Abstract
BACKGROUND
Cowden syndrome is an autosomal-dominant condition associated with mutations in the tumor suppressor gene PTEN. Gynecologic malignancies are common with a 5–10% risk of endometrial cancer and 25–50% risk of breast cancer.
CASE
A 37-year-old woman with a history of breast cancer, other neoplasms, and multiple skin lesions was diagnosed with Cowden syndrome after a germline PTEN mutation was identified. The endometrium had high glucose uptake on positron emission tomography scan and was irregularly thickened on ultrasonography; biopsy revealed endometrial polyps and simple hyperplasia. Fifteen months later, hysteroscopy again confirmed numerous benign endometrial polyps.
CONCLUSION
Recurrent, multiple endometrial polyps portend a high risk of endometrial cancer in women with Cowden syndrome. Monitoring for malignancy and consideration of hysterectomy after childbearing is completed is warranted.
Cowden syndrome is an autosomal-dominant condition characterized by hamartomas arising from all three embryonic layers and an increased lifetime risk of developing several types of cancer.1 The prevalence of Cowden syndrome is thought to be at least one in 200,000. Eighty percent of cases arise from a germline mutation in the PTEN tumor suppressor gene located on chromosome 10q23.3.1 PTEN is an important tumor suppressor gene because it negatively regulates signaling through the PI3K/Akt pathway that normally stimulates cellular growth, proliferation, and migration. PTEN loss may also promote tumorigenesis through other mechanisms because it has been described to stabilize chromosomes, facilitate DNA repair, and stabilize cell cycle functions.2
Common clinical findings of Cowden Syndrome include macrocephaly, mucocutaneous lesions, and cancers of the breast, endometrium, and thyroid (Table 1). The lifetime risk for endometrial cancer is 5–10% and breast cancer is 25–50% in women with Cowden syndrome, compared with a risk in the general population of 2–4% and 12–13%, respectively.1 These risks, together with its heavy female preponderance, make gynecologists central in the management of patients with Cowden syndrome. Surveillance for endometrial cancer and treatment of endometrial polyps in women with Cowden syndrome and a history of breast cancer is a critical aspect of their care (Table 2). We present a case of a 37-year-old woman with a history of breast cancer at an early age that was diagnosed with Cowden syndrome and found to have recurrent endometrial polyps and hyperplasia.
Table 1.
International Cowden Consortium Diagnostic Criteria
| Pathognomonic Criteria | Major Criteria | Minor Criteria |
|---|---|---|
| Adult-Lhermitte-Duclos disease (LDD) | Breast carcinoma | Other thyroid lesions (adenoma or multinodular goiter) |
| Mucocutaneous lesions | Thyroid carcinoma (especially follicular) | Mental retardation (IQ 76 or less) |
| Facial trichilemmomas | Macrocephaly (occipital frontal circumference 97th percentile or greater) | Gastrointestinal hamartomas |
| Acral keratoses | Endometrial carcinoma | Lipomas |
| Papillomatous lesions | Fibrocystic disease of the breast | |
| Uterine fibroids | ||
| Fibromas | ||
| Genitourinary tumors or malformations | ||
| Operational diagnosis | ||
| Mucocutaneous lesions alone in conjunction with | ||
| Six or more facial papules (three or more trichilemmomas) or | ||
| Cutaneous facial papules and oral mucosal papillomatosis or | ||
| Oral mucosal papillomatosis and acral keratoses or | ||
| Six of more palmoplantar keratoses | ||
| Two or more major criteria | ||
| One major criteria and three minor criteria | ||
| Four minor criteria | ||
| For individuals in a family in which one relative is diagnostic for Cowden syndrome: | ||
| A pathognomonic criterion | ||
| Any one major criteria with or without minor criteria | ||
| Two minor criteria | ||
| History of Bannayan-Riley-Ruvalcaba syndrome | ||
Reprinted by permission from Macmillan Publishers Ltd: Blumenthal GM, Dennis PA. PTEN hamartoma tumor syndromes. Eur J Hum Genet 2008;16:1289–300.
Table 2.
Breast and Endometrial Cancer Screening Guidelines in Cowden Syndrome Advocated by the National Comprehensive Screening Network 2007
| Type of Screening | When? | How Often? |
|---|---|---|
| Women | ||
| Breast self-examination | Age 18 y | Monthly |
| Clinical breast examination | Age 25 or 5–10 y before earliest known breast cancer in the family | Every 6 mo |
| Mammogram and breast MRI | Age 30–35 or 5 y before earliest known breast cancer in the family | Every 12 mo |
| Blind endometrial biopsy | Age 30–35 or 5 y before earliest diagnosis of endometrial cancer in family until menopause | Every 12 mo |
| Endometrial ultrasound examination | Postmenopause | Every 12 mo |
MRI, magnetic resonance imaging.
