Abstract
Mycobacterium tuberculosis (Mtb) extracellular DNA (eDNA) gains access to the host cell cytosol via the ESX-1 secretion system. It is puzzling that this eDNA of Mtb does not induce activation of the AIM2-inflammasome since AIM2 recognizes cytosolic DNA. Here we show that non-virulent mycobacteria such as M. smegmatis induce AIM2-inflammasome activation, which is dependent upon their strong induction of IFN-β production. In contrast, Mtb, but not an ESX-1 deficient mutant, inhibits the AIM2-inflammasome activation induced by either M. smegmatis or transfected dsDNA. The inhibition does not involve changes in host cell AIM2 mRNA or protein levels but led to decreased activation of caspase-1. We furthermore demonstrate that Mtb inhibits IFN-β production and signaling, which was partially responsible for the inhibition of AIM2 activation. In conclusion, we report a novel immune evasion mechanism of Mtb that involves the ESX-1-dependent, direct or indirect, suppression of the host cell AIM2-inflammasome activation during infection.
Introduction
IL-1β is important for host immune defense against Mtb, since several studies demonstrated that IL-1β and IL-1-receptor knock-out mice are more susceptible to Mtb infections (1, 2). In macrophages and dendritic cells the production of mature IL-1β is dependent on activation of the inflammasome (3). The NLR (nucleotide-binding and oligomerization domain, leucine-rich-repeat-containing) proteins such as NLRP3 and NLRC4 are one family of cytosolic receptors, which upon ligand binding mediate inflammasome activation. In the case of Mtb the sole NLR capable of inducing inflammasome activation is NLRP3 (1, 2, 4–7).
The significance of type I IFN signaling for activation of inflammasome responses was first reported for Francisella infected macrophages (8). Nevertheless, during the course of Mtb infections IFN-β has the opposite effect and suppresses activation of the NLRP3 inflammasome, suggesting that induction of IFN-β could correlate with increased virulence (9, 10). Mice deficient in IRF-3, a major signaling component of the type I IFN host cell signaling pathway, are much more resistant to Mtb infections (11). The induction of host cell type I IFN signaling after Mtb infection is dependent upon the type VII secretion system (ESX-1) mediated translocation of Mtb extracellular DNA (eDNA) into the host cell cytosol (11, 12). It is confounding that this cytosolic Mtb DNA is not recognized by the host cell inflammasome component Absent In Melanoma 2 (AIM2) which should lead to subsequent inflammasome activation. AIM2 binds to double stranded (ds) DNA of intracellular pathogens such as Francisella and Listeria (13, 14). There is evidence that transfected Mtb dsDNA can interact with AIM2 and activate the AIM2 inflammasome and that AIM2 is important for host resistance to Mtb infection (15).
Material and Methods
Cell culture and animals
C57Bl/6 WT mice were obtained from The Jackson Laboratories. Nlrc4−/−, Nlrp3−/−, Asc−/−, Nlrp6−/−, Nlrp10−/− mice were provided by Dr. R. Flavell and Millennium Pharmaceuticals. Aim2−/− (16) and Aim2/Nlrp3−/− double knockout mice were obtained from Dr. K. A. Fitzgerald. The Ifnar1−/− mice from Dr. A. Sher (NIH). IFN-β−/− mice were provided by Dr. S. N. Vogel. All studies were approved by the IACUC and were conducted in accordance with the National Institutes of Health Guide.
Bacteria
M. smegmatis (mc2155), M. tuberculosis H37Rv (ATCC 25618), H37Ra (ATC25177) were obtained from Dr. W.R. Jacobs Jr. (AECOM). M. fortuitum (ATCC 6841) and M. kansasii strain Hauduroy (ATCC 12478) were obtained from ATCC. Mtb ΔesxA and ΔexoU PAK (P. aeruginosa) were kindly provided by Dr. L. Gao and Dr. V. Lee. M. smegmatis Δesx1, ΔeccCb mutant, ΔeccCb complemented strains were kind gifts of Dr. K. Derbyshire (17). F. tularenesis Live Vaccine Strain (LVS) was obtained from Dr. Kevin McIver.
