Abstract
Background data
There has been an increased focus on the role of rib abnormalities in the development of scoliosis. Rib resection may influence the development of scoliosis. Although scoliosis has been identified in patients after thoracotomy, most of the currently available information is from case reports.
Methods
We examined records of 37 patients who underwent a chest wall or rib resection for rib lesions at our institution during the period of 1992 to 2005. Adequate data was available in 21 patients. We gathered data on demographic information, location of resection, and changes in curvature after resection based on radiograph or scout CT films at the latest follow-up appointment.
Results
Fourteen of 21 patients developed scoliosis with a mean Cobb angle of 25.8° (10°–70°). Eleven of these 14 patients had a progressive spinal deformity after chest wall resection with an average change in curvature of 29° (10°–70°). Eight of those 11 developed a convex toward the resection, while 3/11 developed a convex away from the resection. Seven of the eight patients with resections that included a rib superior to the sixth rib developed scoliosis, while four of 13 with resections below the sixth rib developed scoliosis.
Conclusion
Patients who have had a rib or chest wall resection are at risk for developing scoliosis, particularly if the resection is performed above the sixth rib.
Keywords: Chest wall resection, Rib resection, Chest wall lesion, Rib fusion, Thoracogenic scoliosis
Introduction
A variety of lesions may involve ribs or their surrounding chest wall structures [1]. While the most common malignant rib lesion in childhood is Ewing’s sarcoma [1–4], the types of lesions that may involve the chest wall include a number of benign and malignant entities. The mainstay of treatment is frequently local control with surgical resection [1].
Animal models [5–12] and clinical reports [5, 13–19] have suggested that rib resection and fusion may influence the development of scoliosis. Recently, there has been an increased focus on the role rib abnormalities have on the development of scoliosis, chest wall deformity, and lung function. This includes both our understanding of thoracogenic scoliosis and thoracic insufficiency syndrome as well as our ability to treat it with growth sparing procedures including expansion thoracostomy and vertical expandable prosthetic titanium rib (VEPTR) [17, 20–22]. Although scoliosis has been identified in patients after thoracotomy, most available information on the effects that chest wall surgery may have on the development of scoliosis in children is from case reports or small case series [5, 13–19].
We examine below our experience with a population of patients that have required rib or chest wall resection in order to investigate the potential long-term consequences of this treatment on the development of scoliosis.
Methods and materials
We performed a retrospective review of all patients <18 years of age who had a chest wall or rib resection for rib lesions evaluated at our tertiary referral children’s hospital from 1992 to 2005. Of the 46 patients who had a chest wall lesion, 37 patients had a chest wall or rib resection. Eleven patients were excluded for inadequate clinical or radiographic data, and one patient was excluded based on age criteria. Two patients died with <1 year of follow-up. One patient was excluded for <1 year follow up. One further patient was excluded because this patient had a large scoliosis prior to surgery, and had spinal fusion 6 months later. For the 21 eligible patients with complete imaging studies before and after surgery, we recorded patient demographic information, location and type of resection, degree of scoliosis pre-resection, and the change in curvature after resection based on radiograph (chest film or upright scoliosis film) or scout CT films at latest follow-up, understanding that the supine scout CT film may underestimate the scoliosis compared to upright films.
We then performed an analysis of factors associated with this progressive scoliosis after rib resection. Possible factors included age in years, gender, preexisting scoliosis, procedure superior to the sixth rib and follow-up time in years. Univariate logistic regression was used to assess association between each variable and the likelihood of progression. Factors that were significant or marginally significant in unadjusted analysis were considered in adjusted analysis using multivariate logistic regression. Both ordinary logistic regression and penalized likelihood logistic regression were used. The two methods yielded similar results, but given the properties of penalized logistic regression to reduce bias due to small numbers of events, we used the penalized logistic regression values. For significant predictors, penalized likelihood odds ratios with corresponding 95 % confidence intervals (CI) were estimated. P values <0.05 were considered significant.
Results
The mean age of the 21 patients was 12.3 (2–17) years. Median follow-up for the 21 patients in the current series was 3.5 years after resection (1.1–10.5 years). A flow chart (Fig. 1) demonstrates how patients were classified as having a progressive scoliosis or having no progression after their resection. Demographic, diagnosis, and treatment data are listed in Tables 1 and 2 for patients with progressive scoliosis or no progression of scoliosis, respectively.