Modified by permission from Macmillan Publishers Ltd: Blumenthal GM, Dennis PA. PTEN hamartoma tumor syndromes. Eur J Hum Genet 2008;16:1289–300.
CASE
A 37-year-old woman, para 0, was diagnosed with Cowden syndrome using clinical diagnostic criteria1 after she presented with numerous characteristic skin lesions and a history of multiple neoplasms at younger than expected ages. She was subsequently found to have the PTEN germline mutation that confirmed her diagnosis. Her medical history included classical mixed cellularity Hodgkin’s lymphoma stage IIA diagnosed at the age of 19 years and treated with mantlefield irradiation. She relapsed 6 months after the radiation therapy but subsequently responded fully to alternating Mechlorethamine, Vincristine (Oncovin), Procar-bazine, Prednisone and Doxorubicin (Adriamycin), Bleomycin, Vinblastine, Dacar-bazine1 chemotherapy. She also underwent two partial thyroidectomies at ages 21 and 37 years for benign thyroid neoplasms. She then developed a large stage IIB estrogen receptor-positive, progesterone receptor-positive and HER2-negative left-sided breast cancer at 29 years of age, which was treated with preoperative chemotherapy (neoadjuvant doxorubicin and cyclophosphamide followed by docetaxel) followed by a left-sided mastectomy, which confirmed zero of 23 positive lymph nodes. Postsurgical treatment consisted of radiotherapy and a 5-year course of tamoxifen. A prophylactic mastectomy on the contralateral breast was subsequently performed. As part of her care at the National Institutes of Health, she participated in an 8-week trial on the safety and potential efficacy of sirolimus in decreasing the skin lesions caused by Cowden syndrome with her skin lesions successfully decreasing in size during this brief treatment.
The patient was first referred to the gynecology consult service for an assessment of her endometrium, which was strongly positive for glucose metabolism on positron emission tomography scan performed as part of the clinical trial 2 months after completing the 5-year course of tamoxifen. Because of the strongly positive positron emission tomography scan, a transvaginal ultrasonogram was done. On ultrasonography, a thickened endometrium of 28 mm with irregular contours was noted. By history, she reported experiencing heavy menses, and, in light of her positron emission tomography and ultrasound findings as well as recent tamoxifen use and high risk of endometrial cancer, the patient underwent a vacuum curettage endometrial biopsy. This biopsy demonstrated polyps and simple endometrial hyperplasia without atypia.
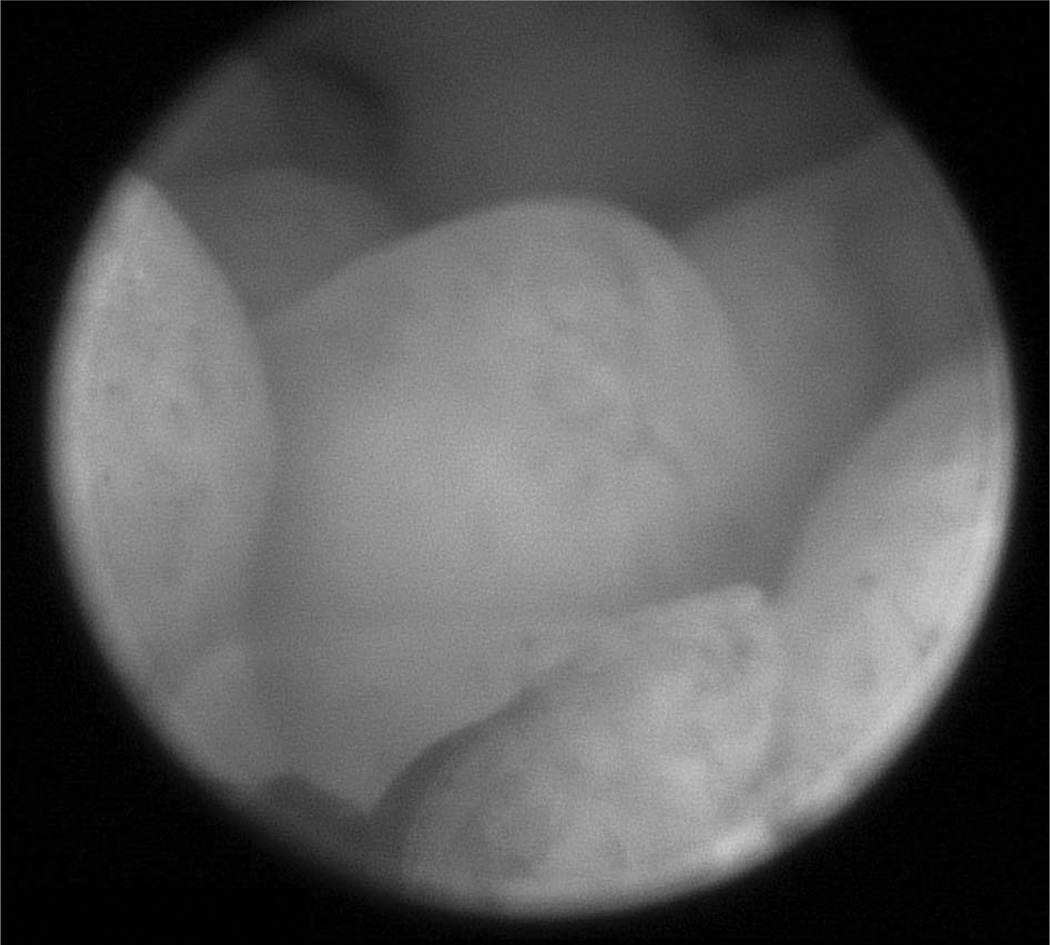
On follow-up with the gynecology consult service 15 months later, her menstrual periods were of normal flow. During this visit, in a discussion regarding childbearing, the patient was unsure whether she wanted to bear children because of her history of breast cancer and endometrial polyps. The risks and benefits of a total abdominal hysterectomy and bilateral salpingo-oophorectomy to lower her risk of both endometrial and ovarian cancer were discussed. A second transvaginal