Abstract
Hepatocellular carcinoma (HCC) continues to represent a major worldwide problem. While treatments such as resection, transplantation and ablation may provide a chance for cure, these options are often precluded because of advanced disease presentation. Palliative treatments include transarterial embolization and systemic therapies. This review will summarize the state of the science for embolic therapies in HCC (conventional and drug-eluting chemoembolization, radioembolization), as well as discuss related topics including HCC staging, assessment of response and ongoing clinical trials.
Keywords: hepatocellular carcinoma, chemoembolization, radioembolization
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 6th most common malignancy diagnosed worldwide.1 Its incidence is on the rise and has now become the 3rd most common cause of cancer-related mortality.2 Late stage presentation, co-morbidities, and limited donor availability enables only 10% of patients to receive curative therapies.3 According to the Barcelona Clinic Liver Cancer (BCLC) staging system, conventional chemoembolization (cTACE) represents the mainstay of treatment for intermediate BCLC B disease. This has now evolved into more controlled delivery of chemotherapy in the form of drug-eluting bead TACE (DEB-TACE). Radioembolization, also an intra-arterial treatment, represents an alternate form of treatment for HCC patients with BCLC B disease. Rather than injecting chemotherapy, micron-sized non-embolic radioactive particles are injected in the hepatic artery. Studies have shown that radioembolization may also have a role in the treatment of patients with early (BCLC A) or advanced stage disease (BCLC C). This review article will focus on cTACE, DEB-TACE and radioembolization, with special discussions on the practical aspects of each modality including scientific rationale, number of treatment sessions, adverse events, clinical outcomes, response assessment and ongoing clinical trials.
DIAGNOSIS AND STAGING OF HCC
HCC is diagnostic in cirrhotic livers when there is an arterially enhancing lesion >1 cm with venous washout. Unless genomic marker analysis is planned, biopsy is generally not necessary, since diagnosis can be made using guidelines from the European and American Association for the Study of the Liver.4, 5 This is followed by a thorough evaluation of the patient including history and physical examination, assessment of laboratory values, imaging and determination of baseline performance status. Selection of a specific therapy by the BCLC staging system is based on the tumor characteristics, Child-Pugh stage and performance status. Although treatment recommendations are available for all BCLC stages, up to 50% of patients cannot receive the recommended treatment modality. As a result, cTACE, DEB-TACE and radioembolization play an important therapeutic role across many BCLC stages. A multidisciplinary team (hepatology, medical/surgical oncology, transplant surgery, interventional radiology) should be considered the optimal model when making treatment recommendations to HCC patients.
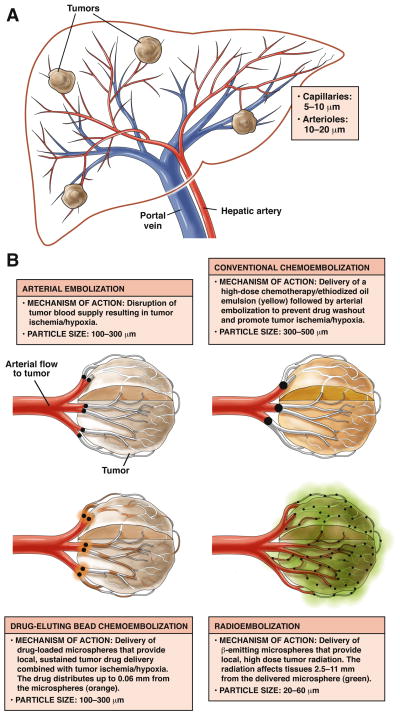
EMBOLOTHERAPY: MECHANISM OF ACTION
Arterial embolotherapies are based on the fact that while the normal hepatic parenchyma derives its blood supply primarily from the portal vein (75%), hepatocellular carcinoma (HCC) derives all of its blood supply from the hepatic artery. Hence, while tumors grow in size, the hepatic arterial blood supply also hypertrophies. Capitalizing on this mechanism, hepatic arterial catheterization can be exploited in order to deliver a therapeutic (drug, radiation), in the hypertrophied vessels, eventually lodging near or within the target, depending on the size of the agent administered (Figure 1). In the case of cTACE, lipiodol is mixed with one or more chemotherapeutics (doxorubicin, cisplatin, mitomycin) and injected within the target vessel. Lipiodol acts as a delivery vehicle for the agents and ultimately lodges near the tumor. DEB-TACE, the evolution of cTACE, involves the loading of drug (doxorubicin) in drug-eluting microspheres; once injected near the tumor, a slow and controlled release of the drug results in anti-tumoral effects. Finally, with radioembolization, 30-micron sized particles are injected and ultimately lodge within the tumor. A low dose-rate brachytherapy is applied to the tumor during the radioactive decay process. While all 3 of these treatments appear to share similarities, there are distinct differences in patient selection, technique, patient monitoring and complications. All have achieved encouraging clinical outcomes in terms of response, time-to-progression (TTP) and overall survival. Given these outcomes, all are gaining acceptance for treating appropriately selected HCC patients.
