Abstract
The retinoic acid receptor-related orphan receptors α and γ (RORα and RORγ), are key regulators of helper T (Th)17 cell differentiation, which is involved in the innate immune system and autoimmune disorders. However, it remains unclear whether environmental chemicals, including pesticides, have agonistic and/or antagonistic activity against RORα/γ. In this study, we investigated the RORα/γ activity of several azole-type fungicides, and the effects of these fungicides on the gene expression of interleukin (IL)-17, which mediates the function of Th17 cells. In the ROR-reporter gene assays, five azole-type fungicides (imibenconazole, triflumizole, hexaconazole, tetraconazole and imazalil) suppressed RORα- and/or RORγ-mediated transcriptional activity as did benzenesulphonamide T0901317, a ROR inverse agonist and a liver X receptor (LXR) agonist. In particular, imibenconazole, triflumizole and hexaconazole showed RORγ inverse agonistic activity at concentrations of 10−6 M. However, unlike T0901317, these fungicides failed to show any LXRα/β agonistic activity. Next, five azole-type fungicides, showing ROR inverse agonist activity, were tested on IL-17 mRNA expression in mouse T lymphoma EL4 cells treated with phorbol myristate acetate and ionomycin. The quantitative RT-PCR analysis revealed that these fungicides suppressed the expression of IL-17 mRNA without effecting RORα and RORγ mRNA levels. In addition, the inhibitory effect of imibenconazole as well as that of T0901317 was absorbed in RORα/γ-knocked down EL4 cells. Taken together, these results suggest that some azole-type fungicides inhibit IL-17 production via RORα/γ. This also provides the first evidence that environmental chemicals can act as modulators of IL-17 expression in immune cells.
Keywords: fungicide, interleukin 17, mouse, reporter gene assay, retinoic acid receptor-related orphan receptor
Introduction
T helper (Th) cells with diverse effector functions differentiate from naïve CD4+ T cells upon stimulation by antigens in the presence of various cytokines produced by cells of the innate immune system. Th17 cells, the T helper cells that produce interleukin 17 (IL-17) and other pro-inflammatory cytokines, were recently identified as a novel Th subset and play important roles in host defense against bacterial and fungal infections, and in a wide variety of autoimmune disease, including psoriasis and rheumatoid arthritis (Harrington et al., 2005; Park et al., 2005; Weaver et al., 2007; Stockinger and Veldhoen, 2007). In Th17 cells, the transcription of IL-17 is mediated by Th17-specific transcriptional regulators of the retinoic acid receptor-related orphan receptors, RORα and RORγ (Ivanov et al., 2006; Yang et al., 2008).
RORα/γ are members of the nuclear hormone receptor superfamily, which contains a signature type II zinc finger DNA binding motif and a hydrophobic ligand binding pocket (Jetten et al., 2001). To date, three subtypes, designated RORα, -β, and -γ (NR1F1–3 or RORA-C), have been identified with distinct tissue distributions and biological activities (Jetten, 2009). In the thymus, RORα and two isoforms, γ1 and γ2 (also named RORγt), have been identified (He et al., 1998), with RORγt differing from the RORγ1 isoform in that it lacks the amino terminus of RORγ1. The RORs bind as monomers to ROR response elements (ROREs) in the promoter regulatory region of the target genes (Giguere et al., 1994; Medvedev et al., 1996). Recent studies have also demonstrated that RORs function as ligand-dependent transcriptional factors. For example, in some cholesterol metabolites, 7α-hydroxycholesterol was reported to be a RORα and RORγ inverse agonist that inhibits the basal transcription mediated by RORα and RORγ, respectively (Wang et al., 2010a), while cholesterol sulfate was reported to function as an RORα agonist (Kallen et al., 2004). In addition, Kumar et al. (2010) provided novel evidence that N-(2,2,2-trifluoroethyl)-N-[4-(2,2,2-trifluoro-1-hydroxy-1-trifluoromethyl-ethyl)-phenyl]-benzene sulfonamide (T0901317), known to be a potent agonist of liver X receptor (LXR, NR1H1–3), was reported to be an inverse agonists of RORα and RORγ. In fact, other previous studies had shown that this compound leads to a decreased severity of experimental autoimmune encephalomyelitis (EAE), which is dependent on Th17 cell function (Hindinger et al., 2006; Xu et al., 2009). Recent novel studies have also provided evidences that a synthetic chemical SR1001, digoxin derivatives, and ursolic acid act as ROR inverse agonists and ameliorate EAE disease in mice (Solt et al., 2011; Huh et al., 2011; Xu et al., 2011). As Th17 cells play an important role in both the immune response to invading pathogens and autoimmunity (Harrington et al., 2005; Park et al., 2005), the identification of ROR modulators, such as ROR agonists and inverse agonists, would be of great value with regard to immunotoxicity against the innate immune system and drug-therapy for autoimmune diseases. However, although exposure to environmental agents can compromise a number of immunological functions, the effects of environmental chemicals against RORs remain unclear.
Pesticides are used globally to control agricultural and indoor pests, and are intentionally released into the environment in large quantities. As pesticides possess a wide variety of chemical structures, they may represent the most likely candidates for ligands against nuclear receptors (Kojima et al., 2010). Transactivation or reporter gene assays, a powerful tool for testing receptor agonists and antagonists among chemicals, have been established as an in vitro method for evaluating the receptor activity of chemicals. We previously developed novel highly sensitive reporter gene assays using Chinese hamster ovary (CHO) cells for detecting human estrogen receptor (ER)α, ERβ and androgen receptor (AR) activities among numerous chemicals (Kojima et al., 2003; 2004), and demonstrated that a number of environmental chemicals, including pesticides, possess ERα- and ERβ-mediated estrogenic activities and/or AR-mediated antiandrogenic activity (Kojima et al., 2004; 2009; Takeuchi et al., 2005; 2009; 2011).
