Abstract
Background
Feeding difficulties and gastroesophageal reflux (GER) are common problems in neonates. The authors hypothesize that GER could be influenced by feeding mechanics by evaluating the effects of feeding volumes, feeding durations, feeding flow rates, and caloric density on the chemical composition and clearance of GER in dysphagic neonates.
Methods
Symptomatic dysphagic neonates (n = 35) underwent evaluation for suspected GER using pH-impedance methods.
Results
The proportions of acid and nonacid GER were different during the first, second, and third postprandial hours (P < .0001). Prolonged feeding duration was significantly associated with decreased total, nonacid GER and BCT (P < .03). Significant positive correlations (P < .05) were detected between feeding flow rate vs frequency of total, nonacid GER and BCT. Significant positive correlation (P = .002) was noted between feeding volume and BCT. BCT decreased with each hourly interval (analysis of variance [ANOVA] P < .05); however, ACT increased with each hourly interval (ANOVA P = .05). Comparison between BCT and ACT at each postprandial hour is remarkable for longer ACT during the second and third hours after the initiation of feed (P ≤ .001). No significant correlation was noted between the milk types (breast milk or formula) or caloric density with regard to the GER characteristics. Oral-fed infants had more GER events than gavage-fed infants.
Conclusions
Prolonged feeding durations and slower flow rates are associated with decreased frequency of GER. Modification of feeding duration and flow rate can be a useful adjunct to ameliorate GER in dysphagic neonates.
Keywords: neonates, dysphagia, feeding strategies, gastroesophageal reflux
Clinical Relevancy Statement
Gastroesophageal reflux (GER) is a common problem in infants. The first step of management often includes advice on feeding strategies and postural intervention. However, the impact of caloric density and milk type relevant to GER is unclear. The findings of this study support that modification of feeding duration and feeding flow rate can significantly reduce the frequency and characteristics of GER events. Nevertheless, there is no effect of milk type (breast milk and formula) or of caloric density on the frequency and characteristics of GER events. Our findings indicate that higher GER rates in oral-fed infants as compared to the gavage-fed group are contrary to the frequent speculation in current clinical practice.
Introduction
Gastroesophageal reflux (GER) is a frequent occurrence in neonates with feeding problems who are convalescing in the neonatal intensive care unit (ICU), so much so that the use of antireflux medications is high. About 48% (range, 10%–90%) of premature neonates are being discharged on acid-suppressive medications from the nursery without objective evidence.1–3 Causal associations between feeding problems, cardiorespiratory concerns, and GER are often implicated without diagnostic certainty.4–6 Furthermore, feeding management choices are often made empirically and may include changes in body position,7,8 modified feeding methods or feeds,9,10 and feeding diversion methods.11 Effect of feeding methods relevant to GER is often debated, although few data have tested the impact of feeding practices on the frequency of GER in premature infants.
Detailed evaluation of the composition of refluxate is now possible with the recent applications of multichannel intraluminal impedance methods.12 The refluxate can be classified into acid (pH <4) and nonacid (pH >4).13,14 It is recognized that the central nervous system and vagal neural outputs regulate feeding abilities and gastrointestinal cyclical rhythms, feeding skills, acid production, and aerodigestive tract protection mechanisms.12,15–19 Knowledge about the characteristics of GER events across the feeding cycle in relation to different feeding strategies in convalescing premature neonates may clarify prevailing myths about neonatal GER and feeding methods.
The objectives of the current study were to evaluate the effects of feeding strategies (feeding volumes, feeding durations, caloric density, milk types, feeding methods, feeding cycles) and the interval between feeding sessions on the chemical composition and clearance of GER in premature neonates that who undergone diagnostic evaluation for GER.
Methods
Patients and Feeding Methods
Neonates (n = 35) with feeding problems and suspected GER underwent esophageal pH-impedance evaluation. Patients did not have any structural, genetic, or chromosomal abnormalities and were not receiving any acid-suppressive medications or prokinetics at evaluation. As part of the standard nursery protocol, patients were fed every 3 hours. Ethical approval was obtained and permission was granted by the institutional review board (IRB) committee at the Nationwide Children’s Hospital Research Institute to report the data.
