numerous studies and found consistent patterns across different ages, different cultures and across different comparison groups including individuals with ASD, DS, and TD individuals (Doyle et al., 2004; Järvinen-Pasley et al., 2008; Jones et al., 2000; Zitzer-Comfort, Doyle, Masataka, Korenberg, & Bellugi, 2007). Additionally, social measures in this inventory have been employed for various behavioral and neuroimaging research (Haas et al., 2010; Järvinen-Pasley et al., 2010). Psychometric properties of the SISQ are outlined in Doyle et al. (2004) and Zitzer-Comfort et al. (2007). Global sociability is calculated with 12 rated items, of which 8 measure social approach behavior and 4 compute social-emotionality. Social approach comprises two subscales: Approach Strangers and Approach Familiars. The Approach Strangers submeasure included 5 items regarding the participant’s actions toward different unfamiliar persons (i.e., adults, same-age peers) and how strangers react to the child. An example item would be: “Compare your child’s tendency to approach strangers with an average child of the same age.” Approach Familiars is an assessment of the participant’s behavior to known peers (i.e., family members, peers, family friends) using 3 items. A sample statement would be: “My child would spontaneously greet or approach members of his/her immediate family.” Social emotionality comprised 4 questions gauging emotional empathetic tendencies, accuracy of emotional evaluations, eagerness to please others, and the capacity to remember names and faces of individuals they meet on first encounter. An example question would be: “How likely is your child to comment on the emotional states of other individuals?”
A verbal assessment was administered by trained experimeners to explore the possibility of linguistic competence in the social and musical phenotype of WS and TD. The Peabody Picture Vocabulary Test 3rd Edition (PPVT-III; Dunn & Dunn, 1997) was administered to all participants. The PPVT-III is commonly used as both a measure of verbal intelligence and as an indicator of receptive language. As expected, a t-test indicated that the verbal intelligence or linguistic comprehension was impaired in WS, M = 76.67, relative to TD, M = 108.84, t(72) = −8.59, p < .001.
Results
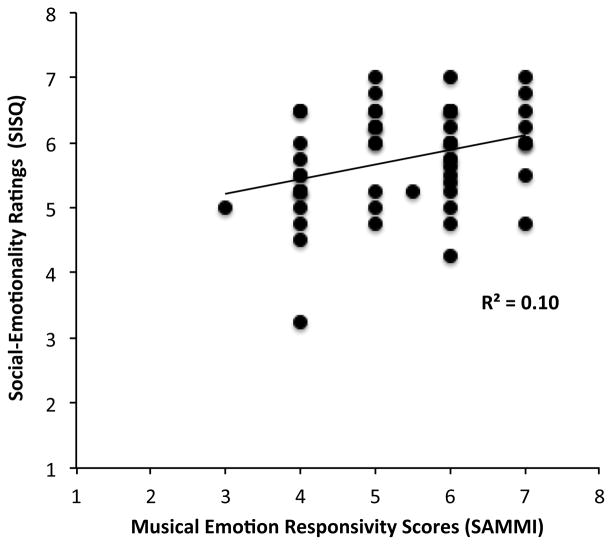
Bivariate Pearson’s correlations were conducted to explore the relation between musical profile (as assessed by the six factors of the SAMMI), sociability (SISQ: social approach, social-emotionality), and PPVT scores in WS and TD (see Table 1 and 2). Figure 1 indicates a significant correlation between emotional responsivity to music and social-emotionality in WS, r = .32, p < .05. Further correlations were computed between emotional responsivity to music from SAMMI with the four independent questions comprising socioemotionality from the SISQ (accuracy in emotional assessment, desire to please, memory for facial affect, verbalization on others’ affect). Within social-emotionality questions, this musical submeasure was most positively associated with the rating for accuracy in emotional assessment of others, r = .30, p < .05, which indicates that the more emotionally expressive and responsive WS individuals are to music, the more sensitive they are at judging the emotions of others in social discourse. Stepwise regression analysis with emotion responsivity, musical creativity, and musical interest from the SAMMI as the independent variables and emotional assessment accuracy from the SISQ as the dependent factor was computed to confirm the strength of this relationship in WS. Results from this regression computation indicated that WS emotion responsivity in music explains 8.7% of the variance in emotional evaluative accuracy, β = .30, F(1, 54) = 5.07, p < .05, whereas the other musical constructs were not significant determinants (musical interest: β = .07, creative frequency: β = .14, all p values > .28. Finally, this analysis was repeated with the three musical constructs as independent factors (musical interest, music creativity, and emotion responsivity) and socioemotionality as the response variable. Results show that emotion responsivity to music is the only significant determinant of socioemotional behaviors, β = .32, p < .05. In WS participants, affective responsivity from the SAMMI explained 10.2% of the variation in socioemotional scores. In contrast, no relationships between music and social submeasures in TD were observed, all p values > .18.
