Abstract
Purpose
Betulinic acid (BA), a naturally occurring pentacyclic triterpenoid, exhibits potent anti-tumor activities, whereas the underlying mechanisms remain unclear. In current study, we sought to determine the role and regulation of lamin B1 expression in human pancreatic cancer pathogenesis and BA-based therapy.
Experimental Design
We used cDNA microarray to identify BA target genes and used tissue microarray to determine the expression levels of lamin B1 in pancreatic cancer tissues and to define their relationship with the clinicopathologic characteristics of pancreatic cancer. We also used in vitro and in vivo models to determine the biological impacts of altered lamin B1 expression on and mechanisms underlying lamin B1 overexpression in human pancreatic cancer.
Results
We found that lamin B1 was significantly downregulated by BA treatment in pancreatic cancer in both in vitro culture and xenograft models. Overexpression of lamin B1 was pronounced in human pancreatic cancer and increased lamin B1 expression was directly associated with low grade differentiation, increased incidence of distant metastasis and poor prognosis of pancreatic cancer patients. Furthermore, knockdown of lamin B1 significantly attenuated the proliferation, invasion and tumorigenicity of pancreatic cancer cells.
Conclusions
Lamin B1 plays an important role in pancreatic cancer pathogenesis and is a novel therapeutic target of BA treatment.
Keywords: Progression, pancreatic cancer, transcription factor, lamin B1, betulinic acid
Introduction
Pancreatic cancer is currently the fourth leading cause of cancer-related deaths worldwide. The median survival duration from diagnosis to death is about 6 months, and the overall 5-year survival rate is less than 5% (1-3). Although surgery remains the best choice for pancreatic cancer treatment, most cases are diagnosed at an advanced stage, making patients poor candidates for surgical resection (4-6). Gemcitabine, the first-line agent for advanced pancreatic cancer currently used in clinic, provides only a marginal survival benefit (7). Furthermore, pancreatic tumor is highly resistant to chemotherapy and radiation therapy. Development of more effective therapeutic agents is urgent.
Betulinic acid (BA) is a phytochemical triterpenoid acid from bark extracts and exhibits significant cytotoxic effects on pancreatic cancer cells (8, 9). A few molecular targets of BA have been documented, including ErbB2, NF-κB, STAT3, HIF-1α, and etc (10-12). Recent reports have shown that degradation of transcription factor Sp1 is a critical mechanism mediating the antitumor activity of BA and that overexpression of Sp1 renders cancer cells resistant to BA (8, 9, 13). However, knockdown of Sp1 expression does not drastically affect the growth of pancreatic cancer cells in vitro, while reduced expression of Sp1 attenuates tumor growth and angiogenesis in animal models (14, 15). In contrast, BA exhibits direct cytotoxic effects against tumor cells in vitro (8). Thus, molecular mechanisms other than interfering with Sp1 signaling should exist underlying the anti-tumor effects of BA.
Lamin B1 is an important member of the lamin protein family (16). Lamins are conserved proteins making up nuclear matrix and involved in a list of fundamental cellular functions, including nuclear stability, chromatin structure and gene expressions (16-18). Vertebrate lamins consist of two types, A and B, and there are two lamin B genes. Mutations of lamin genes, including lamin B1, will cause panoply of human diseases (“laminopathies”) (19). The role of lamin B1 in cancer development and progression is unclear. The expression of lamin B1 is reduced in lung cancer, colon cancer, and gastric cancer (20,21), whereas the expression of lamin B1 is increased in prostate cancer and hepatocellular carcinoma (22-24). The expression pattern of lamin B1 and its clinical significance in pancreatic carcinogenesis is yet to be elucidated.
Importantly, no consensus has been reached regarding lamin B1's functions (19). For example, RNAi-mediated knockdown of lamin B1 in Hela cells arrests cell growth and causes apoptosis (25), and this finding is consistent with the notion that lamin B1 is essential for nuclear integrity, cell survival and normal development (26). However, genetic knockout of lamin B1 in keratinocytes has no effect on cell proliferation or the development of skin and hair (27), and mouse embryonic stem cells apparently do not need any lamins for self-renewal and pluripotency (28). Therefore, the lamin B1 functions in both physiology and cancer biology are unclear (29).
In the current study, we sought to identify lamin B1's functions in pancreatic cancer and define its role in the antitumor activity of BA-based treatment. Previous publications implied that Sp1 is a critical regulator of pancreatic cancer pathogenesis and a therapeutic target of BA (14, 15) and that Sp1 could regulate lamin B1 expression (30, 31). Mechanistically, we have determined whether lamin B1 is essential for the antitumor activity of BA and depends on Sp1. Therefore, our present study would not only further our understanding the role of Sp1-lamin B1 signalling in pancreatic cancer pathogenesis, but also help design potentially more effective therapeutic strategies for pancreatic cancer.
Materials and Methods
Cell culture and BA treatment
The human pancreatic adenocarcinoma cell lines AsPC-1, CaPan-1, CaPan-2, MiaPaCa-2, BxPC-3, Hs766T, PANC-1, and PA-TU-8902 were purchased from the American Type Culture Collection. The pancreatic cancer cell lines MDA Panc-28 and MDA Panc-48 were gifts from Dr. Paul J. Chiao (The University of Texas MD Anderson Cancer Center). All of these cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin and incubated in a 37oC, 5% CO2 humidified atmosphere. FG human pancreatic adenocarcinoma cells were used as reported previously (32).
Total RNA extraction and quantitative RT-PCR
Total RNA from each sample was isolated by TRIzol Reagent (Invitrogen Co., Carlsbad, CA). The cDNA was synthesized by using a RT-PCR Kit (TAKARA, Ohtsu, Shiga, Japan). Quantitative RT-PCR was performed using 2μl of cDNA product and the real-time PCR Master Mix (Toyobo, Osaka, Japan) and an ABI 7500 sequence detection system (Applied Biosystems, Foster City, CA). The PCR primers used were: LMNB1, 5′-ttgcgccagcttgtactcatac-3′ (forward) and 5′-catgaaacgcgcttggtaga-3′ (reverse); ACTIN 5′-caccattggcaatgagcggttc-3′ (forward) and 5′-aggtctttgcggatgtccacgt-3′ (reverse); CCDN1 5′ -cgatgccaacctcctcaacga-3′ (forward) and 5′ -tcgcagacctccagcatcca-3′ (reverse); and CDK4 5′ -caggacctaaggacatatctgga-3′ (forward), 5′ - ctcggtaccagagtgtaacaacc-3′ (reverse).