Infants (n = 20) were tube fed based on physician choice and on the lack of independent oral feeding capabilities in infants. Nineteen were fed with a nasogastric feeding tube and 1 via a gastrostomy tube. Infants were generally fed via pump over 30 minutes. However, in some instances, the duration of gavage feeding was longer. In all cases, the duration of the feeding period was noted and computed for the patient group. In the case of oral-fed patients, duration of feeding time was driven by the infant’s hunger and feeding abilities, and that was also documented.
pH-Impedance Methods
These methods have been described by us and others.5,12,20,21 Briefly, after determining the nares–lower esophageal sphincter (LES) distance using esophageal manometry pull-through, the pH-impedance probe (7 impedance rings and 6 channels, 1 pH channel, model ZIN-S61C01E; Sandhill Scientific, Highlands Ranch, CO) was positioned at the desired location (87% the distance from the nares to the upper border of the LES).12,20
Data Analysis
The acid characteristics of GER events were evaluated for the 24-hour pH-impedance study with the exclusion of meal periods. To understand the effects of feeding methods on GER characteristics in each patient, the acid characteristics of GER events were further analyzed during two 3-hour feeding cycles; the 3-hour feeding cycle was first classified into hourly intervals, as the first postprandial hour (0–60 minutes), second postprandial hour (>60–120 minutes), and third postprandial hour (>120–180 minutes). Next, within each postprandial hour, data were further analyzed in 2-minute epochs. The feeding cycles were selected based on the following criteria: (1) the interval between feeding cycles varied from 2 hours, 45 minutes to 3 hours, 15 minutes. This practical approach was based on infants’ feeding readiness and feeding provider availability. (2) In each patient, 2 feeding cycles were analyzed separately, one from the day and one from the night. This was mainly done to ensure 2 random cycles. (3) Only those feeding cycles that had complete information about feeding methods, feeding volume, and feeding duration were selected.
Analysis of GER composition
Acid GER events were verified and confirmed, and the pH-impedance characteristics were evaluated as described before.12,22–25 GER events were classified as (a) acid GER events (fall in pH <4 or pH decrease of at least 1 pH unit if pH was already <4; Figure 1A) and (b) nonacid reflux events (fall in pH ≤1 unit when pH >4; Figure 1B). As a prerequisite, the change in pH must have lasted more than 5 seconds, and the event must have been associated with a retrograde bolus motion in at least 2 subsequent impedance channels. We analyzed overall acid GER events, reflux index per 24 hours, and Boix-Ochoa score.26 This score factors the following: total time (%) spent in acid reflux in 24 hours, total acid reflux events per 24 hours, longest acid reflux event per 24 hours, and frequency of acid events >5 minutes per 24 hours. The higher the Boix-Ochoa score, the greater the severity of the acid GER.
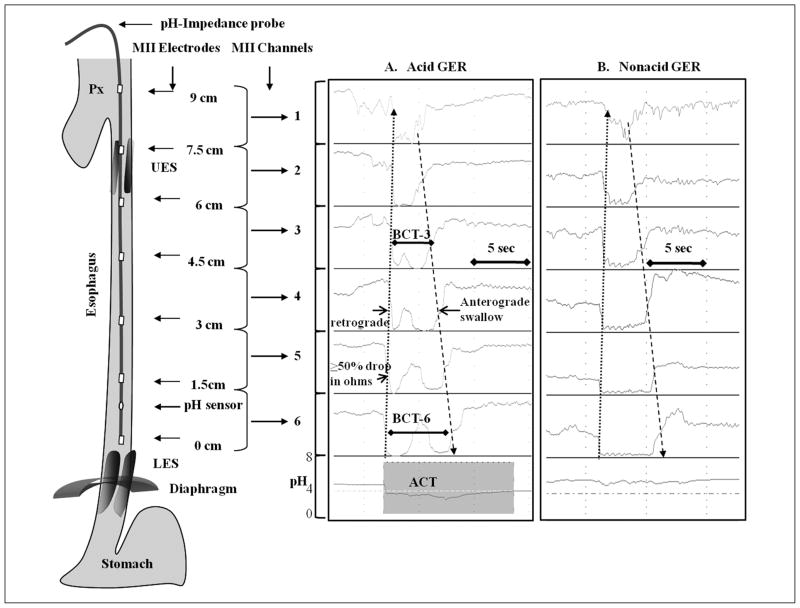
Figure 1.
Impedance characteristics of retrograde propagation bolus (dotted arrow) during (A) acid and (B) nonacid gastroesophageal reflux (GER). Anterograde clearance (hashed arrow), acid clearance time (ACT), and bolus clearance time at channel 3 (BCT-3) and channel 6 (BCT-6) are shown. The location of the pH channel is shown. LES, lower esophageal sphincter; UES, upper esophageal sphincter.