We have now performed a pesticide screen of around 200 compounds using CHO cell-based RORα/γ reporter gene assays, and identified several pesticides possessing RORα/γ activity. In the present study, we show that five azole-type fungicides have RORα and/or RORγ inverse agonistic activity. In addition, we examined whether these fungicides affect IL-17 gene expression in mouse T lymphoma EL4 cells, and found that these fungicides suppress IL-17 mRNA expression. Here, we provide the first evidence that environmental chemicals might affect IL-17 gene transcriptional activity via RORα and/or RORγ.
Materials and Methods
Chemicals
Hexaconazole (>99% pure) and imazalil (>98% pure) were purchased from Wako Pure Chemical Industries Ltd. (Wako; Osaka, Japan). Imibenconazole (>99% pure) and triflumizole (>99% pure) were purchased from Kanto Chemical Company Ltd. (Tokyo, Japan). Tetraconazole (97.5% pure) was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). A synthetic ROR inverse agonist and LXR agonist, T0901317 and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ionomycin was purchased from LKT Laboratories (St. Paul, MN, USA). We used dimethylsulfoxide (DMSO, Wako) as a vehicle, and all compounds were dissolved in DMSO at a concentration of 3 × 10−2 M.
Cell line and cell culture materials
We obtained Chinese hamster ovary (CHO-K1) cells, COS-7 simian kidney cells, and EL4 cells from the American Type Culture Collection. Fetal bovine serum (FBS) and charcoal-dextran treated FBS (CD-FBS) were obtained from Hyclone (Logan, UT, USA). Dulbecco’s modified Eagle medium (DMEM), DMEM plus Ham’s F-12 nutrient mixture (DMEM/F-12) and RPMI-1640 medium were obtained from GIBCO-BRL (Invitrogen Rockville, MD, USA). Glutamine solution and penicillin-streptomycin (antibiotics) solution were obtained from Dainippon Pharmaceutical Co. Ltd. (Osaka, Japan), and 0.25% trypsin/0.02% ethylenediamine tetra-acetic acid (EDTA) disodium salt solution was obtained from Life Technologies (Paisley, UK). CHO-K1 cells were maintained in DMEM/F-12 containing 5% FBS and antibiotics. COS-7 cells were maintained in DMEM containing 10% FBS and antibiotics. EL4 cells were maintained in RPMI-1640 medium containing 10% FBS and antibiotics.
Plasmids
We used the expression plasmids pCMV10-mRORα4 and pCMV10. RORγ1 encoding the full-length receptor protein and the reporter plasmid pGL4.27(RORE)5-Luc (pRORE-Luc) for determining RORα/γ activity (Takeda, Y., unpublished). We used the expression plasmids pTARGET-hLXRα and pTARGET-hLXRβ and fusion protein expression plasmids hLXR-N/pBIND and hLXR-C/pACT for determining LXRα/β activity (Kobayashi et al., 2010). We purchased the internal control plasmid pCMV-βGal from Clontech (Palo Alto, CA, USA).
Transfection of plasmids to cells and reporter gene assays for RORα and RORγ
We plated the host CHO-K1 cells in 96-well microtiter plates (Nalge, Nunc, Denmark) at a density of 8,400 cells per well in DMEM/F-12 containing 5% CD-FBS (complete medium) 1 day before transfection. For detection of RORα or RORγ activity, cells were transfected with 12 ng pCMV-mRORα or pCMV-mRORγ, 48 ng pRORE-Luc, and 12 ng pCMVβ-Gal per well using FuGENE®6 Transfection Reagent (Roche Diagnostics Corp., Indianapolis, IN, USA). After a 3-hr transfection period, cells were dosed with various concentrations of the test compounds or with 0.1% DMSO (vehicle control) in complete medium. After an incubation period of 24 hr, cells were rinsed with phosphate-buffered saline (pH 7.4) and lysed with passive lysis buffer (50 μl/well; Promega, Madison, WI, USA). We measured firefly luciferase activity with a MiniLumat LB 9506 luminometer (Berthold, Wildbad, Germany) in one reaction tube with a 5-μl aliquot of the cell lysate using the Luciferase Assay System (Promega), according to the manufacturer’s instructions. We measured β-galactosidase activity to check the cytotoxicity of the test compounds against transcriptional activity using a fluorescence method as described previously (Takeuchi et al., 2005; 2006). The luciferase activity was normalized against the β-galactosidase activity for each treatment. Results are expressed as mean ± SD from at least three independent experiments performed in triplicate.
To estimate the potency of the receptor-inverse agonistic activity of the compounds tested, the concentration of the compound exhibiting a response equal to 20% that of the vehicle control response was evaluated from a dose-response curve of the luminescence intensity, and expressed as the 20% relative inhibitory concentration (RIC20).
Reporter gene assays for LXRα and LXRβ
The LXRα and LXRβ assays were performed using the same procedure as that for the RORα/γ assays. Namely, COS-7 cells were transfected with 50 ng of either pTAGET-hLXRα or pTARGET-hLXRβ, 50 ng hLXR-N/pBIND, 50 ng hLXR-C/pACT, and 10ng pCMV-βGal per well using the FuGene6 transfection reagent. The LXR agonist, T0901317, was utilized as a positive control in these LXRα/β assays.
EL4 cells experiments, isolation of RNA and reverse transcription
We plated the host EL4 cells in 12-well microtiter plates (Nalge, Nunc, Denmark) at a density of 2 × 106 cells/ml in RPMI-1640 medium containing 10% FBS. Next day, EL4 cells were treated with vehicle or both PMA (5 ng/ml) and ionomycin (1 μM) for induction of IL-17. Simultaneously, the cells were treated with vehicle, 1, 3, 10 or 30 μM of the fungicides or 0.1–10 μM T0901317 as a ROR inverse agonist (positive control), and incubated at 37°C for 6 h. The EL4 cells were removed, rinsed with isotonic saline, and homogenized in TRIzol Reagent (Molecular Research Center, Cincinnati, OH, USA). Total RNA was isolated from the EL4 cells using TRIzol Reagent (1 mL/100 mg of tissue) following the manufacturer’s instructions. The complementary DNA (cDNA) was synthesized using 5 μg of total RNA by Revertra Ace reverse transcriptase (TOYOBO, Osaka, Japan). The cDNAs were then stored at −20°C until analysis.