Figure 1.
Figure 1a. Schema depicting arterial blood supply to hepatocellular carcinoma.
Figure 1b. Demonstration of mechanism of action of bland, chemoembolization, drug-eluting beads and radioembolization.
CONVENTIONAL CHEMOEMBOLIZATION (cTACE)
cTACE is defined as the delivery of one or more chemotherapeutics directly to the tumor via hepatic arterial injection6. This method has been in clinical practice since the 1980s and represents the mainstay of embolotherapy worldwide.7, 8 Although controversial, the most commonly used drugs for cTACE include doxorubicin alone or in combination with mitomycin C and/or cisplatin. The triple-drug combination is the preferred method in the United States.9
Patient Selection
cTACE is indicated in HCC patients with preserved performance status and liver function without vascular invasion or extrahepatic disease. In general, contraindications include intractable systemic infection, leukopenia (white blood cell count <1000/ul), cardiac/renal insufficiency (serum creatinine >2.0 mg/dl), hepatic encephalopathy, performance status >2, hepatofugal flow and biliary obstruction. 10, 11
Procedure
Once a patient has been deemed a candidate for cTACE, a thorough pre-treatment preparation is required. Patients are typically admitted the morning of the procedure for hydration, antibiotics (optional), anti-emetic and narcotic loading. The procedure is performed using a common femoral artery catheterization (same for DEB-TACE and radioembolization), and the lipiodol/chemotherapy emulsion is administered to the hepatic artery perfusing the tumor(s).11 The vehicle used for chemotherapy delivery is lipiodol, a poppy seed oil containing 38% Iodine by weight. In order to obtain an embolic effect and prevent washout of the drug, 100–500 um bland occlusive particles are subsequently injected12. Lipiodol permits the drug to concentrate in the tumor and is retained for weeks; normal hepatocytes excretion is seven days. Immediately following cTACE, a non-contrast CT is obtained demonstrating the proper location of the chemotherapy/lipiodol combination (Figures 2a, 2b). The standard approach to cTACE is for repeated treatments at 2–4 month intervals, depending on the tumor burden and response.
Figure 2.
