Abstract
Cryopreservation of fish sperm has been studied for decades at a laboratory (research) scale. However, high-throughput cryopreservation of fish sperm has recently been developed to enable industrial-scale production. This study treated blue catfish (Ictalurus furcatus) sperm high-throughput cryopreservation as a manufacturing production line and initiated quality assurance plan development. The main objectives were to identify: 1) the main production quality characteristics; 2) the process features for quality assurance; 3) the internal quality characteristics and their specification designs; 4) the quality control and process capability evaluation methods, and 5) the directions for further improvements and applications. The essential product quality characteristics were identified as fertility-related characteristics. Specification design which established the tolerance levels according to demand and process constraints was performed based on these quality characteristics. Meanwhile, to ensure integrity throughout the process, internal quality characteristics (characteristics at each quality control point within process) that could affect fertility-related quality characteristics were defined with specifications. Due to the process feature of 100% inspection (quality inspection of every fish), a specific calculation method, use of cumulative sum (CUSUM) control charts, was applied to monitor each quality characteristic. An index of overall process evaluation, process capacity, was analyzed based on in-control process and the designed specifications, which further integrates the quality assurance plan. With the established quality assurance plan, the process could operate stably and quality of products would be reliable.
Keywords: Cryopreservation, blue catfish sperm, quality assurance plan, specification design, quality control, CUSUM, process capacity
Introduction
Cryopreservation can retain the biological function of animal spermatozoa for years or decades under very low temperatures (−196°C for liquid nitrogen, and usually <−100°C for vapor phase). Cryopreservation technology has been developed in mammals, birds, amphibians, fishes, and some invertebrate species [22]. The development of fish sperm cryopreservation dates back 60 yr [3], and in the past decade alone, there were some 300 publications on fish sperm cryopreservation [33]. With growing interest and applications in cryopreserved fish sperm, laboratory-scale cryopreservation cannot satisfy the demands from aquaculture farms or fish hatcheries. Therefore, cryopreservation of fish sperm at a commercial scale with high-throughput has become an increasingly important research topic [34].
Catfish sperm cryopreservation has been studied for more than 35 yr [15] but mostly in the laboratory environment. Previous large-scale trials have been performed in a dairy improvement center using processing specialized for bull semen [20], although these products were not tested at a commercial level. During the past 4 years, automation of the cryopreservation process has been developed at laboratory and commercial scales [18]. A demonstration production line was developed for cryopreserved blue catfish sperm, but was operated without the quality assurance guidelines necessary to produce reliable products at a scale relevant to commercial application.
The quality assurance concept has been integrated previously into aquaculture research. These include qualitative activities, such as sex identification [19] and separation of spawning-ready females after collection from ponds [12], and quantitative activities, such as selective breeding [4]. However, those activities and their details were seldom placed in written form or uniform formats. From an engineering perspective, all criteria used for decision making in those activities could be described as quality characteristics, and all quality assurance can be documented within two aspects: specification design and quality control. Specification design regulates the acceptance of materials with certain quality characteristics, so that waste and high variation in the process due to less-functional or non-functional components can be avoided [8]. For example, if the quality characteristics were defined by observable body features during selection and hormone injection of females for artificial spawning [12], the specification would be a flat belly (indicating poor potential for spawning); if the quality characteristic is the ultrasonic image features of gonads [24], the specification would be the absence of visible mature eggs. These specifications could help ensure that the final products would meet the basic expectation of farmers (customers). Quality control is used to monitor the variability of products during processing, and to provide suggestions for reduction of variability [23]. In the same example of selecting catfish females, the ultrasound images can provide quantified quality characteristics such as egg size within the ovaries [16]. When statistical quality control is applied, control charts of egg size developed from previous size data can be used to check every new sample and identify samples that exceed the control limits. If the data set exceeds control limits, corrective measurements can be taken to rectify out-of-control products or operation steps by using quality control tools such as a cause-and-effect diagrams [23].
In this study, an initial quality assurance plan for high-throughput sperm cryopreservation of blue catfish (Ictalurus furcatus) was developed and evaluated in practice. The main objectives were to identify: 1) the main quality characteristics; 2) the process features for quality assurance; 3) the internal quality characteristics and their specification designs; 4) the quality control and process capability evaluation methods, and 5) the directions for further improvements and applications. This study was the first application of statistical quality control in aquatic species sperm cryopreservation and potentially the first of its kind for any species.
Main quality characteristics
Voice of customers
The “Voice of Customers” represents the demands for product quality which provide the initial point of quality assurance. Properly satisfying the requirements of customers can improve profitability [13]. Typically voice of customers data are collected from large comprehensive surveys, and represent the customer tolerances on the product. Engineers and designers would convert these data into a production engineering matrix using quality function deployment [13]. Commercial-scale high-throughput cryopreservation in blue catfish sperm is a newly developed process. Customers have not yet provided quantitative expectations on its production. Without comprehensive surveys and quality function deployment support, in this study, the voice of customers was generalized as an overall pursuit of high product quality.
Fertility quality characteristics
The product resulting from this process was cryopreserved sperm contained in sealed plastic straws. With the purpose of making hybrid catfish fry, the quality of product was directly related to the fertility of the sperm cells. In commercial practice with fresh sperm, the number of swim-up fry (a free-feeding developmental stage after hatching) is the most commonly used evaluation method [1]. In laboratories, the ratio of the initial number of eggs to different stages of embryo development is often used for evaluations [2; 18; 20]. Although fry and embryo development are reliable indices for sperm fertility, the evaluation takes 1 to 10 days and introduces other variations such as female condition [11; 19] and hatchery management [31]. Specifically, after sperm is used to fertilize eggs, neurulation is assessed after 27 −30 hr (at 27 −29 °C); embryonic mobility is observed after 48–50 hr [18]; hatching percent or sacfry (newly-hatched) numbers are assessed after 5 d, and swim-up fry are counted after 10 d [1]. In addition, channel catfish females only spawn within a strict temperature range [31]. Therefore, high quality eggs for performing fertility evaluations are typically limited to 6 to 8 weeks per year (during April to June in the southeastern US).
Some of the measureable indices for thawed sperm are motility, membrane integrity and cell concentration. These indices are often used in fish sperm research. Motility represented the progressive sperm swimming behavior by identifying and estimating the proportion within the entire sperm population [28; 32]. Membrane integrity represents the overall viability of sperm cells typically measured by flow cytometery [28]. Concentration indicates sperm numbers before freezing and after thawing. Although, there has been no significant correlation between these three indices of thawed sperm and the numbers of fry produced (due to a lack of experimental standardization and variations at the hatchery level during development such as water quality), studies have suggested that sperm motility is correlated with embryo development [7; 15; 18; 36]. In this study, motility, membrane integrity and cell concentration of thawed sperm were considered as the main quality characteristics for the final product.
Specification design
Catfish culture is the largest foodfish aquaculture industry [35]. Within this industry, hybrid catfish (channel catfish Ictalurus punctatus female × blue catfish Ictalurus furcatus male) are in high demand due to their fast growth rate and disease resistance [12]. Blue catfish males (D&B strain) used in this study were from Baxter Land Company Fish Farm (Arkansas City, Arkansas; 33°34’58.64”N, 91°15’18.45”W). The males were selected at the farm based on observable secondary sexual characteristics (e.g. a well-muscled head and dark coloration) [1] before transportation. An oxygenated hauler was used for transport from the Baxter Land Company Fish Farm to the Aquaculture Research Station of the Louisiana State University Agricultural Center (Baton Rouge; 30°22’07.32”N, 91°10’27.90”W) in spring of 2009 to 2011. The males were held for 2 to 4 wk in aerated outdoor 0.1-acre ponds and fed commercial diets (Aquaxcel, CargillTM, 45% protein), before capture and transfer into indoor tanks with a recirculating system 2 d before processing. The system used bubble-washed bead filters that were back-flushed every 2 d. The water quality parameters were: pH 7.0 – 8.0, total ammonia-nitrogen 0.1 – 0.8 mg/L, nitrite 0.04 – 0.30 mg/L, alkalinity 39 – 125 mg/L, hardness 44 – 126 mg/L, temperature 28 ± 1 °C, and dissolved oxygen 4.3 – 6.5 mg/L. Water quality data were obtained throughout the year. With different levels of biomass maintained in the system, the parameters varied but were maintained within the suitable range for fish. Guidelines from the Institutional Animal Care and Use Committees (IACUC) of Louisiana State University were followed for animal care in this study.