Analysis of GER events in relation to feeding cycle
A feeding cycle was defined as the time interval from the onset of feeding to the onset of the next feeding time. Two feeding cycles were analyzed in greater detail each in 2-minute epochs within the first, second, and third postprandial hours. Frequency and pH-characteristics of GER events, ACT, and BCT were measured. The characteristics of feeding such as feeding volume, feeding duration, feeding flow rate (feeding volume ingested divided by duration of feeding), and caloric density were also noted. ACT was measured as the time taken for esophageal pH to normalize to a pH ≥4.0 for ≥5 seconds (Figure 1A), and BCT was measured in channel 3 (BCT-3 proximal esophagus) and channel 6 (BCT-6 distal esophagus) as the time taken for impedance to return to normal in the proximal impedance channels (Figure 1A).
Analysis of correlation between the caloric density of infant feeds on GER events
The caloric density for human milk was presumed to be 20 cal/oz. Although energy content of human milk is variable, a commonly used value is 67 cal/dL (approximately 20 cal/oz).27 The caloric density of the formula was presumed to be as per specifications given by the manufacturers.
Statistical Analysis
The pH-impedance markers of GER events were analyzed and pairwise comparisons were performed to ascertain the differences within the first, second, and third postprandial hours. Data were adjusted for multiple testing by applying the Bonferroni method. Proportions of acid characteristics of GER during the feeding cycle were analyzed using the Fisher exact test or χ2 testing as appropriate. Parametric tests were applied for within-group comparisons; nonparametric tests (Wilcoxon rank-sum test) were used for data that were not normally distributed (GER characteristics). Spearman’s correlation coefficient was used for nonparametric dependent variables. Median (interquartile range [IQR]), mean ± SD, or % was reported, unless stated otherwise. P values <.05 were considered statistically significant. SPSS version 19.0 (SPSS, Inc, an IBM Company, Chicago, IL) methods were used to perform the analysis.
Results
Patient and Disease Characteristics
Demographic, disease, and symptom characteristics of the neonates are described in Tables 1 and 2, respectively. The primary reason for admission included feeding problems associated with prematurity (n = 28), bronchopulmonary dysplasia (BPD; n = 3), cardiorespiratory concerns related to feedings (n = 3), and perinatal asphyxia (n = 1). Infants were transitioning from gavage tube feeds to oral feeds. Of the 35 neonates, 17 were fed exclusively via oral feeding route. Five were fed fortified human milk (mean ± SD, 25.4 ± 1.9 cal/oz), 3 were fed unfortified human milk (20.0 ± 0.0 cal/oz), and 27 were fed formula feeds (25.0 ± 3.5 cal/oz).
Table 1.
Demographic Characteristics of Infants Referred for Evaluation of Gastroesophageal Reflux Disease
| Characteristics | Study Population (n = 35) |
|---|---|
| GA, mean ± SD, wk | 30.0 ± 4.9 |
| Birth weight, mean ± SD, kg | 1.5 ± 1.1 |
| Male gender, No. (%) | 20 (57) |
| PMA at study, mean ± SD, wk | 43.4 ± 4.5 |
| Weight at study, mean ± SD, kg | 3.6 ± 1.2 |
GA, gestational age; PMA, postmenstrual age.
The mean ± SD of total pH-impedance recording time per patient was 23.6 ± 0.7 hours. To describe the overall group composition, the median (IQR) of GER indices was reported as follows: Boix-Ochoa score for acid GER was 21.5 (24.8), acid reflux index (total acid reflux events per 24 hours) was 6.1% (9.5%), frequency of acid reflux events longer than 5 minutes per 24 hours was 4.3 (8.2), longest acid reflux events per 24 hours were 14.0 (21.4) minutes, total acid reflux events per 24 hours were 46.7 (45.2), average cumulative acid clearance time (ACT) in 24 hours was 128.0 (82.5) seconds, frequency of acid GER events per 24 hours was 18.0 (20.5), and frequency of nonacid GER events per 24 hours was 35.0 (30.0).
Effect of Milk Type and Feeding Methods on GER Events
The median (IQR) of the effect of feeding milk (human milk vs formula milk, respectively) was 58.0 (21.2) vs 20.0 (40.0), P = 1.0, for total GER events; 27.0 (14.3) vs 16.0 (19.0), P = .8, for acid GER events; and 43.5 (29.5) vs 35.0 (29.5), P = 0.8, for nonacid GER events and showed no significant difference during 24 hours. Similarly, effect of caloric density on total GER events (ρ = −0.1, P = .6), acid GER events (ρ = −0.1, P = .5), and nonacid GER events (ρ = −0.05, P = .8) in 24 hours also was not significant.