Sample preparation and affymetrix exon array analysis
PANC-1 cells were treated with 50 μM BA or DMSO for 48 h. Total RNA was isolated from BA- or DMSO- treated PANC-1 cells using a Qiagen kit (Qiagen, Inc., Valencia, CA). To analyse gene expression patterns in BA- or DMSO-treated PANC-1 cells on a whole-genome scale, 1 μg of total RNA from BA- or DMSO- treated PANC-1 cells was processed and labelled using the GeneChip Whole Transcript Sense Target Labelling Assay (Affymetrix, Inc., Santa Clara, CA), and then hybridized to Affymetrix GeneChip Human Exon 1.0 ST Arrays (Affymetrix, Inc.). The array was scanned and processed by Shanghai Life Technologies Corporation (Shanghai, China).
Western Blot analysis
Standard western blot was performed using 35 μg whole-cell protein lysates with primary antibodies against lamin B1 (sc-6216, Santa Cruz Biotechnology) or Sp1 (sc-59, Santa Cruz Biotechnology), and proper secondary antibodies (anti-rabbit IgG and anti-goat IgG, Santa Cruz Biotechnology). Equal protein-sample loading was monitored using an anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (G9545, Sigma-Aldrich, St. Louis, MO).
Transient transfection and siRNA sequences
Transfection of siRNAs into pancreatic cancer cells was performed using Lipofectamine 2000 CD (Invitrogen, Carlsbad, CA). In brief, the cells were seeded in 6-well plates at a confluence of 30%-50% and were subsequently transfected with siRNA at different doses as indicated for 48 hours before functional assays were carried out. The siRNA's final concentration was 40 nM or 80 nM. The siRNAs sequences were: CGCGCUUGGUAGAGGUGGAdTdT (targeting LMNB1) (33) and GAAUUUGUUGCUGCUGUGUCUGCdTdT (targeting Sp1).
Cell wound healing assay
Forty-eight hours after siRNA transfection, a wound was generated by scraping with a 10-μl tip and maintained at 37°C. After 20 h, the cells in the wounded monolayer were photographed and cell migration was assessed by measuring gap sizes at multiple fields.
Cell invasion assay
Cell invasion assay was conducted using a specialized Chemicon invasion chamber, which included a 24-well tissue culture plate with 12 cell culture inserts (Millipore, Bedford, MA). The inserts contained an 8 μm pore size polycarbonate membrane with a precoated thin layer of basement membrane matrix (ECMatrix). Ten percent fetal bovine serum-containing medium was placed in the lower chambers to act as a chemoattractant. 5×104 PANC-1 and 3×105 AsPC-1 in a 300 μL volume of serum-free medium were placed in the upper chambers and incubated at 37°C for 24 h. Invasive cells on the lower surface of the membrane, which had invaded the ECMatrix and had migrated through the polycarbonate membrane, were stained by the staining solution, and counted under a microscope in five randomly selected fields at a magnification of 100×.
Cell proliferation assay
AsPC-1 and PANC-1 cells were transfected with siLMNB1 or siCtrl. After 48 h, six-wells plates were seeded with 2.5×104 AsPC-1 cell per well and 1×104 PANC-1 cells per well. Cell counting was performed daily for 6 days.
Pancreatic cancer xenograft models and drug treatment
Pathogen-free male athymic BALB/C nude mice were purchased from the Institute of Animal Center, Chinese Academy of Sciences, Shanghai, China. Tumor xenografts were established by subcutaneous inoculation of 1 × 106 PANC-1 or AsPC-1 cells into the right scapular region of nude mice. Ten days after tumor injections, the BA (25 mg/kg or 50 mg/kg) was mixed in corn oil and administered via oral gavages 3 times a week. Corn oil was administered as control. The medication was given for a total of 30 days starting 10 days after implantation of the primary xenograft. Then the mice were sacrificed and the xenografts were harvested for further study. All procedures were performed in accordance with institution guidelines and were approved by the Committee of Shanghai Changhai Hospital and MD Anderson Cancer Center on the Use and Care of Animals.
Statistical Analysis
The clinical and follow-up information were verified in all cases by reviewing medical records and/or follow-up. The overall survival (OS) was calculated as the time from the date of diagnosis to the date of death or the date of last follow-up if death did not occur. The Fisher exact tests or the Pearson correlation coefficient were used to compare categorical data. OS curves were constructed using the Kaplan–Meier method, and the log-rank test was used to evaluate the statistical significance of differences. Multivariate Cox regression analysis was used to examine the prognostic significance of lamin B1 and clinicopathologic parameters. The significance of the in vitro and in vivo data was determined using the Student t-test (two-tailed), Mann-Whitney U test (two-tailed), or one-way analysis of variance. P values less than 0.05 were considered significant.
Results
Suppression of lamin B1 expression in pancreatic cancer by BA treatment
To unravel the critical mechanisms underlying BA's cytotoxic effects, we compared the gene expression profiles in PANC-1 cells with or without BA treatment. An array of genes that changed more than two fold was shown (Supplementary Fig. S1 and Supplementary Table S1). Interestingly, lamin B1, an important protein that drives the disassembly of the lamina and the nuclear envelope during mitosis, was among the genes that were significantly downregulated. Reduced expression of lamin B1 was further confirmed at both mRNA and protein levels in three BA treated pancreatic cancer cell lines using real-time PCR and western blot (Fig. 1A).
