Abstract
Purpose
The objective of the present study was to conduct a systematic review and meta-analysis of published literature investigating the survivin expression and its effects on bladder cancer prognosis.
Materials and Methods
We carefully searched online Pubmed, Cochrane Library and SCOPUS database from August 1997 to May 2013.
Results
A total of 14 articles met the eligibility criteria for this systematic review. The eligible studies included a total of 2,165 patients with a median number of 155 patients per study (range: 17–726). Of the 14 studies, nine evaluated immunohistochemistry in formalin-fixed paraffin-embedded tissue blocks. In non-muscle invasive bladder tumor, the pooled hazard ratio (HR) was statistically significant for recurrence-free survival (pooled HR, 1.81; 95% confidence interval [CI], 1.30–2.52), progression-free survival (pooled HR, 2.12; 95% CI, 1.60–2.82), cancer-specific survival (pooled HR, 2.01; 95% CI, 1.32–3.06), and overall survival (pooled HR, 1.53; 95% CI, 1.02–2.29). The overall HRs by survivin status were robust across advanced stages. When only adjusted survival data were included, statistically significant differences were identified for all survival subgroup analyses. There was no between-study heterogeneity in the effect of survivin status on the majority of meta-analyses. There was no clear evidence of publication bias in this meta-analysis.
Conclusions
Survivin expression indicates worse prognosis in patients with bladder cancer but the results should be interpreted with caution. It is necessary that better-designed studies with standardized assays need to provide a better conclusion about the relationship between survivin expression and the outcome of patients with bladder cancer.
Introduction
Bladder cancer is the second most common cancer arising in the genitourinary tract [1], and is characterized by its variable prognosis. In about 70% of patients with non-muscle invasive bladder cancer, tumors recur and some of these patients will eventually show progression towards muscle invasive cancer. Tumors that are muscle invasive have a high risk of progression, despite radical cystectomy and other treatments. One of important focuses in bladder cancer research is the prediction of tumor recurrence and tumor progression. Conventional prognostic factors, like tumor stage and grade, do not accurately predict the clinical outcome of many patients with bladder cancer, because of the inherent heterogeneity of tumor biology and patient characteristics. Additional effective biomarkers are required for explaining variability of outcome in patients with bladder cancer.
The ability of molecular markers to predict recurrence and progression of the disease, response to treatment, and survival has been investigated intensively over the last decades. Although numerous potential bladder tumor markers have been identified, their significance remains controversial. Survivin has been described as the smallest, structurally unique member of the ‘inhibitor of apoptosis’ family [2]. As compared with normal differentiated adult tissues, survivin is frequently overexpressed in tumors [3]. Functionally, survivin displays regulatory functions for control of cell division and inhibition of apoptosis, induces angiogenesis, and plays a pivotal role in cancer progression [4]. Because of this upregulation in malignancy and its functional involvement in apoptosis, as well as proliferation, survivin is attracting considerable interest as a potential cancer biomarker [5]. Generally, high survivin mRNA or protein expression is correlated with aggressive behavior of tumor cells, and survivin expression has been established as a prognostic factor in several tumor types [6]–[8].
Thus, in urothelial carcinoma of the urinary bladder, survivin has been suggested as a promising biomarker for cancer prognosis. Survivin expression has been reported to be indicator of poor prognosis in bladder cancer, whereas some other studies did not show the same results [9]–[11]. Because reports about its prognostic significance in bladder cancer are comparatively few, the combination of these data to reach a reasonable conclusion is fairly necessary at present. The objective of the present study was to conduct a systematic review and meta-analysis of published literature investigating the survivin expression and its effects on bladder cancer prognosis. We also aimed to assess the quality of published studies.
Materials and Methods
Search Strategy and Selection Criteria
We carefully searched online Pubmed, Cochrane Library and SCOPUS database. Since the first survivin article was published in 1997, we searched literatures published from August 1997 to May 2013, to identify relevant studies by combining the keywords [survivin] AND [urinary bladder neoplasms] OR [urinary AND bladder AND neoplasms] OR [bladder AND cancer] OR [bladder cancer]. To be eligible for our meta-analysis, studies had to be English-language published documents dealing with histopathologically confirmed bladder cancer at the time of study inclusion.
The inclusion criteria for our systematic review were, as follows: (i) articles were published in English in the periodical literature; (ii) the histologic type of the tumors was urothelial carcinoma; (iii) expression of the survivin was evaluated in tissues or urines; (iv) the association between survivin expression levels and survival outcome was investigated; and (v) the authors offered the size of the sample, hazard ratios (HRs) and their 95% confidence intervals (CIs) or other information that could help infer the survival results in the paper. When multiple articles were published by the same authors or group, the most recently published or most informative single article was selected to avoid duplication of the patient data. Duplicate reports were included in the specific analyses only if they performed different subgroup analyses. No attempt was made to restrict the search according to more specific methodological characteristics. Accordingly, the following exclusion criteria were used: (i) review articles or letters to the editor; (ii) laboratory studies, such as studies on bladder cancer cell lines and animal models; and (iii) studies which did not provide sufficient data to acquire HR and its standard error.
To minimize the bias and to improve reliability, two independent reviewers (C.W.J and J.H.K.) assessed the eligibility of abstracts identified by the search. If studies seemed appropriate, the full manuscript was scrutinized and the study was deemed “relevant” if it met the inclusion criteria. If the eligibility was unclear from the abstract, the full article was retrieved for clarification. The full text publication was independently screened by two of the authors (C.W.J and J.H.K.). Disagreements between reviewers were resolved by consensus.
Data Extraction and Quality Assessments
The extracted data elements of this review included the following: (i) publication details: country, first author’s last name, publication year, period of recruitment, and study design; (ii) characteristics of the studied population: sample size, mean or median age, gender, inclusion and exclusion criteria, tumor characteristics, treatment, endpoint definition, and follow-up period; (iii) cut-off value of positive expression and the antibodies used for immunohistochemistry (IHC), as well as biologic samples and the type of measurements used to determine survivin status; and (iv) survival curves, the exact data of total and exposed number in case and control groups, as well as HRs and their CIs.
Study quality was assessed independently by two investigators (C.W.J and J.H.K.). Any disagreement was resolved by discussion. Although no standard quality assessment method is currently available, an assessment of study methodology was made according to previously defined criteria. We systematically assessed the quality of all included studies using the predefined form by De Graeff et al [12], which was adapted from Hayes et al [13] and McShane et al [14]. Briefly, the following criteria were investigated: (i) the study reported inclusion and exclusion criteria; (ii) study data were prospectively or retrospectively gathered; (iii) clinical and pathological characteristics of the patients were sufficiently described; (iv) the assay used was sufficiently described; (v) a definition of the study endpoint was provided; (vi) the follow-up time was described; and (vii) the study reported how many patients were lost to follow-up or were not available for statistical analysis.
