Abstract
Purpose
Controversy exists regarding approach to treatment of pediatric patients with fibrous dysplasia.
Methods
We retrospectively reviewed medical records of seven patients who were treated at our institution for fibrous dysplasia by intramedullary rod fixation without bisphosphonate supplementation.
Results
Seven patients with a total of ten fibrous dysplasia lesion sites surgically treated by intramedullary rod fixation were included. Of these ten lesion sites, eight demonstrated pathologic fracture at the time of fixation. Complete fracture healing was observed in all eight sites, with no incidence of recurrent pathologic fractures examined radiographically. There were no major infections or neurologic deficits, and lesions appeared to stabilize.
Conclusions
In this series, intramedullary rod fixation proved to be successful in treatment of acute pathologic fracture and incompletely healed fibrous dysplasia lesions. We observed partial resolution of fibrous dysplasia lesions at all ten sites without significant long-term complications. Following treatment, there were no refractures.
Level of evidence
Level IV, case series.
Keywords: Fibrous dyslplasia, Intramedullary fixations, Biphosphonates, Pathologic fracture, Lesion
Introduction
Fibrous dysplasia of bone is a disease of abnormal bone architecture in which there is an overproduction of disorganized fibrotic bone matrix [1]. It may present as monostotic fibrous dysplasia evidenced by a single dysplastic skeletal lesion, or as polyostotic fibrous dysplasia substantiated by multiple dysplastic lesions throughout the skeleton [2, 3]. Fibrous dysplasia may also be a component of McCune-Albright syndrome, which is characterized by three pathologic manifestations: bone lesions which show osteitis fibrosa on histologic examination; brown non-elevated pigmented areas of the skin (cafe-au-lait spots); and endocrine abnormalities in females that are often associated with precocious puberty [4, 5].
The evolution of fibrous dysplasia is quite variable, and has the potential to cause significant morbidity. Harris et al. [6] remarked that when fibrous dysplasia develops early in life, the natural history is often marked with severe deformities and recurrent fractures [6]. Patients often present with bone pain secondary to fatigue fractures or pathologic fractures, structural deformities, or with a limp [7].
Several treatment methods have been proposed including non-operative management with pamidronate infusions, operative management with casting, curettage and bone grafting, fixation with screws and plates, and intramedullary rod fixation. There is increasing evidence that conservative management with casting and curettage and bone grafting yields unsatisfactory results in long-term treatment of fibrous dysplasia, especially in cases of more severe fractures of the lower extremities [8–13]. Internal fixation with intramedullary nails of pathologic fractures has revealed more promising results with decreased incidence of postoperative deformity of the affected extremity, as well as less risk of recurrent fracture [9–12, 14].
It is apparent from the literature that controversy exists regarding the approach to treatment of pediatric patients with fibrous dysplasia. This is especially true for those presenting with pathologic fracture. This paper is a retrospective cohort review of the successful orthopedic treatment of fibrous dysplasia by intramedullary rod fixation without bisphosphonate supplementation.
Materials and methods
Our Institutional Review Board approved the retrospective chart and radiograph review without patient recall. Seven patients with a total of ten fibrous dysplasia lesion sites surgically treated by intramedullary rod fixation were included in this series. Of these ten lesion sites, eight lesion sites demonstrated pathologic fracture at the time of fixation. Incidence of previous pathologic fractures and incomplete healing was observed at the remaining two lesion sites. Patients 3, 4, and 5 (Table 1) had multiple lesion sites.
Table 1.