Each year channel catfish and blue catfish spawn during overlapping but different 2-to-3-month windows (i.e., spawning seasons) [16]. For the purposes of this study, all blue catfish that spawned during the same season were considered as a single population designated by the year for quality assurance studies. For specification design, all fish from the first season (2010) were considered as the “normal population” (i.e., representative of this species in all characteristics). The features of this “normal population” initiated the quality assurance plan that was used to monitor the test population of the 2nd season (2011). In future work, both populations (2010 and 2011) could be merged to form a more representative “normal population” which would make the quality assurance plan more comprehensive. Sperm samples from 27 males were cryopreserved individually during the 2010 season by use of our established high-throughput protocol [18], and samples from 59 males were processed in the 2011 season. Thawing of straws was performed in a 40°C water bath for 20 sec. Motility assessment and flow cytometric analysis were performed for the individual thawed samples [9]. The processing period overlapped with the hybrid catfish fry production season (April – June) and the males in this study were representative of the blue catfish male populations used commercially in hybrid catfish hatcheries.
The ideal specifications for industrial processing are often set with the three-sigma rule which equals a 99.7% confidence interval [23]. However, with the biological products in this study, a 95% confidence interval was applied, which equaled to two sigmas (1.96 sigmas above and below the mean value exactly) from the mean value. All data were checked to identify outliers, and tested for normality. The range was calculated according to the data distribution and practical considerations (Table 1).
Table 1.
Specifications for the main quality characteristics in blue catfish sperm high-throughput cryopreservation. LSL: lower specification limit; USL: upper specification limit.
| Quality characteristic | Unit | LSL | USL |
|---|---|---|---|
| post-thaw motility | percent | 7 | 100 |
| post-thaw membrane integrity | percent | 70 | 100 |
| post-thaw concentration | cells/ml | 9,880 | 307,511 |
Because sperm with low post-thaw motility (e.g., 18 ± 22%) can fertilize eggs [18], there were no quantitative studies available to define the acceptable level of post-thaw motility. Based on our practical experience, this study used 10% as a threshold. After discarding values under 10%, Kolmogorov-Smirnov normality testing showed the post-thaw motility followed a normal distribution (P = 0.127) with a mean of 25% and standard deviation of 11%. The 95% confidence interval was from 7 – 44%. However, a positive correlation with embryo development suggested that higher the motility was better [18], so the adjusted specification for post-thaw motility was from 7 – 100%.
The post-thaw membrane integrity dataset contained a single outlier (statistical extreme value that was outside normal data range) based on the data distribution. This outlier was associated with a sample that had incomplete records and poor repeatability among quality characteristics possibly due to human error. After eliminating the outlier, Kolmogorov-Smirnov normality testing showed the data followed a normal distribution (P = 0.291) with a mean of 80%, and a standard deviation of 6%. The 95% confidence interval was from 70 – 89%. Because the biological definition of membrane integrity represents viable cells, high membrane integrity was not considered as a negative impact. Therefore, the adjusted specification for post-thaw membrane integrity was 70 – 100%.
The post-thaw sperm concentration was measured by flow cytometry. For the purposes of the statistical quality control process, the actual (unmodified) data collected were used for analysis. Therefore the dilution (1:249) required for flow cytometry yielded reduced cells counts [10]. These values were used directly as inputs for the quality control analysis.. According to statistical analysis of the residual plots of the data distribution, the dataset contained a single outlier. This outlier was associated with a sample that had an extreme value probably due to a pipetting error. After eliminating the outlier, Kolmogorov-Smirnov normality testing showed the data did not follow a normal distribution (P < 0.005) with a mean of 1.04×105 cells/ml, a standard deviation of 1.34×105/ml, and a skewness of 1.12 and kurtosis of –0.16. The best fit was 3-parameter Weibull distribution (P < 0.005) with shape 0.53, scale of 5.92×104, and a threshold of 4.75×103. A large number of random data (500 data sets) were simulated from the same distribution and sorted repeatedly. From the probability density function of these simulated data, the 95% confidence interval was from 9,880 – 307,511 cells/ml.
High-throughput cryopreservation process structure
The entire high-throughput cryopreservation process was divided into 11 operations (Table 2). Each operation could be carried out separately as an assembly line; or a single worker could finish the job by executing the operations in sequence. To ensure product quality, detailed quality assurance activities had to be established and documented. The sperm cells were kept in different forms in different operations during the process. The purpose of the process was to transfer the sperm cells from the fish body to a well-controlled artificial condition for long-term preservation. For Operations 1 to 3, sperm were kept inside the fish body cavity; for Operation 4, sperm were kept inside the extracted gonad; for Operations 5 to 7, sperm were kept in 50-ml tubes of Hanks’ balanced salt solution at 300 mOsmol/kg (HBSS300) with or without cryoprotectant (5% methanol); for Operation 8, sperm were packaged in 0.5-ml straws with HBSS300 and cryoprotectant; for Operations 9 to 11, sperm were frozen in straws, and for Operation 11 large numbers of straws (e.g., 144 straws) were sorted and grouped into 12-compartment daisy goblets for storage.
Table 2.
List of operations in high-throughput cryopreservation processing for blue catfish sperm.
| Operation number | Operation name | Material used |
|---|---|---|
| 1 | Fish handling | Blue catfish males |
| 2 | Fish dissection | Blue catfish males |
| 3 | Testis processing | Blue catfish testes |
| 4 | Filtering sperm suspension | Crushed testes |
| 5 | Sample inspection | Sperm suspensions |
| 6 | Preparing for freezing | Sperm suspensions |
| 7 | Packaging in straws | Sperm suspensions |
| 8 | Freezing and off-line inspection | Nonfrozen straws |
| 9 | Sorting and storage | Frozen straws |
| 10 | Off-line quality check | Frozen straws |
| 11 | Storage preparation | Daisy goblets |
The current process had two distinguishing features. First, it was a continuous process specifically designed for processing of blue catfish sperm whenever the process was running. No other fish species were applied in this process, and process setup remained unchanged through time. Secondly, the process was built based on 100% inspection, wherein each operation, all samples were inspected. The inspection type affected the methods of quality control. For variable (or quantitative) data, this study applied cumulative sum x (CUSUM x) for mean monitoring and cumulative sum x2 (CUSUM x2) for variance monitoring [26], and for attribute (or qualitative) data, binomial cumulative sum (CUSUM) [5] has been recommended.
Related internal quality characteristics and specification design
The major quality characteristics of the final products were influenced by the many internal quality characteristics along the operations of processing. As part of the quality assurance plan, identifying and managing these internal quality characteristics helped to control the process and reduce the variation in product quality.
Defects
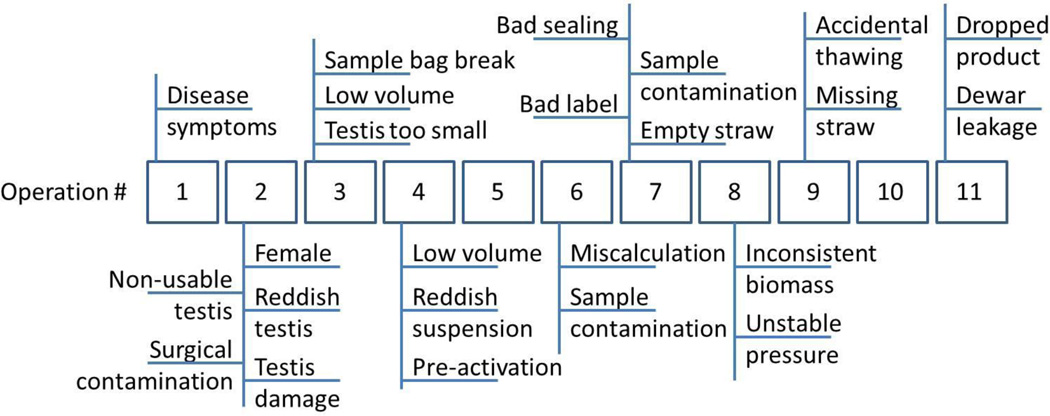
The high-throughput cryopreservation process was identified as a batch process with several material transformations during the process (Table 2). Batch processing meant that directly from the fish materials were handled individually as sperm suspensions. After packaging, the individual materials were distributed into smaller units (straws), but were still identified by their sources as individual fish and were handled together. Throughout the processing, each step of the operation could incur defects that might lead to waste, partial batches or low quality (Figure 1). Some of these defects were related to incoming material quality while others could be caused by operational errors throughout the process.
