Abstract
Purpose
This retrospective cohort study assesses the outcomes of a protocol of management, based on the recommendations of the European Paediatric Orthopaedic Society (EPOS) multi-centre study, for the management of congenital pseudarthrosis of the tibia.
Methods
Utilising an incremental protocol of bracing, intramedullary rods and circular frame fixation with or without bone morphogenetic protein-2 (BMP-2), 11 patients had reached skeletal maturity or had follow up of 5 years from radiological union of the pseudarthrosis. Demographic data, deformity parameters before and after treatment, and functional outcome scores were recorded.
Results
Ten of the 11 patients successfully healed and two sustained a refracture. All deformity parameters improved and a mean leg length discrepancy of 2.5 cm (range 0–7.5 cm) existed at the time of the last follow up. Some pseudarthroses healed with deformity correction and rod insertion alone. Six of the 11 patients had a confirmed diagnosis of neurofibromatosis and nine had sustained a fracture before 4 years of age. Refracture was associated with malalignment after healing.
Conclusion
This method of treatment provides a successful stepwise protocol for the management of this complex disorder, avoiding the use of aggressive limb reconstruction techniques at a young age in some cases.
Level of evidenceCase series Level IV.
Keywords: Pseudarthrosis of the tibia, Treatment, Management
Introduction
Congenital pseudarthrosis of the tibia (CPT) develops spontaneously or after minimal trauma in a segment of abnormal tibial diaphysis. The pseudarthrosis usually develops during the first 2 years of life but has been diagnosed both antenatally and in adulthood [1]. CPT is rare, with an incidence of 1 per 190,000 live births [2]. The aetiology is unclear but there is a strong association between CPT and neurofibromatosis type I (NF-I). Histological examination has shown hyperplasia of fibroblasts within the periosteum [3–5] and a better understanding of the role of NF-1 has suggested that the defect results from failure of osteoblastic differentiation [6, 7]. CPT is challenging to treat and, historically, amputation rates of up to 50 % have been reported [8, 9]. There is the prospect of multiple operations with persistent pseudarthrosis or transient union followed by subsequent fracture, which, together with lower limb deformity and leg length discrepancy, lead to poor limb function [10, 11].
The goal of surgical management of CPT is to obtain and maintain union while minimising deformity. The rarity of the condition makes it difficult for any individual surgeon, except in a specialised centre, to gain significant experience. The philosophy of our treatment has, therefore, been influenced by the results of the European Paediatric Orthopaedic Society (EPOS) multi-centre study [12]. The EPOS study was a collective review of 340 patients originating from 13 countries. It identified that use of the Ilizarov technique and fibula microvascular transfer were the most successful methods of treatment [13, 14], a finding mirrored outside of Europe [15]. The neglect of ankle function and limb alignment were also important findings. We report the results of 11 consecutive children treated for CPT of the tibia by a single surgeon with a protocol evolved from the EPOS findings followed up to skeletal maturity or with a minimum follow up of 5 years from healing.
Materials and methods
A retrospective case series review of children treated between 1998 and 2012 was conducted. All patients had radiological assessment and functional outcome scores including the Activity Scale for Kids (ASK), Oxford Ankle Foot Questionnaire (OAFQ) Child and Teenager version, and the OAFQ Parent version [16, 17]. All cases were classified according to the Boyd system [18]. We describe how they presented, their demographics, associated conditions, tibial deformity, leg length discrepancy, union rate, time to union, modes of treatment, fibula involvement, length of pseudarthrosis segment of the tibia, additional procedures and complications.
Thirteen boys and five girls were treated in our unit for CPT with an incremental protocol of bracing, intramedullary rodding and circular frame with or without bone grafting and bone morphogenetic protein-2 (BMP-2) (Fig. 1). All the procedures were performed by a single surgeon. Bracing with a clam shell plastic brace incorporating the foot was used for the control of simple antero-lateral bowing. Intramedullary nailing with antegrade Rush nails were reserved for those children under the age of 6 years with a frank pseudarthrosis, a fracture or progressive deformity with angulation of more than 40°. The procedure of rod insertion with corrective osteotomies was carried out with minimal resection of bone and periosteum at the apex of the deformity, or the fracture site. Further osteotomies were performed as necessary depending on the pattern of deformity (Fig. 2). The nails were serially exchanged as the patient grew. The majority had Rush pins, although in some cases, Sheffield telescopic rods were used to prevent reoperation associated with relative shortening of the Rush pins with growth.