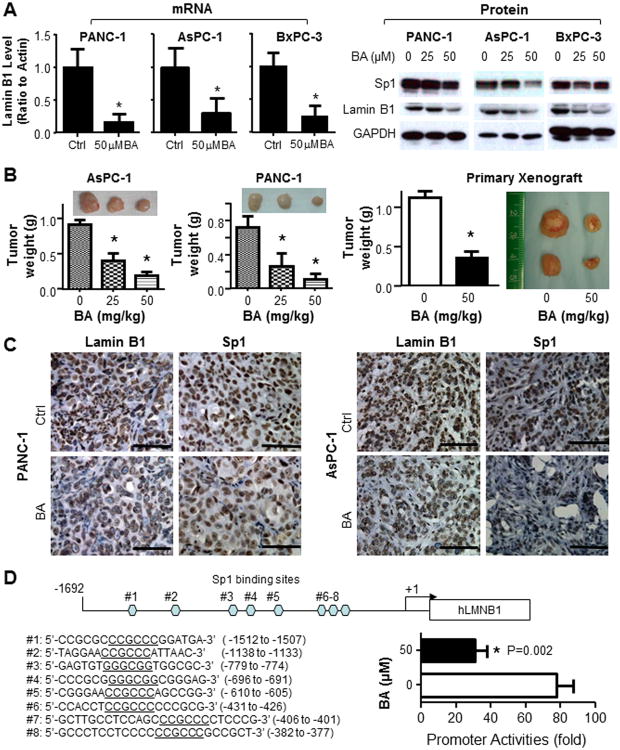
Fig. 1. Identification of lamin B1 as a downstream target of BA in pancreatic cancer.
A. Suppression of lamin B1 expression at the levels of mRNA (qRT-PCR) and protein (Western blot) analysis. B. Dose-dependent antitumor effects of BA in xenograft models of human pancreatic cancer. Shown were gross tumors in mice (6 mice in each group). This was one representative experiment of three with similar results. *P<0.05 as compared with the control mice. The antitumor effect of BA in the primary pancreatic tumor xenograft model. C. Immunohistochemical analysis of the expression levels of lamin B1 and Sp1 in xenograft tumors of PANC-1 and AsPC-1 cells. Representative pictures showed that Sp1 (staining the nuclear) and lamin B1 (staining the nuclear envelope) levels were significantly decreased after BA treatment. D. Schematic structure of the lamin B1 promoter reporter with 8 Sp1 binding sites (CCGCCC or GGGCGG) were identified. BA treatment significantly suppressed the promoter activities (P=0.002) in PANC-1 cells.
Next, we treated AsPC-1 and PANC-1 xenograft tumors with different doses of BA (25mg/kg or 50mg/kg) 3 times a week or corn oil as a control. BA produced dose-dependent antitumor activities in both xenograft tumor models (Fig. 1B). Furthermore, in a primary pancreatic cancer xenograft model and primary cultures, BA showed consistent inhibitory effects against lamin B1 and cell growth in vitro and in vivo (Supplementary Fig. S2 and Fig. 1B). Immunohistochemical analysis showed that BA treatment decreased the expression levels of lamin B1 and Sp1 proteins (Fig. 1C). To further identify the direct suppressive effects against lamin B1 by BA, we constructed a lamin B1 promoter reporter and treatment with BA significantly suppressed the promoter activities (Fig. 1D). Our data showed that BA could suppress lamin B1 expression both in vitro and in vivo, suggesting that lamin B1 be an important contributor to antitumor activities of BA in pancreatic cancer.
Expression pattern of lamin B1 in human pancreatic tissues
We first determined the expression pattern of lamin B1 within pancreatic tissue cells. As shown in Supplementary Fig. S3A, lamin B1 was mostly detected on the nucleus envelop of certain acinar cells in the tumor adjacent normal tissues. In a TMA, no cytoplasmic aggregation of lamin B1 was observed (Supplementary Fig. S3B). Moreover, very little lamin B1 was detected in the islet regions (Supplementary Fig. S3A).
Lamin B1 overexpression and its direct association with pancreatic cancer clinicopathologic characteristics
To determine the effect of lamin B1 on pancreatic cancer development and progression, we investigated the expression of lamin B1 protein in a tissue microarray containing primary pancreatic cancers and paired tumor adjacent normal pancreatic tissues (Supplementary Table S2). Lamin B1 expression was significantly increased on the nuclear envelope of pancreatic cancer cells (P<0.001; Fig. 2A). Presence of aberrant upregulation of lamin B1 expression at both mRNA and protein levels were further confirmed in 5 pancreatic cancer and paired normal pancreatic tissue specimens by using real-time PCR and western blot (Fig. 2B). Because a larger nucleus presents more DNA and proteins involved and usually suggests a more malignant phenotype, we determined the lamin B1 expression intensity among cells with different nucleus sizes. As shown in Supplementary Fig. S4, larger nucleus cells exhibited high lamin B1 expression when compared with those smaller ones. Those results suggested that lamin B1 expression correlate with increased pancreatic malignancy.
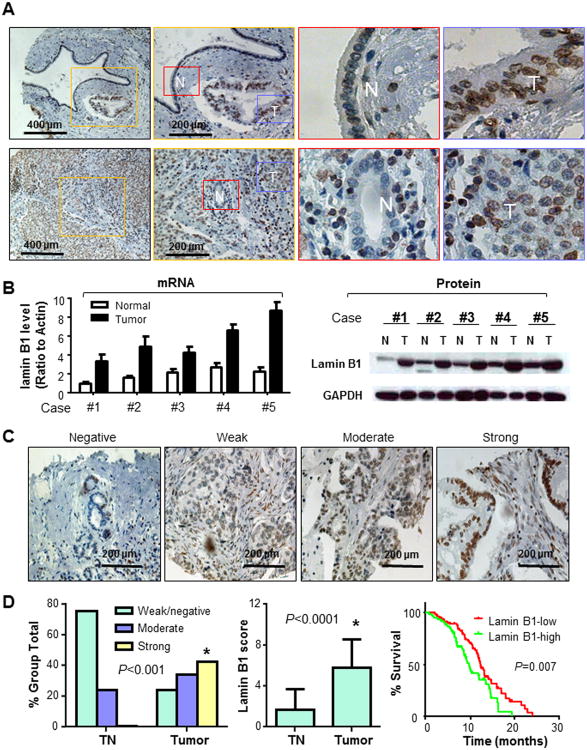
Fig. 2. Overexpression of lamin B1 in human pancreatic cancer tissues.