Fig. 1.
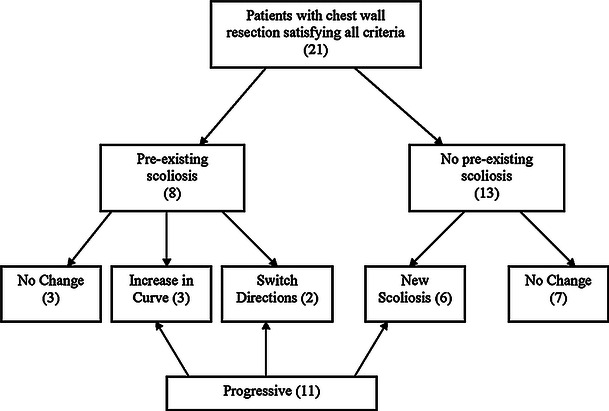
Flow chart of breakdown of patients curves after resection
Table 1.
Characteristics of 11 patients who demonstrated a progressive scoliosis after chest wall resection
| Age at resection | Gender | Procedure | Pre-surgery Cobb/Convex (L/R) | Post-surgery Cobb | Change in Cobb | Curve location (apex) | Diagnosis | Adjuvant therapy | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 | M | R rib 5: resection posterior rib, pleura, intercostal muscles with Gore-Tex reconstruction | 9 L | 14R | 23 | T3–T8 (T5) | Aneurysmal bone cyst | N | 1.6 |
| 2 | 8 | F | R ribs 6, 7, 8: resection rib, pleura, muscle, pleura, wedge resection of lung, Gore-Tex reconstruction | 0 | 19 L | 19 | T5–T10 (T7) | Ewing’s sarcoma | Chemo | 10.2 |
| 3 | 15 | F | Left rib 12: resection rib, muscle, peritoneum, diagphragm, Gore-Tex reconstruction | 14 R | 14 L | 28 | T9–L3 (T11/12) | Osteosarcoma | Chemo | 1.6 |
| 4 | 10 | F | R ribs 4, 5, 6: resection rib, sternum, muscle, pleura, Gore-Tex reconstruction | 0 | 19 L | 19 | T8–L1 (T10) | Chondrosarcoma | N | 7.5 |
| 5 | 12 | F | L rib 5: resection rib, muscle, pleura, Gore-Tex reconstruction | 16 L | 29 L | 13 | T3–T10 (T5/6) | Metastatic osteosarcoma | Chemo | 1.8 |
| 6 | 11 | M | L rib 3, 4, 5: resection rib, muscle, pleura, wedge resection lung, Gore-Tex reconstruction | 0 | 70 L | 70 | T2–T7 (T4) | Ewing’s sarcoma | Chemo | 10.5 |
| 7 | 15 | F | R rib 4, 5, 6: resection rib, muscle, pleura, Gore-Tex reconstruction | 0 | 10 R | 10 | T2–T6 (T4/5) | Osteosarcoma | Chemo | 3.5 |
| 8 | 15 | F | R rib 2, 3, 8, 9, resection rib, muscle, pleura, partial lung resection, RLL wedge resection | 10 R | 25 R | 15 | T2–T12 (T9) | Ewing’s sarcoma | Chemo | 9 |
| 9 | 6 | F | L rib 2, 3, 4: resection rib, muscle, pleura, Gore-Tex reconstruction | 0 | 45 L | 45 | T1–T10 (T3) | Synovial sarcoma | Chemo | 4 |
| 10 | 13 | F | R rib 8, 9, 10: resection rib, muscle, pleura, wedge resection lung, Gore-Tex reconstruction | 0 | 52 R | 52 | T3–L2 (T8/9) | Ewing’s sarcoma | Chemo + radiation | 2 |
| 11 | 11 | M | R rib 8, 9: resection rib, muscle, pleura, diagpragm, wedge resection lung | 15 L | 25 L | 10 | T5–L1 (T8) | Metastatic osteosarcoma | Chemo | 2 |
Table 2.