Reverse transcription polymerase chain reaction (RT-PCR)
The following primers were synthesized: for IL-17A, 5′-TCCAGAAGGCCCTCAGACTA-3′ and 5′-AGCATCTTCTCGACCCTGAA-3′; and for β-actin, 5′-TGTTACCAACTGGGACGACA-3′ and 5′-GGGGTGTTGAAGGTCTCAAA-3′. PCR amplification was conducted on cDNA (20 μL) synthesized from 0.25 μg of the total RNA of EL4 cells treated with T0901317 or a vehicle using Taq DNA polymerase (Promega, USA) for 22 to 35 cycles. The PCR cycle consisted of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. The PCR product was size-fractionated on 1.5% agarose gel and visualized by ethidium bromide staining. The expected sizes of the amplified cDNAs were 239 bp and 165 bp for IL-17A and β-actin, respectively.
Real-time quantitative PCR for IL-17, RORα, RORγ, and β-actin
We measured IL-17, RORα, RORγ, and β-actin mRNA levels using TaqMan® Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). cDNA prepared from the total RNA of the EL4 cells was subjected to real-time quantitative PCR on a 7900HT Fast Real Time PCR System (Applied Biosystems). The following probes for detection were designed by Applied Biosystems: for IL-17A, 5′-FAM d(CTTCATCTGTGTCTCTGATGCTGTT) NFQ-3′; for RORα, 5′-FAM d(AAGGCTGCAAGGGCTTTTTCAGGAG) NFQ-3′; for RORγ, 5′-FAM d(GCTACCAGAGGAAGTCAATGTGGGA) NFQ-3′; and for β-actin, 5′-FAM d(ACTGAGCTGCGTTTTACACCCTTTC) NFQ-3′. The amounts of IL-17, RORα, and RORγ mRNAs were normalized to that of β-actin mRNA.
Establishment of RORα/γ-knockdown EL4 cells using RNA-mediated interference
EL4 cells were maintained in RPMI 1640 medium containing 10% FBS. A RORα and RORγ-knockdown EL4 cell clone (EL4/shRorac) was established by electroporation with the pGPU6/GFP/Neo vector (Shanghai GenePharma, Shanghai, China), bearing short hairpin RNA (shRNA) targeting Rora (5′-GGATCAAACCCGAACCCATAT-3′) and Rorc (5′-GCTACCAGAGGAAGTCAATGT-3′), and subsequent selection in G418-containing medium. Similarly, a control shRNA (nonsilencing; 5′-TTCTCCGAACGTGTCACGT-3′)-transfected EL4 cell line (EL4/shNC) was also established.
Data analysis
We used an analysis of variance (ANOVA) followed by Bonferroni correction to evaluate the differences in transcriptional levels between the control group and each of the chemical groups in the reporter gene assays and quantitative RT-PCR assays. Statistical significance was set at p < 0.05. Data were presented as means ± SD of three triplicate experiments.
Results
Effects of azole-type fungicides on transcriptional activity in the RORα and RORγ assays
Figure 1 shows the chemical structures of T0901317 and five azole-type fungicides tested in this study. We examined the effects of these compounds on RORα- and RORγ-mediated transcriptional activity using CHO cell-based reporter gene assays. As a result, T0901317 and five azole-type fungicides didn’t show any RORα/γ agonistic activity. However, we found that these compounds have RORα and/or RORγ inverse agonistic activity, which inhibits their constitutive activation. Figures 2A and B show the inhibitory effects of five azole-type fungicides together with T0901317 on transcriptional activity via RORα and RORγ, respectively. In the RORα assay, although imazalil and tetraconazole did not show almost any inverse agonistic activity, imibenconazole, triflumizole and hexaconazole were found to be weak RORα inverse agonists similar to T0901317 (Fig. 2A). However, in the RORγ assay, these five fungicides and T0901317 showed inverse agonistic activity in a dose-dependent manner from 1 to 30 μM (Fig. 2B). The order of relative potency for RORγ inverse agonistic activity were T0901317 > Imibenconazole > triflumizole, hexaconazole > tetraconazole, Imazalil. Based on their β-galactosidase activity, none of the compounds tested in this study showed any cytotoxic effects, except for the highest dose (30 μM) of T0901317 and imibenconazole. From these dose-response curves, we estimated the RIC20 values of five azole-type fungicides and T0901317 for RORα and RORγ, and summarized in Table 1.
Fig. 1.
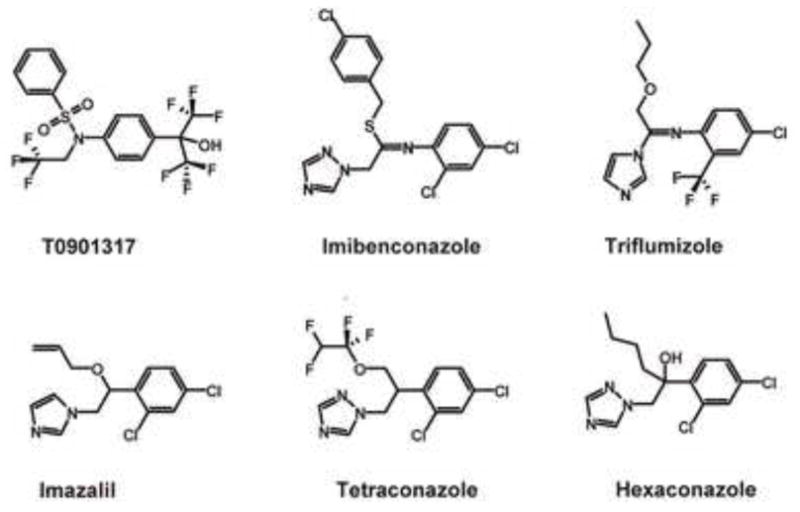
Chemical structures of five azole-type fungicides and T0901317 tested in this study.