Ex vivo infection
Bacterial infections of BMDCs and BMDMs were performed as described (7, 18). For induction of AIM2 inflammasome, BMDCs were pre-treated with 20ng/ml LPS (Invivogen) for 1 hour and then infected with H37Rv for 4 hours. Infected BMDCs were then washed twice with PBS and transfected with 0.5ug/ml poly (dA:dT) (Sigma) using Lipofectamine LTX Plus reagent (Invitrogen) for 2 hours. The transfection was performed according to the manufacturer’s instructions and the supernatants were harvested 2 hours post transfection.
IFN-β Neutralization
BMDCs from C57Bl/6 mice were treated with anti-IFN-β neutralizing antibody 7F-D3 (5 μg/ml) (Abcam) for 1 hour and infected with M. smegmatis at MOI 10:1 for two hours as previously described. Cells were then washed with PBS and incubated for an additional 20 h in DMEM chase media. Supernatants were collected for ELISA.
Cell death assays
The adenylate kinase (AK) release assay, Toxilight®BioAssay (Lonza) was used to quantify necrotic cell death. The assay was performed according to the manufacturer’s instructions.
Cytokine Measurement and Immunoblotting
ELISA was used to measure secreted IL-1β (BD Biosciences) and IFN-β LEGEND MAX™ (BioLegend) respectively. For immunoblotting the cell lysate preparation and western blotting was performed as described earlier (7). The primary antibodies used were: anti-IL-1β (R&D systems) at 0.15μg/ml in 0.1% BSA, anti-caspase 1 (Santa Cruz) at 1:300, anti-AIM2 (Santa Cruz) at 1:500, anti-Tubulin (Cell Signaling) at 1:1000. The above 3 antibodies were diluted in 5% milk with TBST. The secondary antibodies used were: Donkey anti-goat (Jackson) at 1:25,000, goat anti-rabbit (Jackson) at 1:50,000 and goat anti-mouse (Jackson) at 1:50,000 dilutions respectively.
Real time PCR
BMDCs were harvested 8 hours post-infection using TRIzol (Invitrogen). Real time PCR was done using SYBR green PCR master mix (Roche) with GAPDH as the house keeping gene. The primers used were: Aim2: 5′-GTCACCAGTTCCTCAGTTGT-3′ and 5′-CACCTCCATTGTCCCTGTTTTAT-3′ Gapdh: 5′-ATGGGATTTCCATTGATGACA-3′ and 5′-CCACCCATGGCAAATTCC-3′; Mx1: 5′-TGTGCAGGCACTATGAGGAG-3′ and 5′-ACTCTGGTCCCCAATGACAG-3′; PKR: 5′-GCACCGGGTTTTGTATCGA-3′ and 5′-GGAGCACGAAGTACAAGCGC-3′.
Statistical analysis
Statistical analysis was performed on at least three independent experiments using GraphPad Prism 5.0 software and One-way ANOVA with Tukey’s post-test unless otherwise noted in the figure legends. Shown are representative results of triplicate values with standard deviation. The range of p-values is indicated as follows: * 0.01<p<0.05; ** 0.001<p<0.01 and *** 0.0001<p<0.001.