Table 1.
Correlations Among Mean Musical, Social, and Language Scores in Williams Syndrome (N = 55) and Typical Development (N = 19)
| Williams Syndrome | ||||||
|---|---|---|---|---|---|---|
| Musical Interest | Emotion Responsivity | Musical Creativity | Social Approach | Social-Emotionality | PPVT-III | |
|
| ||||||
| Musical Interest | --- | .50** | .27* | −.05 | .21 | .39 |
| Emotional Responsivity | --- | .23 | .05 | .32* | −.36** | |
| Musical Creativity | --- | .08 | .21 | .03 | ||
| Social Approach | --- | .35** | −.24 | |||
| Social-Emotionality | --- | −.11 | ||||
| PPVT-III | --- | |||||
| Typical Development | ||||||
|---|---|---|---|---|---|---|
| Musical Interest | Emotion Responsivity | Musical Creativity | Social Approach | Social-Emotionality | PPVT-III | |
|
| ||||||
| Musical Interest | --- | .74** | .60** | .11 | −.01 | .48* |
| Emotional Responsivity | --- | .42 | −.23 | −.16 | .28 | |
| Musical Creativity | --- | .00 | −.30 | .18 | ||
| Social Approach | --- | .56* | .12 | |||
| Social-Emotionality | --- | −.01 | ||||
| PPVT-III | --- | |||||
Note
Significant at p < .05
Significant at p <.01
Figure 1.
However, the relationship of linguistic competence and musical behaviors in TD and WS showed vast differences. In WS, the PPVT scores were strongly, inversely related to emotional responsivity to music, r = −.36, p < .01, indicating that in WS, the greater linguistic competence acquired, the less emotionally responsive they are. In contrast, musical interest in TD was positively correlated with PPVT scores, r = .48, p < .05, suggesting those with greater linguistic capacity tend to be more interested in music. No significant relationships between language and social measures were observed in both WS and TD groups.
Results suggest that emotion responsivity in music and sensitivity in social interactions may be more intimately related in WS than in TD individuals. Further, verbal competence show diverging relationships in TD versus WS groups, while increased language capacity is related to more musical behaviors in TD, the opposing effect was shown in WS.
Discussion
The music phenotype in individuals with WS is a relative strength characterized by enhanced interest in and affinity for music. While individuals with WS are not typically ‘savants’ in musical skill, the unusual behavior displayed by many in this population is a propensity towards music. To our knowledge, there are no reports of WS having greater capacity for music than TD individuals; however, previous studies indicate WS show greater drive to participate in musical activities (Levitin, 2005; Levitin et al., 2004). It was reported that WS show more interest in music and greater emotional responsivity to music, spend more hours playing instruments, and develop an earlier age of musical interest onset despite having similar degree of musical education than normal healthy individuals. Even when compared to other neurogenetic disorders such as ASD and DS, individuals with WS show greater participation in musical activities, interest in music, and engagement in listening to music (Levitin et al., 2004). Thus, it appears music behaviors in WS are not externally driven, rather it is an intrinsic interest that may be related to their genetic condition.
The current findings further support that there may be a connection between the social phenotype of WS and the musical behaviors displayed by WS individuals. Our findings of a positive association between emotion responsivity in music and emotion sensitivity in social intercourse in WS but not TD suggest that this musicality-sociability relationship may be more entwined and exclusive in WS. Additionally, the negative correlation found between verbal ability in WS and emotional responsivity in music but not social measures suggest that musical emotional expressivity may be less prevalent in those with greater linguistic competence; thus, for WS individuals with greater language capacity, it might be particularly advantageous to instill emotion expressivity using music to aid with their broader affective awareness and understanding.