A. Representative pictures from tissue microarray slides demonstrated the lamin B1 staining in tumor (T, in Blue Square) and paracancerous normal pancreatic tissues (N, in Red square). B. Analyses of lamin B1 expression at levels of mRNA (qRT-PCR) and protein (Western blot) in 5 pancreatic cancer samples (T) and their paired normal pancreatic tissues (N). Actin was used as a control. C. Representative images of IHC showed lamin B1 protein expression at different levels. D. Lamin B1 expression was negative or weak in tumor adjacent normal pancreatic ductal cells, whereas the expression was strong in pancreatic cancer tissues. Kaplan–Meier curve depicting survival of pancreatic cancer patients with high or low levels of lamin B1. The median survival duration of each group was 9.75 or 12.4 months, respectively.
We further analyzed the relationship between clinicopathological parameters and lamin B1 expression levels in pancreatic cancer tissues (Supplementary Table S2; Fig. 2C & 2D). High lamin B1 staining was inversely correlated with the overall survival duration in Kaplan-Meier survival analysis; 60 patients with high lamin B1 expression had a median survival duration of only 9.75 months, whereas the 82 patients with low lamin B1 expression had a median survival duration of 12.4 months (P=0.007; Fig. 2D). Multivariate analysis showed that high level of lamin B1 expression (lamin B1-high) was associated with shorter survival (P=0.04) independent of human gender, age, tumor differentiation, metastasis (Supplementary Table S3).
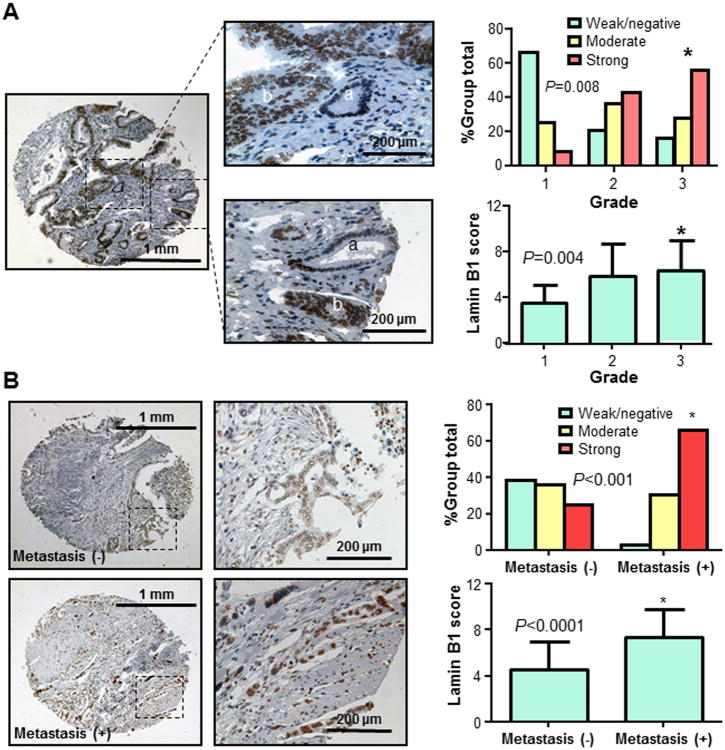
Furthermore, increased lamin B1 expression was correlated with decreased levels of tumor differentiation and significant difference was detected between well (grade 1) and poorly differentiated (grade 3) tumors (Fig. 3A). Lamin B1 expression was significantly up-regulated in late stage of pancreatic cancer (Supplementary Table S2). In addition, lamin B1 expression in specimens with metastasis was significantly higher than that in those without metastasis (Fig. 3B). These data strongly suggested that lamin B1 expression plays critical roles in pancreatic cancer and be a valuable prognosis biomarker for this disease.
Fig. 3. Lamin B1 expression in pancreatic cancer specimens and its association with pancreatic cancer clinicopathologic features.
A. Lamin B1 expression correlated with tumor differentiation. Representative images showed different levels of lamin B1 expression in well (a) and poorly (b) differentiated pancreatic ductal cells (left panel). An increased lamin B1 expression correlated with decreased levels of tumor differentiation and increased grade (between grade 1 and grade 3) of tumors (*P=0.008) (right panel). B. Lamin B1 expression was positively correlated with the presence of distant metastasis (*P<0.001 in a comparison between cases with distant metastasis and cases without distant metastasis) (right panel) and representative images of tumors from patients with or without distant metastasis are shown (left panel).