Results and Discussion
Non-virulent mycobacteria induce AIM2 inflammasome activation
The non-virulent mycobacterial species such as M. smegmatis (Msme) induce a potent pro-inflammatory immune response and host cell apoptosis when compared to more virulent mycobacterial species (18) but the activation of host cell inflammasome mediated by Msme infection has not been analyzed. BMDCs of various mouse strains were infected with Msme and the amount of IL-1β in the supernatant was detected and normalized to IL-1β levels secreted by WT BMDCs (Fig. 1A). There were no differences in cell lysis as determined by AK release assay and pro-IL-1β ELISA (not shown). Surprisingly, in NLRP3−/− cells the amount of secreted IL-1β dropped by less than 50%. This is unexpected because the inflammasome activation in Mtb is completely dependent upon the presence of NLRP3 (1, 2, 4, 5). The NLRP6, NLRP12, NLRC4, and NLRP10 did not significantly contribute to Msme-induced inflammasome activation (Fig.1A). Interestingly however, the deficiency of AIM2, resulted in approximately 40% reduction in IL-1® response in BMDCs (Fig.1A) and almost 75% reduction in BMDMs (Fig.1B). The partial reductions in IL-1β secretion suggested redundancy between AIM2 and NLRP3 pathways. Consistently, the IL-1β production in Aim2/Nlrp3−/− cells was further reduced when compared to any of the single deletions; surprisingly though, Msme infected Aim2/Nlrp3−/− BMDCs were still able to secrete up to 40% of the IL-1β (Fig 1A). This result suggests recognition of Msme cytosolic components by one or more unidentified NLRs. The Mtb-induced activation of NLRP3 is dependent upon the Mtb ESX-1 secretion system (7, 19, 20). The core ESX-1 secretion complex is conserved in Msme making it a compelling model to study the mechanisms of ESX-1-mediated protein secretion (21, 22). Similar to Mtb a functional Msme ESX-1 secretion system is required for maximal response of AIM2/NLRP3 dependent secretion of IL-1β since two different Msme mutants with defective ESX-1 show an almost 50% reduction of IL-1β secretion when compared to WT Msme in WT BMDCs but that difference is abolished in Aim2/Nlrp3−/− BMDCs (Fig. 1C).
FIGURE 1.
M. smegmatis (Msme) activates the AIM2 inflammasome via a partially ESX-1 dependent mechanism. (A) BMDCs and (B) BMDMs from various indicated knockout mice were either left uninfected (UI, grey bars) or infected with Msme (black bars). The cell supernatants were harvested 16 hours post infection (hpi) and analyzed for IL-1β secretion by ELISA. (C) BMDCs from WT and Aim2/Nlrp3−/− mice were infected with different Msme strains (Msme, Δesx1, ΔeccCb, compl) and the supernatants were analyzed for IL-1β secretion. Data are shown as the mean and standard deviation of triplicate measurements of one representative experiment out of three except for 1B and 1C where the data is one representative experiment out of two.
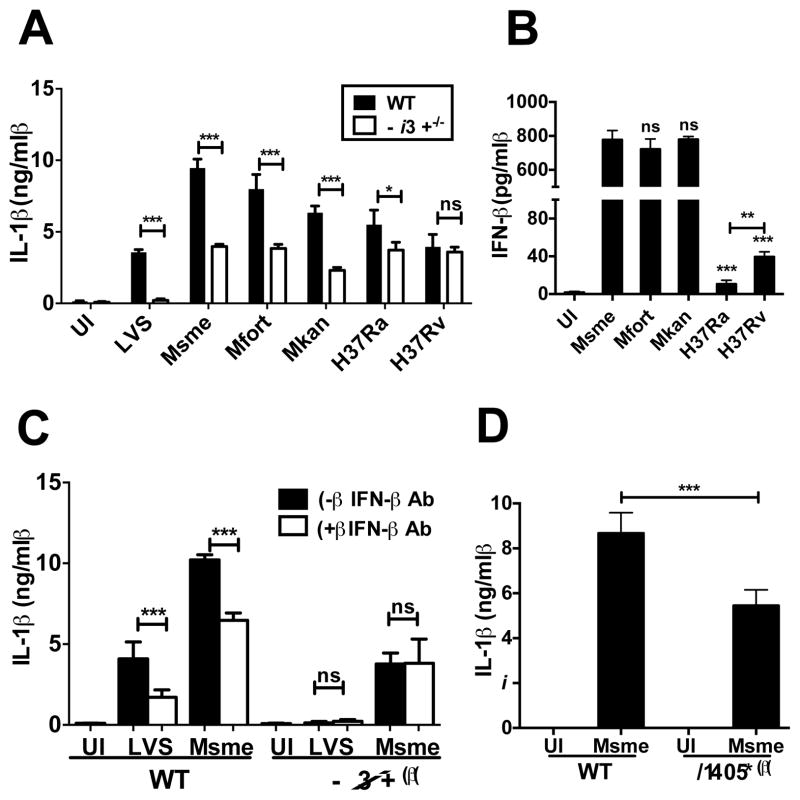