Fig. 1.
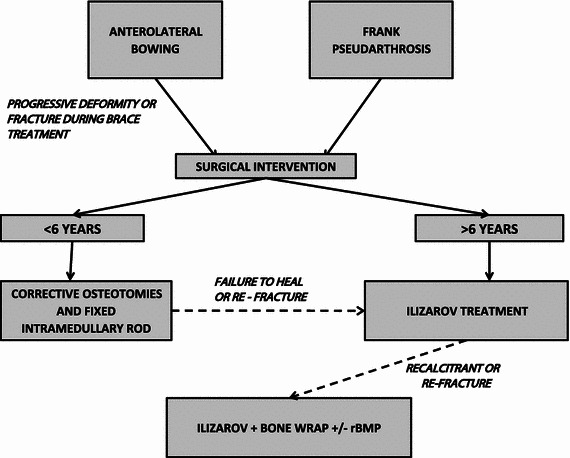
Treatment protocol for the management of congenital pseudarthrosis of the tibia (CPT). rBMP recombinant bone morphogenetic protein
Fig. 2.
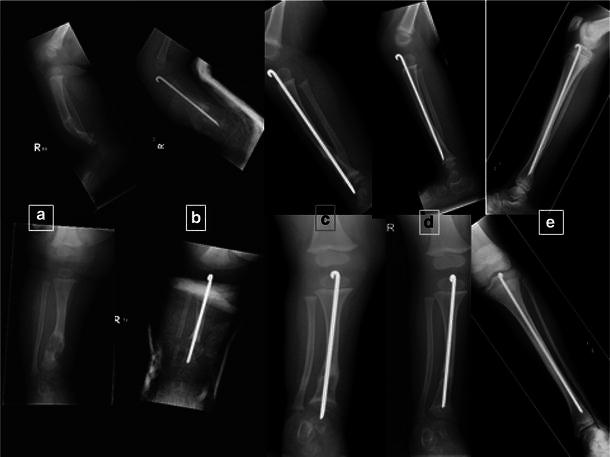
a Fracture through a cystic-type pseudarthrosis. b Managed with Rush pin insertion and realignment. c Rod exchanged with growth. d Pseudarthrosis heals. e Early adolescence showing satisfactory alignment and healing
Those over the age of 6 years who failed to heal with intramedullary nailing went on to have excision of the pseudarthrosis, acute shortening and proximal lengthening with a circular frame (Fig. 3). Circular frames were sometimes combined with an intramedullary nail in order to control alignment and to protect against tibial fracture when the frame was removed. The technique of Ilizarov application involved creating a very stable construct utilising multiple rings and paying attention to correct alignment. Resection was carried out to bleeding bone at the pseudarthrosis site, with compression achieved by creating a ‘pen and inkwell’ docking site. When necessary, the foot and ankle were included in the frame to primarily improve stability and also help ankle alignment and function. Compression was maintained at the pseudarthrosis site and a proximal lengthening was carried out via a percutaneous osteotomy. In those patients who failed to heal with a circular frame, a bone wrap (iliac crest outer table cortical sheet scored to permit bending) was wired around the pseudarthrosis site with BMP-2 (Induct Os; Wyeth Europa, Maidenhead, UK) being placed between the pseudarthrosis and the bone graft [19–23]. The BMP-2 and bone wraps were reserved for resistant cases and were additional to an existing circular external fixator or concurrent with the application of an external fixator. The bone graft wrap and BMP was not used in isolation.
Fig. 3.