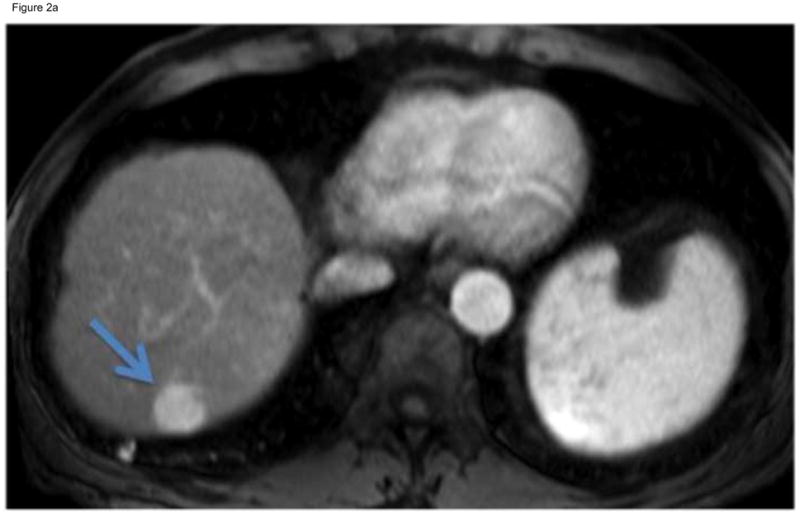
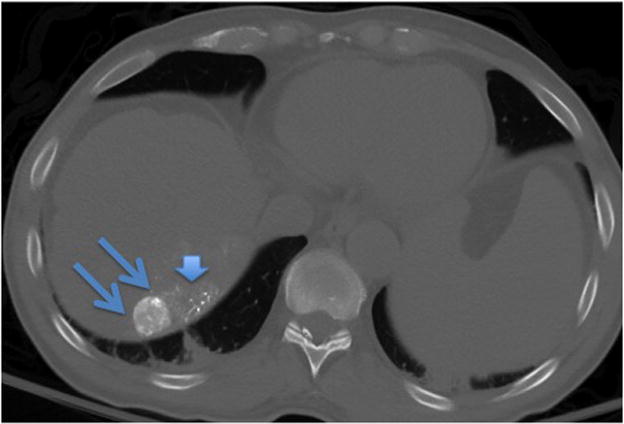
Figure 2a. T1-Weighted gadolinium-enhanced MRI reveals a focal enhancing mass in hepatic segment 7 (arrow). This mass demonstrated venous phase contrast washout, meeting the guidelines for HCC. After discussion at multidiscipline tumor board, this non-operative candidate underwent cTACE.
Figure 2b. A non-contrast CT scan performed immediately after cTACE revealed focal uptake of Lipiodol within the targeted tumor (double arrows). There is some non-target Lipiodol uptake in the non-cancerous hepatic parenchyma (arrow head) adjacent to the tumor.
Clinical Outcomes with cTACE
Lo and Llovet published 2 separate studies establishing the benefit of cTACE in patients in HCC. Both studies used repeated, fixed-interval (intention-to-treat) chemoembolization compared to best supportive care.13, 14 They concluded that cTACE improved survival in patients with unresectable HCC. In a large phase 2 study, Takayasu published data from a large cohort study of 8510 HCC patients treated with cTACE describing liver function, alpha-fetoprotein, tumor size, number of lesions, and portal vein invasion as significant prognosticators of survival.15 These findings were later confirmed with a meta-analysis of seven published randomized control trials concluding that cTACE is an effective palliative treatment modality for unresectable HCC.16 Lewandowski reported on a recent comprehensive imaging and long-term survival analysis in a cohort of 172 patients following cTACE. Median survival was significantly different between patients with BCLC stages A, B and C disease (Stage A: 40.0 months; B: 17.4 months; C: 6.3 months; P<.0001). The study concluded that chemoembolization was safe and effective in patients with HCC; however, TTP and survival was confounded by tumor biology and background cirrhosis.17 Most recently, Takayasu published another large cohort of 4966 HCC patients.18 As opposed to the Lewandowski series, the recent Japanese study excluded patients with vascular invasion, extrahepatic metastases and prior treatment. Applying these selection criteria, excellent results were achieved (median survival 3.3 years), with Child-Pugh class, tumor number, size, alpha-fetoprotein, and des-gamma carboxy-prothrombin levels being independent predictors of survival.