In contrast, comparing the oral-fed group vs the gavage-fed group, respectively, a greater frequency of total GER events (77.0 [55.0] vs 47.0 [23.5], P = .01), acid GER events (24.0 [23.0] vs 14.5 [20.0], P = .04), and nonacid GER events (52.0 [27.5] vs 29.5 [21.8], P = .005) was noted in the oral-fed group. Overall, no significant difference (P = .3) was noted between the oral-fed (131.0 [66.5]) and gavage-fed groups (128.0 [102.0]) with regard to the average cumulative ACT in 24 hours. The median (range) of average oral feeding duration vs gavage feeding duration for 35 neonates was 19.5 (7.4–31.9) minutes vs 30.8 (11.8–180.4) minutes, P = .002. In the gavage-fed group, 1 patient was fed continuously, and 2 were fed for over 2 hours in the feeding cycle. These outliers increased the average feeding duration in the gavage-fed group. Also, the average oral feeding volume vs gavage feeding volume was 60.0 (45.0–132.0) mL vs 54.5 (29.0–88.0) mL, P = .4.
Changes in Acid/Nonacid Profile of GER Events in Relation to Feeding Cycles
Data were analyzed in detail over 2-minute segments from 70 feeding cycles, with each cycle lasting 3 hours, in 35 neonates during which patients were lying supine. There were 562 GER events during these feeding cycles. We tested if the composition of refluxate changed with each postprandial hour by evaluating the frequency and chemical composition of GER occurring hourly over the 3-hour feeding cycle (Figure 2). The percentage distribution of acid GER events was significantly greater with each incremental increase from postprandial hours 1 to 3.
Figure 2.
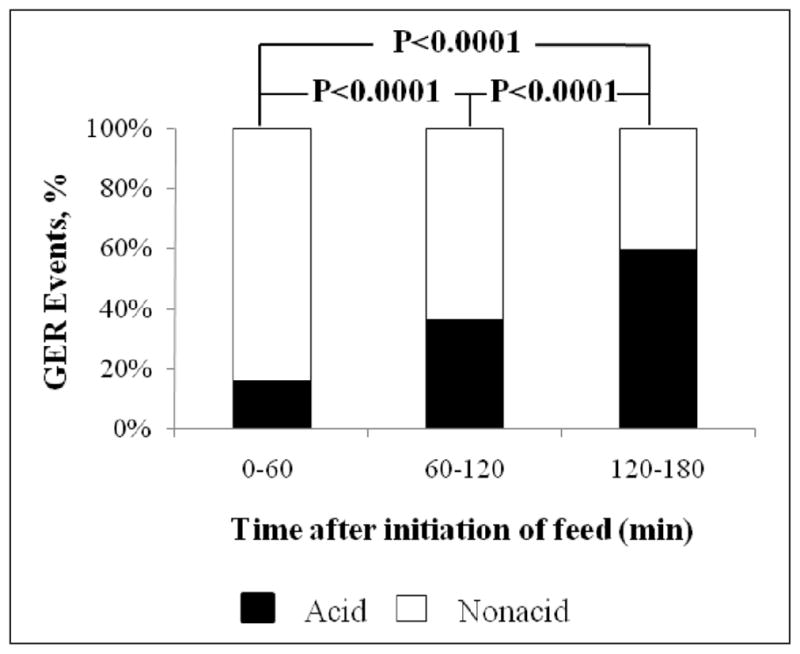
Acid characteristics of the gastroesophageal reflux (GER) during each hour of two 3-hour feeding cycles. The percentage distribution of acid GER events is significantly greater with an increment in time intervals.
Effects of Feeding Duration, Feeding Volume, and Feeding Flow Rates on GER Frequency, Composition, and Clearance Characteristics
During each feeding attempt, the median (IQR) of feeding duration, feeding volume, feeding flow rate, and caloric density was 25.6 (15.4) minutes, 56.0 (20.0) mL, 2.7 (3.0) mL/min, and 24.0 (5.0) cal/oz, respectively.
To test whether there was a correlation between feeding duration (min), feeding volume (mL), flow rate (mL/min), and caloric density (cal/oz) vs total GER events, number of acid GER, number of nonacid GER, BCT, and ACT, we calculated Spearman correlation coefficients (ρ) and P values (Table 3). Significant negative linear correlation (P ≤ .03) was detected between feeding duration with regard to the total GER events, number of nonacid GER, and BCT-3. Conversely, a significant positive linear correlation (P = .002) was found between feeding volume with BCT-3. Also, a significant positive linear correlation (P ≤ .01) was detected between feeding flow rate with regard to the total GER events, number of nonacid GER, and BCT-3 and BCT-6. Importantly, no significant correlation was noted between caloric density to the acid characteristics of GER and clearance.