Next, we wanted to investigate if other mycobacterial species activate the AIM2 inflammasome. We thus infected WT and AIM2-deficient BMDCs with Msme, M. fortuitum (Mfort), M. kansasii (Mkan), the attenuated M. tuberculosis H37Ra and the virulent M. tuberculosis H37Rv. We monitored cell lysis (not shown) and IL-1β secretion for all the infections (Fig. 2A). In general, there was an inverse correlation between virulence of the species and the amount of IL-1β induction, with the least virulent species inducing the most IL-1β in WT BMDCs (Fig. 2A). The infection of AIM2-deficient BMDCs by these mycobacterial species allowed determining the fraction of total IL-1β secretion that was dependent upon presence of AIM2. As expected the Francisella-induced inflammasome activation was completely dependent on AIM2, whereas about 40–50% of the Msme, Mfort and Mkan induction was dependent on AIM2 (Fig. 2A). The attenuated Mtb strain H37Ra induced the lowest amount of AIM2 inflammasome activation and the virulent Mtb strains H37Rv did not induce any AIM2 activation (Fig. 2A).
FIGURE 2.
Non-virulent mycobacteria induce IFN-β dependent AIM2 inflammasome activation. (A) WT and Aim2−/− BMDCs were infected with Msme, M. fortuitum (Mfort), M. kansasii (Mkan), attenuated M. tuberculosis H37Ra and virulent M. tuberculosis H37Rv. Francisella Live Vaccine Strain (LVS) infection is used as a positive control. The secreted IL-1β was measured at 16hpi by ELISA. (B) IFN-β ELISA of supernatants from WT BMDCs infected with indicated mycobacterial species. (C) IL-1β ELISA of supernatants from WT and Aim2−/− Msme infected BMDCs in the presence or absence of IFN-β neutralizing antibodies. LVS is used as a positive control. (D) IL-1β ELISA of supernatants from Ifnar1−/− or WT BMDCs infected with Msme or left uninfected. Data is shown as the mean and standard deviation of triplicate measurements of one representative experiment out of three.
Non-virulent mycobacteria induce IFN-β dependent AIM2 inflammasome activation
The AIM2 inflammasome activation by Francisella is stimulated by IFN-β (8). In order to investigate the potential importance of IFN-β in AIM2-inflammasome activation by non-virulent mycobacteria we analyzed the supernatants of BMDCs infected with various mycobacterial species for IFN-β production. Interestingly, the three species (Msme, Mfort and Mkan) that mediated the strongest AIM2-inflammasome activation also induce a very pronounced IFN-β production of about 800pg/ml which was a 20 fold increase over the amount of IFN-β produced by BMDCs after infection with Mtb H37Rv (Fig. 2B). In order to demonstrate that this IFN-β secretion is important for AIM2-inflammasome activation we infected BMDCs from WT and Aim2−/− mice with F. tularensis LVS and Msme in the absence or presence of neutralizing IFN-β antibodies and measured the IL-1β secretion after 24h (Fig. 2C). The addition of neutralizing IFN-β antibodies significantly reduced the IL-1β secretion after infection with either F. tularensis LVS or Msme (Fig. 2C). Consistently, the production of IL-1β was reduced by similar levels when BMDCs of WT or Ifnar1−/− mice were infected (Fig.2D). The neutralization of IFN-β had no effect on the IL-1β production after F. tularensis LVS or Msme infection of Aim2−/− BMDCs. These results suggest that IFN-β plays a similar role in the induction of AIM2 inflammasome activation by non-virulent mycobacterial species as it does after Francisella infections.