Inhibition of pancreatic cancer growth in vitro and animal models by knockdown of lamin B1 expression
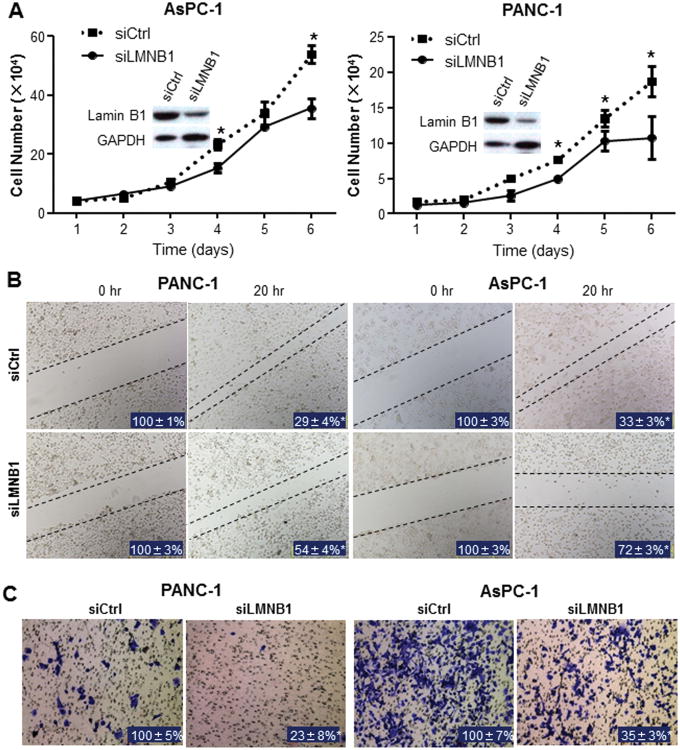
To determine the role of lamin B1 in pancreatic cancer cell proliferation, we treated AsPC-1 and PANC-1 cells with lamin B1 siRNA. Efficiency of knockdown against lamin B1 was confirmed using western blot (Fig. 4A). Knockdown of lamin B1 significantly inhibited the growth of cancer cells (Fig. 4A). To investigate the mechanism by which lamin B1 affects pancreatic cancer growth, we tested the effects of altered lamin B1 expression on cell cycle distribution. As shown in Supplementary Fig. S5, knockdown of lamin B1 induced G1 arrest in both AsPC-1 cells and PANC-1 cells and this cell cycle arrest directly correlated with a significant suppression of the mRNA expression levels of Cyclin D1 and CDK4.
Fig. 4. Influences of lamin B1 silence on pancreatic cancer cell proliferation, migration and invasion.
A. AsPC-1 and PANC-1 cells were transfected with siCtrl or siLMNB1. Lamin B1 silence efficiency were determined 48 hours after transfection and cell proliferation was analysed (data were mean±SEM calculated from three independent experiments). B. For cell scratch wound assay, the cultures were wounded by scratching and maintained at 37°C for additional 20 hours. Cell cultures were photographed and cell migration was assessed by measuring gap sizes (inserted number represented percentage area of mean±SD). C. For cell invasion assay, the transfected cells were maintained at 37 °C for additional 24 hours. Representative tumor cells invaded through Matrigel were photographed, whereas the numbers of invasive cells that penetrated through Matrigel-coated filter were counted in 15 random fields identified within the lower surface of the filters and expressed as percentage of control (inserted numbers). Data represented mean±SD of triplicates. (*P<0.05 in a comparison between the siLMNB1–transfected cells and the siCtrl cells.)
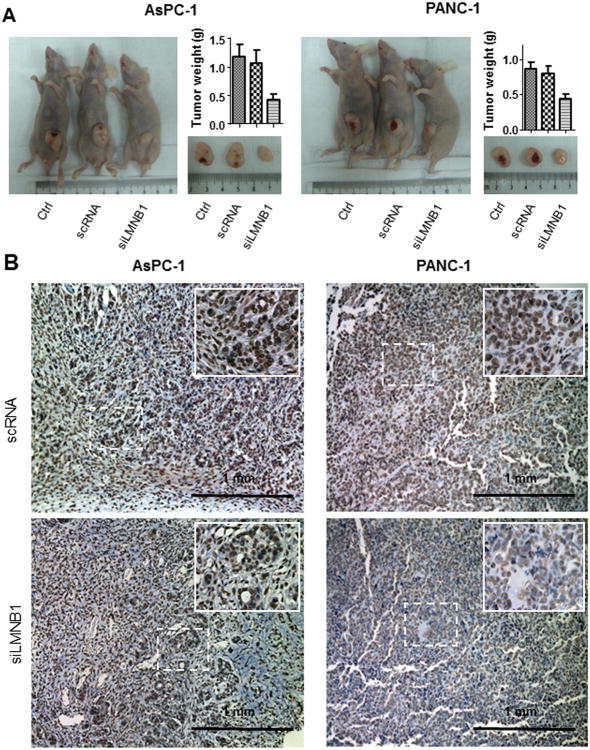
Because increased lamin B1 expression correlated with pancreatic cancer metastases (Fig. 3B), we investigated the role of lamin B1 in pancreatic cancer cells migration and invasion. Wound healing assay showed that silencing lamin B1 in AsPC-1 and PANC-1 cells significantly attenuated the migration and invasion ability of pancreatic cancer cells (P<0.05; Fig. 4B & 4C). Consistently, silencing lamin B1 significantly inhibited pancreatic tumor growth in vivo (Fig. 5A). Immunohistochemical analysis validated the knockdown efficiency against lamin B1 in the xenograft tumors (Fig. 5B). Thus, overexpression of lamin B1 contributes to pancreatic cancer proliferation and invasion.
Fig. 5. Influence of lamin B1 expression on pancreatic cancer growth in vivo.
A. AsPC-1 and PANC-1 cells with lamin B1 knockdwon were injected subcutaneously (1×106 per mouse) into the right scapular region of nude mice. The tumor-bearing mice were sacrificed when they became moribund or on day 28. Shown were gross tumors in the mice. The tumors removed from the mice were weighed (*P<0.05 in a comparison between siLMNB1-transfected tumors and the parental or siCtrl ones): AsPC-1 (left panel) and PANC-1 (right panel). B. Knockdown of lamin B1 expression was observed in the xenograft tissues as determined by immunohistochemistry: AsPC-1 (left panel) and PANC-1 (right panel).