Table 3.
Relationship of Feeding Duration, Feeding Volume, Feeding Flow Rate, and Caloric Density of Feeds on Gastroesophageal Reflux (GER) Characteristics
| Variable of Interest (Per Patient Per Feeding Cycle) | Feeding Duration, min
|
Feeding Volume, mL
|
Flow Rate, mL/min
|
Caloric Density, cal/oz
|
||||
|---|---|---|---|---|---|---|---|---|
| ρ | P Value | ρ | P Value | ρ | P Value | ρ | P Value | |
| Total GER events | −0.5 | .006* | 0.2 | .2 | 0.4 | .01* | −0.1 | .5 |
| Number of acid GER events | −0.1 | .5 | 0.2 | .2 | 0.1 | .4 | −0.1 | .5 |
| Number of nonacid GER events | −0.5 | .002* | 0.2 | .3 | 0.5 | .003* | −0.05 | .8 |
| BCT-3 | −0.4 | .03* | 0.5 | .002* | 0.6 | .001* | −0.08 | .7 |
| BCT-6 | −0.2 | .2 | 0.3 | .09 | 0.4 | .04* | −0.2 | .3 |
| ACT | −0.03 | .9 | 0.01 | .9 | −0.03 | .9 | 0.07 | .7 |
ACT, acid clearance time; BCT-3, bolus clearance time at channel 3; BCT-6, bolus clearance time at channel 6.
Significant correlation.
Impact of the BCT and ACT in Relation to Feeding Cycles During GER Events
The mean ± SD (median, IQR) of BCT and ACT for 2 feeding cycles during the first, second, and third postprandial hours is described in Table 4. At an hourly interval, the BCT-3 significantly decreased with time (analysis of variance [ANOVA] P < .05). Comparing the hourly intervals, ACT had increased with time (ANOVA P = .05). Comparing BCT-3 and ACT by each postprandial hour, a longer ACT was noted during the second and third hours after the initiation of feed (P ≤ .001; Table 4).
Table 4.
Bolus Clearance Time and Acid Clearance Time During Each Hour of the 3-Hour Feeding Cycle
| Postprandial Hour | BCT, s | ACT, s | P Value |
|---|---|---|---|
| 1 | 26.2 ± 30.9 (18.7, 36.3) | 218.4 ± 716.7 (0, 7.4) | .9 |
| 2 | 15.3 ± 23.0 (4.9, 21.7) | 419.1 ± 940.8 (0, 271.1) | .001 |
| 3 | 13.5 ± 24.1 (1.8, 18.0) | 589.6 ± 709.2 (432.4, 863.7) | <.0001 |
Values are shown as mean ± SD (median, IQR). ACT, acid clearance time; BCT, bolus clearance time.
Discussion
The aerodigestive symptoms pertinent to dysphagia in ICU neonates are heterogeneous, and GER is a frequent diagnosis often made empirically. This approach results in significant use of acid-suppressive treatments, albeit adding to further drug-related risks.2,28 Furthermore, feeding strategies (ie, methods of administration of feeds) are frequently altered in the convalescing neonates owing to the complications speculated to be due to GER. However, the impact of feeding methods on the prevalence of GER events has not been clear. In this study, we tested the effects of feeding methods, feeding volumes, feeding duration, feeding flow rates, postprandial phases, feeding type (human milk or formula), and caloric density on the frequency and acid characteristics of GER events.
The significant findings of this study are as follows. (1) The profile of symptom characteristics of dysphagic infants was predominantly aerodigestive in nature. (2) The proportion of acid GER events increased and nonacid GER events decreased with an increment in each postprandial hour (P < .0001). (3) The BCT-3 within the feeding cycle significantly decreased with an incremental increase in postprandial time intervals (ANOVA P < .05). The ACT within the feeding cycle was significantly longer with an incremental increase in time intervals (ANOVA P = .05). (4) Prolonged feeding duration resulted in a decrease in the number of total GER events, a decrease in the number of nonacid GER events, and a lower BCT-3. (5) Reduced feeding volume decreased average BCT-3. (6) A decrease in the flow rate of feeds resulted in a decrease in the number of total GER events, a decrease in the number of non-acid GER, and a lower BCT-3 and BCT-6. (7) Importantly, no significant difference was noted between the milk types (breast milk or formula) or in caloric density with regard to the GER characteristics. (8) The oral-fed group had a higher frequency of acid and nonacid GER events than the gavage-fed group during the 24-hour study.