ultrasonogram was performed, which again revealed a thickened endometrium of 18 mm with an irregular contour. Given the thickened endometrium on ultrasonography, the prior biopsy results, continued high risk of endometrial abnormalities and cancer, and desire to know whether the endometrium contained one or many polyps, the patient underwent a hysteroscopy to view and sample the endometrial cavity (Fig. 1). Bipolar electrocautery resectoscope and subsequent curettage were used to remove as many of the endometrial polyps as possible. The uterus was sharply anteverted making hysteroscopic removal of polyps from the anterior wall of the uterus technically difficult. Pathological review confirmed simple endometrial hyperplasia with no atypia.
Fig. 1.
Multiple polyps seen on hysteroscopic view of the uterus.
Kalin. Cowden Syndrome, Polyps, and Breast Cancer. Obstet Gynecol 2013.
Because many polyps were noted on hysteroscopy, the patient was counseled that the polyps would again recur soon, so the optimal time to attempt pregnancy was right after hysteroscopic resection. The patient and her husband may forgo attempting pregnancy. However, she is not yet ready to undergo hysterectomy. Given the recent history of breast cancer and the proclivity for having other tumors because of the PTEN mutation, the decision was made to avoid use of an extended course of progestins because this might increase her risk of breast cancer recurrence. Annual monitoring of transvaginal ultrasonography and sampling by hysteroscopy and biopsy was planned.
COMMENT
This patient presented in her late 30s with a history of breast cancer, other tumors, and recently diagnosed, recurrent endometrial polyps with simple hyperplasia without atypia in the setting of Cowden syndrome. The finding of multiple polyps on hysteroscopy underscores the field effect of PTEN loss in uterine tissue that promotes cellular proliferation and the need for active management of these patients. Thus, the screening options considered for endometrial cancer included close observation with annual endometrial sampling while she is premenopausal and annual pelvic ultrasonography if she becomes postmenopausal but has not undergone hysterectomy. Treatment options considered for recurrent polyps and to limit endometrial cancer risk included annual hysteroscopic polypectomy, progestin therapy, or hysterectomy with bilateral salpingo-oophorectomy. Childbearing in the setting of recurrent polyps, a history of breast cancer, and Cowden syndrome were also addressed.
Given the autosomal-dominant inheritance pattern of Cowden syndrome, it is important to consider the risk of any offspring developing the condition. Thus, each child of an affected parent has a 50% chance of inheriting the PTEN mutation and developing the syndrome. Hence, the involvement of genetic consultants and counselors in helping affected patients decide about future pregnancies is important. The use of donor eggs or in vitro fertilization followed by preimplantation genetic testing is an option for couples who opt to have children but do not want to risk passing on the PTEN mutation.