Suppression of lamin B1 by BA is independent on Sp1
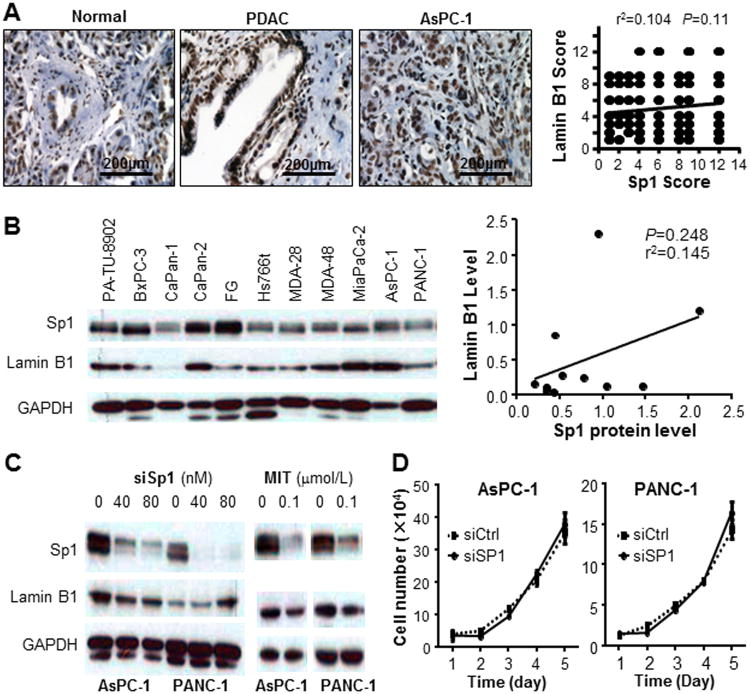
Previous studies have implied that Sp1 be an important transcription factor that regulates the expression of lamin B1 (30,35), and that multiple Sp1-binding sites were identified within human lamin B1 promoter region (Fig. 1D). To determine whether suppression of lamin B1 by BA was dependent on Sp1 signaling, we first quantitated the expression level of Sp1 in the TMA using immunohistochemistry. Consistent with our previous results, Sp1 expression is significantly higher in pancreatic cancer tissues compared with the normal control. Sp1 is also overepxressed in xenograft tumors sampled from nude mice (Fig. 6A). There was a direct correlation between lamin B1 and Sp1 expression levels, while no statistical significance was observed (Fig. 6A, P=0.11). We also evaluated Sp1 and lamin B1 expression levels in 11 pancreatic cancer cell lines (Fig. 6B), showing no linear correlation (r2=0.145, P=0.248) (Fig. 6B). Furthermore, knockdown of Sp1 did not caused a drastic reduction of lamin B1 expression (Fig. 6C). Interestingly, mithramycin A, a commonly used Sp suppressor, showed inhibitory effects on lamin B1 (Fig. 6C), which suggested that BA's suppression to lamin B1 did not work through Sp1, but other members of the Sp family proteins. Finally, knockdown of lamin B1 attenuated pancreatic cancer cell proliferation in vitro, while knockdown of Sp1 has little effect on pancreatic cancer growth in vitro (Fig. 6D), suggesting that Sp1 signaling was dispensable for lamin B1 expression and function.
Fig. 6. Suppression of lamin B1 expression by BA is independent on Sp1.
A. Immunohistochemistry showed overexpression of Sp1 in human pancreatic cancer and xenograft tumors when compared with normal pancreatic ductal cells. However, no significant correlation between lamin B1 and Sp1 expressions was detected in pancreatic cancer samples. B. Lamin B1 and Sp1 expression in pancreatic cancer cell lines and their relationship. Western blot analysis of the lamin B1 and Sp1 protein expression in pancreatic cell lines was performed and GAPDH was used as a control. Correlation analysis was done between lamin B1 and Sp1 expressions in pancreatic cancer cell lines (Pearson's correlation test). C. Lamin B1 expression after knockdown of Sp1 and treatment with MIT. AsPC-1 and PANC-1 were transfected with siRNA targeting Sp1. The Sp1 knockdown efficiency was validated using western blot, whereas lamin B1 expression was not downregulated neither in AsPC-1 nor in PANC-1 cells. However, treatment of PANC-1 and AsPC-1 cells with indicated dose of MIT for 48 hours suppressed the expression of both Sp1 and lamin B1. D. Sp1 was knocked down in AsPC-1 and PANC-1 cells and proliferation assays were performed at indicated time points (data were mean ± SEM calculated from three independent experiments).
Discussion
In current study, we discovered four lines of evidence supporting the critical roles of lamin B1 in mediating anti-tumor activities of BA in pancreatic cancer. First, BA treatment significantly suppressed lamin B1 expression in pancreatic cancer cells in culture and in animal models. Second, an elevated expression of lamin B1 directly correlated with the progression of pancreatic cancer. Increased expression of lamin B1 predicted poor differentiation, high metastatic potential and short patient survival duration. Third, knockdown of lamin B1 attenuated the growth and migration and invasion of pancreatic cancer in vitro and in animal models. Fourth, consistent with that LMNB1 is a Sp1-downstream gene as predicted by promoter sequence analysis (30,31), Sp1 might regulate the expression of lamin B1. However, inhibition of lamin B1 by BA appeared to be independent on Sp1 signalling, suggesting that lamin B1 be a novel target critically mediating the anti-cancer effects of BA.
BA has significant antitumor activities (13,36,37) and is undergoing development with assistance from the Rapid Access to Intervention Development program of the National Cancer Institute. Of particular interest is its direct and relatively selective cytotoxic effect on tumor cells versus normal or nonneoplastic cells (37). However, the molecular basis of BA antitumor activity is unclear. Recent studies have identified Sp1 as a target molecule mediating the antitumor activity of BA (9,13), and its overexpression renders pancreatic cancer cells resistant to BA cytotoxicity (8). However, Sp1 knockdown does not drastically affect the growth of pancreatic cancer cells in vitro (14). A study using genetic mouse model also indicates that Sp1 is dispensable for cell growth and differentiation (38). Thus, molecular mechanisms other than Sp1 should exist underlying the anti-tumor effects of BA. Our current study has shown for the first time that BA downregulated the expression of lamin B1 protein, which plays important roles in cellular functions, including DNA replication, the formation of the mitotic spindle, chromatin organization and regulation of gene expression, and is essential for cell survival. Thus, we have uncovered a novel mechanism underlying BA's anti-tumor effects and our study may help develop potentially more effective BA-based targeted therapeutics.