The acid characteristics of GER were also differently distributed within the feeding cycle, suggesting that feeds neutralize or modify the acidity of refluxate and that gastric acid secretion is upregulated prior to the feed.29–31 An increase in acid GER events may be due to an increase in acidity in the third postprandial hour that may have resulted from gastric emptying of the previously ingested milk, as well as a sustained increase in gastric acid output prior to the next feeding session. This finding is expected. However, there were no differences between the human milk–fed and formula-fed groups. This may because the neonates on human milk are not receiving pure human milk feeds but are on cow milk additives to boost nutrients that make this different than earlier findings.32 In addition, it was speculated in a previous study that the presence of an intragastric tube stents the LES, resulting in more acid GER events.33 Interestingly, tube-fed infants in the current study had fewer GER events (overall and acid) than the exclusively oral-fed group. This is contrary to frequent speculation of higher GER rates in gavage-fed infants. Thus, prophylactic use of antireflux strategies in gavage-fed infants should be discouraged without objective evidence because the implications and side effects of such therapies have far-reaching effects.34 Clinical symptoms of GER feeding in the first hour of the feeding cycle are more likely due to nonacid GER events because feeds neutralize the acidity from the stomach and contribute to nonacid reflux.
We did not evaluate the association of GER-related symptoms in this article. However, Salvatore et al35 reported that a significant higher association between symptoms and nonacid reflux was seen in the first 6 months of life. We speculate that increased GER observed in oral-fed infants may be due to gastric distention or fundal stimulation from an increased volume of swallowed milk and air. Swallow-associated LES relaxation or transient LES relaxation may be the mechanisms responsible for GER events during feeding or the postprandial period, respectively. At the same time, oral-fed infants burp gas out of their stomachs during or after oral feedings and therefore can be more prone to physiological GER events. The pathophysiological significance of such events in dysphagic infants is unclear. This contrasts with the fact that gavage-fed infants did not have an increase in GER events during feeding, suggesting that the integrity of the LES remains intact, thus protecting the aerodigestive tract. Increased frequency of GER and longer BCT in the first postprandial hour in oral-fed infants would then suggest important implications<@151>that in symptomatic infants, a decreased feeding volume and postural interventions may be most effective if implemented immediately following a feed. Transient LES relaxation is the most common mechanism for GER events and occurs due to fundal stimulation. Significantly, the average feeding duration in the gavage-fed group was 2.6 times longer than that of the oral-fed group. However, the average feeding volume in the gavage-fed group was 0.8 times vs the feeding volume for the oral-fed group but was not statistically significant. Therefore, it is conceivable that gastric distention can occur faster in the oral-fed infants.
However, esophageal stimulation can also result in LES relaxation by different mechanisms (deglutition response and LES relaxation or secondary peristalsis and LES relaxation), as demonstrated by our group.36
The relationships between body position and GER7 or gastric emptying and GER37 have been studied before. In the former study, patients were fed orally or via orogastric tube, inserted and removed at each meal, and body position varied with each meal. In the latter study, positions were varied in a predesigned manner within a feeding period. These studies did not differentiate the effects of feeding strategies, as was done in our study. Our study is different in several respects: (1) because body position is an important variable for the occurrence of GER events, in the current study, we compared data gathered during those feeding cycles when neonates were supine; (2) neonates were on maximal prescribed feeds per kg at the time of evaluation; and (3) characteristics of GER classified on the chemical nature of refluxate and acid clearance duration are described with different feeding strategies.
In summary, this is the first study to evaluate the impact of feeding variables on the acid and nonacid characteristics of GER events in relation to the feeding cycles and feeding strategies in human neonates. Important clinical implications for consideration in suspected GER in infants include the following: (1) longer feeding duration and slower milk intake or slower milk flow rates are associated with fewer GER events, (2) gavage-fed infants are not naturally at increased risk of GER because of an indwelling tube, and (3) type of milk (breast milk or formula) or the composition of milk (caloric density) has no increased effects on GER frequency or characteristics.
Table 2.
Symptom Characteristics
| No. (%) | |
|---|---|
| Dysphagia | 35 (100) |
| Emesis | 9 (26) |
| Bradycardia and desaturation | 8 (23) |
| Feeding aversion | 8 (23) |
| Irritability and arching | 6 (17) |
| Choking events needing resuscitation | 5 (14) |
| Cough or stridor | 4 (11) |
| Aspiration | 1 (3) |
Acknowledgments
We are grateful to Juan Peng, MAS, for statistical advice and Walter Kim, BS, MPH, for technical support with this project.
Footnotes
Financial disclosure: This study is supported in part by grant funding from NIH RO1 DK 068158 (Jadcherla).