A second factor for this patient to consider in planning pregnancy was her developing multiple polyps approximately 1 year after prior treatment. The rapid recurrence of a currently benign multiple neoplasms illustrates an abnormal endometrium that may compromise establishing a pregnancy. Timing polypectomy just before attempting pregnancy may be advised. The use of a pregnancy surrogate may also be considered.
Another factor this patient faces in considering pregnancy is her history of breast cancer and risks faced for breast cancer recurrence. If she and her husband were to opt for fertility treatments involving ovulation induction, ovarian stimulation resulting in high levels of estradiol may directly stimulate breast cancer cells and is therefore considered unsafe in estrogen-sensitive breast cancer.3 Alternatives such as gonadotropins combined with tamoxifen and third-generation aromatase inhibitors (letrozole) are effective in inducing ovulation while showing no significant increase in breast cancer recurrence rates.4
The usual risk of progression of endometrial hyperplasia to endometrial cancer ranges from less than 2% in simple hyperplasia to approximately 20% in severe hyperplasia with atypia.5 The overall risk is higher in patients with Cowden syndrome who have endometrial hyperplasia. Indeed, animal studies have shown that 22% of pten+/− mice with endometrial hyperplasia progressed to endometrial carcinoma.6 Furthermore, although atypical endometrial hyperplasia can be effectively treated with progestins, management of endometrial hyperplasia occurring in recurrent, multiple endometrial polyps in women with Cowden syndrome and a history of breast cancer is more complex. Because progestins have been implicated in the pathogenesis of breast cancer, the extended use of local or systemic progestins in such patients would be contraindicated.7 Thus, in light of the patient’s history of breast cancer and need for extended use of progestins, hormone therapy was considered and rejected. Close follow-up with annual ultrasonograms and endometrial sampling was the preferred management.
Given the high risk of endometrial cancer in Cowden syndrome, patients with recurrent endometrial hyperplasia and polyps may consider having total hysterectomy with bilateral salpingo-oophorectomy for surgical treatment. Postponing surgery until childbearing decisions are addressed is important. The higher risk of morbidity and mortality in women who have undergone oophorectomy before age 45 years and not taken hormone replacement suggests that surgery may be postponed until age 45 years.8 Estrogen therapy after surgery may be considered in those having surgery before age 45 years.
Women with Cowden syndrome are at high risk of both breast and endometrial cancer, which complicates their management, because treatment options for one may affect the other. Considerations regarding the desire for childbearing in the context of an inherited condition, recurrent endometrial polyps, risk of endometrial cancer, and history of breast cancer make gynecologists central to the management of patients with Cowden syndrome. In addition to an important role in annual monitoring for malignancy using ultrasonography, hysteroscopy, and sampling the endometrium, gynecologists also have a unique opportunity to diagnose Cowden syndrome in patients who present with early-onset breast cancer or multiple endometrial polyps.
Acknowledgments
Funded, in part, by the Intramural Program of the National Institutes of Health, National Cancer Institute, Clinical Center, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health Intramural Office of Rare Diseases, the National Human Genome Research Institute, and clinical trial NCT00971789. Dr Kalin was supported by Magdalen College, Oxford, UK.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Blumenthal GM, Dennis PA. PTEN hamartoma tumor syndromes. Eur J Hum Genet. 2008;16:1289–1300. doi: 10.1038/ejhg.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Planchon SM, Waite KA, Eng C. The nuclear affairs of PTEN. J Cell Sci. 2008;121:249–253. doi: 10.1242/jcs.022459. [DOI] [PubMed] [Google Scholar]
- 3.Maltaris T, Weilgel M, Mueller A, Schmidt M, Seufert R, Fischl F, et al. Cancer and fertility preservation: fertility preservation in breast cancer patients. Breast Cancer Res. 2008;10:206. doi: 10.1186/bcr1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotrophin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery BE, Daum GS, Dunton CJ. Endometrial hyperplasia: a review. Obstet Gynecol Surv. 2004;59:368–378. doi: 10.1097/00006254-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Stambolic V, Tsao M-S, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden Syndrome in pten+/− mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- 7.Aupperlee M, Osuch J, Haslam S. Progestins and breast cancer. Breast Dis. 2005–2006;24:37–57. doi: 10.3233/bd-2006-24104. [DOI] [PubMed] [Google Scholar]
- 8.Berek JS, Chalas E, Edelson M, Moore DH, Burke WM, Cliby WA, et al. Prophylactic and risk-reducing bilateral salpin-go-oophorectomy: recommendations based on risk of ovarian cancer. Obstet Gynecol. 2010;116:733–743. doi: 10.1097/AOG.0b013e3181ec5fc1. [DOI] [PubMed] [Google Scholar]