Statistical Analysis
Primary analysis
The recommended summary statistics for meta-analysis of time-to-event data are the logHR and its variance, which account for both the time it takes for an event to occur, as well as censoring. For each trial, this HR was estimated by a method depending on the data provided in the publications. The simplest method consisted in the direct collection of HR and their 95% CI from the original article. If those data were not available, previously reported indirect methods were utilized for extracting the logHR and variance, due to the paucity of prognostic literature directly reporting these values [15]–[17]. A random-effect model was used to obtain the summary HRs and 95% CIs. An observed HR >1 indicated worse outcome for the study group relative to the reference group, and would be considered statistically significant if the 95% CI did not overlap, with p<0.05.
Subgroup analysis
Subsequently, we assessed the effect of unadjusted HR on the survivin results in patients with non-muscle invasive bladder tumor. First, attempt was made to use only adjusted survival data as part of this meta-analysis. Studies that did not report an adjusted HR for survival after controlling for potential confounding clinical variables in a multivariable analysis (e.g. Cox regression analysis including important clinical factors, such as age, grade, and/or performance status) were excluded, since the accuracy of HRs estimated from Kaplan-Meier survival curves without a multivariate analysis was uncertain [18]–[20]. These data were applied in a subgroup, and meta-analyses were performed to test the stability of our conclusions.
Sensitivity analysis
We performed sensitivity analyses in patients with non-muscle invasive bladder tumor. Through sensitivity analyses, we examined if our pooled estimate of the prognostic value of survivin status was largely influenced by the method for determination of survivin expression. Studies using immunohistochemical (IHC) expression were included in sensitivity analyses.
Assessment of heterogeneity
Heterogeneity was assessed using the chi-square test for heterogeneity, with a p value of <0.05 taken to reflect the presence of significant heterogeneity [21]. The I2 statistic was calculated to quantify the degree of heterogeneity [22].I2 describes the proportion of total variation in meta-analysis estimates, which is due to inter-study heterogeneity, rather than sampling error, and is measured from 0% to 100%, with increasing I2 values indicating a larger effect of between-study heterogeneity in the meta-analysis.
Publication bias
For those meta-analyses including 10 or more studies, we assessed the possibility of publication bias. Publication bias was evaluated using the funnel plot. In the absence of bias, the graph should resemble a symmetrical inverted funnel; conversely, in the presence of bias, the plot should appear skewed and asymmetrical.
The meta-analysis was undertaken using Review Manager (RevMan) software version 5.0 (RevMan 5; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark).
Results
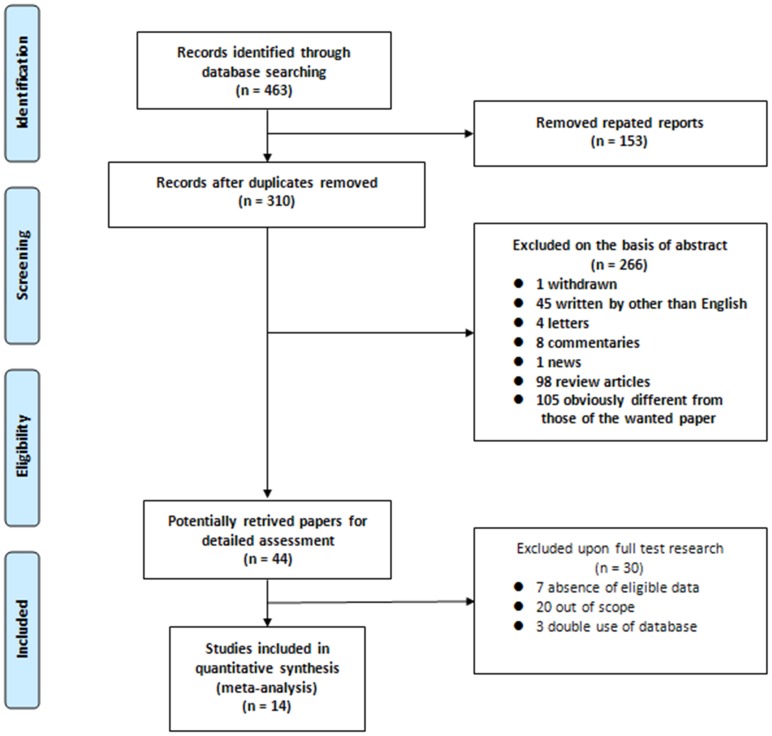
Our search strategy identified 463 articles. Following deduplication, two reviewers independently screened the identified titles and abstracts. They subsequently agreed that 44 articles should be retrieved for detailed review; for these manuscripts, full texts were obtained. On careful review of study methodologies, 31 were excluded for the following reasons: 20 studies had no formal investigation of outcomes [23]–[42]. Instead, these studies assessed only the predictive ability and included the detection validity in the diagnosis of bladder cancer or based their results on association tests; seven studies provided incomplete information for HRs and 95% CIs [43]–[49]; and three studies were excluded because it contained duplicate data [9], [50], [51]. Thus, a total of 14 articles met the eligibility criteria for this systematic review [10], [11], [52]–[63]. A flow diagram of the study selection process is presented in Fig. 1.
Figure 1. Methodological flow chart of the systematic review.
Table 1 outlines the main characteristics of the included studies. Considering the selected studies, one was carried out in the United States, nine in Europe, three in Asia, and one was multinational. None of selected studies was prospective study. Patient tissues were the mostly common samples used to detect survivin, but in two studies [54], [57] the authors used urine specimens to assess survivin mRNA. Four (44.4%) of nine evaluated IHC staining in formalin-fixed paraffin-embedded tissue blocks did not define the primary antibody used [55], [56], [61], [62]. A wide range of dilutions was used (1/50 to 1/1,600). The definition of survivin overexpression also varied among studies. The cutoff value used to define survivin overexpression was 10% in most studies, whereas in the remaining two studies, the cut-off value was 8% and 20%, respectively [55], [59]. Immunopositive cells were defined according to the percentage of nuclear [55], [58], [60], cytoplasmic [53] or both [56], [59], [62], [63] staining. Four studies documented whether staining assessment was blinded to outcome status [53], [58], [60], [61]. The median quality score was recorded as 5 (range: 3–6). There was no significant correlation between study size and quality scores (Spearman’s r = 0.472, p = 0.210).