Fig. 2. Effects of five azole-type fungicides and T0901317 on RORα/γ-mediated transcriptional activity.
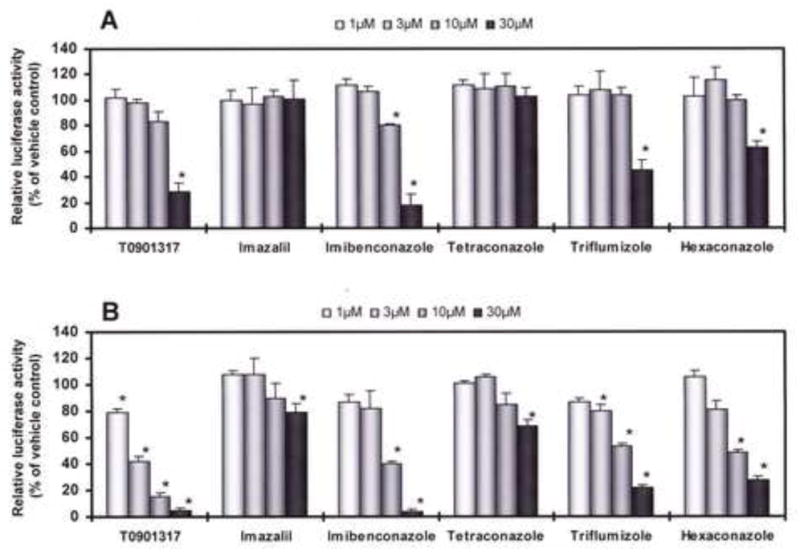
CHO-K1 cells were transiently cotransfected with an expression plasmid for mouse RORα (A) or RORγ (B) as well as a reporter-responsive firefly luciferase plasmid and a constitutively active β-galactosidase expression plasmid. Cells were treated with increasing concentrations of the five fungicides or T0901317, a RORα/γ inverse agonist. The firefly luciferase activity was normalized against β-galactosidase activity. Values represent the means ± SD of three independent experiments and are presented as percentage induction, with 100% activity defined as the constitutive activity by the vehicle control.
Table 1.
RIC20 for RORα/γ inverse agonistic activities of five azole-type fungicides.
| Compound | RORα assay | RORγ assay |
|---|---|---|
|
| ||
| RIC20 (M) | RIC20 (M) | |
| T0901317 | 1.2×10−5 | 9.7×10−7 |
| Imazalil | > 3.0×10−5 | 2.9×10−5 |
| Imibenconazole | 1.0×10−5 | 3.3×10−6 |
| Tetraconazole | > 3.0×10−5 | 1.5×10−5 |
| Triflumizole | 1.8×10−5 | 3.0×10−6 |
| Hexaconazole | 2.1×10−5 | 3.2×10−6 |
RIC20 (20% relative inhibitory concentration) is represented as the concentration of the compound exhibiting a response equal to 20% that of the vehicle control response.
Effects of azole-type fungicides in the LXRα/β transactivation assays
As T0901317 is a potent LXR agonist, we investigated whether the five azole-type fungicides also possess LXR agonistic activity similar to T0901317. Figures 3A and B show LXRα- and LXRβ-mediated transactivation by the fungicides and T0901317. Although T0901317, as expected, induced LXRα and LXRβ activation at low concentrations of 10−7 M order, none of the five fungicides showed any LXRα or LXRβ agonistic activity even at a high dose of 1 × 10−5 M.
Fig. 3. Effects of five azole-type fungicides in hLXRα and hLXRβ transactivation assays.
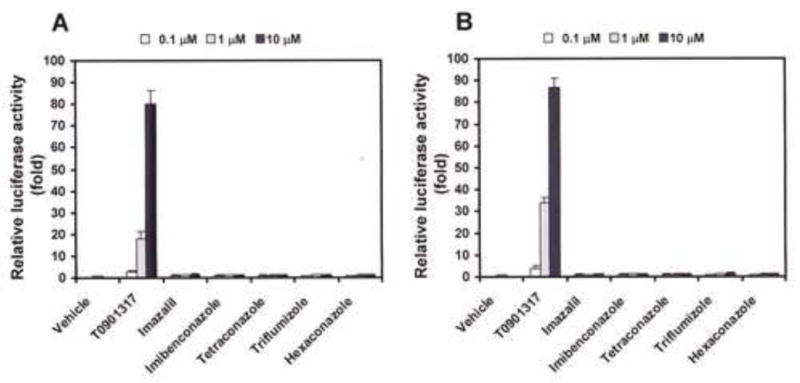
COS-7 cells were transiently cotransfected with an expression plasmid for mouse LXRα (A) or LXRβ (B) as well as two fusion protein expression plasmids and a constitutively active β-galactosidase expression plasmid. Cells were treated with increasing concentrations of the five fungicides or T0901317, a potent LXR agonist. The firefly luciferase activity was normalized against β-galactosidase activity. Values represent the means ± SD of three independent experiments and are presented as the mean n-fold induction over the vehicle control.
Effects of T0901317 on in vitro expression of IL-17A mRNA in mouse T lymphoma EL4 cells
Since induction of IL-17A via ROR is a specific positive marker in Th17 cell proliferation pathway, we examined whether azole-type fungicides affect IL-17A level in EL4 cells. Figure 4 shows the inhibitory effect of T0901317 on IL-17A gene expression in PMA/ionomycin-stimulated EL4 cells. Although neither T0901317 nor the vehicle induced the expression of IL-17A gene in non-stimulated EL4 cells, T0901317 inhibited IL-17A mRNA expression in a dose-dependent manner in PMA/ionomycin-stimulated EL4 cells (Fig. 4A). Real-time PCR analysis revealed that T0901317 significantly inhibited IL-17A mRNA expression at concentrations of 0.1, 1, and 10 μM, although it did not affect RORα and RORγ mRNA expressions (Fig. 4B).