Characteristics of 10 patients who did not develop scoliosis after chest wall resection
| Age at resection | Gender | Site | Pre-surgery Cobb/Convex (L/R) | Post-Surgery Cobb | Change in Cobb | Curve location | Diagnosis | Adjuvant therapy | Follow-up (yr) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | M | Ribs 5–7: resection rib, pleura, muscle, Gore-Tex reconstruction | 11 L | 10 L | 1 | T4–T9 | Aneurysmal bone cyst | N | 3.8 |
| 2 | 15 | F | R anterior rib 5: rib resection | 15 R | 16 R | 1 | T4–T10 | Enchondroma | N | 1.1 |
| 3 | 17 | M | L ribs 8, 9, 10: resection rib, pleura, muscle, wedge resection of lung, Gore-Tex reconstruction | 0 | 0 | 0 | n/a | Ewing’s sarcoma | Chemo | 1.8 |
| 4 | 16 | F | L ribs 8, 9: rib resection | 0 | 0 | 0 | n/a | Slipping rib syndrome | N | 1.8 |
| 5 | 11 | F | L posterior ribs 9, 10, 11: resection rib, pleura, muscle, wedge resection lung | 0 | 0 | 0 | n/a | Metastatic synovial sarcoma | Chemo | 5.8 |
| 6 | 15 | F | R posterior rib 6: resection rib, pleura, muscle | 13 R | 15 R | 2 | T9–L1 | Fibrous dysplasia | N | 2 |
| 7 | 2 | F | L rib 10, resection rib | 0 | 0 | 0 | n/a | Vascular malformation | N | 2 |
| 8 | 16 | F | L rib 6, 7, 8: resection rib, pleura, muscle, wedge resection lung, Gore-Tex reconstruction | 0 | 0 | 0 | n/a | Ewing’s sarcoma | Chemo | 8.5 |
| 9 | 13 | F | L rib 7, 8, 9: resection rib, pleura, muscle, Gore-Tex reconstruction | 0 | 0 | 0 | n/a | Ewing’s sarcoma | Chemo | 8 |
| 10 | 12 | M | L rib 10, 11: resection rib, pleura, muscle, diaphragm, Gore-Tex reconstruction | 0 | 0 | 0 | n/a | Ewing’s sarcoma | Chemo | 7 |
Ten of the 11 patients with a progressive scoliosis had a malignant rib lesion. Nine of these patients received chemotherapy and one of these patients received additional radiation. Five of the 11 patients who did not have a progressive scoliosis had a malignant diagnosis, and all five of these patients received adjuvant chemotherapy.
Curvature of the spine was noted prior to resection in 8/21 patients with a mean Cobb angle of 13.2 (9 –16°). After resection with a median follow up of 1.9 (1.1–9) years, the preexisting scoliosis increased in three patients and switched directions in two patients. Three patients had no change in a preexisting scoliosis.
Six patients developed a new scoliosis after resection at median follow-up of 5.8 (2–10.5) years. Seven patients maintained a straight spine at a median follow-up of 5.8 (1.8–7) years. At last follow-up, the mean Cobb angle of the major curve in the 14 patients with scoliosis was 25.8° (10 –70°).
Of the 11 patients who had a change in the curvature (six new, three progressive, two switched directions) the average change in curvature was 29° (range 10 –70°). Eight of 11 patients developed a curve convex toward the resection; 3/11 developed a curve convex away from the resection. The average age in the 11 patients who had a change in curvature and ten who did not was 11.5 (6–15) and 13.2 (2–17) years, respectively.
In the progressive group, all had a true chest wall resection with violation of the chest cavity, and involved resection of muscle, rib, and pleura, with or without partial lung resection (Table 1). In the non-progressive group, 3/10 patients had a rib resection only with no violation of the pleura. Nine of 11 patients who had a progressive deformity had a Gore-Tex reconstruction, while 5/10 patients who did not develop deformity had a Gore-Tex reconstruction. In those with Gore-Tex reconstruction and progressive deformity, seven patients developed a curve apex towards the resection, and two patients developed a curve convex away from the resection.