Patient results
| Patient | Diagnosis | Age at operation | Fracture site(s) | Index procedure | Type of IM rod | Length of follow-up (years) | Post-op fracture status | Pain | ROM in adjacent joints | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MFD | 6 | L tibial diaphysis | Closed reduction and IM antegrade reamed nailing | 7.5 × 190 mm IM reconstruction nail w/locking screw | 6 | Complete healing | None | Full | Stress fracture at screw head occuring 6 years post-op; screw removed, local bone graft placed in screw and stress fractures sites. Good healing observed at 1 mo post-op |
| 2 | MFD | 7 | L humeral diaphysis | Closed reduction and IM retrograde nailing | Three (3) IM flexible titanium nails | 1 | Complete healing | None | Full | None |
| 3* | PFD | 9 | R tibial diaphysis | Closed reduction and IM antegrade reamed nailing | 9.5 × 230 mm IM reconstruction nail w/locking screw | 5.67 | Complete healing | None | Full | None |
| 11 | R subtrochanteric femur | Open reduction and IM retrograde reamed nailing w/osteotomy and allograft | 13 mm IM reconstruction nail w/locking screws | 4 | Complete healing | None | Full | None | ||
| 4* | PFD | 17 | L radial diaphysis | Closed reduction and IM retrograde nailing | Three (3) 2.0 IM flexible titanium nails | 10 | Complete healing | None | Full | None |
| 17 | L humerus lesion; Incomplete healing of previous fracture | Prophylactic IM retrograde nailing | Two (2) 3.0 mm and two (2) 2.0 mm IM flexible titanium nails | 10 | Complete healing | None | Full | None | ||
| 5* | PFD | 12 | L distal femur | Closed reduction and IM antegrade reamed nailing | 15 × 360 mm IM reconstruction nail w/locking screws | 7.8 | Complete healing | None | Full | Patient demonstrated antalgic gait w/a Trendelenburg limp 14 mo post-op. L leg measured 0.75in shorter than R leg 1.75 yr post-op, underwent epiphysiodesis of R knee. L leg 0.5in shorter than R, 4 years post-op |
| 13 | L tibial lesion; Incomplete healing of previous fracture | Prophylactic IM antegrade reamed nailing | 12 × 285 IM reconstruction nail | 7.25 | Complete healing | None | Full | None | ||
| 6 | PFD | 8 | L proximal femur | Closed reduction and IM retrograde nailing | One (1) 3.5 mm and two (2) 3.0 mm IM flexible titanium nails | 3.5 | Complete healing | None | Full | Knee pain and swelling, nails removed 10.5 months post-op; no pain reported 1.5 months post-removal |
| 7 | PFD | 10 | L distal femur | Closed reduction and IM retrograde nailing | Two (2) 4.0 mm flexible titanium IM nails | 7.75 | Complete healing | None | Full | None |
| Avg: | 11.00 | 6.30 |
MFD monostotic fibrous dysplasia, PFD polyostotic fibrous dysplasia, IM intramedullary
* Patient underwent two operative procedures
Patients were initially diagnosed clinically and radiographically, and confirmed histopathologically, with one of three types of fibrous dysplasia. Considered in this study are two patients with monostotic fibrous dysplasia and five with polyostotic fibrous dysplasia. The average age of each patient at the time of surgery was 11.0 years (range 7–17). All acute pathologic fractures involved the long bones; four involved the femur, two the tibia, one the humerus, and one the radius. Of the two fibrous dysplasia lesion sites demonstrating previous fracture and subsequent incomplete healing, one involved the tibia and one the humerus. Table 1 details the age of each patient at the time of presentation and operation, the index procedure, the type, size, and number of intramedullary rods used in each operation as well as the implantation of locking screws.
Closed reduction was performed in seven operations and open reduction was utilized in one operation in those cases of recent fracture.
The surgical procedure in a subtrochanteric femoral fracture (Patient 3, Table 1; Fig. 1) consisted of a femoral osteotomy to correct bowing of the femur, followed by reaming the medullary canal and retrograde placement of an interlocking reconstruction nail and locking screws. Crushed cancellous bone allograft was placed into the fracture site. One distal femoral fracture (Patient 5) was managed by reaming the medullary canal with subsequent antegrade placement of an interlocking reconstruction nail with proximal and distal locking screws. A second distal femur fracture (Patient 7) was treated with closed reduction and retrograde placement of two 4.0 mm flexible titanium nails. One proximal femoral fracture (Patient 6) was stabilized by closed reduction and retrograde intramedullary fixation with three flexible titanium nails. Pre-, intra-, and postoperative imaging can be seen in Figs. 2, 3, 4.