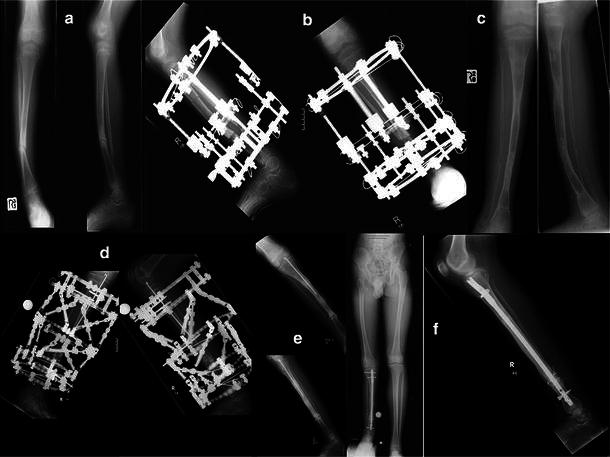
a Atrophic pseudarthrosis with fracture. b Managed with ring fixator incorporating compression and proximal lengthening. c Refracture with underlying sagittal and coronal malalignment. d Ring fixator with bone wrap and rBMP, proximal lengthening takes place over a Rush pin. e Post removal of ring fixator. f Final result at skeletal maturity following further refracture, treated by locked intramedullary nailing showing significant limb length discrepancy with healed pseudarthrosis
Results
Of the 18 patients treated, the outcomes of 11 with long-term follow up are presented. Eight had reached skeletal maturity; a further three having 5 years of follow up from union are also presented. The remaining seven patients, six of whom are currently united, are excluded due to having less than 5 years of follow up being available.
Of the 11 patients, 10 are united. One patient, despite going on to union in 13 months with an Ilizarov frame, elected to have a below-knee amputation after suffering two fractures in close succession, an insufficiency supracondylar fracture of the ipsilateral distal femur during ring fixator treatment managed surgically and a refracture at the pseudarthrosis site after frame removal. This patient had previously been treated elsewhere with a telescopic intramedullary nail. The patient was offered further limb reconstruction but elected for surgery with a certain outcome. One other patient sustained two refractures (Fig. 3). In both cases, refractures were associated with persistent coronal malalignment, with radiographic evidence of bridging bone on four cortices at the previous pseudarthrosis site.
Eight of the 11 patients had sustained a fracture through the abnormal tibia before the age of 4 years. Five had already been treated in other institutions before referral for a persistent pseudarthrosis despite surgical treatment.
A total of seven patients had treatment with a circular frame. Frames were used in a variety of ways: to excise and compress the CPT while lengthening (P), for deformity correction alone (D), for lengthening alone (L) or a combination of lengthening and deformity correction (Table 1). Five patients had frame treatment for a pseudarthrosis. Of these, two had one treatment with a frame, two had two treatments with a frame, the second treatment being for deformity correction and lengthening, and one had three treatments with a frame, the second frame was for the correction of deformity and lengthening, and the third for a further pseudarthrosis excision after a refracture. Two patients had frames for either deformity correction (D) or lengthening (L) alone. In this group of five patients with six excisions of pseudarthrosis combined with lengthening, the mean time in the frame was 12.3 months (range 9–17 months). Three patients had bone grafting and one of these also had BMP-2. Ten patients were treated by intramedullary pinning at least once during their treatment. From the eight subjects to have reached skeletal maturity, only one patient was treated by rodding alone. Overall, in the group of 11 patients, a mean leg length discrepancy of 2.5 cm (range 0–7.5 cm) existed at the time of the last follow up. Coronal deformity was more successfully corrected than sagittal deformity, although an improvement was seen in alignment in all patients (Table 2). Surgeries other than rodding and circular frame treatment involved the correction of residual ankle deformity or attempts to improve leg length discrepancies. Only one of the cohort has outstanding surgery, in the form of a contralateral epiphysiodesis.
Table 1.
Demographics and deformity treatment for the patient cohort
| Patient number | Age/sex | Age at first fracture (years) | Follow up from union/years | Location of pseudarthrosis | Percentage of tibia with abnormal bone involvement | Fibula involvement | Boyd classification | Presence of neurofibromatosis | Number of rodding procedures | Ring fixator treatments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 M | >4a | 5 H | Distal 1/3 | 11 | Yes | IV | Yes | 0 | 14M-P, 6M-D/L |
| 2 | 17 M | <4a | A | Distal 1/3 | 25 | Yes | II | No | 1 + (1T) | 13M-P |
| 3 | 16 M | <4a | 6 H | Mid shaft | 31 | Yes | II | No | 1 + (1T) | 6M-D |
| 4 | 16 F | <4a | 3 H | Mid shaft | 66 | Yes | IV | Yes | 2 | 17M-P |
| 5 | 16 M | <4 | 6 H | Distal 1/3 | 29 | Yes | IV | Yes | (1T) | 6M-L |
| 6 | 16 F | <4a | 5 H | Distal 1/3 | 23 | No | II | Yes | (1T) + (1T) | 10M-P, 6M-D/L |
| 7 | 16 M | >4 | 7 H | Mid shaft | 33 | Yes | IV | Yes | (1T) | N/A |
| 8 | 15 M | >4 | 1 H | Distal 1/3 | 26 | No | IV | No | 1 + 2b + (1L) | 11M-P, 10M-D/L, 9M-P (+BMP-2) |
| 9 | 14 M | <4 | 5 H | Distal 1/3 | 28 | Yes | II | No | 1 + (1T) | N/A |
| 10 | 13 M | <4 | 8 H | Distal 1/3 | 31 | Yes | II | No | 2 | N/A |
| 11 | 12 M | <4 | 8 H | Distal 1/3 | 25 | No | II | Yes | 2 | N/A |
H Healed, A amputation, T telescopic, L custom locking nail, Rush rod if unspecified, M time in fixator/months, D deformity correction, L lengthening, P pseudarthrosis treatment
aTreatment initiated at other hospital, treatments performed elsewhere highlighted in bold
bRush rods used in conjunction with ring fixation for control of lengthening segment
Table 2.