In the group that had a change in curvature, 8/11 had a multiple ribs resection while 7/10 in the group that did not have a change in curvature had multiple rib resections. Seven of 11 patients in the progressive deformity group had a resection that included a rib superior to the sixth rib, while only 1/10 in the group who did not have a change in spinal alignment had a resection that included a rib superior to the sixth rib. In other words, 7/8 patients with a resection above the sixth rib developed progressive scoliosis while a progressive deformity occurred in 4/13 with resection below the sixth rib (Fig. 1). Logistic regression analysis determined that a procedure superior to the sixth rib was the only significant independent predictor of progression. Subjects with procedures superior to the sixth rib had ten times the odds of progression compared to those who did not (OR = 10.6; 95 %CI 1.6–125.2; p = 0.01) (Table 3). Figure 2a, b presents an example of a patient that had resection of the third to fifth ribs with a severely progressive scoliosis.
Table 3.
Summary characteristics by outcome
| Characteristic | Progressive (n = 11) | Non-progressive (n = 10) | P | All subjects |
|---|---|---|---|---|
| Gender (% male) | 3 (27 %) | 3 (30 %) | 0.89 | 6 (29 %) |
| Pre-existing scoliosis | 5 (46 %) | 3 (30 %) | 0.47 | 8 (38 %) |
| Superior sixth rib | 7 (64 %) | 1 (10 %) | 0.01 | 8 (38 %) |
| Age (years; mean ± SD) | 12.0 ± 2.9 | 15.8 ± 4.6 | 0.34 | 12.7 ± 3.8 |
| Follow-up time (years; mean ± SD) | 4.9 ± 3.7 | 4.2 ± 2.9 | 0.62 | 4.6 ± 3.3 |
P values are based on unadjusted univariate logistic regression models. Bold entries indicate significance at the 5 % level. Data are presented as frequency (%), unless stated otherwise
Fig. 2.
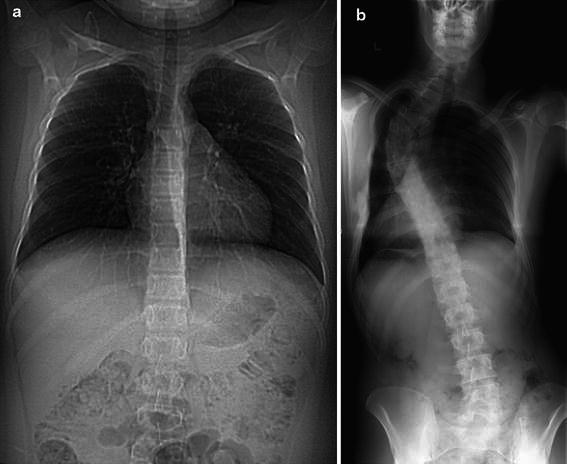
a Scout CT image of spine prior to chest wall resection. b Radiographic image of spine curvature post chest wall resection
Discussion
There are a number of animal models in which various rib-based procedures utilize rib and thoracic vertebrae resection to induce a secondary scoliosis [5–8, 12]. Intact ribs on the side opposite the resection exert more load against the spinal column, causing it to move laterally and rotate, as the rib cage has important biomechanical functions related to the spine [12, 23, 24] and is a proposed mechanism for developing scoliosis.
Elongation of ribs has been shown to induce scoliosis experimentally in rabbits [5, 10, 11]. Resection of ribs on the concave side has been shown to control progression of curves in chickens with scoliosis as a result of pineal gland resection when compared to control animals [24, 25]. From experimental models it appears that posterior rib resection, specifically including the costovertebral joints and the number of ribs resected is relatively consistent factors in the development of scoliosis [5–8, 12]. However, attempts to create a similar scoliosis model in upright primates have been less consistent and have produced mixed results [9, 26].