Figure 1.
Common defects along the entire 11-step processing pathway for blue catfish sperm high-throughput cryopreservation.
Identification of quality characteristics
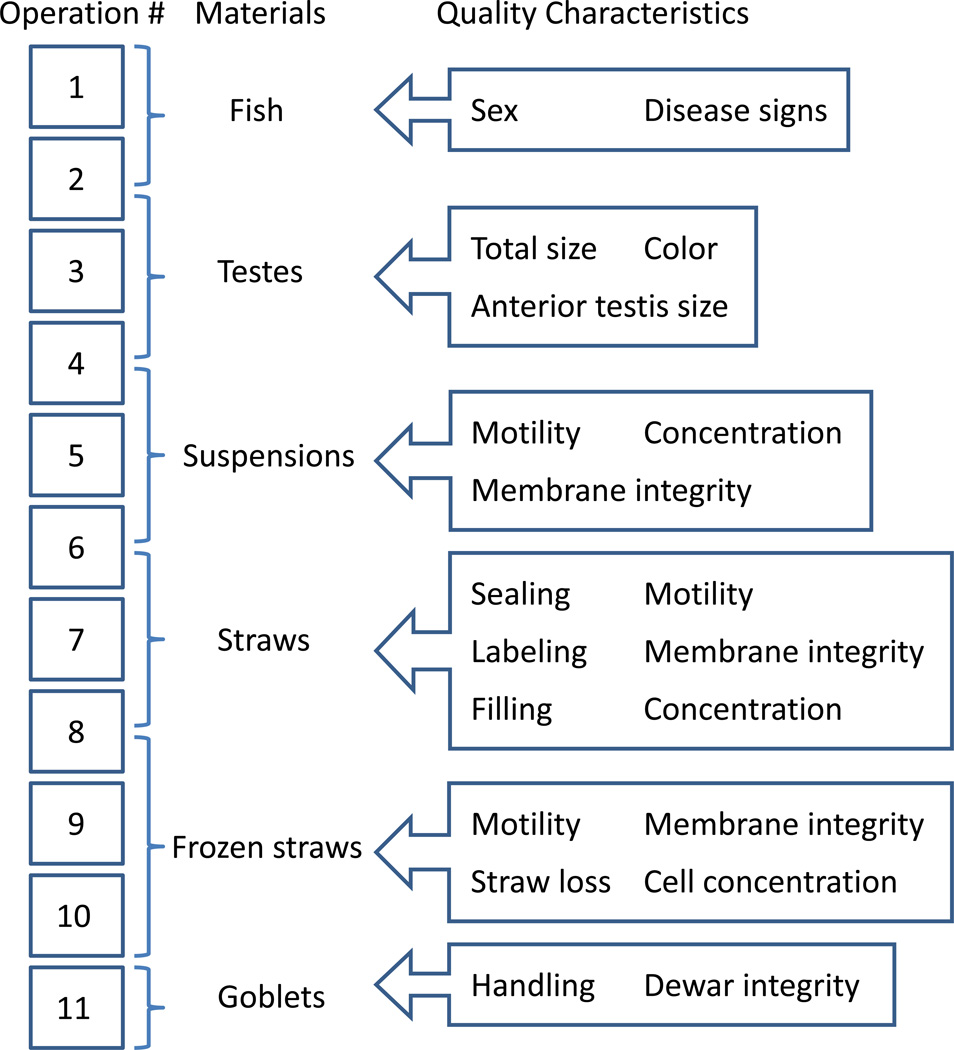
Quality characteristics are identifiable characteristics that can affect product quality. Generally, all possible defects can be considered as quality characteristics. However, tracking of too many characteristics will increase work, and the cost of inspection. Therefore, only key quality characteristics were targeted to be regularly checked. In the process, three inspections were included as routine tasks. Sperm motility, membrane integrity, and sperm concentration were measured through inspections in Operations 5, 8, and 10. Sperm motility and sperm cell membrane integrity reflected sperm quality [28]; concentration reflected sample volumes [32]. Any defects (Figure 1) that directly led to changes in those three characteristics, which included contamination, low sperm volume, or freezing and sorting errors, were eliminated as secondary characteristics. The independent defects (Figure 2) along with motility, membrane integrity, and concentration were key quality characteristics of this process (more discussion on these characteristics is provided in following sections).
Figure 2.
Key quality characteristics with their target materials along the entire 11-step processing pathway for of blue catfish sperm high-throughput cryopreservation.
Key internal qualitative quality characteristics
Among the internal quality characteristics, some were measurable or quantifiable, others were not. In general, those non-measurable quality characteristics were defined by qualitative specifications. Even measurable characteristics can use qualitative specifications according to manufacturer’s recommendations [8]. Qualitative specifications set standards for defect identification, such as occurrence of specific phenomena, appearance, or a certain numerical levels. In this study, six key quality characteristics were applied with qualitative specifications (Table 3).
Table 3.
Qualitative specifications for key quality characteristics.
| Inspection Level |
Quality characteristic | Standard for defect | Justification |
|---|---|---|---|
| Fish | Disease | Signs, symptoms | Health issues, biosecurity |
| Fish | Sex | Female | No sperm |
| Testis | Total size | No usable testis | No sperm |
| Testis | Color | Pink or reddish | Undeveloped gonad |
| Anterior testis | Size | < 3 grams | Too small to work with |
| Concentration | Initial concentration | < 1×109 cells/ml | Does not match required density |
| Motility | Initial motility | < 40% | Low motility, low quality |
| Shipping | Handling | Dropped products | Damaged products |
| Shipping | Dewar integrity | Leakage | Damaged products |
Key internal quantitative quality characteristics
To further reduce the number of quality characteristics that needed to be inspected, a correlation was performed among quantitative quality characteristics. The sperm samples taken from intact testes were labeled as “intact”; the sperm samples taken from crushed sperm suspension were labeled as “crushed sample”; the sperm samples taken from sperm suspensions after a 30-min equilibration with cryoprotectant were labeled as “equilibration”; and the samples taken from thawed sperm suspensions were labeled as “post-thaw”. The membrane integrity measurements used in correlation analysis were from Operation 3 (intact membrane integrity), Operation 5 (crushed sample membrane integrity), Operation 8 (equilibration membrane integrity), and Operation 10 (post-thaw membrane integrity). The motility measurements used in correlation analysis were from Operation 5 (initial motility), Operation 8 (equilibration motility), and Operation 10 (post-thaw motility). The concentration measurements used in correlation analysis were from Operation 3 by flow cytometer (intact concentration), Operation 5 by spectrophotometer (initial concentration), Operation 5 by flow cytometer (crushed sample concentration), Operation 8 by flow cytometer (equilibration concentration), and Operation 10 by flow cytometer (post-thaw concentration). To reduce the size of the correlation matrix, related indices were combined: because concentration represented the number of live cells in each ml of suspension, concentration combined with motility indicated the number of motile sperm per ml; concentration combined with membrane integrity indicated the number of viable cells (membrane-intact cells) per ml (Table 4). Those new indices were justifiable with biological meanings. All statistics were performed by use of Minitab (version 14.12.0, State College, PA, USA).
Table 4.