Mtb inhibits AIM2 dependent IL-1β production
Finally, we addressed the hypothesis that Mtb can actively inhibit AIM2 inflammasome activation by performing mixed infection experiments. BMDCs from Nlrp3−/− mice were infected with Mtb or the esxA deletion Mtb mutant (MtbΔesxA) and either 4 or 8 hours post infection (hpi) the supernatants were harvested for analysis of IL-1β secretion (Fig. 3A and B). In Msme-infected BMDCs we detected about 100 pg/ml after 4hpi and 3000 pg/ml after 8hpi. Interestingly, when BMDCs, which had been infected with Mtb, were challenged with Msme we observed a 2–3fold reduction in the amount of secreted IL-1β when compared to BMDCs infected only with Msme. The Mtb mediated inhibition was dependent on functional ESX-1 secretion system since infection with the MtbΔesxA mutant did not inhibit IL-1β secretion after challenge with Msme (Fig. 3A and B). The rate of infection of Msme was not affected by the prior infection with Mtb as analyzed via flow cytometry using GFP-labeled Msme (Fig. S1A). A similar inhibition by Mtb was also observed for IL-18 induced by Msme infections (Fig.S1B). There were no differences in necrosis induction at 8hpi (Fig. 3B). To confirm the specific inhibition of the AIM2 inflammasome, we transfected the Mtb or MtbΔesxA infected Nlrp3−/− BMDCs with 0.5μg/ml of poly(dA:dT) in the absence (Fig. 3C) or presence (Fig. 3D) of LPS pre-treatment. Consistent with the previous finding, analysis of IL-1β showed that Mtb does inhibit the activation of the AIM2 inflammasome when compared to MtbΔesxA infected cells. In addition, we determined that Mtb was not able to inhibit NLRC4 inflammasome activation by Pseudomonas aerigunosa (Fig. S2).
FIGURE 3.
Mtb inhibits AIM2 dependent IL-1β production. Nlrp3−/− BMDCs were first infected with Mtb or the Mtb ΔesxA mutant and then with Msme. Secreted IL-1β (blue, filled bars) was measured by ELISA at (A) 4hpi (B) 8hpi. Necrotic cell death (red, striped bars) was assayed and is represented as fold change over uninfected (UI). Nlrp3−/− BMDCs in the (C) absence or (D) presence of LPS pre-treatment were first infected with Mtb or the Mtb ΔesxA mutant and then transfected with poly (dA:dT) for 2 hours. Both secretion of IL-1β and necrosis were measured as before. Data is shown as the mean and standard deviation of triplicate measurements of one representative experiment out of three.
Finally, to further support our hypothesis that Mtb mediates inhibition of AIM2 inflammasome activation we started to investigate the mechanism of this inhibition. First we determined that there were no significant changes of Aim2 transcription mediated by Mtb infection (not shown). Consistently, immunoblots of cell lysates from infected BMDCs at 6hpi showed that there is no difference in AIM2 protein expression (Fig. 4A). Also, the protein expression of pro-IL-1β and pro-caspase-1 was not affected by Mtb. However, immunoblots of the corresponding supernatants showed that there is decreased secretion of p10 fragment of caspase-1 and the mature IL-1β (p17) fragment in Mtb infected cells challenged with Msme when compared to Msme or MtbΔesxA/Msme infected cells (Fig. 4A). Interestingly, Mtb infection reduced the amount of Msme-induced IFN-β secretion in an ESX-1-dependent manner (Fig.4B). Even the addition of high amounts of exogenous IFN-β (400ng/ml) could only partially overcome the Mtb-mediated inhibition of Msme-induced IL-1β secretion (Fig. 4C). These results demonstrate that Mtb is able to limit IFN-β production in infected host cells, which may explain some of its capacity to inhibit the IFN-β-dependent AIM2 inflammasome activation. To investigate if Mtb may also inhibit IFN-β signaling we used IFN-β−/− BMDCs and infected them with Mtb and MtbΔesxA followed by treatment with IFN-β and the transcription of IFN-β–inducible genes Mx1 and PKR was analyzed by qRT-PCR (Fig. 4D+E). In both cases Mtb infection reduced the IFN-β mediated increase in transcription but this inhibition was not dependent upon ESX-1, since the MtbΔesxA mutant showed a similar reduction. The inhibition of IL-1β production by Mtb has been reported before (23) but the inhibition of IFN-β signaling by Mtb has not been shown previously to our knowledge.