Fig. 4. Qualitative and quantitative analysis of IL-17A mRNA expression in the EL4 cells treated with T0901314.
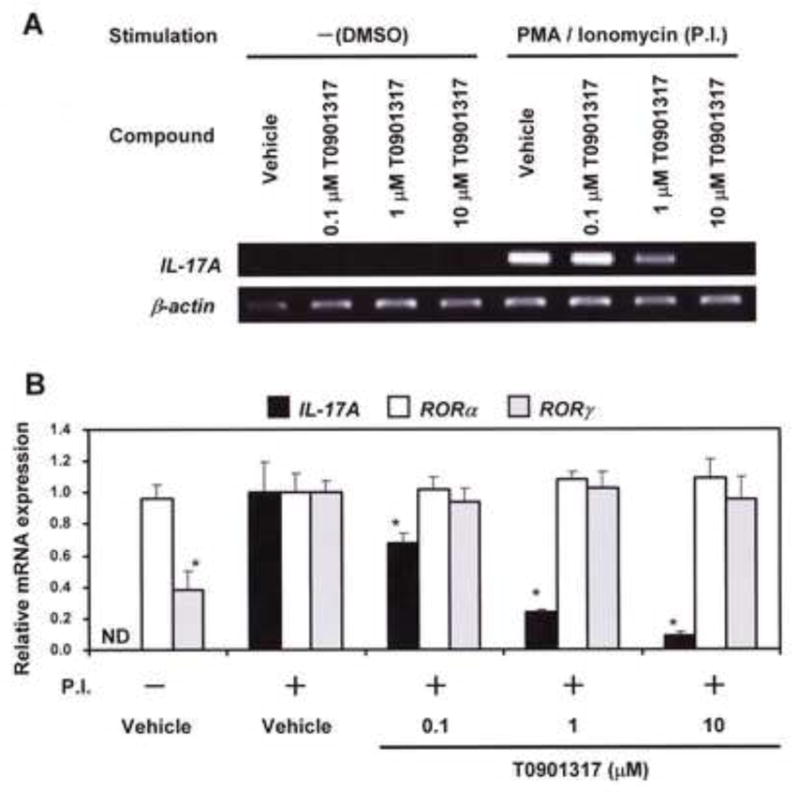
(A) EL4 cells were treated with vehicle (no stimulation) or both PMA (5 ng/ml) and ionomycine (1 μM) for induction of IL-17A. Simultaneously, the cells were treated with 0.1% DMSO (vehicle control), 0.1, 1, or 10 μM of T0901317 at 37°C for 6 h. IL-17A and β-actin mRNA expressions were amplified by qualitative RT-PCR using specific primers, and amplified products (5 μL) were separated by electrophoresis on 1.5% agarose gels and stained with ethidium bromide. β-actin was used as a housekeeping gene. (B) IL-17A, RORα, and RORγ mRNA expressions in the cells were amplified by real-time RT-PCR and normalized to the expression of a housekeeping gene β-actin. Values are expressed relative to vehicle control with PMA/ionomycin stimulation (taken as 1) and represent the mean ± SD of three experiments. ND, not detected (< 0.005). * Significant difference (p < 0.05; ANOVA) from vehicle control with PMA/ionomycine stimulation.
Effects of five azole-type fungicides on in vitro expressions of IL-17A, RORα, and RORγ mRNA in EL4 cells
Based on the real-time PCR analysis in PMA/ionomycin-stimulated EL4 cells, we investigated the effects of the five azole-type fungicides on IL-17A, RORα, and RORγ expressions. As shown in Fig. 5, we found that five azole-type fungicides possessed inhibitory effects of PMA/ionomycin-stimulated IL-17A mRNA expression. In particular, imibenconazole (10 μM) strongly suppressed IL-17A mRNA expression similar to T0901317 (Fig. 5B), and triflumizole and hexaconazole significantly inhibited it at a concentration of 10 μM (Figs. 5D and E). In addition, treatment with the highest doses (30 μM) of imazalil and tetraconazole significantly suppressed IL-17 mRNAs compared with the vehicle (Figs. 5A and C). On the other hand, these fungicides failed to affect the expression levels of RORα and of RORγ mRNAs (Fig. 5A–E).
Fig. 5. Suppression of IL-17 mRNA expression by azole-type fungicides in EL4 cells treated with PMA and ionomycin.
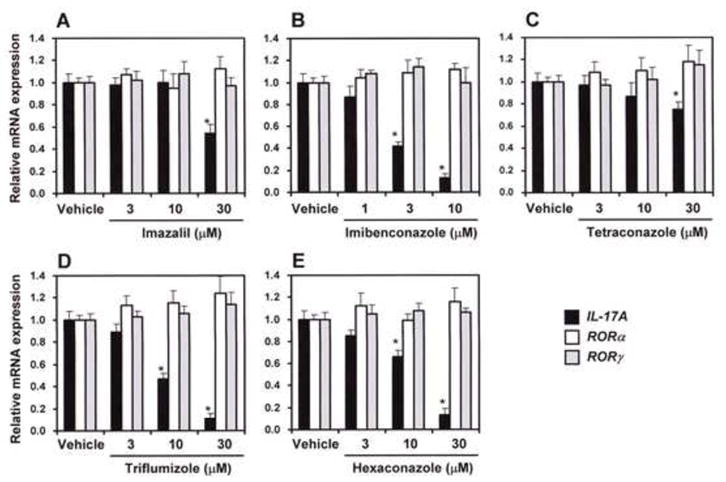
EL4 cells were treated with PMA (5 ng/ml) and ionomycin (1 μM) for IL-17 gene induction. Simultaneously, the cells were treated with vehicle, 1, 3, 10 or 30 μM of the fungicide, and incubated at 37°C for 6 h. IL-17A, RORα, and RORγ mRNA expressions in the cells were amplified by real-time RT-PCR and normalized to the expression of a housekeeping gene β-actin. Values are expressed relative to vehicle control with PMA/ionomycin stimulation (taken as 1) and represent the mean ± SD of three experiments. * Significant difference (p < 0.05; ANOVA) from vehicle control with PMA/ionomycine stimulation.