Table 1. Main characteristics of the eligible studies.
| Study | Year | Country | Recruitment period | Study design | Inclusion and exclusion criteria | Consecutive Patients | Specimen | Method | Compartment | Cut-off | Definitionof survival | Blindassessment | Quality Assessment(0–8) |
| Gazzaniga10 | 2003 | Italy | 1996–1998 | retrospective | no | NA | tissue | RT-PCR | − | − | yes | NA | 5 |
| Schultz52 | 2003 | Netherlands | NA | retrospective | no | NA | tissue | real-time RT-PCR | − | 0.26* | yes | NA | 5 |
| Ku53 | 2004 | Korea | 1993–1997 | retrospective | no | no | tissue | IHC | cytoplasm | 20% | yes | blind | 5 |
| Schultz54 | 2004 | Netherlands | NA | retrospective | no | NA | urine | real-time RT-PCR | − | 0.13* | yes | NA | 3 |
| Yin55 | 2006 | China | NA | retrospective | no | yes | tissue | IHC | nuclear | 8% | no | NA | 4 |
| Karam56 | 2007 | USA | 1995–2003 | retrospective | no | NA | tissue | IHC | nuclear or cytoplasm | 10% | yes | NA | 3 |
| Pina-Cabral57 | 2007 | Portugal | NA | retrospective | no | NA | urine | RT-PCR | − | − | yes | NA | 3 |
| Skagias58 | 2009 | Greece | 1998–2005 | retrospective | no | NA | tissue | IHC | nuclear | 10% | yes | blind | 5 |
| Weiss59 | 2009 | Germany | 1982–2004 | retrospective | no | no | tissue | IHC | nuclear or cytoplasm | 20% | yes | NA | 5 |
| Gradilone11 | 2010 | Italy | NA | retrospective | yes | NA | tissue | RT-PCR | − | − | no | NA | 4 |
| Fristrup(Denmark)60 | 2012 | Denmark | 1979–2007 | retrospective | no | NA | tissue | IHC | nuclear | 10% | yes | blind | 5 |
| Fristrup(validation1)60 | 2012 | Sweden | 1984–2005 | retrospective | no | NA | tissue | IHC | nuclear | 10% | yes | blind | 5 |
| Fristrup(validation2)60 | 2012 | Spain | 1994–2008 | retrospective | no | NA | tissue | IHC | nuclear | 10% | yes | blind | 5 |
| Xi61 | 2013 | China | 2000–2006 | retrospective | yes | no | tissue | IHC | NA | 10% | yes | blind | 6 |
| Shariat62 | 2009 | Multination | 1983–2005 | retrospective | yes | no | tissue | IHC | nuclear or cytoplasm | 10% | yes | NA | 4 |
| Als63 | 2007 | Denmark | 1995–2004 | retrospective | yes | NA | tissue | IHC,microarray | cytoplasm with an intensity of 2 or 3 | 10% | no | NA | 6 |
survivin mRNA copy number/cyclophilin mRNA copy number.
NA: not available, RT-PCR: reverse transcriptase-polymerase chain reaction, IHC: immunohistochemistry.
The 14 eligible studies included a total of 2,165 patients, with a median number of 155 patients per study (range: 17–726). Basic sociodemographic information, such as sex and age, was missing from 28.6% and 28.6% of studies, respectively. Other characteristics such as the patient and tumor characteristics are summarized in the Table S1 and S2 (in File S1). Of the 1,755 patients available in the present study, survivin overexpression was detected in 846 (48.2%). There were higher frequencies of survivin overexpression with tumor grade were higher. However, no relationship was found between survivin expression and T stage (Table S3 in File S1).
Table 2 summarizes the methods for estimation of HR. nine (64.3%) studies reported the cofactors used in the multivariate models, which varied widely, even for a given endpoint. Twenty-three clinicopathologic factors were incorporated in one or more of the included studies’ multivariate analyses. The most common cofactors in the studies that used multivariate analysis to assess the risk of mortality were grade (n = 6) and pT stage (n = 6).
Table 2. Estimation of the hazard ratio.
| Study | Survival analysis | HR estimation | Co-factors | Analysis results |
| Gazzaniga10 | recurrence-free | p value, event number (univariate) | − | not significant |
| Schultz52 | recurrence-free | absence of eligible data | − | significant |
| progression-free | p value, event number (univariate) | − | not significant | |
| cancer-specific | absence of eligible data | − | not significant | |
| Ku53 | recurrence-free | HR, 95% CI (multivariate) | age, sex, size, number, architecture, grade, T stage | significant |
| Schultz54 | recurrence-free | p value, event number (univariate) | − | significant |
| Yin55 | progression-free | HR, 95% CI (multivariate) | age, grade, T stage, grade and stage, ki67, BIRC5-C | significant |
| cancer-specific | HR, 95% CI (multivariate) | age, grade, T stage, grade and stage, ki67, BIRC5-C | significant | |
| Karam56 | recurrence-free | HR, 95% CI (multivariate) | grade, T stage, intravesical therapy | significant |
| progression-free | HR, 95% CI (multivariate) | grade, T stage, intravesical therapy | significant | |
| cancer-specific | HR, 95% CI (multivariate) | grade, T stage, intravesical therapy | not significant | |
| Pina-Cabral57 | recurrence-free | p value, event number (univariate) | − | significant |
| Skagias58 | recurrence-free | HR, 95% CI (multivariate) | grade, T stage | Significant |
| overall | HR, 95% CI (multivariate) | grade, T stage | not significant | |
| Weiss59 | recurrence-free | p value, event number (univariate) | − | significant |
| progression-free | p value, event number (univariate) | − | not significant | |
| cancer-specific | p value, event number (univariate) | − | not significant | |
| Gradilone11 | recurrence-free | HR, 95% CI (multivariate) | circulating tumor cell | not significant |
| Fristrup (Denmark)60 | progression-free | HR, 95% CI (multivariate) | cathepsin E, maspin, PIK1 | significant |
| cancer-specific | HR, 95% CI (multivariate) | cathepsin E, maspin, PIK1 | significant | |
| overall | HR, 95% CI (multivariate) | cathepsin E, maspin, PIK1 | significant | |
| Fristrup (validation)60 | progression-free | HR, 95% CI (multivariate) | cathepsin E, maspin, PIK1 | significant |
| Xi61 | progression-free | HR, 95% CI (multivariate) | grade, T stage, livin | significant |
| Shariat62 | recurrence-free | HR, 95% CI (multivariate) | Age, sex, grade, pT stage, N stage, surgical margin, LVI,concomitant CIC, ACH | significant |
| cancer-specific | HR, 95% CI (multivariate) | Age, sex, grade, pT stage, N stage, surgical margin, LVI,concomitant CIC, ACH | significant | |
| Als63 | overall | HR, 95% CI (multivariate) | visceral metastasis, emmprin | significant |
HR: hazard ratio, CI: confidence interval, BIRC5-C: cytoplasmic staining of survivin, LVI: lymphovascular invasion, CIS: carcinoma in situ, ACH: adjuvant chemotherapy.