The location of excision may influence the severity of scoliosis and may have some clinical support. Ohara reported on pediatric patients where the ribs were resected anteriorly prior to the age of ten, which resulted with 64 % of patients with a rib deformity [14]. Thoracic scoliosis occurred in 4/16 patients although the Cobb angle was small and clinically insignificant, ranging from 12 to 14° [14]. In contrast, Piggot [27] reviewed 25 cases of posterior rib resections on the concave side in children with scoliosis and reported a reduced progression in curves after treatment and limited reversal in some cases. DeRosa [13] studied six pediatric patients that underwent a partial chest wall resection and concluded that posterior resections have a more significant impact on scoliosis than do anterior resections. Unfortunately, these results have not been consistent. Barnes, in a series of large curves with Cobb angles ranging from 41 to 140°, failed to demonstrate a difference between rib resection plus bracing vs bracing alone in progressive infantile scoliosis, suggesting that rib resection did not prove to be an efficient treatment strategy for scoliosis [28].
The risk of scoliosis after repair of esophageal atresia is 13-fold higher compared to the general population; however, many of these patients have vertebral anomalies that confound the cause of the scoliosis [29]. Scoliosis after treatment of esophageal atresia or tracheoesophageal fistula tends to be concave toward the side of the chest incision, often as a result of rib fusion [16, 18, 19, 29]. In one series, 8/82 patients developed scoliosis, and all eight also had complications related to their index procedure. [19] The long-term consequences of the secondary scoliosis may not be significant. This was demonstrated in a series of 100 patients treated in infancy through long-term follow-up, resulting in 56 % having scoliosis, but only 11 % with scoliotic Cobb of >20° and no patients requiring treatment for their scoliosis [29].
There are a number of reports of scoliosis that are secondary to multiple rib resections and/or chest wall resection [5, 15, 18, 19, 29–32]. This population historically includes patients with tuberculosis treated with thoracoplasty, but also contains patients treated for chest wall tumors [5]. The incidence of scoliosis in children who have undergone a chest wall resection is unclear from the available literature. Most studies focus on patients with both a chest wall resection and resulting scoliosis, but do not necessarily look at patients who had a chest wall resection but did not develop scoliosis [5]. In a series of 11 patients with scoliosis secondary to multiple rib resections, the patients had an average Cobb angle of 48° and nine of these patients were <18 years of age at the time of the rib resection [5]. In a series of 17 children who underwent a chest wall resection, only one patient developed a scoliosis of 40° [15]. Our current series suggests a higher risk of scoliosis after chest wall or rib resection; as 11/21 patients demonstrated a change in the curvature, and three required surgical intervention for their scoliosis.
Patients who undergo a chest wall or rib resection at a younger age may theoretically be at more risk for developing secondary scoliosis, when compared to adult populations, due to the greater growth potential of the spinal column and rib cage. Some series have suggested that chest wall resection done at an early age may increase the risk of scoliosis, but others have not confirmed this relationship [5, 13, 14]. Age appeared similar in this series for patients with progressive and non-progressive scoliosis after resection. Comparing this group of patients to an adult series with similar operations would likely be useful in studying age as a risk factor for scoliosis.
Patients who have chest wall resections may develop a different type of scoliosis than those who have had treatment of esophageal atresia, as seen in a series of 11 patients with scoliosis and chest wall resection where all developed curves convex toward the resection [5]. We found a mixed morphology of curves in the current study, with 8/11 patients developing curves convex toward and 3/11 developing curves convex away from the location of chest surgery, respectively. DeRosa [13] has suggested that any process that led to a pleural thickening such as recurrent tumor, irradiation, scarring or pulmonary metastases creates a convex curve toward the normal side and may respond to removal of this tether. Gore-Tex may have an influence on the development of scoliosis, but no conclusions can be drawn from the current data, which is additionally confounded by the fact that larger, more complicated resections are more likely to require a Gore-Tex reconstruction. In cases of rib resection without scarring, scoliosis that develops away from the location of the chest surgery is likely a result of altered biomechanics of the chest wall, although this has not been proven.
Previous literature has not addressed both the location of the rib resection and the risk of scoliosis and may be the most interesting finding of this current series. In studying this patient cohort, the risk of developing scoliosis is increased in patients who had a chest wall or rib resection involving ribs higher in the thoracic cage compared to those in whose ribs are resected lower in the thoracic cage. While this was found in the current study, there is no evidence in the literature to suggest why this may have been the case.