Given the augmented interest to music in WS, participating in musical activities may be therapeutic and beneficial in multiple ways. Individuals with WS encounter a wide variety of maladaptive symptoms — with anxiety as the most prevalent symptom. This condition has been associated with overly anxious tendencies including excessive fear and phobias (Dykens, 2003). Anxiety has been observed to be persistent in this population (Woodrudd-Borden, Kistler, Henderson, Crawford, & Mervis, 2010). However, a study by Dykens and colleagues using parental questionnaires found a decrease in maladaptive behaviors alongside lower levels of anxiety when individuals with WS engaged in musical activities (Dykens, Rosner, Ly, & Sagun, 2005; Sellinger, Hodapp, & Dykens, 2006). In addition to affective behaviors, music has also been used as a teaching tool to enhance areas of cognitive and social deficits. For example, Reis and colleagues (2003) used music to teach mathematics, a cognitive deficit typically associated with WS; post-test results revealed an overall gain in the comprehension of fractions in 94% of the WS participants. Thus, music may have implications on skill improvement across multiple domains in WS.
Music therapy has been most notable in treating emotional and social disturbances across neurodevelopmental disorders. In ASD, a condition with many similar social communicative deficits akin to WS (Klein-Tasman, Phillips, Lord, Mervis, & Gallo, 2009), music therapy has been shown to be effective in social skills interventions. In this population, music has been shown to aid in increasing various socially relevant skills including affective understanding (Katagiri, 2009), emotional and social responsiveness (Finnigan & Starr, 2010; Kim, Wigram, & Gold, 2009), and speech production (Lim, 2010). In line with our findings, WS may particularly find musical interventions more beneficial with their unique affinity to music and association between musical and social-emotional behaviors. It is possible that emotional expressivity through music may be a method for WS individuals to relieve their emotional distress, soothe self, and understand emotions in a broader level to diminish anxious and affective disturbances. Altogether, given the affective problems WS individuals face, music may be a valuable form of outlet to relieve and understand emotions.
The current results provide the first piece of evidence that musicality and socioemotionality is associated in WS individuals. Yet, it is unknown whether this relationship is specific to WS population or extends to others with similar cognitive capacity. The inclusion of a developmentally-matched contrast group would aid in further distinguising the relationship between cognitive ability, socioemotionality, and musicality. Consequently, later studies should incorporate both TD and DD comparison groups with larger sample sizes to provide greater statistical power and to clarify the extent this relationship is unique to WS population.
In sum, our findings indicate that music may be a promising avenue to improve on emotional understanding and sensitivity in WS, which in turn may enrich ability to socially interact in an appropriate manner. More significantly, however, is that these results show an atypical intimate association between music and social behaviors, suggesting that this population may particularly benefit from musical interventions. While our preliminary study evidently has limited participant pools, it sheds light on the possible connections between genetics, music and the social phenotype in WS. Future research should continue to explore music as a possible therapeutic outlet for those with WS.
Acknowledgments
We thank all the participants and families that took part in this study and the Williams Syndrome Association. This research was supported in part by NICHD P01 HD33113, NINDS P50 NS22343, and The Oak Tree Philanthropic Foundation to UB and by the Social Sciences and Humanities Research Council of Canada (SSHRC) to DL.
Contributor Information
Rowena Ng, Laboratory for Cognitive Neuroscience, Salk Institute for Biological Studies, La Jolla, California.
Philip Lai, SDSU/UCSD Joint Doctoral Program in Language and Communicative Disorders.
Daniel J. Levitin, Department of Psychology, McGill University
Ursula Bellugi, Laboratory for Cognitive Neuroscience, Salk Institute for Biological Studies, La Jolla, California.