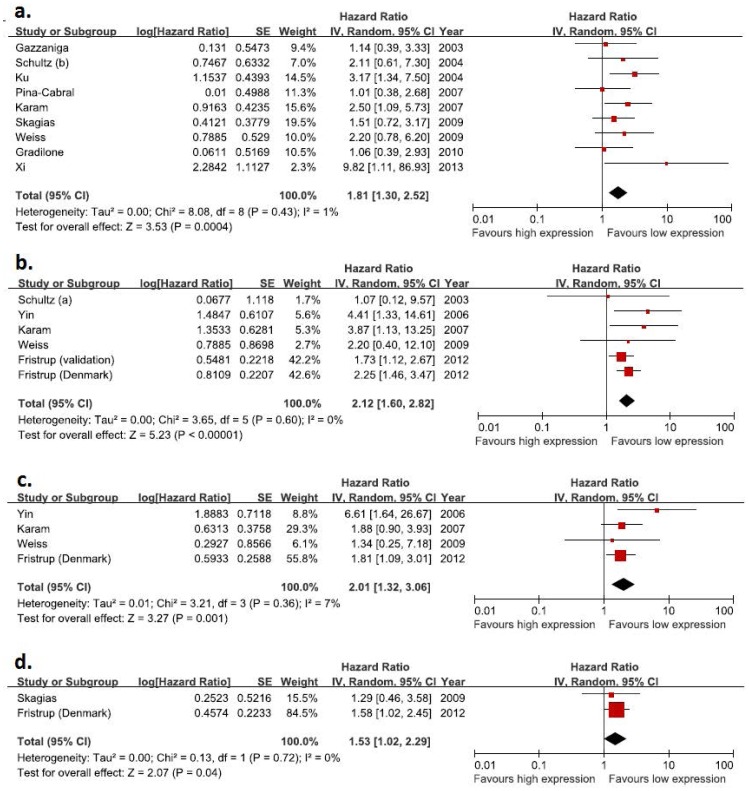
Forrest plots of the primary meta-analyses can be seen in Fig. 2. Fig. 2 reports the average (pooled) HR and its 95% CI for each of the meta-analysis in muscle invasive bladder tumor. Each figure represents HR of survivin for recurrence-free survival (Fig. 2a), progression-free survival (Fig. 2b), and cancer-specific survival (Fig. 2c) and overall survival (Fig. 2d). The pooled HRs were statistically significant for recurrence-free survival (pooled HR, 1.81; 95% CI, 1.30–2.52), progression-free survival (pooled HR, 2.12; 95% CI, 1.60–2.82), cancer-specific survival (pooled HR, 2.01; 95% CI, 1.32–3.06), and overall survival (pooled HR, 1.53; 95% CI, 1.02–2.29).
Figure 2. Forest plots of hazard ratios with random effects model for survivin in patients with non-muscle invasive bladder tumor.
(A) Recurrence-free survival. (B) Progression-free survival. (C) Cancer-specific survival. (D) Overall survival.
In muscle invasive and advanced bladder tumors, the HRs were also statistically significant for recurrence-free survival (HR, 1.46; 95% CI, 1.18–1.82), cancer-specific survival (HR, 1.54; 95% CI, 1.21–1.96), and overall survival (HR, 2.46; 95% CI, 1.63–3.71). The results are shown in Fig. S1 and S2.
Only adjusted survival data were sufficient articles available to compare survival analyses according to survivin expression (Table 3), although this subgroup analysis only includes 2 studies with overall survival data available. Statistically significant differences were identified for all survival subgroup analyses. Survivin overexpression was significantly associated with adverse survival in the pooled patient group. In addition, sensitivity analyses confirm that our estimate of the overall HR of recurrence-free survival, progression-free survival, cancer-specific survival and overall survival by survivin status is robust when IHC was chosen for the method for determination of survivin expression (Table 4).
Table 3. Subgroup analysis in non-muscle invasive bladder tumor.
| No. of included articles | No. of cases | Pooled HR (95% CI) | I2 | Chi2 (p value) | |
| Recurrence-free survival | 5* | 368 | 2.09 (1.27–3.45) | 27% | 5.45 (0.24) |
| Progression-free survival | 4** | 868 | 2.17 (1.59–2.97) | 8% | 3.27 (0.35) |
| Cancer-specific survival | 3† | 458 | 2.17 (1.26–3.73) | 33% | 2.99 (0.22) |
| Overall survival | 2‡ | 363 | 1.53 (1.02–2.29) | 0% | 0.13 (0.72) |
Table 4. Sensitivity analysis in non-muscle invasive bladder tumor.
| No. of included articles | No. of cases | Pooled HR (95% CI) | I2 | Chi2 (p value) | |
| Recurrence-free survival | 5* | 362 | 2.32 (1.53–3.52) | 0% | 3.52 (0.48) |
| Progression-free survival | 5** | 916 | 2.15 (1.62–2.86) | 0% | 3.27 (0.51) |
| Cancer-specific survival | 4† | 506 | 2.01 (1.32–3.06) | 7% | 3.21 (0.36) |
| Overall survival | 2‡ | 363 | 1.53 (1.02–2.29) | 0% | 0.13 (0.72) |
Despite our attempts to limit between-study heterogeneity through our strict inclusion criteria, heterogeneity between overall survival results still remains within each subgroup and results should be interpreted cautiously.