Deformity parameters pre- and post-treatment, leg length discrepancy at last review and other procedures performed or outstanding. Note initial measurements are for the initial assessment at our unit, as opposed to initial presentation
| Patient number | Age/sex | Tibial coronal deformity, (°) apex medial | Tibial sagittal deformity, (°) apex anterior | Radiographic leg length discrepancy (cm) | Other procedures | ||
|---|---|---|---|---|---|---|---|
| Presentation | Final review | Presentation | Final review | ||||
| 1 | 17 M | 21 | 4 | 11 | 0 | 0 | Medial distal tibial hemi epiphysiodesis Corrective osteotomy distal tibia |
| 2 | 17 M | 15 | N/A | 15 | N/A | N/A | Posteromedial release Distal femoral fracture fixation Below knee amputation |
| 3 | 16 M | 20 | 2 | 15 | 0 | 3 | Hemi epiphysiodesis distal medial femur Ankle arthrodesis |
| 4 | 16 F | 20 | 15 | 41 | 0 | 1 | Epiphysiodesis contralateral proximal tibia |
| 5 | 16 M | 11 | 10 | 9 | 0 | 3.5 | |
| 6 | 16 F | 10 | 7 | 12 | 11 | 2.5 | |
| 7 | 16 M | 0 | 8 | 30 | 4 | 2 | |
| 8 | 15 M | 35 | 10 | 33 | 0 | 7.5 | |
| 9 | 14 M | 9 | 8 | 13 | 0 | 4 | Epiphysiodesis contralateral distal femur |
| 10 | 13 M | 40 | 0 | 8 | 0 | 2.5 | Epiphysiodesis pending |
| 11 | 12 M | 38 | 3 | 34 | 8 | 1.5 | |
One Sheffield rod became infected, which was exchanged after surgical debridement, later going on to union with a circular frame. A T-piece fracture occurred in another patient with a Sheffield rod. In a third patient, although the pseudarthrosis healed, the rod pulled through the growth plate, resulting in a growth arrest that subsequently required leg lengthening with a frame.
The functional scores recorded at the last follow up showed a mean Activities Scale for Kids (ASK©) score of 78.4 (range 54.2–98.5) for all 18 cases. For the 11 patients for which data are presented, they had an Oxford Ankle Foot Questionnaire for Children (OxAFQ-C) mean score of 34 (range 12–50) and an Oxford Ankle Foot Questionnaire for Parent (OxAFQ-P) mean score of 32 (range 13–50).
Discussion
CPT is a heterogeneous condition which requires treatment tailored to the individual. The EPOS multi-centre study on pseudarthrosis of the tibia was limited by the collective nature of the data, with varying methods of treatment around Europe. Despite these limitations, this sort of study of a rare condition with large numbers of cases is helpful to surgeons trying to establish a successful treatment protocol. Many small case series exist advocating a variety of treatments, but there are little data available comparing treatment methods and comparisons are further complicated by the variable severity of CPT. The EPOS study suggested to the senior author that the results of treatment were better after the age of 6 years and that the best results were obtained with the Ilizarov method or vascularised fibula transfer [12]. This interpretation, together with a view that the foot and ankle function was often neglected, lead to the development of the incremental protocol of management described in Fig. 1.