List of indices combinations for correlation testing in blue catfish sperm high-throughput cryopreservation (operation steps and numbers provided in Table 2).
| Operation number |
Index name |
Combined index |
New index abbreviation |
|---|---|---|---|
| 3 | intact membrane integrity | intact concentration | Int.live/ml |
| 5 | crushed sample membrane integrity | crushed sample concentration | Cr.live/ml |
| 5 | initial motility | initial concentration | Ini.mot/ml |
| 8 | equilibration membrane integrity | equilibration concentration | Eq.live/ml |
| 8 | equilibration motility | equilibration concentration | Eq.mot/ml |
| 10 | post-thaw membrane integrity | post-thaw concentration | Po.live/ml |
| 10 | post-thaw motility | post-thaw concentration | Po.mot/ml |
A total of 7 combined indices were tested (Table 5). The Int.live/ml (intact membrane integrity divided by intact concentration) had no correlation with other indices. The fractions of testis sampled for this index “intact sample” were largely affected by sampling technique due to small sample size, therefore high variation occurred comparing to other inspections later in the process. The Ini.mot/ml (initial motility divided by initial concentration) was correlated with Cr.live/ml (crushed sample membrane integrity divided by crushed sample concentration); however, the equipment required for measuring Cr.live/ml (flow cytometer) was more complex than the use of a microscope or spectrophotometer. In addition, at Operation 5, motility and concentration testing were built as on-line inspections, but flow cytometer testing was an off-line inspection. To reduce inspection costs, the flow cytometer testing at Operation 5 could be optional. The same reasoning applied to Operation 8 off-line inspection. Because Eq.mot/ml (equilibration motility divided by equilibration concentration) and Eq.Live/ml (equilibration membrane integrity divided by equilibration concentration) were correlated, motility test results were sufficient to represent the quality in that operation. Reduction of the flow cytometer inspection at Operation 8 was based on economics. Operation 10 off-line inspection had two correlated indices. To measure the main quality characteristics more accurately, the flow cytometer was recommended. With suggestions from the correlation results, the key internal quality characteristics for quantitative specifications design were therefore initial motility, initial concentration, and equilibration motility.
Table 5.
Correlations among combined indices. Int.Live/ml: live cell concentration in testis fractions; Cr.Live/ml: live cell concentration in sperm suspension; Ini.mot/ml: motile cell concentration in sperm suspension; Eq.Live/ml: live cell concentration in equilibrated sperm; Eq.mot/ml: motile cell concentration in equilibrated sperm; Po.Live/ml: live cell concentration in post-thaw sperm; Po.mot/ml: motile sperm concentration in post-thaw sperm.
| Int.Live/ml | Cr.Live/ml | Ini.mot/ml | Eq.Live/ml | Eq.mot/ml | Po.Live/ml | |
|---|---|---|---|---|---|---|
| Cr.Live/ml | −0.151 | |||||
| 0.387 | ||||||
| Ini.mot/ml | −0.309 | 0.436 | ||||
| 0.071 | 0.006 | |||||
| Eq.Live/ml | 0.274 | 0.725 | 0.137 | |||
| 0.123 | 0.000 | 0.407 | ||||
| Eq.mot/ml | 0.014 | 0.750 | 0.270 | 0.837 | ||
| 0.943 | 0.000 | 0.122 | 0.000 | |||
| Po.Live/ml | 0.161 | −0.193 | −0.260 | −0.016 | −0.166 | |
| 0.423 | 0.334 | 0.158 | 0.934 | 0.398 | ||
| Po.mot/ml | 0.078 | −0.046 | −0.245 | 0.188 | 0.084 | 0.807 |
| 0.725 | 0.835 | 0.219 | 0.369 | 0.688 | 0.000 |
The calculation of the specifications was performed following the method of the main quality characteristics described previously:
The initial motility data were screened and samples with values lower than 40% were discarded based on qualitative quality characteristics specifications. Kolmogorov-Smirnov normality testing showed that the remaining initial motility data did not follow a normal distribution (P = 0.007) with a mean of 56% and a standard deviation of 9%. The best fit distribution was Weibull (P < 0.010) with a shape of 7.74, and scale of 60.05. A large number of random data (500 data sets) was simulated from the same distribution and sorted repeatedly. From the probability density function of those simulated data, the 95% confidence interval was from 43 – 70%. In addition, this characteristic was considered to be a one-sided specification, so the adjusted specification was set at 43 – 100%.
The initial concentration data were screened and values lower than 1 × 109 cells/ml were discarded based on qualitative quality characteristics specifications. Eight outliers based on the data distribution were identified from the data sets of initial concentration. All outliers were associated with samples that had extreme low values and were eliminated from this study in later steps. After the outliers were eliminated, Kolmogorov-Smirnov normality testing showed that the data did not follow a normal distribution (P = 0.006) with a mean of 2.68×109 cells/ml, a standard deviation of 1.13×109 cells/ml, a skewness of 0.579 and a kurtosis of −0.549. The best fit distribution was Lognormal (P = 0.338) with location of 21.62, and a scale of 0.43. A large number of random data (500 data sets) were simulated from the same distribution and sorted repeatedly. From the probability density function of those simulated data, the tolerance ranged from 1.23×109 - 5.00×109 cells/ml.
Kolmogorov-Smirnov normality testing showed that equilibration motility followed a normal distribution (P = 0.231) with the mean of 48% and standard deviation 10%. The 95% confidence interval was from 32 – 64%. With initial motility, this characteristic was considered to be a one-sided specification, so the adjusted specification was 32 – 100%.
Design specifications
The specifications for each internal quality characteristic defined the foundations for quality assurance (Table 6). They defined the quality at the accepted level for each operation, such that unnecessary rejection of the final product could be avoided based on the main quality characteristics (Table 1). Those specifications also determined the Quality Control Points for inspections for the key quality characteristics during the process. Therefore quality assurance actions took place at each Quality Control Point to collect and monitor quality characteristics.
Table 6.
Internal quality characteristics and specifications in blue catfish sperm high-throughput cryopreservation.
| Quality characteristic | Specification type | Reject condition |
|---|---|---|
| Disease signs | Qualitative | Symptoms, signs |
| Sex | Qualitative | Female |
| Total size | Qualitative | No usable testis |
| Testis color | Qualitative | Pink or reddish (blood contamination) |
| Anterior testis size | Qualitative | < 3 g |
| Initial concentration | Quantitative | < 1.23×109 or > 5×109 cells/ml |
| Initial motility | Quantitative | < 40% |
| Equilibration motility | Quantitative | < 32% |
| Handling | Qualitative | Dropped products |
| Dewar integrity | Qualitative | Leakage |
Control Charts and Process Capability
Besides designing specifications for quality characteristics, quality control is the other important part of a quality assurance plan. Quality control can monitor each quality characteristic and identify the out-of-control samples based on shifts of mean and variation [21]. Quality control began with the manufacturing industry [23], and was applied to other fields such as food production [14] and hospital management [17; 29].
Quality control of a high-throughput cryopreservation process has unique features. Firstly, the biological material has inconsistent quality variation. If the quality characteristics were statistically stable over seasons (years), one quality control chart generated from a beginning stage could continuously monitor the process. However, if the quality characteristics vary year by year, each year should be monitored on its own by setting a new chart at the beginning of each year. Secondly, the process has a mixture of qualitative and quantitative quality characteristics with 100% inspection. The quality control chart setup depends on the data type and inspection sample size. Use of 100% inspection (rather than use of sampling) is a special case in quality control. Recent research indicates that the cumulative sum (CUSUM) control charts performed better than Shewhart control charts for 100% inspection [26]. Generally, qualitative characteristics should apply binomial CUSUM for analysis [5; 29] and quantitative characteristics should apply ordinary or robust CUSUM (insensitive to normal distribution assumptions) [26; 27]. In this study, one qualitative characteristic and two quantitative characteristics were selected to demonstrate the use of the above-mentioned control charts using collected data.