Effect of Imibenconazole and T0901317 on IL-17A mRNA expression in the RORα/γ knockdown EL4 cells
We established EL4/shRorac and EL4/shNC cells as endogenous RORα and RORγ double-knockdown EL4 and control EL4 cell lines, respectively, using shRNA technology. As shown in Figs. 6A and B, RORα and RORγ mRNA expression in EL4/shRorac cells respectively decreased at about 50 and 30 % of those in EL4/shNC cells when the cells were treated with PMA/ionomycin. Figure 6C shows the effects of imibenconazole and T0901317 (each 10 μM) on IL-17A mRNA levels in EL4/shRorac and EL4/shNC cells treated with PMA/ionomycin. The IL-17A mRNA expression in EL4/shRorac cells was found to potently decrease in comparison with that in EL4/shNC cells. Although imibenconazole and T0901317 showed inhibitory effects on IL-17A mRNA expression in EL4/shNC cells, these compounds also suppressed IL-17 mRNA expression in EL4/shRorac cells.
Fig. 6. Comparison of inhibitory effects for IL-17A mRNA expression by imibenconazole using EL4/shRorc cells and EL4/shNC cells.
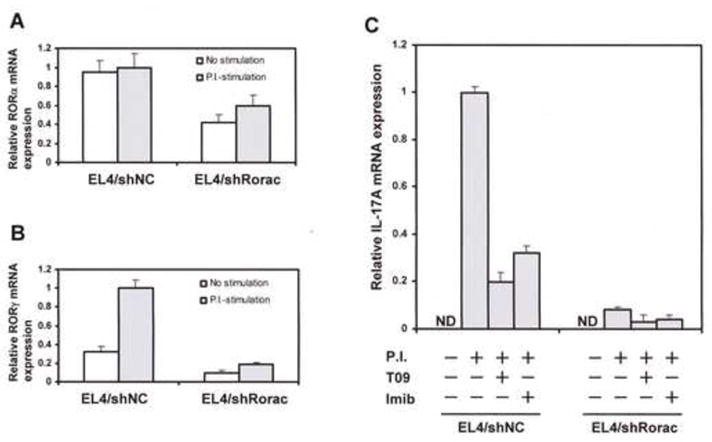
RORα (A) and RORγ (B) mRNA expressions in EL4/shRorac cells and EL4/shNC cells, with or without PMA/ionomycine (P.I.) stimulation, were amplified by real-time RT-PCR and normalized to the expression of a housekeeping gene β-actin, respectively. (C) IL-17 mRNA expression was measured in EL4/shRorac cells and EL4/shNC cells in the presence of T0901317 (T09; 10 μM) or imibenconazole (Imib; 10 μM), with or without PMA/ionomycine stimulation, and normalized to the expression of a housekeeping gene β-actin. Values are expressed relative to vehicle control with PMA/ionomycin stimulation in EL4/shNC cells (taken as 1) and represent the mean ± SD of three experiments. ND, not detected (< 0.01).
Discussion
Unlike steroid hormone receptors, apo-RORα and -γ are constitutively active and retain the ability to interact with a range of coactivator NR box peptides in the absence of binding to any ligand (Jetten et al., 2001). In fact, RORs are orphan receptors whose endogenous ligands are still unclear. However, recent studies have reported that the binding of cholesterol metabolites (e.g., 7-oxygenated sterols) to RORα/γ reduces the level of this constitutive interaction, resulting in the repression of the transcriptional output of the receptors (Wang et al., 2010a). In addition, Kumar et al. (2010) provided evidence that the synthetic chemical T0901317, which is also a potent LXR agonist, directly binds to RORα and RORγ with high affinity (Ki = 132 and 51 nM, respectively), and represses RORα/γ-dependent transactivation of RORE reporter genes. Therefore, these cholesterol metabolites and T0901317 are classified as ROR inverse agonists that inhibit ROR basal transcriptional activity in the absence of agonist, and are distinct from ROR antagonists that themselves do not effect the receptor equilibrium but are capable of competitive antagonism of both agonists and inverse agonists (Klein et al., 1996). In this study using CHO-K1 cell-based reporter gene assays, we found that T0901317 decreased RORα- and RORγ-dependent luciferase induction at a RIC20 of 12 μM and 0.97 μM, respectively (Table 1), thereby supporting the results obtained by Kumar et al. (2010). More recently, they have reported that synthetic compounds derived from the benzenesulfonamide scaffold, SR1078 and SR1001, act as a RORα/γ agonist and RORα/γ inverse agonist, respectively (Wang et al., 2010b; Solt et al., 2011). This suggested the possibility that small molecule compounds, such as environmental chemicals, may also act as ligands of ROR.
We investigated the potential agonistic and inverse agonistic activity of about 200 pesticides against RORα and RORγ using CHO-K1 cell-based reporter gene assays. As a result, we have found that five azole-type fungicides inhibited constitutive activation of RORα and RORγ in a dose-dependent manner similar to T0901317 (Fig. 2). In particular, imibenconazole, triflumizole and hexaconazole were found to inhibit basal RORγ-mediated transactivation at concentrations of 10−6 M order. According to a recent study by Martin et al. (2010), hexaconazole has been listed as a potent ROR inhibitor together with other 13 pesticides, supporting our results. As shown in Fig. 1, five fungicides acting as ROR inverse agonists have structures similar to each other and resembling T0901317, although the RORα/γ activities of these fungicides were weaker than those of T0901317.