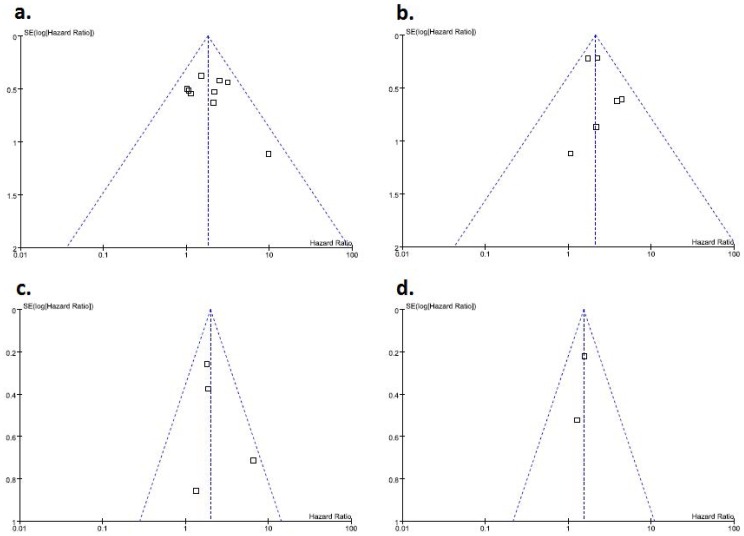
There was no clear evidence of funnel plot asymmetry for outcomes, and thus, there was no clear evidence of publication bias (Fig. 3). However, due to the small number of studies in most meta-analyses, it was not sensible to examine the potential for publication bias in meta-analysis, which did not contain 10 studies.
Figure 3. Funnel graphs of the assessment of potential publication bias in studies of survivin expression in patients with non-muscle invasive bladder tumor.
(A) Recurrence-free survival. (B) Progression-free survival. (C) Cancer-specific survival. (D) Overall survival.
Discussion
Currently, expression of survivin is being used as a novel prognostic factor in several human neoplasms. The rationale for investigating survivin as a prognostic marker in bladder cancer is based on its ability to inhibit apoptosis, promote proliferation and enhance angiogenesis, as well as its predominantly tumor-specific expression in adult tissues. In spite of suggested pivotal role of survivin as a prognostic marker, there are relatively few studies available exploring the role of survivin in bladder cancer, and some of them are controversial. In addition, the power of most individual studies was limited, due to low sample size. To date, no meta-analysis had been undertaken for any studies evaluating survivin as a prognostic marker in bladder cancer.
In this meta-analysis, which enrolled all the eligible studies comparing the survival of bladder cancer patients according to the tumor expression of survivin, survivin is a prognostic factor in bladder cancer. Our results showed that survivin overexpression is strongly predictive of recurrence, progression and mortality in bladder cancer.
Generally, meta-analysis based on individual data is considered as a gold standard [64]. However, meta-analysis of prognostic literature is associated with a number of inherent limitations. One of these key limitations is the general prevalence of retrospective study design in this setting. None of the studies included in the current meta-analysis specified a prospective design. It is difficult to draw any precise conclusions when studies are not conducted prospectively and when not all relevant data are available. Alongside this, an additional hindrance to meta-analysis of prognostic literature is the general lack of multivariable survival data in many of studies, although the REMARK guidelines state the investigation must include established clinicopathologic prognostic factors as part of a multivariate model, and report the resulting HRs regardless of statistical significance [14]. If the authors did not report the individual HR together with its variance, we calculated it from the survival comparison statistics and its variance, whenever possible. The estimated HR might be less reliable than the one obtained directly from published statistics. This is also attributable to the fact that the number of patients included in each study is typically small. However, when analyzing the overall relationship between individual study size and methological quality scores in the present study, there was no significant trend towards superior methodological quality in larger studies.
Although the specimens and methods used for the assessment of survivin expression in patients with bladder cancer differed among these studies, many of the eligible studies used IHC to detect survivin expression. IHC results should be interpreted with caution, because of varying specificity of the antibodies used, different concentration of the antibody used, lack of standardized technology, different approaches for storing and processing tissue, and the absence of a uniform definition of positive staining, leading to different results when using different cutoff points [65]. When defining survivin overexpression, the threshold in IHC varied from 8% to 20% among these studies. In patients with bladder cancer, there is no common threshold value in defining positive expression of survivin, but it is important that a common or standard threshold in the assessment of some biomarker should be set to make a comparatively accurate evaluation of its real function in clinical practice.
Survivin exists in two subcellular pools and this is consistent with its function in the regulation of both cell viability and cell division [66], [67]. Therefore, another problem with IHC is the determination of nuclear or cytoplasmic expression of survivin. Some studies pointed out the fact that survivin could be expressed in either cytoplasm or nuclei. For example, one study showed that survivin nuclear, but not cytoplasmic staining, correlated with tumor grade, stage, and patient outcome in patients with bladder cancer [55]. However, IHC results may sometimes lead to misjudgment or misinterpretation of the expression pattern of survivin in normal or cancerous tissues, due to inappropriate processing of either tissues or images [68]. In a review of the literature, Li et al [68] identified 19 publications that measured nuclear survivin in human tumors, and reported that conflicting findings existed on the relationship between nuclear survivin and prognosis. Among 19 publications, 9 showed that nuclear survivin expression is an unfavorable prognostic marker, whereas 5 proposed an opposing notion, i.e. that the nuclear survivin expression represented a favorable prognostic marker. The remaining 5 publications did not focus on studying the significance of survivin nuclear expression in disease outcome. Most eligible studies did not investigate the differential predictive value of nuclear versus cytoplasmic staining of survivin. At present, it remains uncertain as to whether there is a difference when distinguishing between cytoplasmic or nuclear staining for survivin.
Moreover, urine specimens were used to assess survivin mRNA in some studies [54], [57]. Since urine samples may contain variable numbers of tumor cells, the measured survivin levels might not truly represent tumor levels.
Although there was no heterogeneity for survival analysis, caution is perhaps advised, as there were only 14 studies with a relatively small sample size of patients in the analysis. Heterogeneity may be caused by other factors, such as inclusion criteria, different tumor stage, type of treatment, sample storage, primary antibody and dilution, method of measuring survivin, survivin cutoff levels, and adjustment for cofactors. It is also very difficult to examine or explain heterogeneity, due to the variability in clinical characteristics across patients within studies. In addition, there are few reports in the literature with respect to the prognostic impact of survivin in more advanced bladder cancer patients. Especially, only one study examined whether survivin overexpression might be a predictive marker for overall survival to cisplatin-based chemotherapy in patients with advanced (T4b and N2–N3) or metastatic (M1) bladder cancer [56].
Another potential source of bias is related to Language. This review was totally limited to literatures published in English because other languages were not accessible for the investigators. The restriction to English language articles possibly favors the positive results [69]. In addition, we did not extend the search to unpublished data that would likely include increased proportions of null results. Furthermore, the pooled risks of survivin for recurrence-free survivial or overall survival in non-muscle invasive bladder tumor, although statistically significant, were not strong, with pooled HRs of 1.81 and 1.53, respectively. Empirically, HR >2 is considered strongly predictive [70]. Finally, given the complexity of the molecular abnormalities associated with bladder cancer, combinations of independent, complementary markers might provide a more accurate prediction of outcome than a single marker [28], [50], [63].