Data collection and analysis
High-throughput sperm cryopreservation processing was operated in 2010 and 2011 [18]. Data were collected from inspections throughout the process (Table 2). There were 53 sets of records from 2010, and 59 sets from 2011. The shipping inspection (for quality characteristics “handling” and “dewar”) was performed in research separate from this study, so these data were not included in this report. Microsoft Excel 2007 was used for data organization and calculation. The development of quality control charts included two phases: Phase 1 was to use normal group data (in-control data in QC nomenclature) to produce a control chart; Phase 2 was to test the data of interest (e.g., data from processes under monitoring) using the established chart.
Case study: qualitative data
The testes weight data were presented as:
The CUSUM statistics were:
S0 = 0
Si = max(0, Si–1 + Vi – k) i = 1,2,… [6],
Where , h is the control limit, p0 is the original occurrence probability, and p1 is the shifted occurrence probability.
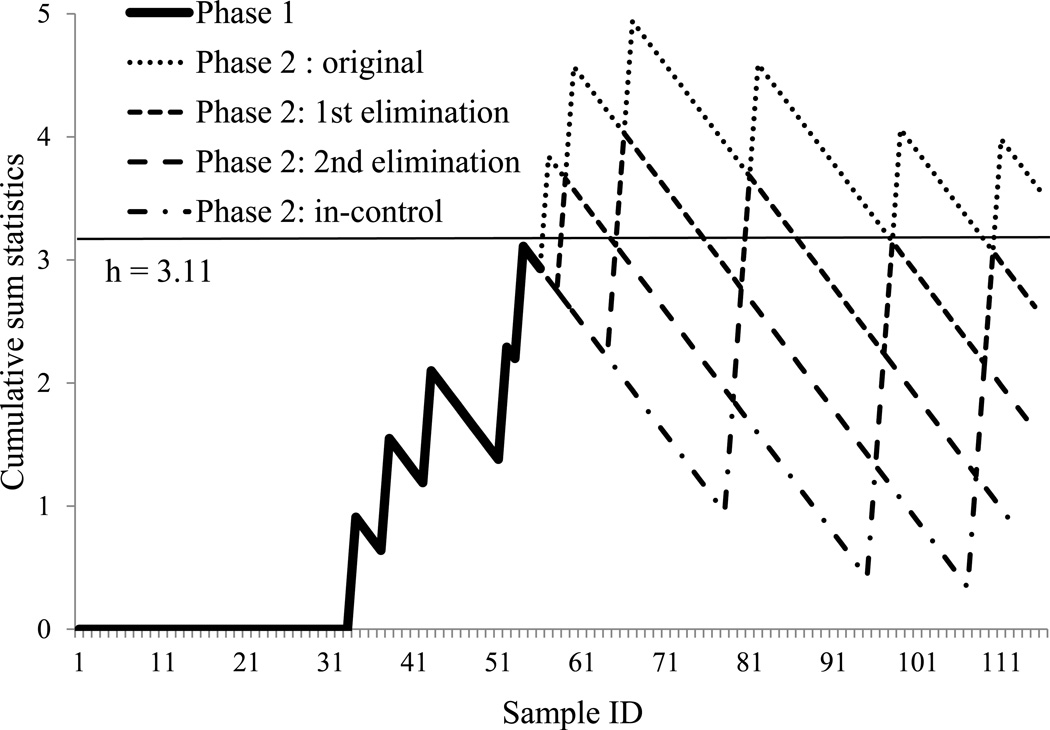
In Phase 1, from the 2010 data, p0 = 0.08. Based on field experience, if one fish out of ten males had a testis that was not usable, the population of fish had questionable gonad development. Therefore, assume p1 = 0.1, and k = 0.090. Because the 2010 data were considered as a normal group, the highest CUSUM value for the 2010 data was set as the h value at 3.11 (Figure 3). After the 2011 data were applied to the CUSUM chart in Phase 2, at each out-of-control occurrence, the first out-of-control data point was eliminated and the CUSUM chart was reset (calculated from the first data point again) until all data sets were in-control. After elimination of 3 out-of-control data sets, all processing was in-control. Those three datasets were identified and the products were separated for further quality inspection and investigation of causes contributing to the out-of-control status. All of the out-of-control datasets were within the lowest 5% of testis weight. Two of these had no detectable testes. The CUSUM chart accurately detected these extreme quality variations.
Figure 3.
Binomial cumulative sum control charts for 2010 and 2011 testis weight data. By 100% inspection, all testes that weighed less than 3 g were assigned a value of 1; all others were assigned a value of 0. The chart was set up with 2010 data as Phase 1, and the control limit (straight line) kept all Phase 1 in-control (no data set exceed the line). The tested 2011 data were Phase 2. The chart presented the approach of eliminating each first out-of-control point until all Phase 2 data were in-control. h: control limit.
Case study: quantitative data indifferent from year to year
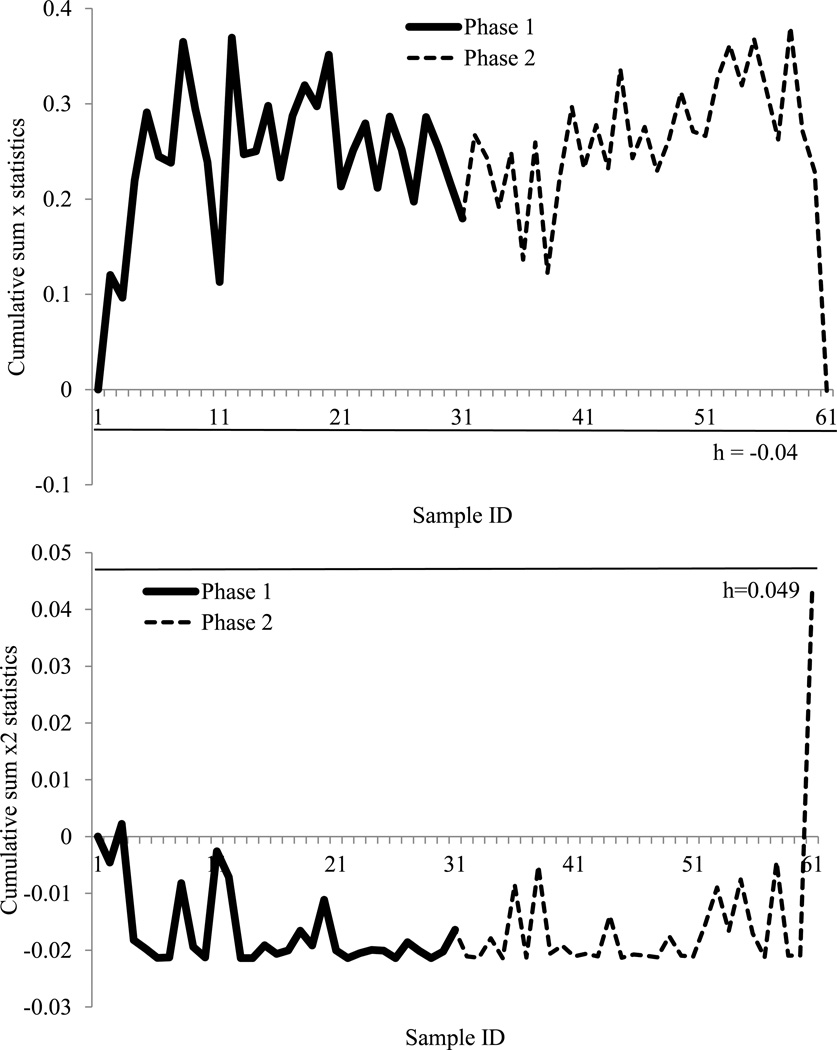
Use of T-test showed that the post-thaw motility was not different from 2010 to 2011 (P = 0.398). In practice, a value of lower than 10% post-thaw motility was not recommended for production. However, even applying the 10% threshold to both years, there was still no difference (P = 0.064). Because high post-thaw motility was not considered to be a negative impact (Table 1), the one-sided CUSUM x chart statistics were more appropriate:
Where , Cμ = |μ1 – μ0|/σ0, and out-of-control signal will be triggered if ;
For variance monitoring, the statistics of CUSUM x2 chart were calculated as:
Where , Cσ = σ1/σ0, n=1 and out-of-control signal will be triggered if