It is topical that, in recent immunological studies, a novel Th subset, termed Th17, which preferentially produces IL-17, has been identified as a distinct Th lineage mediating tissue inflammation (McGeachy and Cua, 2008). Th17 cells are defined by their secretion of the pro-inflammatory cytokine IL-17, which mediates the recruitment of inflammatory cells and the induction of pro-inflammatory mediators by endothelial cells (Ouyang et al., 2008). Therefore, Th17 cells and their effector cytokines mediate the host defense mechanisms to various infections, especially extracellular bacteria infections, and are involved in the pathogenesis of many autoimmune diseases (Ouyang et al., 2008; Stockinger and Veldhoen, 2007). Several mechanism studies have revealed that RORα and RORγ, and particularly the RORγt isoform, act as Th17 lineage-specific master transcription factors (Ivanov et al., 2006; Yang et al., 2008). For example, Yang et al. (2008) demonstrated that RORα and RORγ co-expression synergistically leads to greater IL-17 production in Th17 cells, whereas double deficiencies in RORα and RORγ globally impair IL-17 production and completely protect mice against EAE. Thus, it is important to investigate whether chemicals having ROR activity affect Th17 differentiation, and to identify ROR agonists and antagonists. In this study, we examined the effects of ROR inverse agonistic fungicides on IL-17 gene expression in mouse T lymphoma EL4 cells treated with PMA/ionomycin. Interestingly, as with T0901317, five azole-type fungicides (imibenconazole, triflumizole, hexaconazole, tetraconazole and imazalil) were found to inhibit IL-17 gene expression in a dose-dependent manner (Figs. 5A–E). This result suggests that ROR inverse agonistic activities in reporter gene assays well corresponded to the potency of IL-17 inhibitory effects in EL4 cells.
A synthetic compound, T0901317, is well known as a potent LXR agonist. T0901317 has been reported to suppress Th17 function in a mouse autoimmune model of EAE (Xu et al., 2009), supporting our results regarding the inhibition of IL-17 expression by T0901317. However, Cui et al. (2011) have recently reported that the LXR agonistic activity of T0901317 is involved in the repression of IL-17 production, and leads to EAE prevention. Therefore, we examined whether these fungicides show LXR agonistic activity, and confirmed that they did not act as LXR agonists (Fig. 3). This suggests that the five fungicides affect IL-17 gene expression via ROR, not LXR, unlike T0901317, a potent LXR agonist. Thus, our findings indicate that environmental chemicals, such as pesticides, may act as regulatory molecules of Th17 cell function as ROR inverse agonists, leading to potential effects on the immune system in whole organisms.
The EL4 cells constitutively express RORα (encorded by Rora), RORγ (Rorc) and IL-17A (Il17a) (Ichiyama et al., 2008). Next, we established the RORα/γ knockdown EL4 (EL4/shRorac) cells using shRNA technology. As a result, we found that IL-17A mRNA expression was clearly suppressed in EL4/shRorac cells when they were treated with PMA/ionomycin. This suggests that RORα/γ is a critical regulator of IL-17 gene expression in EL4 cells, as pointed out by other studies (Ichiyama et al., 2008; Solt et al., 2011). More importantly, although treatment of EL4/shNC cells with imibenconazole and T0901317 potently suppressed IL-17A mRNA expression, treatment of EL4/shRorc cells with those also resulted in decrease. This may suggest that the remained RORα/γ are involved in suppression of IL-17A mRNA expression by imibenconazole and T0901317.
Azole fungicides are widely used in agriculture and in human mycosis (Zarn et al., 2003; Giavini and Menegola, 2010). Their antifungal activity is based on their ability to inhibit fungal CYP51 (lanosterol 14α-demethylase), a key enzyme in the formation of the fungal wall. In addition, several azoles are also used in antiestrogen therapy such as in breast cancer treatment, as they inhibit CYP19 (aromatase), a key enzyme in the estrogen biosynthesis (Zarn et al., 2003). Although five azole-type fungicides showed ROR inverse agonistic activity, triazole or imidazole structures, which play an important role in antifungal activity, might be not necessary for ROR activity as other azole fungicides (bitertanol, triadimefon, propiconazole, and prochloraz) were inactive (data not shown). Thus, further studies on the structural property of azole-type fungicides are required for elucidating their ROR inverse agonistic activity.
The present study also indicates the structural design of potent and selective ROR ligands with potential application in the treatment of metabolic and immune disorders (Kumar et al., 2010; Solt et al., 2010). The development for ROR inhibitors represents a promising therapeutic strategy in the treatment of Th17-mediated diseases. Thus, RORα/γ inverse agonists, such as imibenconazole, might be useful as lead compounds for drug development in autoimmune diseases involving Th17 cells. On the other hand, inhibiting RORα/γ might adversely affect the beneficial functions of Th17 cells in fighting pathogens. Although the physiological concentrations reached during exposure to these fungicides might be lower than the concentrations by which those affect ROR activity, unexpected additive exposure to numerous environmental chemicals should be concerned about their suppressive effect of the immune response, rather than their therapeutic merit for autoimmune disease.
The ROR inverse agonistic activity obtained from our ROR assays well corresponded with the data for IL-17A mRNA expression obtained from RT-PCR analysis. Therefore, this study also indicates that our CHO-K1 cell-based reporter gene assay is highly suitable for identifying the effects on IL-17 production, via ROR, in immune cells by a large number of chemicals. Recently, a synthetic chemical SR1078 has been reported to be an agonist of RORα and RORγ (Wang et al., 2010b). Thus, in addition to identifying ROR inverse agonists, the study for identifying ROR agonists among numerous environmental chemicals is also required to prevent the acceleration of immune inflammation by environmental factors.
Acknowledgments
We would like to thank Dr. Kaoru Kobayashi (Faculty of Pharmaceutical Sciences, Chiba University, Japan) for providing the plasmids for LXRα/γ assays. This study was supported by Grant-in-Aid for Scientific Research (C) (21590675) from the Japan Society for the Promotion of Science (JSPS).