Adverse Events and Complications
Post-embolization syndrome, manifest by pain, nausea and vomiting, is managed during the hospitalization (1–3 days). Other complications may include: a) biliary duct injury (up to 5.3%), 19,20 b) liver abscesses in patients following biliary interventions (stents, sphincterotomy), 19 c) duodenal or gastric ulcers from inadvertent deposition of the chemotherapeutic agents, 19 d) vascular injury such as spasm/dissection from repeated chemotherapy injection in the arterial system, 21 e) and tumor rupture (<1%).19
DRUG-ELUTING BEAD CHEMOEMBOLIZATION (DEB-TACE)
The concept of DEB-TACE builds on the rationale for cTACE. Through a drug-loading process, the microspheres are able to absorb the chemotherapeutic agent. These unique properties permit for release of drug in a controlled and sustained manner. This leads to a significant reduction of peak plasma concentration when compared to cTACE.26 The mechanism of drug-elution is based on a strong drug-bead interaction that can be attributed to an ionic exchange process between anionic drug moieties and the hydrogel sulfonate or carboxyl counter ions22, 23 .
Patient Selection
In general, patients in the intermediate BCLC B category may be considered for DEB-TACE, provided they have preserved performance status and liver function. However, in accordance with clinical trials that have been completed with DEB-TACE, ideal patients should have HCC that can be isolated angiographically, such that selective (as opposed to lobar) injections can be performed. Similar to cTACE, the microspheres are infused slowly while the delivery to tumor is performed. Treatment guidelines for DEB-TACE recommend up to 4 treatments within 6 months to the entire treatment field.24
Clinical Outcomes with DEB-TACE
There have been several studies reporting on outcomes with DEB-TACE. 25, 26 As mentioned earlier, the rationale is one of increased intra-tumoral retention and decreased bioavailability, translating into lower toxicity rates. In an early study, Poon et al reported a 63% response using the modified RECIST criteria.27 A recent randomized controlled trial on 212 patients comparing conventional TACE with DEBs failed to show an improvement in response using the more controlled drug-eluting methodology. However, in a subset analysis, more advanced patients were better able to tolerate DEB-TACE compared with cTACE. 28 In a recent retrospective analysis, Dhanasekran et al concluded that transcatheter therapy with DEB-TACE offered a survival benefit over conventional chemoembolization in patients with unresectable HCC.29 There were fewer adverse events when compared to cTACE, further supporting the safety profile of DEB-TACE.
Varela et al reported on a small 27 patient series of DEB-TACE in HCC and Child-Pugh A cirrhosis. They demonstrated that response rate was 75% by CT at 6 months, with systemic doxorubicin levels significantly lower than cTACE. One and 2-year survival rates were 92.5% and 88.9% respectively, with a median follow up of 27.6 months. They concluded that chemoembolization using DEBs is an effective procedure with a favorable pharmacokinetic profile.30 The same group recently reported on a 104-patient cohort of hyperselected (preserved synthetic function and performance status) treated with DEB-TACE. They reported a median survival of 48 months, challenging current thinking on the 20–22 month expected outcomes in intermediate BCLC B patients31. The combination of Sorafenib and DEB-TACE was also shown to be safe in a recent 35 patient cohort, resulting in a response rate of 58% by necrosis criteria.32
Recently, Malagari et al performed a prospective randomized trial comparing DEB-TACE to bland embolization. Although a partial imaging response to therapy was similar between the groups, TTP was longer in the DEB-TACE arm, establishing that the chemotherapy, along with embolization, plays an important role in the cytotoxic effects.33 The same group also expanded their analysis into a 173 patient cohort and a 5-year survival analysis. Outcomes replicated those reported by other investigators, with median survival exceeding 43 months.34 Clinical outcomes of cTACE and DEB-TACE are summarized in Table 1.
Table 1.