Despite the inherent limitations of meta-analyzing prognostic literature, the findings from the present study suggest that survivin represents the consistently reproducible molecular marker with prognostic value in bladder cancer. Our strengths lie within the broad, unbiased search of the literature and the application of standardized systematic review and meta-analysis techniques to objectively identify manuscripts containing data sufficiently robust to be summarized. Strict inclusion/exclusion criteria were used to select the studies included in the present meta-analysis, thus limiting the potential bias. In cases where part or all of the same patients series was included in more than one publication, only the more recent or more complete study was included in the analysis, in order to avoid duplicating the same patient data. When considering the overall effects of potential publication bias in this analysis, the funnel plots for survival analysis were not indicative of any strong publication bias.
Conclusions
In conclusion, our meta-analysis has yielded significant association between survivin expression and bladder cancer recurrence, progression, and mortality, although these findings need to be interpreted with caution. It is difficult to draw any reliable conclusion for the current meta-analysis of survivin for overall survival in bladder cancer, due to the limited number of evaluable studies. Survivin determination might help identify patients with bladder cancer at high risk of disease recurrence, progression and poor prognosis, who might benefit from closer follow-up or more aggressive therapy. However, simplified, quantitative and reproducible assays need to be developed and validated for the detection of survivin. In addition, it is rather necessary that better designed studies need to be enrolled into such kind of analysis in the future, to provide a better conclusion about the relationship between survivin expression and the outcome of patients with bladder cancer. The value of survivin for molecular staging of bladder cancer also needs to be confirmed in controlled trials involving larger number of patients with longer follow-up, before any definitive conclusions can be made.
Supporting Information
Forest plots of hazard ratios with random effects model for survivin in patients with muscle invasive bladder tumor. (A) Recurrence-free survival. (B) Cancer-specific survival. (will be attached by *.TIF File)
(TIF)
Forest plots of hazard ratios with random effects model for survivin in patients with advanced or metastatic bladder tumor (overall survival). (will be attached by *.TIF File)
(TIF)
Supporting tables. Table S1. Patient characteristics. Table S2. Tumor characteristics. Table S3. Survivin expression according to pathological features.
(DOC)
PRISMA checklist part 1.
(TIF)
PRISMA checklist part 2.
(TIF)
Funding Statement
No current external funding sources for this study.
References
- 1. Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2. Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3: 917–921. [DOI] [PubMed] [Google Scholar]
- 3. Margulis V, Lotan Y, Shariat SF (2008) Survivin: a promising biomarker for detection and prognosis of bladder cancer. World J Urol 26: 59–65. [DOI] [PubMed] [Google Scholar]
- 4. Pennati M, Folini M, Zaffaroni N (2008) Targeting survivin in cancer therapy. Expert Opin Ther Targets 12: 463–476. [DOI] [PubMed] [Google Scholar]
- 5. Duffy MJ, O’Donovan N, Brennan DJ, Gallagher WM, Ryan BM (2007) Survivin: a promising tumor biomarker. Cancer Lett 249: 49–60. [DOI] [PubMed] [Google Scholar]
- 6. Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, et al. (2000) Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res 6: 127–134. [PubMed] [Google Scholar]
- 7. Kappler M, Kotzsch M, Bartel F, Füssel S, Lautenschläger C, et al. (2003) Elevated expression level of survivin protein in soft-tissue sarcomas is a strong independent predictor of survival. Clin Cancer Res 9: 1098–1104. [PubMed] [Google Scholar]
- 8. Rödel F, Hoffmann J, Distel L, Herrmann M, Noisternig T, et al. (2005) Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res 65: 4881–4887. [DOI] [PubMed] [Google Scholar]
- 9. Shariat SF, Bolenz C, Godoy G, Fradet Y, Ashfaq R, et al. (2009) Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J Urol 182: 78–84. [DOI] [PubMed] [Google Scholar]
- 10. Gazzaniga P, Gradilone A, Giuliani L, Gandini O, Silvestri I, et al. (2003) Expression and prognostic significance of LIVIN, SURVIVIN and other apoptosis-related genes in the progression of superficial bladder cancer. Ann Oncol 14: 85–90. [DOI] [PubMed] [Google Scholar]
- 11. Gradilone A, Petracca A, Nicolazzo C, Gianni W, Cortesi E, et al. (2010) Prognostic significance of survivin-expressing circulating tumour cells in T1G3 bladder cancer. BJU Int 106: 710–715. [DOI] [PubMed] [Google Scholar]
- 12. de Graeff P, Crijns AP, de Jong S, Boezen M, Post WJ, et al. (2009) Modest effect of p53, EGFR and HER-2/neu on prognosis in epithelial ovarian cancer: a meta-analysis. Br J Cancer 101: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayes DF, Bast RC, Desch CE, Fritsche H Jr, Kemeny NE, et al. (1996) Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst 88: 1456–1466. [DOI] [PubMed] [Google Scholar]
- 14. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, et al. (2005) Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 16. Williamson PR, Smith CT, Hutton JL, Marson AG (2002) Aggregate data meta-analysis with time-to-event outcomes. Stat Med 21: 3337–3351. [DOI] [PubMed] [Google Scholar]
- 17. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duchateau L, Collette L, Sylvester R, Pignon JP (2000) Estimating number of events from the Kaplan-Meier curve for incorporation in a literature-based meta-analysis: what you don’t see you can’t get! Biometrics. 56: 886–892. [DOI] [PubMed] [Google Scholar]
- 19. Michiels S, Piedbois P, Burdett S, Syz N, Stewart L, et al. (2005) Meta-analysis when only the median survival times are known: a comparison with individual patient data results. Int J Technol Assess Health Care 21: 119–125. [DOI] [PubMed] [Google Scholar]