References
- 1.Clark RH, Spitzer AR. Patience is a virtue in the management of gastroesophageal reflux. J Pediatr. 2009;155:464–465. doi: 10.1016/j.jpeds.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Malcolm WF, Gantz M, Martin RJ, Goldstein RF, Goldberg RN, Cotten CM. Use of medications for gastroesophageal reflux at discharge among extremely low birth weight infants. Pediatrics. 2008;121:22–27. doi: 10.1542/peds.2007-0381. [DOI] [PubMed] [Google Scholar]
- 3.Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 4.Di Fiore J, Arko M, Herynk B, Martin R, Hibbs AM. Characterization of cardiorespiratory events following gastroesophageal reflux in preterm infants. J Perinatol. 2010;30:683–687. doi: 10.1038/jp.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peter CS, Sprodowski N, Bohnhorst B, Silny J, Poets CF. Gastroesophageal reflux and apnea of prematurity: no temporal relationship. Pediatrics. 2002;109:8–11. doi: 10.1542/peds.109.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Wheatley E, Kennedy KA. Cross-over trial of treatment for bradycardia attributed to gastroesophageal reflux in preterm infants. J Pediatr. 2009;155:516–521. doi: 10.1016/j.jpeds.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corvaglia L, Rotatori R, Ferlini M, Aceti A, Ancora G, Faldella G. The effect of body positioning on gastroesophageal reflux in premature infants: evaluation by combined impedance and pH monitoring. J Pediatr. 2007;151:591–596. 596.e1. doi: 10.1016/j.jpeds.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Omari TI, Rommel N, Staunton E, et al. Paradoxical impact of body positioning on gastroesophageal reflux and gastric emptying in the premature neonate. J Pediatr. 2004;145:194–200. doi: 10.1016/j.jpeds.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Wenzl TG, Schneider S, Scheele F, Silny J, Heimann G, Skopnik H. Effects of thickened feeding on gastroesophageal reflux in infants: a placebo-controlled crossover study using intraluminal impedance. Pediatrics. 2003;111:e355–e359. doi: 10.1542/peds.111.4.e355. [DOI] [PubMed] [Google Scholar]
- 10.Rudolph CD, Mazur LJ, Liptak GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001;32:S1–S31. doi: 10.1097/00005176-200100002-00001. [DOI] [PubMed] [Google Scholar]
- 11.Babbitt C. Transpyloric feeding in the pediatric intensive care unit. J Pediatr Gastroenterol Nutr. 2007;44:646–649. doi: 10.1097/MPG.0b013e318030d7d2. [DOI] [PubMed] [Google Scholar]
- 12.Jadcherla SR, Gupta A, Fernandez S, et al. Spatiotemporal characteristics of acid refluxate and relationship to symptoms in premature and term infants with chronic lung disease. Am J Gastroenterol. 2008;103:720–728. doi: 10.1111/j.1572-0241.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Alonso M, Moya MJ, Cabo JA, et al. Twenty-four-hour esophageal impedance-pH monitoring in healthy preterm neonates: rate and characteristics of acid, weakly acidic, and weakly alkaline gastroesophageal reflux. Pediatrics. 2006;118:e299–e308. doi: 10.1542/peds.2005-3140. [DOI] [PubMed] [Google Scholar]
- 14.Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024–1031. doi: 10.1136/gut.2003.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burklow KA, Phelps AN, Schultz JR, McConnell K, Rudolph C. Classifying complex pediatric feeding disorders. J Pediatr Gastroenterol Nutr. 1998;27:143–147. doi: 10.1097/00005176-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Hans DM, Pylipow M, Long JD, Thureen PJ, Georgieff MK. Nutritional practices in the neonatal intensive care unit: analysis of a 2006 neonatal nutrition survey. Pediatrics. 2009;123:51–57. doi: 10.1542/peds.2007-3644. [DOI] [PubMed] [Google Scholar]
- 17.Helm JF, Dodds WJ, Riedel DR, Teeter BC, Hogan WJ, Arndorfer RC. Determinants of esophageal acid clearance in normal subjects. Gastroenterology. 1983;85:607–612. [PubMed] [Google Scholar]