In a tertiary centre, patients may present after initial treatment elsewhere has been unsuccessful and any subsequent treatment has to take account of this and the particular difficulties of the case. However, for the previously untreated patient, our protocol was to brace using a clamshell type brace incorporating the foot for antero lateral bowing without fracture. Should the bowing be progressive on follow up or the patient presented with a fracture, then our protocol was to correct and maintain tibial alignment by Rush pinning by a minimal bone resection just sufficient to permit correction of the deformity, reserving Ilizarov treatment as the definitive treatment for patients 6 years of age or older.
Ten patients were treated by intramedullary pinning at least once during their treatment. From the eight subjects who reached skeletal maturity, only one patient was treated by rodding alone. Our practice was to initially use a Rush pin inserted antegrade through the knee and then to change the pin for growth. It is important to correct the deformity so that abnormal mechanical alignment does not contribute to persistence of the pseudarthrosis and ankle function is maintained; this requires care with the insertion of the rod. The rod has to traverse the proximal growth plate and repeated passes of a reamer may cause damage and the track that is formed proximally will be the one that will be used for revision procedures. These subsequent procedures will be facilitated by care and accuracy with the initial rodding. We have found that a technique of patella splitting facilitates access. If the patella is cartilaginous, it can be cut with a scalpel and if bony, with a sharp thin osteotome. At the conclusion of the procedure, the patella is repaired with a heavy absorbable figure of eight suture and the limb protected in a back slab cast for 3 to 4 weeks. Repeated exchanges of the Rush pin led to a consideration of the use of a telescopic rod and, in this series, the Sheffield rod [24] was used.
In practice, the Sheffield rod proved to be difficult to insert particularly distally, and less able to resist deformation from the persisting pseudarthrosis than simple Rush rods. A total of three Sheffield rods were inserted at our unit, with a further two inserted prior to referral to us for treatment. On the basis of this poor experience and because the Sheffield rods are relatively thin and may bow, we prefer to use Rush pins at present. We have insufficient experience of Fassier–Duval rods to make any comment at this stage, but it is important to remember that, should the pseudarthrosis fail to unite with rodding, an Ilizarov frame may have to be applied around an intramedullary device and the device may require removal. Under these circumstances, the simple Rush pin has some advantages [25].
Five patients went on to frame treatment with resection and compression of the pseudarthrosis and proximal lengthening; four of these had residual or recurrent pseudarthrosis after the age of 6 years, despite treatment with an intramedullary nail. Compression was maintained at the pseudarthrosis site using the frame. In the management of pseudarthrosis, it is important to use a very stable frame and to correct alignment. In a distal pseudarthrosis, the frame may have to be extended to the foot for stability. Weight-bearing was encouraged in all patients. Whilst regenerate formation at the lengthening site is easy to identify on radiographs, it can be difficult to be certain whether union is occurring at the pseudarthrosis site. Dynamising the frame or removing some rods between the rings spanning the pseudarthrosis can be helpful in assessing union and when it is difficult to obtain good radiographs due to the presence of the frame, screening the limb in various positions under image intensification is helpful. In six patients, these five and one healed case (patient 5), the proximal lengthening was carried out over an existing Rush pin or over one inserted at the time of surgery, as this guards against the risk of regenerate deformity or fracture after frame removal in bone which may be potentially abnormal.
Similarly good results have been obtained historically with fibula transfer [26–30] as with the Ilizarov method [31–34], despite the EPOS suggesting that the circular frame was the ‘gold standard’. Some surgeons advocate extensive debridement of all periosteum within the area of the pseudarthrosis [35], a technique that we do not reproduce nor feel necessary. The other main EPOS finding regarding Ilizarov treatment was that surgery is more successful in older children, although this is disputed by some studies who have found age at surgery not to be predictive of failure [36]. Based on the knowledge at the time of establishment of our protocol, circular frame treatment was reserved for those over 6 years olds. In this series, one patient was treated with BMP and a bone graft wrap for failure to heal with Ilizarov treatment. The concept of the bone graft wrap is to bridge the pseudarthrosis site, analogous to the concept of bypass grafting, and to thicken the bone diameter, whilst the BMP encourages union. We believe that, in addition to this biological stimulus, it is important to maintain stability, mobility and alignment with a stable frame. BMP is currently unlicensed in the UK, although we obtained approval from our hospital Drugs and Therapeutics Committee. We reserved the use of BMP for failure to heal after Ilizarov treatment, having ensured that the frame was stable and correctly aligned. As some patients will heal with Ilizarov treatment, it is difficult to know when to add BMP and the bone graft wrap to the treatment. In our first case, we used BMP after failure of the first Ilizarov frame, but the success of that case has lead us to adopt a more individualised approach and our current protocol is to use BMP if, after 6 weeks of treatment, there is no sign of healing at the pseudarthrosis site. It is possible that, with further research and a more prognostic classification of the condition, it will be possible to identify a sub-group of patients among whom it would be appropriate to treat with BMP at the outset.