- 20. Hirooka T, Hamada C, Yoshimura I (2009) A note on estimating treatment effect for time-to-event data in a literature-based meta-analysis. Methods Inf Med 48: 104–112. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hausladen DA, Wheeler MA, Altieri DC, Colberg JW, Weiss RM (2003) Effect of intravesical treatment of transitional cell carcinoma with bacillus Calmette-Guerin and mitomycin C on urinary survivin levels and outcome. J Urol 170: 230–234. [DOI] [PubMed] [Google Scholar]
- 24. Wang H, Xi X, Kong X, Huang G, Ge G (2004) The expression and significance of survivin mRNA in urinary bladder carcinomas. J Cancer Res Clin Oncol 130: 487–490. [DOI] [PubMed] [Google Scholar]
- 25. Wu Y, Wang G, Wei J, Wen X (2005) Survivin protein expression positively correlated with proliferative activity of cancer cells in bladder cancer. Indian J Med Sci 59: 235–242. [PubMed] [Google Scholar]
- 26. Mowla SJ, Emadi Bayegi M, Ziaee SA, Nikpoor P (2005) Evaluating expression and potential diagnostic and prognostic values of survivin in bladder tumors: a preliminary report. Urol J 2: 141–147. [PubMed] [Google Scholar]
- 27. López-Knowles E, Hernández S, Kogevinas M, Lloreta J, Amorós A, et al. (2006) EPICURO Study Investigators. The p53 pathway and outcome among patients with T1G3 bladder tumors. Clin Cancer Res 12: 6029–6036. [DOI] [PubMed] [Google Scholar]
- 28. Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y (2007) Association of cyclin D1 and E1 expression with disease progression and biomarkers in patients with nonmuscle-invasive urothelial cell carcinoma of the bladder. Urol Oncol 25: 468–475. [DOI] [PubMed] [Google Scholar]
- 29. Margulis V, Shariat SF, Ashfaq R, Thompson M, Sagalowsky AI, et al. (2007) Expression of cyclooxygenase-2 in normal urothelium, and superficial and advanced transitional cell carcinoma of bladder. J Urol 177: 1163–1168. [DOI] [PubMed] [Google Scholar]
- 30. Schultz IJ, De Kok JB, Witjes JA, Babjuk M, Willems JL, et al. (2007) Simultaneous proteomic and genomic analysis of primary Ta urothelial cell carcinomas for the prediction of tumor recurrence. Anticancer Res 27: 1051–1058. [PubMed] [Google Scholar]
- 31. Schultz IJ, Wester K, Straatman H, Kiemeney LA, Babjuk M, et al. (2007) Gene expression analysis for the prediction of recurrence in patients with primary Ta urothelial cell carcinoma. Eur Urol 51: 416–422. [DOI] [PubMed] [Google Scholar]
- 32. Nouraee N, Mowla SJ, Ozhand A, Parvin M, Ziaee SA, et al. (2009) Expression of survivin and its spliced variants in bladder tumors as a potential prognostic marker. Urol J 6: 101–108. [PubMed] [Google Scholar]
- 33. Atlasi Y, Mowla SJ, Ziaee SA (2009) Differential expression of survivin and its splice variants, survivin-DeltaEx3 and survivin-2B, in bladder cancer. Cancer Detect Prev 32: 308–313. [DOI] [PubMed] [Google Scholar]
- 34. Pollard C, Nitz M, Baras A, Williams P, Moskaluk C, et al. (2009) Genoproteomic mining of urothelial cancer suggests {gamma}-glutamyl hydrolase and diazepam-binding inhibitor as putative urinary markers of outcome after chemotherapy. Am J Pathol 175: 1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yildirim U, Erdem H, Kayikci A, Sahin AF, Uzunlar AK, et al. (2010) Cyclooxygenase-2 and survivin in superficial urothelial carcinoma of the bladder and correlation with intratumoural microvessel density. J Int Med Res 38: 1689–1699. [DOI] [PubMed] [Google Scholar]
- 36. Birkhahn M, Mitra AP, Williams AJ, Lam G, Ye W, et al. (2010) Predicting recurrence and progression of noninvasive papillary bladder cancer at initial presentation based on quantitative gene expression profiles. Eur Urol 57: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shariat SF, Youssef RF, Gupta A, Chade DC, Karakiewicz PI, et al. (2010) Association of angiogenesis related markers with bladder cancer outcomes and other molecular markers. J Urol 183: 1744–1750. [DOI] [PubMed] [Google Scholar]
- 38. Dong ZL, Lu ZP, Wang HZ, Zhang LY, Wang ZP, et al. (2011) Detection of nuclear matrix protein 22 and survivin baseline level in patients after radical cystectomy. Urol Int 87: 445–449. [DOI] [PubMed] [Google Scholar]
- 39. Patschan O, Shariat SF, Chade DC, Karakiewicz PI, Ashfaq R, et al. (2012) Association of tumor-associated trypsin inhibitor (TATI) expression with molecular markers, pathologic features and clinical outcomes of urothelial carcinoma of the urinary bladder. World J Urol 30: 785–794. [DOI] [PubMed] [Google Scholar]
- 40. Jaiswal PK, Goel A, Mandhani A, Mittal RD (2012) Functional polymorphisms in promoter survivin gene and its association with susceptibility to bladder cancer in North Indian cohort. Mol Biol Rep 39: 5615–5121. [DOI] [PubMed] [Google Scholar]
- 41. Xi RC, Sheng YR, Chen WH, Sheng L, Gang JJ, et al. (2013) Expression of survivin and livin predicts early recurrence in non-muscle invasive bladder cancer. J Surg Oncol 107: 550–554. [DOI] [PubMed] [Google Scholar]
- 42. Sun YW, Xuan Q, Shu QA, Wu SS, Chen H, et al. (2013) Correlation of tumor relapse and elevated expression of survivin and vascular endothelial growth factor in superficial bladder transitional cell carcinoma. Genet Mol Res 12: 1045–1053. [DOI] [PubMed] [Google Scholar]
- 43. Lehner R, Lucia MS, Jarboe EA, Orlicky D, Shroyer AL, et al. (2002) Immunohistochemical localization of the IAP protein survivin in bladder mucosa and transitional cell carcinoma. Appl Immunohistochem Mol Morphol 10: 134–138. [DOI] [PubMed] [Google Scholar]
- 44. Weikert S, Christoph F, Schrader M, Krause H, Miller K, et al. (2005) Quantitative analysis of survivin mRNA expression in urine and tumor tissue of bladder cancer patients and its potential relevance for disease detection and prognosis. Int J Cancer 116: 100–104. [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Zhu Z, Zeng F, Wang L, Wu Y, et al. (2007) Expression and prognostic significance of survivin in the progression of bladder transitional cell cancer. J Huazhong Univ Sci Technolog Med Sci 27: 444–447. [DOI] [PubMed] [Google Scholar]