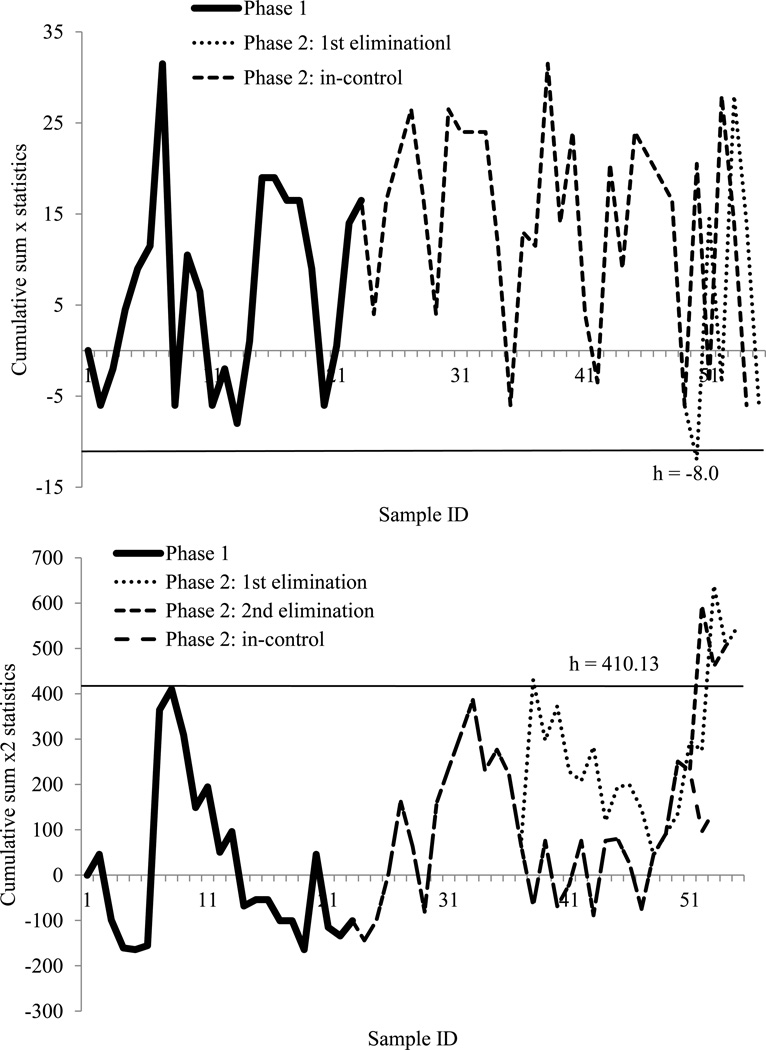
In Phase 1, 2010 data had a mean µ0 of 27, a standard deviation σ0 of 10.12. To identify the mean shift to the lowest acceptable value, µ1 was set at 10, whereas to identify the variation shift that possibly reached the lowest acceptable value, σ1 was set at 17. To keep all 2010 data in-control (they were a “normal group” by experience), and Therefore, the critical parameters of both control charts, and the control limits, were hcx=0.79 and hcx2 =4.00. After being applied to the 2011 data in Phase 2, one data set was identified as out-of-control in the CUSUM x chart (Figure 4). In the CUSUM x2 chart, there were several out-of-control points appearing in the first trial. After eliminating the first out-of-control dataset, there were in total two out-of-control points from the data of 2011, and the rest remained in-control (Figure 4). The out-of-control dataset in the CUSUM x chart was one of the lowest post-thaw motility values. In addition, referring to other parameters of the same sample, it also had the lowest initial motility, which reinforced the observations of the quality control chart. The two out-of-control points from the CUSUM x2 chart were the highest values of post-thaw motility. Therefore, both out-of-control signals were not considered to be quality threats.
Figure 4.
Cumulative sum X and X square (X2) control charts for 2010 and 2011 post-thaw motility data. With 100% inspection, the cumulative sum X chart (above) monitored the mean shifts and the cumulative sum X2 chart (below) monitored the variation changes. The charts were set up with 2010 data as Phase 1, and the control limit (straight line) kept all Phase 1 in-control. The tested 2011 data were Phase 2. The charts presented the process of eliminating every first out-of-control point until all Phase 2 data were in-control. h: control limit.
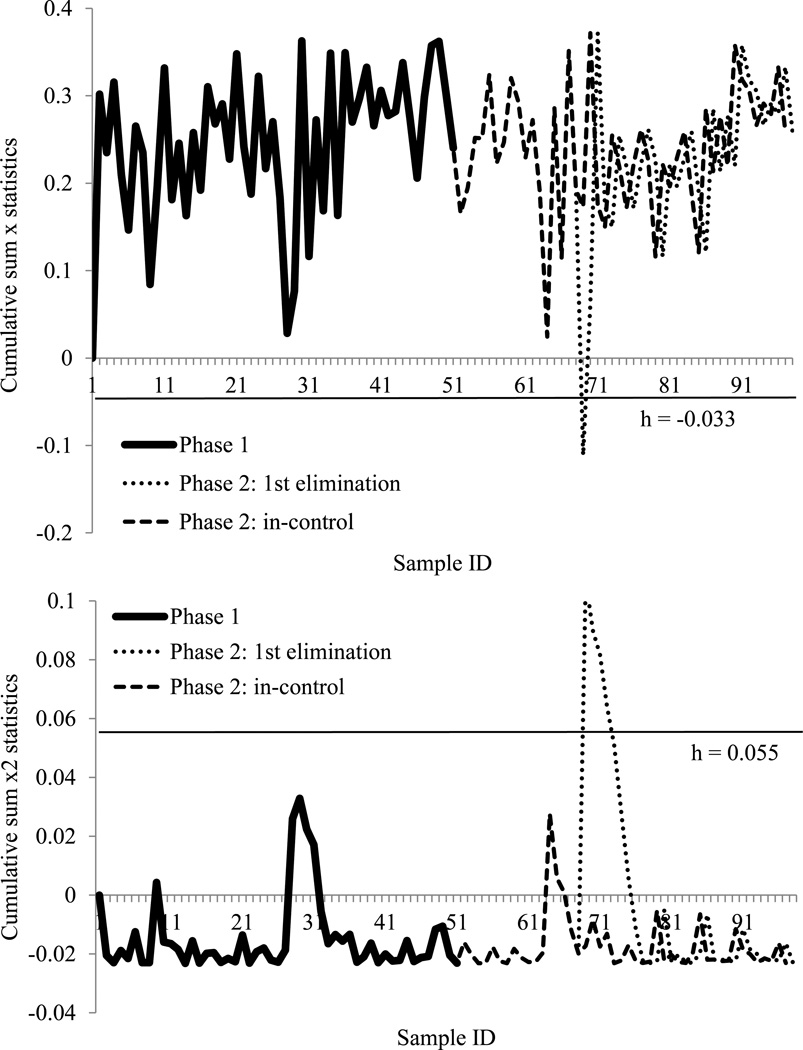
Case study: quantitative data different from year to year
Analysis by t-test showed post-thaw membrane integrity was different from 2010 to 2011 (P = 0.000). Therefore, the 2010 data could serve as the normal group for use in Phase 1, and the data for each year were monitored separately. Because the available data were insufficient to be divided to show Phase 1 and Phase 2 practices, the following strategy was pursued: 1) identify the data distribution using production data early the year, 2) randomly generate data for the quality control chart (Phase 1), and 3) test the production data with an established control chart (Phase 2). With the use of Minitab 14.12.0 (State College, PA, USA), the 2010 data were fit into a 3-parameter Weibull distribution (P = 0.084) with a shape of 550.73, a scale of 32.31, and a threshold of −31.49. In Phase 1, 30 new datasets were simulated from the same distribution and a CUSUM x chart was generated based on them. The simulated data had a mean µ0 of 0.778, a standard deviation σ0 of 0.074; to detect a 50% change in motility µ1 was set at 0.278. After calculation, the lower limit for was −0.041. Repeating the same calculation as the last case study, the control limit was hcx=0.55. All simulated data were in-control. In Phase 2, after applying the 2010 data, all data were in-control. For the CUSUM x2 chart, to detect a large standard deviation in the simulated data σ1 was set at 0.5, the upper limit for was 0.049 and the control limit was hcx2=8.95. In Phase 2, all data were in-control (Figure 5).