References
- American Academy of Pediatric Committee on Genetics. Policy Statement. 5. Vol. 107. Elkgrove, IL: American Academy of Pediatrics; 2001. May, Health care supervision for children with Williams syndrome; pp. 1192–1204. (RE0034) [PubMed] [Google Scholar]
- Bhatara A, Quintin EM, Levy B, Bellugi U, Fombonne E, Levitin DJ. Perception of emotion in musical performance in adolescents with autism spectrum disorders. Autism Research. 2010;3:214–225. doi: 10.1002/aur.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CBS Broadcasting Incorporation. 60 Minutes: A Very Special Brain [News] United States: 1997. [Google Scholar]
- Chedd G. Scientific American Frontiers series. United States: The Chedd-Angler Production Company Inc; Nov 13, 2001. Growing Up Different. [Google Scholar]
- Doyle TF, Bellugi U, Korenberg JR, Graham J. “Everybody in the world is my friend” hypersociability in young children with Williams syndrome. American Journal of Mental Retardation. 2004;124a:263–273. doi: 10.1002/ajmg.a.20416. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Dykens EM. Anxiety, fears, and phobias in persons with Williams syndrome. Developmental Neuropsychology. 2003;23:291–316. doi: 10.1080/87565641.2003.9651896. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Rosner BA, Ly T, Sagun J. Music and anxiety in Williams syndrome: A harmonious or discordant relationship? American Journal on Mental Retardation. 2005;110(5):346–358. doi: 10.1352/0895-8017(2005)110[346:MAAIWS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Finnigan E, Starr E. Increasing social responsiveness in a child with autism. A Comparison of music and non-music interventions. Autism. 2010;14:321–348. doi: 10.1177/1362361309357747. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Wang PP, Bellugi U, Rossen M. Cytoarchitectonic anomalies in a genetically based disorder: Williams syndrome. Neuroreport. 1994;5:753–757. doi: 10.1097/00001756-199403000-00004. [DOI] [PubMed] [Google Scholar]
- Haas BW, Hoeft F, Searcy YM, Mills D, Bellugi U, Reiss AL. Individual differences in social behavior predict amygdala response to fearful facial expressions in Williams syndrome. Neuropsychologia. 2010;48:1283–1288. doi: 10.1016/j.neuropsychologia.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille K, Gust K, Bitz U, Kammer T. Associations between music education, intelligence, and spelling ability in elementary school. Advances in Cognitive Psychology. 2011;7:1–6. doi: 10.2478/v10053-008-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Bellugi U, Reilly J, Mills DL, Galaburda A, Reiss AL, Korenberg JR. Defining the social phenotype in Williams syndrome: A model for linking gene, the brain, and behavior. Development and Psychopathology. 2008;20:1–35. doi: 10.1017/S0954579408000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Vines BW, Hill KJ, Yam A, Grichanik M, Mills D, Bellugi U. Cross-modal influences of affect across social and non-social domains in individuals with Williams syndrome. Neuropsychologia. 2010;48:456–466. doi: 10.1016/j.neuropsychologia.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Bellugi U, Lai Z, Chiles M, Reilly J, Lincoln A, Adolphs R. II. Hypersociability in Williams syndrome. Journal of Cognitive Neuroscience. 2000;12:30–46. doi: 10.1162/089892900561968. [DOI] [PubMed] [Google Scholar]
- Katagiri J. The effect of background music and song texts on the emotional under standing of children with autism. Journal of Music Therapy. 2009;46:15–31. doi: 10.1093/jmt/46.1.15. [DOI] [PubMed] [Google Scholar]
- Kent J. Embraceable [Documentary] United States: Kent Creative; 2011. [Google Scholar]
- Kim J, Wigram T, Gold C. Emotional, motivational, and interpersonal responsiveness of children with autism in improvisional music therapy. Autism. 2009;13:389–409. doi: 10.1177/1362361309105660. [DOI] [PubMed] [Google Scholar]
- Klein-Tasman BP, Mervis CB. Distinctive personality characteristics of 8-, 9-, and 10-year-olds with Williams syndrome. Developmental Neuropsychology. 2003;23:269–90. doi: 10.1080/87565641.2003.9651895. [DOI] [PubMed] [Google Scholar]
- Klein-Tasman BP, Phillips KD, Lord C, Mervis CB, Gallo FJ. Overlap with the autism spectrum in young children with Williams syndrome. Journal of Developmental and Behavioral Pediatrics. 2009;30:289–299. doi: 10.1097/DBP.0b013e3181ad1f9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenberg JR, Chen XN, Hirota H, Bellugi U, Burian D, Roe B, Matsuoka R. Genome structure and cognitive map of Williams syndrome. Journal of Cognitive Neuroscience. 2000;12:89–107. doi: 10.1162/089892900562002. [DOI] [PubMed] [Google Scholar]