Fig. 1.
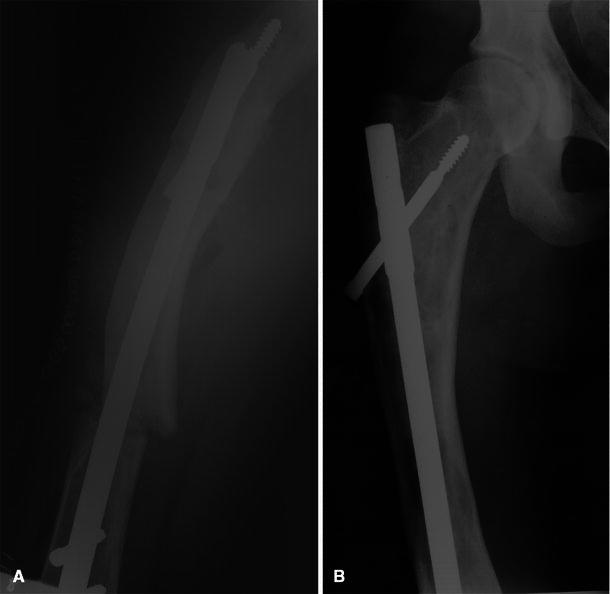
Patient 3: lateral postoperative X-ray (a) shows osteotomy with placement of intramedullary nail through lesion. Radiograph at 4-year follow-up (b) reveals complete healing and residual lesions along the lateral cortex
Fig. 2.
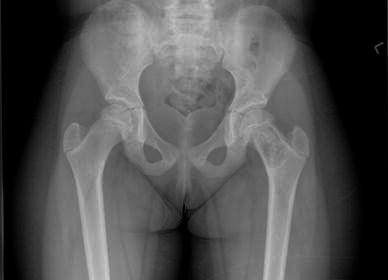
Patient 6: fibrous lesion involving the left femur viewed pre-operatively on plain radiograph
Fig. 3.
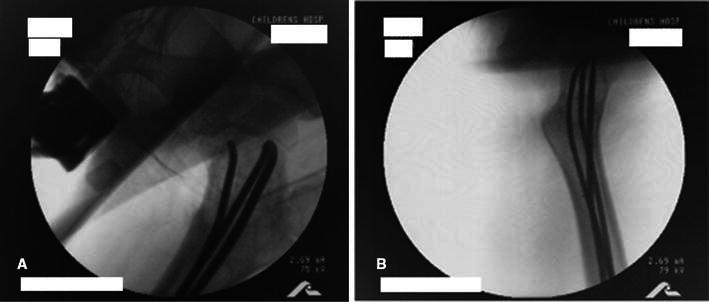
Patient 6: intraoperative placement of intramedullary rods (a, b) observed under fluoroscopy
Fig. 4.
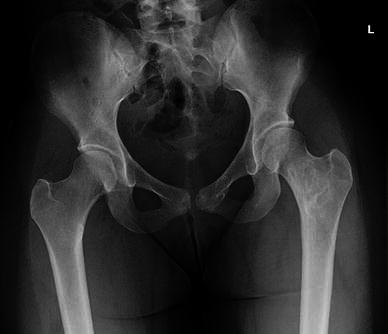
Patient 6: complete healing of fracture and stabilization of lesions at 9.3 years follow up (note interim hardware removal)
Two tibial fractures (Patients 1, 3) involved reaming of the medullary canal followed by antegrade placement of an intramedullary humeral nail with proximal locking screw. One additional fibrous dysplasia tibial lesion (Patient 4) with previous pathologic fracture and incomplete healing following conservative management with casting was treated prophylactically by reaming and antegrade insertion of an intramedullary tibial nail.