Figure 5.
Cumulative sum X and X square (X2) control charts for 2010 post-thaw membrane integrity data. With 100% inspection, the cumulative sum X chart (above) monitored mean shifts and the cumulative sum X2 chart (below) monitored variation changes. The charts were set up with simulated data from the same distribution of the 2010 data as Phase 1, and the control limit (straight line) kept all Phase 1 data in-control. The 2010 data tested were Phase 2. The cumulative sum X chart and the cumulative sum X2 chart were all in-control. h: control limit.
The 2011 data was fit into 3-parameter Weibull distribution (P = 0.266) with a shape of 486.46, a scale of 32.39, and a threshold of −31.65. In Phase 1, 50 new datasets were simulated from the same distribution and a CUSUM x chart was generated based on them. The simulated data had a mean µ0 of 0.717, and a standard deviation σ0 of 0.078, to detect a 50% change in motility µ1 was set at 0.217. After calculation, the lower limit for was −0.033, and the control limit was hcx=0.42. After applying the 2011 data in Phase 2, one data set was out-of-control (Figure 6). To detect a large standard deviation in simulated data in the CUSUM x2 chart σ1 was set at 0.5, the calculated upper limit for was 0.055, and control limit hcx2 was 9.04. In Phase 2, the same dataset that was out-of-control in the CUSUM x chart also appeared out-of-control in the CUSUM x2 chart (Figure 6). The out-of-control sample had the lowest membrane integrity as well as the lowest initial and post-thaw motility. According to the results from the quality control chart, this sample in 2011 was discarded due to unreliable quality.
Figure 6.
Cumulative sum X and X square (X2) control charts for 2011 post-thaw membrane integrity data. With 100% inspection, the cumulative sum X chart (above) monitored mean shifts and the cumulative sum X2 chart (below) monitored variation changes. The charts were set up with simulated data from same distribution of 2011 data as Phase 1, and the control limit (straight line) kept all Phase 1 in-control. The tested 2011 data were Phase 2. The charts represented the approach of eliminating every first out-of-control point until all Phase 2 in-control. h: control limit.
Process capability
There were a total of 13 quality characteristics in the current process, and each had specifications based on customer expectations [8], which was independent to the process operation. Therefore, although the process was in a state of statistical control with the help of quality control [23], it still exceeded specification limits occasionally and did not always produce reliable products. The process capability indicated that the operating process was meeting the specifications by comparing the width of the process variation with the width of the specifications. A higher value of process capability meant a better process with respect to the specifications. For example, in a no-mean-shift normal distribution of quality characteristics, when process capability was one, the process was under a three-sigma level of control, which meant only a 0.3% chance of failure [8].
To carry out process capability analysis, the process must be in-control [23]. If the process is not in control, it is not stable and cannot be described by one distribution. Without a representative distribution, the process variation cannot be predicted during process capability calculations. After the process was in-control, there were different capability indices for different data distributions. For binomial data, a standard normal distribution for historical defect rates was used to test the average defect rate of in-control data and generated the process Z value. For normally distributed data, the short-term process index of process capability (Cp) represented the ratio of the specification width (USL - LSL) to the process width (6 * estimated standard deviation). For other distributions, long-term capability of process (Pp) represented the ratio of the specification width (USL - LSL) to the process width that was the distance between the 99.87th and the 0.13th percentiles in the respective distribution. In general, when the process was under the three-sigma level of control, process Z was equal to 3, Cp was equal to 1, and Pp was equal to 1. With the use of Minitab 14.12.0 (State College, PA, USA), the process capabilities of all quality characteristics were calculated when Phase 2 of quality control was in-control (Table 7). Some quality characteristics were not listed because they did not have enough data. Only post-thaw motility, equilibration motility, and 2011 initial motility were able to achieve 3-sigma capability, others required improvement by either increasing the width of specifications if appropriate, or by reducing the width of quality variation.
Table 7.
Process capability of quality characteristics in blue catfish sperm high-throughput cryopreservation. Pp: long-term capability of process; Process Z: Z-value using standard normal distribution for average defect rate; Cp: short-term process capability.
| Quality characteristic | Year* | Data distribution | Index | Value |
|---|---|---|---|---|
| Post-thaw motility | Smallest extreme value | Pp | 1.18 | |
| Post-thaw membrane integrity | 2010 | Weibull | Pp | 0.60 |
| 2011 | Weibull | Pp | 0.53 | |
| Post-thaw concentration | 2010 | Weibull | Pp | 0.13 |
| 2011 | Logistic | Pp | 0.08 | |
| Sex** | 2011 | Binomial | Process Z | 1.64 |
| Size | Binomial | Process Z | 2.10 | |
| Color | Binomial | Process Z | 1.24 | |
| Anterior testis size | Binomial | Process Z | 1.40 | |
| Initial concentration | 2010 | Weibull | Pp | 0.16 |
| 2011 | Normal | Cp | 0.71 | |
| Initial motility | 2010 | Smallest extreme value | Pp | 0.76 |
| 2011 | Weibull | Pp | 1.01 | |
| Equilibration motility | Weibull | Pp | 1.46 |
When 2010 and 2011 data were statistically different, quality control was performed separately.
2010 data showed no defects, therefore, quality control was only performed on 2011 data.
Conclusions
This project has presented an initiative taken to formulate a quality assurance plan for a newly developed high-throughput cryopreservation process of fish sperm. Key quality characteristics for final and in-process products were identified and their specifications were designed based on data collected during one year of operation (2010, normal group). Different customers could have different quality requirements. For example, use in genetic evaluation programs (e.g., in diallel crosses) would require lower levels of sperm fertility (sufficient only to produce enough offspring for analysis). In such case, the specifications could be less strict than those presented in this report for commercial production scenarios. Even for a particular customer, the requirements could vary on based on specific needs. For example, if all fish were below the specification quality in a certain year (perhaps due to adverse weather conditions) [25], the cryopreservation processer and the customer both face the risk of a complete shut down for that year or season. In such a case, the specifications could be redesigned to allow processing of lower quality material to balance the interests of the processer and customers. Overall, specifications played an important role in process standardization. Development of specifications for biological material processing will present new challenges and ideas for research.
It is recommended that the current high-throughput cryopreservation process be monitored by use of a CUSUM chart for 100% inspection. With proper design of control charts, any out-of-control signals would automatically reject the sample. The control chart could provide more information on operational activities. For example, if out-of-control points appeared in equilibration motility, quality control personnel could check errors during the previous operation (cryoprotectant or filling preparation). A well-developed quality control system can stabilize variation and increase product quality and market competitiveness.
An important feature of this quality assurance plan was the currently intrinsic variation introduced by biological source materials. In this study, five of eight key qualitative quality characteristics were used to select proper materials before cryopreservation. Therefore, the quality of incoming materials (live fish) is extremely important. Catfish processing plants inspect the quality of incoming material by sampling before transport [30]. A similar acceptance-sampling approach is also recommended for high-throughput cryopreservation. If the acceptance sampling can determine the quality of the materials before transport to the cryopreservation processing facility [23], the chance of waste due to the poor quality of biological materials will be reduced.
The outcome of this study indicates that the basic structure of the quality assurance plan developed in this initiative was functional. With the development of aquatic germplasm cryopreservation, more research on process control will be needed in high-throughput processing. This work can serve as a foundation for the expansion of interests in commercialization of cryopreservation processes in other aquatic species, and opens a new vista for ideas in this topic area.