There is very little comparative data on ankle and foot functional outcomes in pseudarthrosis of the tibia; our results show scores well below normal, but it is difficult to make further comment. Our results are, however, a baseline by which we can compare the results of improvements in treatment. Restoring correct alignment of the tibia is challenging and the majority of our patients had some residual malalignment which may increase the risk of late refracture and affect ankle and foot function. In our experience, refracture after apparent healing of a pseudarthrosis tends to occur when there is residual malalignment with a narrow segment at the pseudarthrosis site. This is one reason why the combination of a bone graft wrap and BMP is potentially attractive, as it thickens the bone at the level of the healed pseudarthrosis, as shown in Fig. 3f. Correction and maintenance of alignment can be achieved by locked intramedullary nailing at skeletal maturity, but this is technically challenging and osteotomy is an unattractive prospect due to the risk of complications in some of the more difficult cases. However, it may be justifiable in some cases, perhaps in combination with BMP and grafting.
The EPOS study examined 108 subjects treated with an Ilizarov frame and showed an overall union rate of 75 % (81 % if the frame included the foot). We attribute our results to the use of BMP in selected cases and attention given to the stability and alignment of the circular frame. The union rate of those having reached skeletal maturity is 88 % (7 out of 8), with one patient going on to have an amputation. It is important to note that five patients had previously been treated elsewhere and, so, this cohort contains a higher proportion of difficult cases. Whilst we accept that follow up to skeletal maturity is important because of the risk of refracture, we believe that our results so far demonstrate that our protocol is logical and effective in the management of this difficult condition.
Acknowledgments
The concept of a bone graft wrap was suggested to the senior author by Professor Michael Weber, American Hospital, Dubai.
Conflict of interest
None.
Ethical approval
BMP-2 is not currently licensed in the UK for the management of pseudarthrosis of the tibia in children. Approval for the use of BMP-2 in resistant pseudarthrosis of the tibia at our unit has been granted by the Drugs and Therapeutics Committee of the hospital.
References
- 1.Hefti F, Bollini G, Dungl P, Fixsen J, Grill F, Ippolito E, Romanus B, Tudisco C, Wientroub S. Congenital pseudarthrosis of the tibia: history, etiology, classification, and epidemiologic data. J Pediatr Orthop B. 2000;9(1):11–15. doi: 10.1097/01202412-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Andersen KS. Congenital angulation of the lower leg and congenital pseudarthrosis of the tibia in Denmark. Acta Orthop Scand. 1972;43:539–549. doi: 10.3109/17453677208991276. [DOI] [PubMed] [Google Scholar]
- 3.Boyd HB. Pathology and natural history of congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 1982;166:5–13. [PubMed] [Google Scholar]
- 4.Crawford AH, Schorry EK. Neurofibromatosis in children: the role of the orthopaedist. J Am Acad Orthop Surg. 1999;7(4):217–230. doi: 10.5435/00124635-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Wientroub S, Grill F. Congenital pseudarthrosis of the tibia: part 1. European Pediatric Orthopaedic Society multicenter study of congenital pseudoarthrosis. J Pediatr Orthop B. 2000;9(1):1–2. doi: 10.1097/01202412-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Lee DY, Cho TJ, Lee HR, Lee K, Moon HJ, Park MS, Yoo WJ, Chung CY, Choi IH. Disturbed osteoblastic differentiation of fibrous hamartoma cell from congenital pseudarthrosis of the tibia associated with neurofibromatosis type I. Clin Orthop Surg. 2011;3(3):230–237. doi: 10.4055/cios.2011.3.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Hoss J, Sullivan K, Cheng T, Yu NY, Bobyn JD, Peacock L, Mikulec K, Baldock P, Alexander IE, Schindeler A, Little DG. A murine model of neurofibromatosis type 1 tibial pseudarthrosis featuring proliferative fibrous tissue and osteoclast-like cells. J Bone Miner Res. 2012;27(1):68–78. doi: 10.1002/jbmr.528. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy RE. Amputation for congenital pseudarthrosis of the tibia. Indications and techniques. Clin Orthop Relat Res. 1982;166:58–61. [PubMed] [Google Scholar]