Outcomes of cTACE and DEB-TACE in Hepatocellular Carcinoma
| Takayasu et al15 | Lewandowski et al17 | Takayasu et al17 | Lammer et al28 | Malagari et al33 | Malagari et al31 | Pawlik et al32 | Burrel et al31 | |
|---|---|---|---|---|---|---|---|---|
| Purpose | Analyze survival after TACE | Determine imaging and survival outcome after TACE | Discern the best indication and appropriateness of the current treatment algorithm for TACE | Compare response rate and toxicities of cTACE with DEB-TACE | Compare DEB-TACE with bland embolization | Report on the 5-year survival of HCC patients treated with DEB-TACE on schedule | Report on outcomes of patients treated with DEB-TACE and Sorafenib | Discern survival of selected patients (preserved liver function, absence of symptoms, extrahepatic spread or vascular invasion) treated with DEB-TACE |
| Patients | 8510 | 172 | 4966 | cTACE:108 DEB-TACE: 93 |
DEB-TACE: 41 Bland: 43 |
173 | 35 | 104 |
| Response rate | - | WHO: 31% EASL: 64% |
- | cTACE: 22% DEB-TACE:27% |
DEB-TACE:73.1% Bland: 60% |
EASL CR:23% EASL PR: 48% |
EASL: 58% | - |
| TTP (months) | - | 7.9 | - | - | DEB-TACE: 10.6 Bland embolization:9.1 |
- | - | - |
| Survival | 1-year: 82% 3-year: 47% 5-year: 47% |
BCLC A: 40.0 mo BCLC B: 17.4 mo BCLC C: 6.3 mo |
1-year: 87% 3-year: 55% 5-year: 34% |
- | No difference between two groups within 1 year | 1-year: 93.6% 3-year: 62% 5-year: 22.5% |
- | Overall: 48.6 mo BCLC A: 54.2 mo BCLC B: 47.7 mo |
Abbreviations: BCLC: Barcelona Clinic Liver Cancer; cTACE: conventional transarterial chemoembolization; DEB-TACE: drug-eluting bead transarterial chemoembolization; EASL: European Association for the Study of the Liver; TACE: transarterial chemoembolization; WHO: World Health Organization
Complications
Recent studies, including a 237 patient cohort, have reported on the safety profile of DEB-TACE. Although the systemic exposure is reduced with controlled release of drug in the tumor microenvironment, adverse events seen with DEB-TACE are similar to (but lower in frequency than) cTACE. These include pain, nausea, vascular injury, hepatic failure, abscess formation and tumor rupture.35, 36
RADIOEMBOLIZATION
The technique of radioembolization involves the delivery of high dose radiation via the hepatic arterial system. This is distinctly different from external beam radiation therapy, where dose limitations and hepatic radiosensitivity limit the amount that can be delivered to hepatic tissue before the development of radiation-induced liver disease (ascites, anicteric hepatomegaly, elevation of alkaline phosphatase).3, 37, 38 High dose 30-micron sized radioactive particles are delivered to the tumor at the segmental or lobar level. In contradistinction to cTACE/DEB-TACE, vessel occlusion is not the intent with radioembolization. Rather, the microspheres lodge without causing occlusion at the macroscopic level and emit beta radiation39. Consequently, since vessel occlusion does not occur, hospitalization is not required. Patients are discharged 2–6 hours on the same day following radioembolization.
Patient Selection
As more experience with radioembolization has been garnered over the last decade, certain clinical observations have been made. First, although the concept of segmental injections in HCC is the standard for most embolotherapies, radioembolization, with an improved toxicity profile, appears to play a role in more advanced disease40–42. This includes patients with performance status 1–2, multifocal disease with or without PVT. Since segmental injections are not mandated, lobar infusions treating larger territory of disease is routine43. Second, while cTACE/DEB-TACE requires inpatient hospitalization, radioembolization has shifted the embolotherapy paradigm into one of outpatient therapies, translating in better quality-of-life41, 42. Finally, in contradistinction to routine, scheduled embolizations with cTACE/DEB-TACE, radioembolization patients receive 1 treatment, with follow-up sessions on an as-needed basis.
Clinical Outcomes with Radioembolization
Several large phase 2 studies have been published describing long-term outcomes with radioembolization. A 291-patient comprehensive cohort was the first to describe toxicity, imaging and survival outcomes stratified by BCLC, United Network for Organ Sharing, tumor stage and Child-Pugh44. This study was also the first to describe TTP outcomes in granular detail, serving as background data for comparative studies. Subsequently, a 108-patient study from Germany validated these outcomes in advanced patients, confirming the reproducibility of this technique and equivalent outcomes to cTACE/DEB-TACE45. A 325-patient study followed, further confirming long-term survival outcomes stratified by BCLC stages46. Finally, most recently, a phase 2 study from the group in Italy described the role of radioembolization in intermediate/advanced patients. This last report served to launch a randomized phase 3 study comparing radioembolization to Sorafenib47.