- Lai PT. Master’s thesis. San Diego State University; San Diego, CA: 2009. Music and emotion in Williams syndrome. [Google Scholar]
- Lenhoff HM, Wang PP, Greenberg F, Bellugi U. Williams syndrome and the brain. Scientific American. 1997;277:68–73. doi: 10.1038/scientificamerican1297-68. [DOI] [PubMed] [Google Scholar]
- Levitin DJ. Musical behavior in a neurogenetic developmental disorder: Evidence from Williams syndrome. Annals of the New York Academy of Sciences. 2005;1060:325–334. doi: 10.1196/annals.1360.027. [DOI] [PubMed] [Google Scholar]
- Levitin DJ. What does it mean to be musical? Neuron. 2012;73:633–637. doi: 10.1016/j.neuron.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Bellugi U. Rhythm, timbre and hyperacusis in Williams-Beuren syndrome. In: Morris C, Lenhoff H, Wang P, editors. Williams-Beuren Syndrome: Research, Evaluation, and Treatment. Baltimore, MD: John Hopkins University Press; 2006. pp. 343–358. [Google Scholar]
- Levitin DJ, Cole K, Chiles M, Lai Z, Lincoln A, Bellugi U. Characterizing the musical phenotype in individuals with Williams syndrome. Child Neuropsychology. 2004;10:223–247. doi: 10.1080/09297040490909288. [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Cole K, Lincoln A, Bellugi U. Aversion, awareness, and attraction: Investigating claims of hyperacusis in the Williams syndrome phenotype. Journal of Child Psychology and Psychiatry. 2006;46:514–523. doi: 10.1111/j.1469-7610.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Menon V, Schmitt JE, Eliez S, White CD, Glover GH, Reiss AL. Neural correlates of auditory perception in Williams syndrome: an fMRI study. Neuroimage. 2003;18:74–82. doi: 10.1006/nimg.2002.1297. [DOI] [PubMed] [Google Scholar]
- Lim HA. Effect of “developmental speech and language training through music” on speech production in children with autism spectrum disorders. Journal of Music Therapy. 2010;47:2–26. doi: 10.1093/jmt/47.1.2. [DOI] [PubMed] [Google Scholar]
- Losh M, Bellugi U, Reilly J, Andersen D. Narrative as a social engagement tool: The excessive use of evaluation in narratives from children with Williams syndrome. Narrative Inquiry. 2000;10:1–26. [Google Scholar]
- Quintin E-M, Bhatara A, Poissant H, Fombonne E, Levitin DJ. Processing of musical structure by high functioning adolescents with autism spectrum disorders. Child Neuropsychology. 2012 doi: 10.1080/09297049.2011.653540. [DOI] [PubMed] [Google Scholar]
- Quintin E-M, Bhatara A, Poissant H, Fombonne E, Levitin DJ. Emotion perception in music in high-functioning adolescents with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2011;41(9):1240–1255. doi: 10.1007/s10803-010-1146-0. [DOI] [PubMed] [Google Scholar]
- Reilly J, Klima ES, Bellugi U. Once more with feeling: Affect and language in atypical populations. Development and Psychopathology. 1990;2:367–391. [Google Scholar]
- Reis S, Schader R, Milne H, Stephens R. Minds & music: Using a talent development approach for young adults with Williams Syndrome. Exceptional Children. 2003;69(3):293–313. [Google Scholar]
- Sellinger MH, Hodapp RM, Dykens EM. Leisure activities of individuals with Prader-Willi, Williams, and Down Syndromes. Journal of Developmental & Physical Disabilities. 2006;18(1):59–71. [Google Scholar]
- Tager-Flusberg H, Sullivan K. A componential view of theory of mind: Evidence from Williams syndrome. Cognition. 2000;76:59–90. doi: 10.1016/s0010-0277(00)00069-x. [DOI] [PubMed] [Google Scholar]
- Thornton-Wells TA, Cannistraci CJ, Anderson AW, Kim CY, Eapen M, Gore JC, Dykens EM. Auditory attraction: Activation of visual cortex by music and sound in Williams syndrome. American Journal on Intellectual and Developmental Disabilities. 2010;115:172–189. doi: 10.1352/1944-7588-115.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrudd-Borden J, Kistler DJ, Henderson DR, Crawford N, Mervis CB. Longitudinal course of anxiety in children and adolescents with Williams syndrome. American Journal of Medical Genetics. 2010;154C:277–290. doi: 10.1002/ajmg.c.30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzer-Comfort C, Doyle T, Masataka N, Korenberg J, Bellugi U. Nature and nurture: Williams syndrome across cultures. Developmental Science. 2007;10:755–762. doi: 10.1111/j.1467-7687.2007.00626.x. [DOI] [PubMed] [Google Scholar]