- 46. Kitsukawa S, Aoyagi T, Noda K, Ito T, Yamamoto Y, et al. (2008) Quantitative analysis of survivin mRNA expression in bladder transitional cell carcinomas. Hinyokika Kiyo 54: 101–106. [PubMed] [Google Scholar]
- 47. Gonzalez S, Aubert S, Kerdraon O, Haddad O, Fantoni JC, et al. (2008) Prognostic value of combined p53 and survivin in pT1G3 urothelial carcinoma of the bladder. Am J Clin Pathol 129: 232–237. [DOI] [PubMed] [Google Scholar]
- 48. Jang TJ, Lee KS (2009) The expression of cyclooxygenase-2 and survivin in urinary bladder transitional cell carcinoma Korean J Pathol. 43: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koga F, Yoshida S, Tatokoro M, Kawakami S, Fujii Y, et al. (2011) ErbB2 and NFκB overexpression as predictors of chemoradiation resistance and putative targets to overcome resistance in muscle-invasive bladder cancer. PLoS One 6: e27616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karam JA, Lotan Y, Karakiewicz PI, Ashfaq R, Sagalowsky AI, et al. (2007) Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol 8: 128–136. [DOI] [PubMed] [Google Scholar]
- 51. Shariat SF, Ashfaq R, Karakiewicz PI, Saeedi O, Sagalowsky AI, et al. (2007) Survivin expression is associated with bladder cancer presence, stage, progression, and mortality. Cancer 109: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 52. Schultz IJ, Kiemeney LA, Witjes JA, Schalken JA, Willems JL, et al. (2003) Survivin mRNA expression is elevated in malignant urothelial cell carcinomas and predicts time to recurrence. Anticancer Res 23: 3327–3331. [PubMed] [Google Scholar]
- 53. Ku JH, Kwak C, Lee HS, Park HK, Lee E, et al. (2004) Expression of survivin, a novel inhibitor of apoptosis, in superficial transitional cell carcinoma of the bladder. J Urol 171: 631–635. [DOI] [PubMed] [Google Scholar]
- 54. Schultz IJ, Kiemeney LA, Karthaus HF, Witjes JA, Willems JL, et al. (2004) Survivin mRNA copy number in bladder washings predicts tumor recurrence in patients with superficial urothelial cell carcinomas. Clin Chem 50: 1425–1428. [DOI] [PubMed] [Google Scholar]
- 55. Yin W, Chen N, Zhang Y, Zeng H, Chen X, et al. (2006) Survivin nuclear labeling index: a superior biomarker in superficial urothelial carcinoma of human urinary bladder. Mod Pathol 19: 1487–1497. [DOI] [PubMed] [Google Scholar]
- 56. Karam JA, Lotan Y, Ashfaq R, Sagalowsky AI, Shariat SF (2007) Survivin expression in patients with non-muscle-invasive urothelial cell carcinoma of the bladder. Urology 70: 482–486. [DOI] [PubMed] [Google Scholar]
- 57. Pina-Cabral L, Santos L, Mesquita B, Amaro T, Magalhães S, et al. (2007) Detection of survivin mRNA in urine of patients with superficial urothelial cell carcinomas. Clin Transl Oncol 9: 731–736. [DOI] [PubMed] [Google Scholar]
- 58. Skagias L, Politi E, Karameris A, Sambaziotis D, Archondakis A, et al. (2009) Survivin expression as a strong indicator of recurrence in urothelial bladder cancer. Predictive value of nuclear versus cytoplasmic staining. Anticancer Res 29: 4163–4167. [PubMed] [Google Scholar]
- 59. Weiss C, von Römer F, Capalbo G, Ott OJ, Wittlinger M, et al. (2009) Survivin expression as a predictive marker for local control in patients with high-risk T1 bladder cancer treated with transurethral resection and radiochemotherapy. Int J Radiat Oncol Biol Phys 74: 1455–1460. [DOI] [PubMed] [Google Scholar]
- 60. Fristrup N, Ulhøi BP, Birkenkamp-Demtröder K, Mansilla F, Sanchez-Carbayo M, et al. (2012) Cathepsin E, maspin, Plk1, and survivin are promising prognostic protein markers for progression in non-muscle invasive bladder cancer. Am J Pathol 180: 1824–1834. [DOI] [PubMed] [Google Scholar]
- 61. Xi RC, Sheng YR, Chen WH, Sheng L, Gang JJ, et al. (2013) Expression of survivin and livin predicts early recurrence in non-muscle invasive bladder cancer. J Surg Oncol 107: 550–554. [DOI] [PubMed] [Google Scholar]
- 62. Shariat SF, Karakiewicz PI, Godoy G, Karam JA, Ashfaq R, et al. (2009) Survivin as a prognostic marker for urothelial carcinoma of the bladder: a multicenter external validation study. Clin Cancer Res 15: 7012–7019. [DOI] [PubMed] [Google Scholar]
- 63. Als AB, Dyrskjøt L, von der Maase H, Koed K, Mansilla F, et al. (2007) Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res 13: 4407–4414. [DOI] [PubMed] [Google Scholar]
- 64. Stewart LA, Parmar MK (1993) Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 341: 418–422. [DOI] [PubMed] [Google Scholar]
- 65. Altman DG, Lausen B, Sauerbrei W, Schumacher M (1994) Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 86: 829–835. [DOI] [PubMed] [Google Scholar]
- 66. Fortugno P, Wall NR, Giodini A, O’Connor DS, Plescia J, et al. (2002) Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J Cell Sci 115: 575–585. [DOI] [PubMed] [Google Scholar]
- 67. Li F, Ling X (2006) Survivin study: an update of “what is the next wave”? J Cell Physiol 208: 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li F, Yang J, Ramnath N, Javle MM, Tan D (2005) Nuclear or cytoplasmic expression of survivin: what is the significance? Int J Cancer 114: 509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Egger M, Zellweger-Zähner T, Schneider M, Junker C, Lengeler C, et al. (1997) Language bias in randomised controlled trials published in English and German. Lancet 350: 326–329. [DOI] [PubMed] [Google Scholar]
- 70. Hayes DF, Isaacs C, Stearns V (2001) Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia 6: 375–392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plots of hazard ratios with random effects model for survivin in patients with muscle invasive bladder tumor. (A) Recurrence-free survival. (B) Cancer-specific survival. (will be attached by *.TIF File)
(TIF)
Forest plots of hazard ratios with random effects model for survivin in patients with advanced or metastatic bladder tumor (overall survival). (will be attached by *.TIF File)
(TIF)
Supporting tables. Table S1. Patient characteristics. Table S2. Tumor characteristics. Table S3. Survivin expression according to pathological features.
(DOC)
PRISMA checklist part 1.
(TIF)
PRISMA checklist part 2.
(TIF)