A humeral diaphysis fracture (Patient 2) was treated with intramedullary fixation by passage of three flexible titanium nails retrograde. One patient with a fibrous dysplasia lesion (Patient 4) involving the humeral diaphysis and proximal metaphysis suffered a proximal humeral pathologic fracture that failed conservative treatment with casting. The patient underwent intramedullary fixation by retrograde advancement of four flexible titanium nails through the pathologic diaphysis and proximal metaphysis. This was performed during the same operation in which a pathologic fracture of the ipsilateral radius was treated by retrograde intramedullary fixation with three flexible titanium nails.
In all cases, nail position was visualized by fluoroscopic guidance. Aggregates of bone and surrounding tissue were collected intra-operatively and the diagnosis of fibrous dysplasia was confirmed histopathologically. Patients were not treated with bisphosphonates prior to or during treatment.
Radiographic analysis entailed comparison of pre-operative lesion to postoperative lesion status at last follow-up. Lesions were evaluated by observation of radiolucency of bone, cystic appearance, endosteal scalloping, and cortical contour and thickness. Postoperative lesion status was classified into one of four possible outcomes: progression of lesion, no change, partial resolution, or complete resolution. Progression of lesion was defined by increased radiolucency and cystic appearance, irregular cortical contour and decreased cortical thickness with or without the development of endosteal scalloping. Partial resolution was characterized by decreased radiolucency and cystic components, thickened cortices, lessened irregular cortical contour, and an attenuation in the degree of endosteal scalloping if present pre-operatively. Lesions were deemed completely resolved if there were no discernible radiographic abnormalities, including no lytic or cystic areas, and with normal cortical borders and thickness.
Results
Following the intramedullary fixation, several parameters were assessed at follow-up including fracture healing and recurrence, progression or partial resolution of lesions at last follow-up, recurrence of lesions, pain, range of motion in adjacent joints of affected long bones, and complications. The last postoperative follow-up visit and assessment ranged from 3.5 to 10 years in eight of the nine cases. One recent case with 1 year follow-up was included because complete fracture healing and significant resolution of the lesion was already observed. The overall mean length of follow-up was 6.3 years.
Complete fracture healing was observed in all eight fracture cases with no incidence of recurrent pathologic fractures when examined radiographically. Cortices of the affected long bones were thickened in all cases, all patients had full range of motion in adjacent joints, and all patients remained pain-free at the last follow-up visit. In all cases, lesions were stabilized.
There were no major complications of infection or neurologic deficit. A few minor complications were encountered. One patient incurred a stress fracture of the proximal tibia at the site of a prominent locking screw 6 years postoperatively. The screw was removed and local bone graft was placed in the screw site and site of the stress fracture. One month later there was significant healing at the fracture site and no progression of any lesions. Another complication resulting from femoral fracture fixation involved soft tissue irritation at the nail insertion site on the distal femur, requiring removal of the flexible nails after the lesion had stabilized 10.5 months postoperatively. In this case, the pain was completely resolved 1.5 months following nail removal. Additionally, films visualizing the femur obtained without concurrent orthopedic clinical assessment confirm continued resolution 9.3 years postoperatively (excluded from analysis due to lack of corresponding clinical measures). In one case, a patient developed an antalgic gait with a Trendelenburg limp 14 months after intramedullary rod fixation of a left distal femoral fracture. The same patient had a 0.75 in. leg length discrepancy 1.75 years postoperatively which required surgical equalization by epiphysiodesis of the right knee. Subsequently, the limp resolved, and the affected leg was 0.5 in. shorter than contralateral leg 4 years postoperatively.