- 9.Morrissy RT, Riseborough EJ, Hall JE. Congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 1981;63(3):367–375. doi: 10.1302/0301-620X.63B3.6790551. [DOI] [PubMed] [Google Scholar]
- 10.Tudisco C, Bollini G, Dungl P, Fixen J, Grill F, Hefti F, Romanus B, Wientroub S. Functional results at the end of skeletal growth in 30 patients affected by congenital pseudoarthrosis of the tibia. J Pediatr Orthop B. 2000;9(2):94–102. doi: 10.1097/01202412-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Inan M, El Rassi G, Riddle EC, Kumar SJ. Residual deformities following successful initial bone union in congenital pseudoarthrosis of the tibia. J Pediatr Orthop. 2006;26(3):393–399. doi: 10.1097/01.bpo.0000217716.64986.f0. [DOI] [PubMed] [Google Scholar]
- 12.Grill F, Bollini G, Dungl P, Fixsen J, Hefti F, Ippolito E, Romanus B, Tudisco C, Wientroub S. Treatment approaches for congenital pseudarthrosis of tibia: results of the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop B. 2000;9(2):75–89. doi: 10.1097/01202412-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Romanus B, Bollini G, Dungl P, Fixsen J, Grill F, Hefti F, Ippolito E, Tudisco C, Wientroub S. Free vascular fibular transfer in congenital pseudoarthrosis of the tibia: results of the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop B. 2000;9(2):90–93. doi: 10.1097/01202412-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Keret D, Bollini G, Dungl P, Fixsen J, Grill F, Hefti F, Ippolito E, Romanus B, Tudisco C, Wientroub S. The fibula in congenital pseudoarthrosis of the tibia: the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop B. 2000;9(2):69–74. doi: 10.1097/01202412-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Ohnishi I, Sato W, Matsuyama J, Yajima H, Haga N, Kamegaya M, Minami A, Sato M, Yoshino S, Oki T, Nakamura K. Treatment of congenital pseudarthrosis of the tibia: a multicenter study in Japan. J Pediatr Orthop. 2005;25(2):219–224. doi: 10.1097/01.bpo.0000151054.54732.0b. [DOI] [PubMed] [Google Scholar]
- 16.Plint AC, Gaboury I, Owen J, Young NL. Activities scale for kids: an analysis of normals. J Pediatr Orthop. 2003;23(6):788–790. doi: 10.1097/01241398-200311000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Morris C, Doll HA, Wainwright A, Theologis T, Fitzpatrick R. The Oxford ankle foot questionnaire for children: scaling, reliability and validity. J Bone Joint Surg Br. 2008;90:1451–1456. doi: 10.1302/0301-620X.90B11.21000. [DOI] [PubMed] [Google Scholar]
- 18.Boyd HB. Pathology and natural history of congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 1982;166:5–13. [PubMed] [Google Scholar]
- 19.Anticevic D, Jelic M, Vukicevic S. Treatment of a congenital pseudarthrosis of the tibia by osteogenic protein-1 (bone morphogenetic protein-7): a case report. J Pediatr Orthop B. 2006;15(3):220–221. doi: 10.1097/01.bpb.0000194439.75378.ac. [DOI] [PubMed] [Google Scholar]
- 20.Spiro AS, Babin K, Lipovac S, Stenger P, Mladenov K, Rupprecht M, Rueger JM, Stuecker R. Combined treatment of congenital pseudarthrosis of the tibia, including recombinant human bone morphogenetic protein-2: a case series. J Bone Joint Surg Br. 2011;93(5):695–699. doi: 10.1302/0301-620X.93B5.25938. [DOI] [PubMed] [Google Scholar]
- 21.Richards BS, Oetgen ME, Johnston CE. The use of rhBMP-2 for the treatment of congenital pseudarthrosis of the tibia: a case series. J Bone Joint Surg Am. 2010;92(1):177–185. doi: 10.2106/JBJS.H.01667. [DOI] [PubMed] [Google Scholar]