The role of lamin B1 in cancer development and progression is unclear. Apparently, different tumor types exhibited distinct patterns of lamin B1 expression (29). Among all the epithelial neoplasms, there are no consistent patterns of lamin B1 expression (21). A reduced expression of lamin B1 occurs frequently in various carcinomas, including gastric cancer, colon cancer, squamous and adenocarcinoma of the oesophagus, cervical and uterine body cancers, breast cancer, and also bronchial carcinoma. In contrast, an increased expression pattern was found in other cancers, including prostate cancer and liver cancer (22-24). In pancreatic cancer, the expression levels of lamin B1 increased in 2 of 3 cases examined (21). In our current study of 142 pancreatic cancer specimens and matched normal pancreatic specimens, we determined the clinical significance of lamin B1 expression. We observed that lamin B1 expression was significantly higher in pancreatic cancer tissues than in the corresponding tumor adjacent normal tissues. Higher lamin B1 expression was significantly associated with poor prognostic factors, such as the presence of distant/lymph node metastasis and low grade of differentiation. More importantly, lamin B1-positive staining was associated with poor overall survival rates. Therefore, we provided the first evidence that lamin B1 can be used as a novel biomarker for outcome in patients with pancreatic cancer. In another word, at the time of initial diagnosis of pancreatic cancer, lamin B1 expression can be used not only to design optimal, individualized treatment but also to distinguish patients who would benefit from close monitoring after surgery from those who would not. Our notion is further supported by a recent publication, which has shown that lamin B1 mRNA was present in plasma of hepatocellular carcinoma patients and was able to detect early stage HCCs with a sensitivity of 76% and a specificity of 82% (24). Therefore, lamin B1 may be potentially used for early detection of pancreatic cancer.
Because a modulation of “biomarker” expression can usually produce therapeutic effects (39), we further evaluated the biological functions of lamin B1 within pancreatic cancer. Our work showed that knockdown of lamin B1 significantly attenuated the proliferation, invasion and tumorigenesis of pancreatic cancer cells. These findings are consistent with our clinical data, which showed that lamin B1-positive expression closely associated with poor differentiation and high metastatic potential. Thus targeted inhibition of lamin B1 expression and/or function may constitute a novel therapeutic strategy for pancreatic cancer. Given that the fundamental roles of lamin B1 in physiology and cancer biology remain controversial, further investigation into the interactions between lamin B1 signalling and other major oncogenic signalling pathways is warranted.
Prior studies have also demonstrated that Sp1 plays critical roles in pancreatic carcinogenesis (14, 15) and also identified multiple Sp1 binding sites within the promoter region of lamin B1. Both Sp1 and lamin B1 proteins were knocked down by BA treatment, suggesting lamin B1 might be a further downstream target of Sp1. However, no significant correlations between Sp1 and lamin B1 proteins (neither cell lines nor tissue specimens) were identified in the present study. Furthermore, Sp1 knockdown using siRNA has only marginal impacts on lamin B1 expression, whereas or mithramycin A, a commonly used Sp1-targeted drug did inhibited lamin B1 expression. All these data established the notion that overexpression of lamin B1 in pancreatic cancer appeared to be independent on Sp1 signalling, but other protein members of Sp family, suggesting a unique role of lamin B1 in pancreatic tumorigenesis. Thus, BA may simultaneously suppress both Sp1 and lamin B1 and generate synergistic antitumor effects in pancreatic cancer.
In summary, we have demonstrated that BA targeted both Sp1 and lamin B1, which appeared to be distinct from each other in signalling, thus uncovering a novel mechanism underlying the potent anti-tumor effects of BA in pancreatic cancer. We have also showed that lamin B1 expression closely correlated with the clinical feature of pancreatic cancer patients and regulated the proliferation, invasion and metastasis of pancreatic cancer cells, thus providing critical insight into the role of lamin B1 in the progression of pancreatic cancer. Therefore, lamin B1 expression in pancreatic cancer highlights its potential as a novel molecular biomarker and therapeutic target. The role and regulation mechanisms of lamin B1 in pancreatic cancer development and progression warrant more studies to ensure an eventual translation into clinical uses.
Supplementary Material
Statement of Translational Relevance.
We have used pancreatic cancer tissue microarray and molecular biology and animal models to evaluate the role and regulation of lamin B1 as a therapeutic target of betulinic acid (BA) in human pancreatic cancer. Our clinical and mechanistic findings indicate that lamin B1 is a novel therapeutic target of BA and that increased lamin B1 expression significantly associated with the clinicopathologic characteristics of and predicted poor prognosis for patients with pancreatic cancer. Moreover, lamin B1 positively regulates pancreatic cancer cell migration, invasion and growth, suggesting a novel cellular basis for the critical role of lamin B1 expression in pancreatic cancer development and progression and the deregulated lamin B1 signaling could be a promising new molecular target for designing novel preventive/therapeutic strategies to control this malignancy. Therefore, our findings may have a significant effect on clinical management of pancreatic cancer patients.