Discussion
Fibrous dysplasia is regarded as a non-inherited genetic disease [1, 15]. It is characterized by expanding fibrous lesions consisting of bone mesenchymal cells that produce a matrix of disorganized collagen fiber deposition and islands of woven bone [15]. As part of the McCune-Albright syndrome, the proposed pathogenic basis resides in somatic mutations of the Gs(α) gene early in embryogenesis resulting in mosaicism [15]. The mutation triggers increased activity of the α subunit of the G stimulatory protein (Gs), resulting in constitutive activation of adenylate cyclase and subsequent increased cyclic adenosine 3′,5′-monophosphate (cAMP) in various tissues, which may be responsible for the abnormalities seen in patients with McCune-Albright syndrome [15].
A more recent analysis of osteoblastic cells expressing the Gs(α) gene mutation showed a twofold to threefold increase in cellular proliferation compared to normal osteoblastic cells in the same patient [1]. The study also demonstrated increased proliferation of osteoblastic cells in more severe fibrotic bone lesions compared to less severe ones which may explain the overproduction of a disorganized fibrotic bone matrix seen in polyostotic fibrous dysplasia and monostotic fibrous dysplasia [1]. There is also evidence that increased activity of osteoclasts are responsible for the expanding fibrous lesions which suggests treatment with bisphosphonates may be beneficial by inhibiting osteoclastic resorption of bone [16].
Radiographic features in fibrous dysplasia were well summarized in a paper by DiCaprio and Enneking [7]. They described the lesions as radiolucent with a grayish “ground-glass” pattern, occasionally with cystic areas. Lesions often have a distinct rim of reactive bone on its boundaries, irregular cortical contour, and variations in cortical thickness that may be secondary to “endosteal scalloping”, referring to the slow resorption of the endosteal surface [7].
Curettage and bone grafting has long been a frequently employed modality of treatment for fibrous dysplasia. There is much controversy in the literature regarding the long-term success of this intervention. In a study by Guille et al. [13], 13 of 27 femoral lesions were treated by curettage and bone grafting. They propose that curettage further disrupts what little trabecular support remains in the bone as no lesions were eradicated or decreased in size and all bone grafts were eventually resorbed. Ippolito et al. [9] reported the persistence of dysplastic tissue and resorption of bone grafts of femoral lesions following curettage and bone grafting, and, thus, recommend avoiding this treatment modality in patients with polyostotic fibrous dysplasia and McCune-Albright syndrome. Stephenson et al. [8] concluded that curettage and bone grafting of lower extremity lesions associated with fibrous dysplasia yielded unsatisfactory results in 25 of 31 patients under the age of 18.
Pamidronate infusion therapy of fibrous dysplasia has shown varying results. Several studies show significant decrease in pathologic fracture rate and pain associated with the bone lesions [17–24]. However, when lesion status was assessed radiographically, the results varied widely. One study reported only two out of eight patients achieved a reduction in size of the bone lesion [18], and another study revealed that only 29 out of 58 patients exhibited a reduction in lesion size [21]. Results from a study by Plotkin et al. [17] of 18 children and adolescents with polyostotic fibrous dysplasia who received pamidronate therapy for at least 1 year (up to 9 years) disclosed no radiographic evidence of filling of lytic lesions or thickening of the bone cortex surrounding the lesions in any patient. Chan and Zacharin [25] actually reported expansion of lesions in three patients with McCune-Albright Syndrome treated with long-term pamidronate. Histomorphometric analysis of dysplastic bone tissue revealed no difference between those patients that received pamidronate versus those without any treatment [17]. Alendronate, an oral bisphosphonate used in osteoporosis and children with osteogenesis imperfecta, has been shown in recent case reports to have similar efficacy in treatment of fibrous dysplasia [26, 27].
Methods of internal fixation most commonly employed include plates and screws, and intramedullary nails. Stephenson et al. [8] described satisfactory outcomes in 18 of 21 patients under the age of 18 who had lower extremity lesions treated by internal fixation. Guille et al. [13] proposed internal fixation (with no distinct advantage of one type of internal fixation over another) with valgus osteotomy early in the course of the disease as the best treatment approach to patients with fibrous dysplasia of the proximal femur with varus deformity.