- 22.Lee FY, Sinicropi SM, Lee FS, Vitale MG, Roye DP, Jr, Choi IH. Treatment of congenital pseudarthrosis of the tibia with recombinant human bone morphogenetic protein-7 (rhBMP-7). A report of five cases. J Bone Joint Surg Am. 2006;88(3):627–633. doi: 10.2106/JBJS.D.02201. [DOI] [PubMed] [Google Scholar]
- 23.Fabeck L, Ghafil D, Gerroudj M, Baillon R, Delincé P. Bone morphogenetic protein 7 in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 2006;88(1):116–118. doi: 10.1302/0301-620X.88B1.16619. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson JM, Scott BW, Clarke AM, Bell MJ. Surgical stabilisation of the lower limb in osteogenesis imperfecta using the Sheffield Telescopic Intramedullary Rod System. J Bone Joint Surg Br. 1998;80(6):999–1004. doi: 10.1302/0301-620X.80B6.8667. [DOI] [PubMed] [Google Scholar]
- 25.Mathieu L, Vialle R, Thevenin-Lemoine C, Mary P, Damsin JP. Association of Ilizarov’s technique and intramedullary rodding in the treatment of congenital pseudarthrosis of the tibia. J Child Orthop. 2008;2(6):449–455. doi: 10.1007/s11832-008-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korompilias AV, Lykissas MG, Soucacos PN, Kostas I, Beris AE. Vascularized free fibular bone graft in the management of congenital tibial pseudarthrosis. Microsurgery. 2009;29:346–352. doi: 10.1002/micr.20649. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto A, Yoshida T, Uchida Y, Kojima T, Kubota H, Iwamoto Y. Long-term follow-up on the use of vascularized fibular graft for the treatment of congenital pseudarthrosis of the tibia. J Orthop Surg Res. 2008;3:13. doi: 10.1186/1749-799X-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiland AJ, Weiss AP, Moore JR, Tolo VT. Vascularized fibular grafts in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Am. 1990;72:654–662. [PubMed] [Google Scholar]
- 29.Pho RW, Levack B, Satku K, Patradul A. Free vascularised fibular graft in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 1985;67(1):64–70. doi: 10.1302/0301-620X.67B1.3968148. [DOI] [PubMed] [Google Scholar]
- 30.Heikkinen ES, Poyhonen MH, Kinnunen PK, Seppänen UI. Congenital pseudarthrosis of the tibia. Treatment and outcome at skeletal maturity in 10 children. Acta Orthop Scand. 1999;70(3):275–282. doi: 10.3109/17453679908997807. [DOI] [PubMed] [Google Scholar]
- 31.Ghanem I, Damsin JP, Carlioz H. Ilizarov technique in the treatment of congenital pseudarthrosis of the tibia. J Pediatr Orthop. 1997;17(5):685–690. doi: 10.1097/01241398-199709000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Boero S, Catagni M, Donzelli O, Facchini R, Frediani PV. Congenital pseudarthrosis of the tibia associated with neurofibromatosis-1: treatment with Ilizarov’s device. J Pediatr Orthop. 1997;17(5):675–684. doi: 10.1097/01241398-199709000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Kristiansen LP, Steen H, Terjesen T. Residual challenges after healing of congenital pseudarthrosis in the tibia. Clin Orthop Relat Res. 2003;414:228–237. doi: 10.1097/01.blo.0000076800.53006.c9. [DOI] [PubMed] [Google Scholar]
- 34.Paley D, Catagni M, Argnani F, Prevot J, Bell D, Armstrong P. Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop Relat Res. 1992;280:81–93. [PubMed] [Google Scholar]
- 35.Thabet AM, Paley D, Kocaoglu M, Eralp L, Herzenberg JE, Ergin ON. Periosteal grafting for congenital pseudarthrosis of the tibia: a preliminary report. Clin Orthop Relat Res. 2008;466(12):2981–2994. doi: 10.1007/s11999-008-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joseph B, Somaraju VV, Shetty SK. Management of congenital pseudarthrosis of the tibia in children under 3 years of age: effect of early surgery on union of the pseudarthrosis and growth of the limb. J Pediatr Orthop. 2003;23(6):740–746. doi: 10.1097/01241398-200311000-00011. [DOI] [PubMed] [Google Scholar]