References
- Cui G, Qin X, Wu L, Zhang Y, Sheng X, Yu Q, Sheng H, Xi B, Zhang JZ, Zang YQ. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest. 2011;121:658–670. doi: 10.1172/JCI42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavini E, Menegola E. Are azole fungicides a teratogenic risk for human conceptus? Toxicol Lett. 2010;198:106–111. doi: 10.1016/j.toxlet.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994;8:538–553. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- He YW, Deftos ML, Ojala EW, Bevan MJ. RORγt, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9:797–806. doi: 10.1016/s1074-7613(00)80645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindinger C, Hinton DR, Kirwin SJ, Atkinson RD, Burnett ME, Bergmann CC, Stohlman SA. Liver X receptor activation decreases the severity of experimental autoimmune encephalomyelitis. J Neurosci Res. 2006;84:1225–1234. doi: 10.1002/jnr.21038. [DOI] [PubMed] [Google Scholar]
- Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RRV, Chow J, Manel N, Ciofani M, Kim SV, Cuesta A, Santori FR, Lafaille JJ, Xu HE, Gin DY, Rastinejad F, Littman DR. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Mckenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jetten AM. Retinoid-related orphan receptors (RORs): critical role in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:1–32. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2. [DOI] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. Crystal structure of the human RORα ligand binding domain in complex with cholesterol sulfate at 2.2 Å. J Biol Chem. 2004;279:14033–14038. doi: 10.1074/jbc.M400302200. [DOI] [PubMed] [Google Scholar]
- Klein ES, Pino ME, Johnson AT, Davies PJA, Nagpal S, Thacher SM, Krasinski G, Chandraratna RAS. Identification and functional separation of retinoic acid receptor neutral antagonists and inverse agonists. J Biol Chem. 1996;271:22692–22696. doi: 10.1074/jbc.271.37.22692. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Saito K, Takagi S, Chiba K. Ligand-dependent assembly of pregnane X receptor, constitutive androstane receptor and liver X receptor is applicable to identify ligands. Drug Metab Lett. 2010;4:88–94. doi: 10.2174/187231210791292744. [DOI] [PubMed] [Google Scholar]
- Kojima H, Iida M, Katsura E, Kanetoshi A, Hori Y, Kobayashi K. Effects of a diphenyl ether-type herbicide, chlornitrofen, and its amino derivative on androgen and estrogen receptor activities. Environ Health Perspect. 2003;111:497–502. doi: 10.1289/ehp.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect. 2004;112:524–531. doi: 10.1289/ehp.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Takeuchi S, Nagai T. Endocrine-disrupting potential of pesticides via nuclear receptors and aryl hydrocarbon receptor. J Health Sci. 2010;54:374–386. [Google Scholar]
- Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ Health Perspect. 2009;117:1210–1218. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez RD, Burris TP, Griffin PR. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluormethyl)ethyl]-b enzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-α/γ inverse agonist. Mol Pharmacol. 2010;77:228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MT, Dix DJ, Judson RS, Kavlock RJ, Reif DM, Richard AM, Rotroff DM, Romanov S, Medvedev A, Poltoratskaya N, Gambarian M, Moeser M, Makarov SS, Houck KA. Impact of environmental chemicals on key transcription regulators and correlation to toxicity end points within EPA’s ToxCast program. Chem Res Toxicol. 2010;23:578–590. doi: 10.1021/tx900325g. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. Th17 cell differentiation: The long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Kolls J, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood I, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt LA, Griffin PR, Burris TP. Ligands regulation of retinoic acid receptor-related orphan receptors: implications for development of novel therapeutics. Curr Opin Lipidol. 2010;21:204–211. doi: 10.1097/MOL.0b013e328338ca18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidović D, Schürer SC, Xu J, Wagoner G, Drew PD, Griffin PR, Burris TP. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, Kojima H. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors α and β, and androgen receptor. Toxicology. 2005;210:223–233. doi: 10.1016/j.tox.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Matsuda T, Kobayashi S, Takahashi T, Kojima H. In vitro screening of 200 pesticides for agonistic activity via mouse peroxisome proliferator-activated receptor (PPAR)α and PPARγ and quantitative analysis of in vivo induction pathway. Toxicol Appl Pharmacol. 2006;217:235–244. doi: 10.1016/j.taap.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Shiraishi F, Kitamura S, Kuroki H, Jin K, Kojima H. Characterization of steroid hormone receptor activities in 100 hydroxylated polychlorinated biphenyls, including congeners identified in humans. Toxicology. 2011;289:112–121. doi: 10.1016/j.tox.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takahashi T, Sawada Y, Iida M, Matsuda T, Kojima H. Comparative study on the nuclear hormone receptor activity of various phytochemicals and their metabolites by reporter gene assays using Chinese hamster ovary cells. Biol Pharm Bull. 2009;32:195–202. doi: 10.1248/bpb.32.195. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kumar N, Solt LA, Richardson TI, Helvering LM, Crumbley C, Garcia-Ordonez RD, Stayrook KR, Zhang X, Novick S, Chalmers MJ, Griffin PR, Burris TP. Modulation of retinoic acid receptor-related orphan receptor α and γ activity by 7-oxygenated sterol ligands. J Biol Chem. 2010a;285:5013–5025. doi: 10.1074/jbc.M109.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kumar N, Nuhant P, Cameron MD, Istrate MA, Roush WR, Griffin PR, Burris TP. Identification of SR1078, a synthetic agonist for the orphan nuclear receptor RORα and RORγ. ACS Chem Biol. 2010b;5:1029–1034. doi: 10.1021/cb100223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Xu J, Wagoner G, Douglas JC, Drew PD. Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J Leukoc Biol. 2009;86:401–409. doi: 10.1189/jlb.1008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wang X, Zhong B, Nurieva RI, Ding S, Dong C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORγt protein. J Biol Chem. 2011;286:22707–22710. doi: 10.1074/jbc.C111.250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Pappu B, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns K, Watowich SS, Tian Q, Jetten AM, Dong C. TH17 lineage differential is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarn JA, Brüschweiler BJ, Schlatter JR. Azole fungicides affect mammalian steroidogenesis by inhibitiong sterol 14α-demethylase and aromatase. Environ Health Perspect. 2003;111:255–261. doi: 10.1289/ehp.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]