There are limitations to the current study. The number of patients is small, which is due to the rare instances in which rib or chest wall resections are required. The diversity of procedures, location and degree of resection performed in this population does confound the results. Also, some patients do not have follow-up to maturity, which is an important endpoint when considering their overall risk in developing a curve. Additionally, several patients in the group that did not show curve progression had a relatively short follow-up. Available imaging was a difficulty in our search for patients and as a result, several of our images were based on supine CT scout films or chest radiographs, which may underestimate the scoliotic deformity. By including patients with scoliosis prior to resection, it is impossible to distinguish between deformity progressions related to natural history of scoliosis in contrast to progression due to the chest wall resection. However, these patients were included, so the study represented the entire population that underwent a resection. Interestingly, some patients had a change in direction of their curvature. Also, in the non-progressive group, four patients had rib resection which did not violate the chest cavity, which may represent a procedure with a different inherent risk of scoliosis.
Reviewing pediatric patients who have had chest wall resection offers an opportunity to further study the relationship between chest wall abnormalities and the development of scoliosis. Based on the results of this study, we recommend that patients undergoing a chest wall resection, especially patients who have had resection of ribs from the upper part of their thoracic cage, be followed until skeletal maturity for the development of scoliosis.
Conflict of interest
None.
References
- 1.Kim S, Lee S, Arsenault DA, et al. Pediatric rib lesions: a 13 year experience. J Pediatr Surg. 2008;43:1781–1785. doi: 10.1016/j.jpedsurg.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 2.Kozlowski K, Campbell J, Morris L, et al. Primary rib tumours in children (report of 27 cases with short literature review) Australas Radiol. 1989;33:210–222. doi: 10.1111/j.1440-1673.1989.tb03277.x. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SE, Lorentzon R. The incidence of malignant primary bone tumours in relation to age, sex and site. A study of osteogenic sarcoma, chondrosarcoma and Ewing’s sarcoma diagnosed in Sweden from 1958 to 1968. J Bone Joint Surg Br. 1974;56B:534–540. [PubMed] [Google Scholar]
- 4.Shamberger RC, Tarbell NJ, Perez-Atayde AR, et al. Malignant small round cell tumor (Ewing’s-PNET) of the chest wall in children. J Pediatr Surg. 1994;29:179–184. doi: 10.1016/0022-3468(94)90314-X. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami N, Winter RB, Lonstein JE, et al. Scoliosis secondary to rib resection. J Spinal Disord. 1994;7:522–527. [PubMed] [Google Scholar]
- 6.Langenskiold, Michelsson JE. Experimental progressive scoliosis in the rabbit. J Bone Joint Surg Br. 1961;43:116–120. doi: 10.1302/0301-620X.43B1.116. [DOI] [PubMed] [Google Scholar]
- 7.Langenskiold A, Michelsson JE. The pathogenesis of experimental progressive scoliosis. Acta Orthop Scand Suppl. 1962;59:1–26. [PubMed] [Google Scholar]
- 8.Pal GP. Mechanism of production of scoliosis. A hypothesis. Spine. 1991;16:288–292. doi: 10.1097/00007632-199103000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Thomas S, Dave PK. Experimental scoliosis in monkeys. Acta Orthop Scand. 1985;56:43–46. doi: 10.3109/17453678508992978. [DOI] [PubMed] [Google Scholar]
- 10.Sevastik J, Agadir M, Sevastik B. Effects of rib elongation on the spine. I. distortion of the vertebral alignment in the rabbit. Spine. 1990;15:822–825. [PubMed] [Google Scholar]
- 11.Sevastik J, Agadir M, Sevastik B. Effects of rib elongation on the spine. II. correction of scoliosis in the rabbit. Spine. 1990;15:826–829. [PubMed] [Google Scholar]