FIGURE 4.
Mechanism of AIM2 inflammasome inhibition. Nlrp3−/− BMDCs were infected first with Mtb or the Mtb ΔesxA mutant and then with Msme. In (A) Western blots of supernatants (SN) showing active cleavage fragments of caspase-1 (p10) and IL-1β (p17) and cell lysates (CL) detecting pro-caspase-1 (p45), pro-IL-1β (p35), tubulin (p55) and AIM2 (p38) protein levels. (B) Secreted IFN-β was measured from supernatants collected 8hpi by ELISA. (C) Secreted IL-1β from supernatants of untreated Nlrp3−/− BMDCs or those treated with IFN-β (400ng/ml) and then infected as indicated was measured 8hpi by ELISA. (D+E) IFN-β−/− mice were infected with indicated bacteria and treated or not with IFN-β (700pg/ml) and the mRNA levels of Mx1 and PKR were analyzed after 4h via qRT-PCR. Data is of one representative experiment out of three.
The precise molecular mechanism of the Mtb-mediated AIM2 inflammasome inhibition remains to be elucidated. It seems unlikely that limiting IFN-β production is the only pathway for Mtb to suppress AIM2 inflammasome activation since external addition of IFN-β did not induce IL-1β secretion in Mtb infected Nlrp3−/− BMDCs (Fig.4C) and only partially restored the Mtb-mediated inhibition of Msme induced IL-1β. Hence, Mtb may secrete another effector that could inhibit signaling of the IFN-α/β receptor and/or directly modify AIM2 inflammasome activation. Indeed we provide evidence that Mtb inhibits IFN-β signaling. A detailed analysis of the large number of IFN-β regulated genes may reveal a subset whose expression can only be inhibited by Mtb with a functional ESX-1 system. This subset of genes would be the most likely to contain candidates for mediating the AIM2-inflammasome inhibition. Overall, the co-secretion into the host cell cytosol of Mtb eDNA and a putative AIM2-inhibitor and/or IFN-β signaling inhibitor via the ESX-1 system may allow Mtb to take advantage of the type I IFN-mediated inhibition of the NLRP3-inflammasome without the Mtb eDNA inducing activation of the AIM2-inflammasome. The recent finding that Aim2−/− mice are very susceptible to Mtb infections supports the potential role of AIM2 inflammasome inhibition for optimal virulence of Mtb (15). Our discovery of a novel immune evasion mechanism engaged by Mtb opens the door for investigations into the identification of the Mtb genes involved in this inhibition and subsequent analysis of their importance for virulence of Mtb.
Supplementary Material
Acknowledgments
The work was supported by the NIH grants AI072584 (S.S., A.B., V.B.), AI087630 (F.S.S.), AI083713 (K.A.F.), AI067497 (K.A.F.), AI018797 (S.N.V.), T32 AI09621 (S.E.A.) and a Veterans Administration Merit Review Grant IBX000167A (F.S.S.)
We thank Mrs. Vickie Knepper-Adrian and Lisa Waggoner for technical assistance.