Acknowledgements
We thank J. Christensen, N. Novelo, R. Cuevas-Uribe and H. Yang for assistance during data collection and writing. We thank H. Yang, C Green, J Lynn, and H Blackburn for review and comments. We thank the USDA Catfish Genetics Research Unit in Stoneville, MS and B. Bosworth for providing fish and assistance in data collection. We thank Baxter Land Company for providing fish. This work was supported in part by funding from the Louisiana Sea Grant College Program, USDA special grants, USDA-SBIR, and the National Institutes of Health Office of Research Infrastructure Programs. This manuscript has been approved for publication by the Director of the Louisiana Agricultural Experiment Station as number (2013-244-8304).
This work was supported in part by funding from the Louisiana Sea Grant College Program, USDA special grants, USDA-SBIR, and the National Institutes of Health Office of Research Infrastructure Programs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avery J, Steeby J, Bosworth BG, Small BC. Producing hybrid catfish fry: workshop manual. Stoneville, MS: Mississippi State University, Delta Branch Experiment Station; 2005. [Google Scholar]
- 2.Bart AN, Wolfe DF, Dunham RA. Cryopreservation of blue catfish spermatozoa and subsequent fertilization of channel catfish eggs. Transactions of the American Fisheries Society. 1998;127:819–824. [Google Scholar]
- 3.Blaxter JHS. Sperm storage and cross-fertilization of spring and autumn spawning herring. Nature. 1953;172:1189–1190. [Google Scholar]
- 4.Bondari K. Response to bidirectional selection for body weight in channel catfish. Aquaculture. 1983;33:73–81. [Google Scholar]
- 5.Bourke PD. Sample size and the Binomial CUSUM Control Chart: the case of 100% inspection. Metrika. 2001;53:51–70. [Google Scholar]
- 6.Chang TC, Gan FF. Cumulative sum charts for high yield processes. Statistica Sinica. 2001;11:791–805. [Google Scholar]
- 7.Ciereszko A, Dabrowski K. Relationship between biochemical constituents of fish semen and fertility: the effect of short-term storage. Fish Physiology and Biochemistry. 1994;12:357–367. doi: 10.1007/BF00004300. [DOI] [PubMed] [Google Scholar]
- 8.Creveling CM. Tolerance design: a handbook for developing optimal specifications. New Jersey: Prentice Hall, Upper Saddle River; 1997. [Google Scholar]
- 9.Cuevas-Uribe R. A general approach for vitrification of fish sperm. Baton Rouge, LA: School of Renewable Natural Resources, Louisiana State University; 2011. p. 256. [Google Scholar]
- 10.Daly J, Tiersch TR. Sources of variation in flow cytometric analysis of aquatic species sperm: The effect of cryoprotectants on flow cytometry scatter plots and subsequent population gating. Aquaculture. 2012;370–371:179–188. doi: 10.1016/j.aquaculture.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorman LW, Torrans L. A.C.E. Service. Channel catfish brood stock-selection and management. University of Arkansas at Pine Bluff & United States Fish and Wildlife Service; 1987. [Google Scholar]
- 12.Dunham RA, Masser M. Production of hybrid catfish. Department of Fisheries and Allied Aquacultures, Auburn University & Department of Wildlife and Fisheries Sciences, Texas A&M University; 2012. [Google Scholar]
- 13.Griffin A, Hauser JR. The Voice of the customer. Marketing Science. 1993;12:1–27. [Google Scholar]
- 14.Grigg NP. Statistical process control in UK food production: an overview. International Journal of Quality & Reliability Management. 1998;15:223–238. [Google Scholar]
- 15.Guest WC, Avault JW, Roussel JD. Preservation of channel catfish sperm. Transactions of the American Fisheries Society. 1976;105:469–474. [Google Scholar]
- 16.Guitreau AM, Eilts BE, Novelo ND, Tiersch TR. Fish handling and ultrasound procedures for viewing the ovary of submersed, nonanesthetized, unrestrained channel catfish. North American Journal of Aquaculture. 2012;74:182–187. [Google Scholar]
- 17.Howley P, Hancock S, Ford M. Monitoring clinical indicators, 3rd annual applied statistics education and research collaboration research conference. Newcastle, N.S.W.: Applied Statistics Education and Research Collaboration; 2009. [Google Scholar]
- 18.Hu E, Yang H, Tiersch TR. High-throughput cryopreservation of spermatozoa of blue catfish (Ictalurus furcatus): Establishment of an approach for commercial-scale processing. Cryobiology. 2011;62:74–82. doi: 10.1016/j.cryobiol.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly AM. Channel catfish broodfish management. Southern Illinois University Fisheries and Illinois Aquaculture Center; 2004. [Google Scholar]
- 20.Lang PR, Riley KL, Chandler JE, Tiersch TR. The use of dairy protocols for sperm cryopreservation of blue catfish (Ictalurus furcatus) Journal of the World Aquaculture Society. 2003;34:66–75. [Google Scholar]
- 21.Lee PG. Process control and artificial intelligence software for aquaculture. Aquacultural Engineering. 2000;23:13–36. [Google Scholar]
- 22.Mazur P, Leibo SP, Seidel GE., Jr Cryopreservation of the germplasm of animals used in biological and medical research: importance, impact, status, and future directions. Biology of Reproduction. 2008;78:2–12. doi: 10.1095/biolreprod.107.064113. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery DC. Introduction to statistical quality control sixth edition. New York City, New York: Wiley; 2008. [Google Scholar]
- 24.Novelo N, Tiersch TR. A review of the use of ultrasonography in fish reproduction. North American Journal of Aquaculture. 2012;74:169–181. [Google Scholar]
- 25.Pawiroredjo PA. Temperature effects on spawning and fingerling production of channel catfish Ictalurus punctatus. Baton Rouge, LA: The School of Renewable Natural Resources, the Louisiana State University; 2004. p. 135. [Google Scholar]
- 26.Reynolds, Marion R, Stoumbos, Zachary GG. American Society for Quality. Milwaukee, WI: ETATS-UNIS; 2004. Should observations be grouped for effective process monitoring?. pp. 343–366. [Google Scholar]
- 27.Reynolds MR, Stoumbos ZG. Robust CUSUM charts for monitoring the process mean and variance. Quality and Reliability Engineering International. 2010;26:453–473. [Google Scholar]
- 28.Rurangwa E, Kime DE, Ollevier F, Nash JP. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture. 2004;234:1–28. [Google Scholar]
- 29.Sego LH. Applications of control charts in medicine and epidemiology, Department of Statistics. Blacksburg, Virginia: Virginia Polytechnic Institute and State University; 2006. p. 149. [Google Scholar]
- 30.Silva JL, Ammerman GR, Dean S. Processing channel catfish. Mississippi State University Department of Food Science and Technology & Food and Fiber Center; 2001. [Google Scholar]
- 31.Steeby J, Avery J. Channel catfish broodfish and hatchery management. Stoneville, Mississippi: Mississippi State University National Warmwater Aquaculture Center; 2005. [Google Scholar]
- 32.Suquet M, Omnes MH, Normant Y, Fauvel C. Assessment of sperm concentration and motility in turbot (Scophthalmus maximus) Aquaculture. 1992;101:177–185. [Google Scholar]
- 33.Tiersch TR. Introduction to the second edition. In: Tiersch TR, Green CC, editors. Cryopreservation in Aquatic Speices, 2nd Edition. Baton Rouge, Louisiana: World Aquaculture Society; 2011. [Google Scholar]
- 34.Tiersch TR, Yang H, Hu E. Outlook for development of high-throughput cryopreservation for small-bodied biomedical model fishes. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2011;154:76–81. doi: 10.1016/j.cbpc.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.USDA-NASS. 2005 Census of aquaculture. Washington, D. C: United States Department of Agriculture National Agricultural Statistics Service; 2005. [Google Scholar]
- 36.Vermeirssen ELM, de Quero CM, Shields RJ, Norberg B, Kime DE, Scott AP. Fertility and motility of sperm from Atlantic halibut (Hippoglossus hippoglossus) in relation to dose and timing of gonadotrophin-releasing hormone agonist implant. Aquaculture. 2004;230:547–567. [Google Scholar]