Several studies have reported good outcomes by treatment of fibrous dysplasia with intramedullary rod fixation [9–12, 14]. Ippolito et al. [9] advocate intramedullary rod fixation as the best treatment option for patients with polyostotic fibrous dysplasia or McCune-Albright syndrome, specifically with extensive femoral or other long limb bone involvement. They concluded it provides long-term stabilization of the bone as well as preventing recurrent fracture and deformity [9]. In a recent comprehensive review of fibrous dysplasia, DiCaprio and Enneking [7] concluded that when surgical correction is required to correct deformity or to prevent or stabilize a pathologic or fatigue fracture, cortical allograft or intramedullary fixation of the entire long bone are superior treatment options.
In the present study, intramedullary rod fixation proved to be very successful in treatment of both acute pathologic fracture and in stabilizing incompletely healed fibrous dysplasia lesions. Following treatment, there were no pathologic fractures, and lesions were stabilized. All patients had full clinical recovery without residual symptoms. It is important to note that this was obtained without the use of bisphosphonates as adjunct therapy. These results indicate intramedullary rod fixation following pathologic fracture provides definitive long-term treatment in children and adolescents with fibrous dysplasia. We advocate early surgical intervention in such patients.
Conflict of interest
None of the authors received financial support for this study.
References
- 1.Marie PJ, de Pollak C, Chanson P, et al. Increased proliferation of osteoblastic cells expressing the activating Gs alpha mutation in monostotic and polyostotic fibrous dysplasia. Am J Pathol. 1997;150:1059–1069. [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtenstein L. Polyostotic fibrous dysplasia. Arch Surg. 1938;36:874–879. doi: 10.1001/archsurg.1938.01190230153012. [DOI] [Google Scholar]
- 3.Lichtenstein L, Jaffe HL. Fibrous dysplasia of bone: a condition affecting one, several or many bones, the graver cases of which may present abnormal pigmentation of skin, premature sexual development, hyperthyroidism, and still other extraskeletal abnormalities. Arch Path. 1942;33:777–797. [Google Scholar]
- 4.Albright F, Butler AM, Hampton AO, et al. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females. N Engl J Med. 1937;216:727–746. doi: 10.1056/NEJM193704292161701. [DOI] [Google Scholar]
- 5.McCune DJ, Bruch H. Progress in pediatrics: osteodystrophia fibrosa. Am J Dis Child. 1937;54:806–848. doi: 10.1001/archpedi.1937.01980040110009. [DOI] [Google Scholar]
- 6.Harris WH, Dudley HR, Jr, Barry RJ. The natural history of fibrous dysplasia. An orthopaedic, pathological, and roentgenographic study. J Bone Joint Surg Am. 1962;44-A:207–233. [PubMed] [Google Scholar]
- 7.DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87:1848–1864. doi: 10.2106/JBJS.D.02942. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson RB, London MD, Hankin FM, et al. Fibrous dysplasia. An analysis of options for treatment. J Bone Joint Surg Am. 1987;69:400–409. [PubMed] [Google Scholar]
- 9.Ippolito E, Bray EW, Corsi A, et al. Natural history and treatment of fibrous dysplasia of bone: a multicenter clinicopathologic study promoted by the European Pediatric Orthopaedic Society. J Pediatr Orthop B. 2003;12:155–177. doi: 10.1097/01.bpb.0000064021.41829.94. [DOI] [PubMed] [Google Scholar]
- 10.Santori FS, Manili M, Falez F, et al. The prevention and treatment of skeletal deformities in fibrous dysplasia. Arch Putti Chir Organi Mov. 1990;38:388–393. [PubMed] [Google Scholar]