Abbreviations
- eDNA
extracellular DNA
- BMDM
bone marrow-derived macrophages
- BMDC
bone marrow-derived dendritic cells
- hpi
hours post infection
- MOI
multiplicity of infection
- AK
adenylate kinase
- WT
wild-type
References
- 1.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElvania Tekippe E, Allen IC, Hulseberg PD, Sullivan JT, McCann JR, Sandor M, Braunstein M, Ting JP. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS One. 2010;5:e12320. doi: 10.1371/journal.pone.0012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, Anes E. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046–1063. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 5.Dorhoi A, Nouailles G, Jorg S, Hagens K, Heinemann E, Pradl L, Oberbeck-Muller D, Duque-Correa MA, Reece ST, Ruland J, Brosch R, Tschopp J, Gross O, Kaufmann SH. Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur J Immunol. 2011 doi: 10.1002/eji.201141548. [DOI] [PubMed] [Google Scholar]
- 6.Wong KW, Jacobs WR., Jr Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell Microbiol. 2011;13:1371–1384. doi: 10.1111/j.1462-5822.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdalla H, Srinivasan L, Shah S, Mayer-Barber KD, Sher A, Sutterwala FS, Briken V. Mycobacterium tuberculosis infection of dendritic cells leads to partially caspase-1/11-independent IL-1β and IL-18 secretion but not to pyroptosis. PLoS ONE. 2012 doi: 10.1371/journal.pone.0040722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novikov A, Cardone M, Thompson R, Shenderov K, Kirschman KD, Mayer-Barber KD, Myers TG, Rabin RL, Trinchieri G, Sher A, Feng CG. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1beta production in human macrophages. J Immunol. 2011;187:2540–2547. doi: 10.4049/jimmunol.1100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe. 2012;11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 13.Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7:412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M, Yamamoto M, Takeda K. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int Immunol. 2012 doi: 10.1093/intimm/dxs062. [DOI] [PubMed] [Google Scholar]
- 16.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flint JL, Kowalski JC, Karnati PK, Derbyshire KM. The RD1 virulence locus of Mycobacterium tuberculosis regulates DNA transfer in Mycobacterium smegmatis. Proc Natl Acad Sci U S A. 2004;101:12598–12603. doi: 10.1073/pnas.0404892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohsali A, Abdalla H, Velmurugan K, Briken V. The non-pathogenic mycobacteria M. smegmatis and M. fortuitum induce rapid host cell apoptosis via a caspase-3 and TNF dependent pathway. BMC Microbiol. 2010;10:237. doi: 10.1186/1471-2180-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanley SA, Raghavan S, Hwang WW, Cox JS. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci U S A. 2003;100:13001–13006. doi: 10.1073/pnas.2235593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurenuma T, Kawamura I, Hara H, Uchiyama R, Daim S, Dewamitta SR, Sakai S, Tsuchiya K, Nomura T, Mitsuyama M. The RD1 locus in the Mycobacterium tuberculosis genome contributes to activation of caspase-1 via induction of potassium ion efflux in infected macrophages. Infect Immun. 2009;77:3992–4001. doi: 10.1128/IAI.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Converse SE, Cox JS. A protein secretion pathway critical for Mycobacterium tuberculosis virulence is conserved and functional in Mycobacterium smegmatis. J Bacteriol. 2005;187:1238–1245. doi: 10.1128/JB.187.4.1238-1245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohol YM, Goetz DH, Chan K, Shiloh MU, Craik CS, Cox JS. Mycobacterium tuberculosis MycP1 protease plays a dual role in regulation of ESX-1 secretion and virulence. Cell Host Microbe. 2010;7:210–220. doi: 10.1016/j.chom.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu YC, Simmons DP, Li X, Abbott DW, Boom WH, Harding CV. TLR2 signaling depletes IRAK1 and inhibits induction of type I IFN by TLR7/9. J Immunol. 2012;188:1019–1026. doi: 10.4049/jimmunol.1102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.