- 18.Jadcherla SR, Klee G, Berseth CL. Regulation of migrating motor complexes by motilin and pancreatic polypeptide in human infants. Pediatr Res. 1997;42:365–369. doi: 10.1203/00006450-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Woodley FW, Fernandez S, Mousa H. Diurnal variation in the chemical clearance of acid gastroesophageal reflux in infants. Clin Gastroenterol Hepatol. 2007;5:37–43. doi: 10.1016/j.cgh.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A, Jadcherla SR. The relationship between somatic growth and in vivo esophageal segmental and sphincteric growth in human neonates. J Pediatr Gastroenterol Nutr. 2006;43:35–41. doi: 10.1097/01.mpg.0000226368.24332.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenzl TG, Silny J, Schenke S, Peschgens T, Heimann G, Skopnik H. Gastroesophageal reflux and respiratory phenomena in infants: status of the intraluminal impedance technique. J Pediatr Gastroenterol Nutr. 1999;28:423–428. doi: 10.1097/00005176-199904000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Castell DO, Vela M. Combined multichannel intraluminal impedance and pH-metry: an evolving technique to measure type and proximal extent of gastroesophageal reflux. Am J Med. 2001;111:157S–159S. doi: 10.1016/s0002-9343(01)00826-9. [DOI] [PubMed] [Google Scholar]
- 23.Tutuian R, Castell DO. Use of multichannel intraluminal impedance to document proximal esophageal and pharyngeal nonacidic reflux episodes. Am J Med. 2003;115:119S–123S. doi: 10.1016/s0002-9343(03)00209-2. [DOI] [PubMed] [Google Scholar]
- 24.Tutuian R, Vela MF, Shay SS, Castell DO. Multichannel intraluminal impedance in esophageal function testing and gastroesophageal reflux monitoring. J Clin Gastroenterol. 2003;37:206–215. doi: 10.1097/00004836-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Wenzl TG, Schenke S, Peschgens T, Silny J, Heimann G, Skopnik H. Association of apnea and nonacid gastroesophageal reflux in infants: Investigations with the intraluminal impedance technique. Pediatr Pulmonol. 2001;31:144–149. doi: 10.1002/1099-0496(200102)31:2<144::aid-ppul1023>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Boix-Ochoa J, Lafuenta JM, Gil-Vernet JM. Twenty-four hour esophageal pH monitoring in gastroesophageal reflux. J Pediatr Surg. 1980;15:74–78. doi: 10.1016/s0022-3468(80)80407-6. [DOI] [PubMed] [Google Scholar]
- 27.Kleinman RE. Pediatric Nutrition Handbook. 6. Elk Grove Village, IL: American Academy of Pediatrics; 2009. [Google Scholar]
- 28.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 29.Armand M, Hamosh M, Mehta NR, et al. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res. 1996;40:429–437. doi: 10.1203/00006450-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Cavell B. Postprandial gastric acid secretion in infants. Acta Paediatr Scand. 1983;72:857–860. doi: 10.1111/j.1651-2227.1983.tb09830.x. [DOI] [PubMed] [Google Scholar]
- 31.Omari TI, Davidson GP. Multipoint measurement of intragastric pH in healthy preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:F517–F520. doi: 10.1136/fn.88.6.F517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomomasa T, Hyman PE, Itoh K, et al. Gastroduodenal motility in neonates: response to human milk compared with cow’s milk formula. Pediatrics. 1987;80:434–438. [PubMed] [Google Scholar]
- 33.Peter CS, Wiechers C, Bohnhorst B, Silny J, Poets CF. Influence of nasogastric tubes on gastroesophageal reflux in preterm infants: a multiple intraluminal impedance study. J Pediatr. 2002;141:277–279. doi: 10.1067/mpd.2002.126298. [DOI] [PubMed] [Google Scholar]
- 34.Mertens V, Blondeau K, Vanaudenaerde B, et al. Gastric juice from patients “on” acid suppressive therapy can still provoke a significant inflammatory reaction by human bronchial epithelial cells. J Clin Gastroenterol. 2010;44:e230–e235. doi: 10.1097/MCG.0b013e3181d47dc4. [DOI] [PubMed] [Google Scholar]
- 35.Salvatore S, Arrigo S, Luini C, Vandenplas Y. Esophageal impedance in children: symptom-based results. J Pediatr. 2010;157:949–954. e941–e942. doi: 10.1016/j.jpeds.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 36.Pena EM, Parks VN, Peng J, et al. Lower esophageal sphincter relaxation reflex kinetics: effects of peristaltic reflexes and maturation in human premature neonates. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1386–G1395. doi: 10.1152/ajpgi.00289.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Wijk MP, Benninga MA, Dent J, et al. Effect of body position changes on postprandial gastroesophageal reflux and gastric emptying in the healthy premature neonate. J Pediatr. 2007;151:585–590. e581–e582. doi: 10.1016/j.jpeds.2007.06.015. [DOI] [PubMed] [Google Scholar]