Acknowledgments
Financial Support: Supported by National Nature Science Foundation of China, No. 30971344 and Key projects from Science and Technology Commission of Shanghai Municipality, No. 11JC1416402 (to Y. Du) and by grants R01-CA129956, R01-CA148954, and R01-CA152309 (to K. Xie) from the National Cancer Institute, National Institutes of Health
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
References
- 1.Jemal A, Center MM, Ward E, Thun MJ. Cancer occurrence. Methods Mol Biol. 2009;471:3–29. doi: 10.1007/978-1-59745-416-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Kindler HL. Pancreatic cancer: an update. Curr Oncol Rep. 2007;9:170–6. doi: 10.1007/s11912-007-0018-z. [DOI] [PubMed] [Google Scholar]
- 3.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–94. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 5.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–9. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225:621–33. doi: 10.1097/00000658-199705000-00018. discussion 33-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Jia Z, Kong X, Li Q, Chang DZ, Wei D, et al. Combining betulinic acid and mithramycin a effectively suppresses pancreatic cancer by inhibiting proliferation, invasion, and angiogenesis. Cancer Res. 2011;71:5182–93. doi: 10.1158/0008-5472.CAN-10-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chintharlapalli S, Papineni S, Liu S, Jutooru I, Chadalapaka G, Cho SD, et al. 2-cyano-lup-1-en-3-oxo-20-oic acid, a cyano derivative of betulinic acid, activates peroxisome proliferator-activated receptor gamma in colon and pancreatic cancer cells. Carcinogenesis. 2007;28:2337–46. doi: 10.1093/carcin/bgm189. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Jutooru I, Lei P, Kim K, Lee SO, Brents LK, et al. Betulinic acid targets YY1 and ErbB2 through cannabinoid receptor-dependent disruption of microRNA-27a:ZBTB10 in breast cancer. Mol Cancer Ther. 2012;11:1421–31. doi: 10.1158/1535-7163.MCT-12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan Y, Wu YL, Lian LH, Xie WX, Li X, Ouyang BQ, et al. The anti-fibrotic effect of betulinic acid is mediated through the inhibition of NF-kappaB nuclear protein translocation. Chem Biol Interact. 2012;195:215–23. doi: 10.1016/j.cbi.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Shin J, Lee HJ, Jung DB, Jung JH, Lee EO, Lee SG, et al. Suppression of STAT3 and HIF-1 alpha mediates anti-angiogenic activity of betulinic acid in hypoxic PC-3 prostate cancer cells. PLoS One. 2011;6:e21492. doi: 10.1371/journal.pone.0021492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chintharlapalli S, Papineni S, Ramaiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67:2816–23. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 14.Yuan P, Wang L, Wei D, Zhang J, Jia Z, Li Q, et al. Therapeutic inhibition of Sp1 expression in growing tumors by mithramycin a correlates directly with potent antiangiogenic effects on human pancreatic cancer. Cancer. 2007;110:2682–90. doi: 10.1002/cncr.23092. [DOI] [PubMed] [Google Scholar]
- 15.Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–8. doi: 10.1158/0008-5472.can-03-1945. [DOI] [PubMed] [Google Scholar]
- 16.Dittmer TA, Misteli T. The lamin protein family. Genome Biol. 2011;12:222. doi: 10.1186/gb-2011-12-5-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dauer WT, Worman HJ. New messages in the nuclear envelope. Cell Cycle. 2010;9:645–6. [PubMed] [Google Scholar]
- 18.Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Dev Cell. 2009;17:626–38. doi: 10.1016/j.devcel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119:1825–36. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broers JL, Raymond Y, Rot MK, Kuijpers H, Wagenaar SS, Ramaekers FC. Nuclear A-type lamins are differentially expressed in human lung cancer subtypes. Am J Pathol. 1993;143:211–20. [PMC free article] [PubMed] [Google Scholar]
- 21.Moss SF, Krivosheyev V, de Souza A, Chin K, Gaetz HP, Chaudhary N, et al. Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut. 1999;45:723–9. doi: 10.1136/gut.45.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coradeghini R, Barboro P, Rubagotti A, Boccardo F, Parodi S, Carmignani G, et al. Differential expression of nuclear lamins in normal and cancerous prostate tissues. Oncol Rep. 2006;15:609–13. [PubMed] [Google Scholar]
- 23.Lim SO, Park SJ, Kim W, Park SG, Kim HJ, Kim YI, et al. Proteome analysis of hepatocellular carcinoma. Biochem Biophys Res Commun. 2002;291:1031–7. doi: 10.1006/bbrc.2002.6547. [DOI] [PubMed] [Google Scholar]
- 24.Sun S, Xu MZ, Poon RT, Day PJ, Luk JM. Circulating Lamin B1 (LMNB1) biomarker detects early stages of liver cancer in patients. J Proteome Res. 2010;9:70–8. doi: 10.1021/pr9002118. [DOI] [PubMed] [Google Scholar]
- 25.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–65. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 26.Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- 27.Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y, et al. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet. 2011;20:3537–44. doi: 10.1093/hmg/ddr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, et al. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334:1706–10. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster CR, Przyborski SA, Wilson RG, Hutchison CJ. Lamins as cancer biomarkers. Biochem Soc Trans. 2010;38:297–300. doi: 10.1042/BST0380297. [DOI] [PubMed] [Google Scholar]
- 30.Maeno H, Sugimoto K, Nakajima N. Genomic structure of the mouse gene (Lmnb1) encoding nuclear lamin B1. Genomics. 1995;30:342–6. doi: 10.1006/geno.1995.9868. [DOI] [PubMed] [Google Scholar]
- 31.Lin F, Worman HJ. Expression of nuclear lamins in human tissues and cancer cell lines and transcription from the promoters of the lamin A/C and B1 genes. Exp Cell Res. 1997;236:378–84. doi: 10.1006/excr.1997.3735. [DOI] [PubMed] [Google Scholar]
- 32.Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143–54. [PubMed] [Google Scholar]
- 33.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, et al. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–80. [PubMed] [Google Scholar]
- 35.Lin F, Worman HJ. Structural organization of the human gene (LMNB1) encoding nuclear lamin B1. Genomics. 1995;27:230–6. doi: 10.1006/geno.1995.1036. [DOI] [PubMed] [Google Scholar]
- 36.Jung GR, Kim KJ, Choi CH, Lee TB, Han SI, Han HK, et al. Effect of betulinic acid on anticancer drug-resistant colon cancer cells. Basic Clin Pharmacol Toxicol. 2007;101:277–85. doi: 10.1111/j.1742-7843.2007.00115.x. [DOI] [PubMed] [Google Scholar]
- 37.Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995;1:1046–51. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 38.Marin M, Karis A, Visser P, Grosveld F, Philipsen S. Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell. 1997;89:619–28. doi: 10.1016/s0092-8674(00)80243-3. [DOI] [PubMed] [Google Scholar]
- 39.Jackson DB. Serum-based microRNAs: are we blinded by potential? Proc Natl Acad Sci U S A. 2009;106:E5. doi: 10.1073/pnas.0809999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.