- 11.Ozcan O, Boya H, Baran O, et al. Treatment of femoral fractures associated with fibrous dysplasia: a case report. Acta Orthop Traumatol Turc. 2002;36:442–445. [PubMed] [Google Scholar]
- 12.Lejman T, Sulko J. Orthopedic management in children with fibrous dysplasia of bone. Chir Narzadow Ruchu Ortop Pol. 1999;64:303–310. [PubMed] [Google Scholar]
- 13.Guille JT, Kumar SJ, MacEwen GD. Fibrous dysplasia of the proximal part of the femur. Long-term results of curettage and bone-grafting and mechanical realignment. J Bone Joint Surg Am. 1998;80:648–658. doi: 10.2106/00004623-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Ippolito E, Caterini R, Farsetti P, et al. Surgical treatment of fibrous dysplasia of bone in McCune-Albright syndrome. J Pediatr Endocrinol Metab. 2002;15(Suppl 3):939–944. [PubMed] [Google Scholar]
- 15.Weinstein LS, Shenker A, Gejman PV, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 16.Schoenau E, Rauch F. Fibrous dysplasia. Horm Res. 2002;57(Suppl 2):79–82. doi: 10.1159/000058106. [DOI] [PubMed] [Google Scholar]
- 17.Plotkin H, Rauch F, Zeitlin L, et al. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003;88:4569–4575. doi: 10.1210/jc.2003-030050. [DOI] [PubMed] [Google Scholar]
- 18.Pfeilschifter J, Ziegler R. Effect of pamidronate on clinical symptoms and bone metabolism in fibrous dysplasia and McCune-Albright syndrome. Med Klin (Munich) 1998;93:352–359. doi: 10.1007/BF03044679. [DOI] [PubMed] [Google Scholar]
- 19.Lala R, Matarazzo P, Bertelloni S, et al. Pamidronate treatment of bone fibrous dysplasia in nine children with McCune-Albright syndrome. Acta Paediatr. 2000;89:188–193. doi: 10.1111/j.1651-2227.2000.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 20.Zacharin M, O’Sullivan M. Intravenous pamidronate treatment of polyostotic fibrous dysplasia associated with the McCune Albright syndrome. J Pediatr. 2000;137:403–409. doi: 10.1067/mpd.2000.107836. [DOI] [PubMed] [Google Scholar]
- 21.Chapurlat RD, Hugueny P, Delmas PD, et al. Treatment of fibrous dysplasia of bone with intravenous pamidronate: long-term effectiveness and evaluation of predictors of response to treatment. Bone. 2004;35:235–242. doi: 10.1016/j.bone.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Kos M, Luczak K, Godzinski J, et al. Treatment of monostotic fibrous dysplasia with pamidronate. J Craniomaxillofac Surg. 2004;32:10–15. doi: 10.1016/j.jcms.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 23.O’Sullivan M, Zacharin M. Intramedullary rodding and bisphosphonate treatment of polyostotic fibrous dysplasia associated with the McCune-Albright syndrome. J Pediatr Orthop. 2002;22:255–260. [PubMed] [Google Scholar]
- 24.Lala R, Matarazzo P, Andreo M, et al. Bisphosphonate treatment of bone fibrous dysplasia in McCune-Albright syndrome. J Pediatr Endocrinol Metab. 2006;19(Suppl 2):583–593. doi: 10.1515/jpem.2006.19.s2.583. [DOI] [PubMed] [Google Scholar]
- 25.Chan B, Zacharin M. Pamidronate treatment of polyostotic fibrous dysplasia: failure to prevent expansion of dysplastic lesions during childhood. J Pediatr Endocrinol Metab. 2006;19:75–80. doi: 10.1515/jpem.2006.19.1.75. [DOI] [PubMed] [Google Scholar]
- 26.Aragao AL, Silva IN. Oral alendronate treatment for severe polyostotic fibrous dysplasia due to McCune-Albright syndrome in a child: a case report. Int J Pediatr Endocrinol. 2010;2010:432060. doi: 10.1186/1687-9856-2010-432060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayaraman M, Karikumar K, Verma A, et al. Alendronate therapy in polyostotic fibrous dysplasia presenting with pathologic fracture. Am J Orthop (Belle Mead NJ) 2011;40:E48–E50. [PubMed] [Google Scholar]