- 12.Deguchi M, Kawakami N, Kanemura T, et al. Experimental scoliosis induced by rib resection in chickens. J Spinal Disord. 1995;8:179–185. doi: 10.1097/00002517-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 13.DeRosa GP. Progressive scoliosis following chest wall resection in children. Spine. 1985;10:618–622. doi: 10.1097/00007632-198509000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Ohara K, Nakamura K, Ohta E. Chest wall deformities and thoracic scoliosis after costal cartilage graft harvesting. Plast Reconstr Surg. 1997;99:1030–1036. doi: 10.1097/00006534-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Soyer T, Karnak I, Ciftci AO, et al. The results of surgical treatment of chest wall tumors in childhood. Pediatr Surg Int. 2006;22:135–139. doi: 10.1007/s00383-005-1537-z. [DOI] [PubMed] [Google Scholar]
- 16.Wong-Chung J, France J, Gillespie R. Scoliosis caused by rib fusion after thoracotomy for esophageal atresia. Report of a case and review of the literature. Spine. 1992;17:851–854. doi: 10.1097/00007632-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Tsirikos AI, McMaster MJ. Congenital anomalies of the ribs and chest wall associated with congenital deformities of the spine. J Bone Joint Surg Am. 2005;87:2523–2536. doi: 10.2106/JBJS.D.02654. [DOI] [PubMed] [Google Scholar]
- 18.Dunlay RP, Jones KB, Weinstein SL. Scoliosis caused by rib fusion following thoracotomy for tracheoesophageal fistula: case report. Iowa Orthop J. 2007;27:95–98. [PMC free article] [PubMed] [Google Scholar]
- 19.Gilsanz V, Boechat IM, Birnberg FA, et al. Scoliosis after thoracotomy for esophageal atresia. AJR Am J Roentgenol. 1983;141:457–460. doi: 10.2214/ajr.141.3.457. [DOI] [PubMed] [Google Scholar]
- 20.Mehta HP, Snyder BD, Baldassarri SR, et al. Expansion thoracoplasty improves respiratory function in a rabbit model of postnatal pulmonary hypoplasia: a pilot study. Spine. 2010;35:153–161. doi: 10.1097/BRS.0b013e3181c4b8c7. [DOI] [PubMed] [Google Scholar]
- 21.Campbell RM, Jr, Smith MD, Mayes TC, et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85:399–408. doi: 10.1302/0301-620X.85B3.13429. [DOI] [PubMed] [Google Scholar]
- 22.Campbell RM, Jr, Smith MD, Mayes TC, et al. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2004;86:1659–1674. doi: 10.2106/00004623-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Michelsson JE. The development of spinal deformity in experimental scoliosis. Acta Orthop Scand Suppl. 1965;81:1–91. doi: 10.3109/ort.1965.36.suppl-81.01. [DOI] [PubMed] [Google Scholar]
- 24.Deguchi M, Kawakami N, Kanemura T. Correction of scoliosis by rib resection in pinealectomized chickens. J Spinal Disord. 1996;9:207–213. [PubMed] [Google Scholar]
- 25.Machida M, Dubousset J, Imamura Y, et al. An experimental study in chickens for the pathogenesis of idiopathic scoliosis. Spine. 1993;18:1609–1615. doi: 10.1097/00007632-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Robin GC, Stein H. Experimental scoliosis in primates. Failure of a technique. J Bone Joint Surg Br. 1975;57:142–145. [PubMed] [Google Scholar]
- 27.Piggott H. Posterior rib resection in scoliosis. A preliminary report. J Bone Joint Surg Br. 1971;53:663–671. [PubMed] [Google Scholar]
- 28.Barnes J. Rib resection in infantile idiopathic scoliosis. J Bone Joint Surg Br. 1979;61:31–35. doi: 10.1302/0301-620X.61B1.422632. [DOI] [PubMed] [Google Scholar]
- 29.Sistonen SJ, Helenius I, Peltonen J, et al. Natural history of spinal anomalies and scoliosis associated with esophageal atresia. Pediatrics. 2009;124:e1198–e1204. doi: 10.1542/peds.2008-3704. [DOI] [PubMed] [Google Scholar]
- 30.Dwork RE, Dinken H, Hurst A. Post thoracoplasty scoliosis. Arch Phys Med Rehabil. 1951;32:722–729. [PubMed] [Google Scholar]
- 31.Loynes RD. Scoliosis after thoracoplasty. J Bone Joint Surg Br. 1972;54:484–498. [PubMed] [Google Scholar]
- 32.Stauffer ES, Mankin HJ. Scoliosis after thoracoplasty. A study of thirty patients. J Bone Joint Surg Am. 1966;48:339–348. [PubMed] [Google Scholar]