While there has been no randomized study comparing radioembolization to chemoembolization, a comparative effectiveness report described outcomes in a 245-patient cohort. The authors reported that adverse events, clinical toxicities, response rate and TTP were improved with radioembolization when compared with cTACE. However, overall survival was no different, likely as a result of competing risks of death of HCC and cirrhosis. The study also challenged the concept of TTP being a surrogate of survival in HCC. On post-hoc analyses, it was concluded that a sample size of >1000 patients would be required to establish survival equivalence between cTACE and radioembolization40. The improvement in TTP was also confirmed by another comparative report, demonstrating that radioembolization outperformed cTACE in downstaging to transplantation (Figures 3a, 3b).48 Finally, Kulik reported on the niche clinical application of radioembolization in PVT, reporting on the safety profile in this advanced patient population. The authors confirmed that radioembolization could be used in vascular invasion without the risk of ischemic hepatitis43, 49. These findings were recently further confirmed by the same group49. Clinical outcomes of radioembolization are summarized in Table 2.
Figure 3.
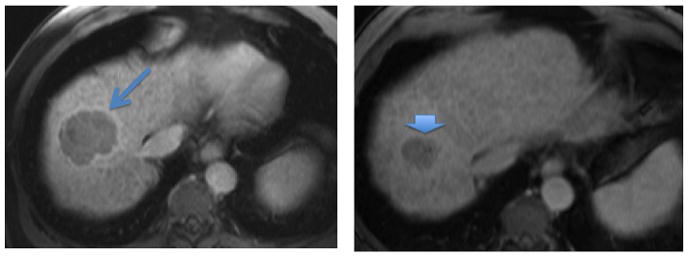
Figure 3a. Gadolinium-enhanced MRI (venous phase) showing a 6 cm mass in the hepatic dome (arrow). This tumor showed early phase arterial enhancement, consistent with HCC. This tumor is outside of the Milan transplant criteria.
Figure 3b. Gadolinium-enhanced MRI 6 months following Y90 radioembolization. The tumor is now 3 × 3 cm (arrowhead) and is within Milan transplant criteria. This patient underwent liver transplantation.
Table 2.
Outcomes of radioembolization in Hepatocellular Carcinoma
| Salem et al44 | Hilgard et al45 | Sangro et al46 | Salem et al40 | Mazzaferro et al41 | |
|---|---|---|---|---|---|
| Purpose | Assess clinical outcomes of patients treated with Y90 | Assess clinical outcomes of patients treated with Y90 in Europe | Evaluate prognostic factors of survival after Y90 in European patients | Compare the effectiveness of Y90 vs. cTACE | Provide a prospective assessment of safety and efficacy for Y90 treatment of patients with intermediate and advanced disease |
| Patients | 291 | 108 | 325 | Y90: 123 TACE: 122 |
52 |
| Response rate | WHO: 42% EASL:57% |
EASL: 40% | - | Y90: 49% TACE: 36% |
WHO: 40% EASL: 40% |
| TTP (months) | 7.9 | 10 | - | Y90: 13.3 TACE: 8.4 |
11 |
| Survival (months) | Child-Pugh A:17.2 Child-Pugh B:7.7 |
16.4 | BCLC A: 24.4 BCLC B: 16.9 BCLC C: 10.0 |
Y90:20.5 TACE: 17.4 |
15 |
Abbreviations: BCLC: Barcelona Clinic Liver Cancer; EASL: European Association for the Study of the Liver; RECIST: Response Evaluation Criteria in Solid Tumors; TACE: transarterial chemoembolization; TTP: Time-to-progression; WHO: World Health Organization
Complications
Adverse events from radioembolization are distinctly different than other embolotherapies. The dominant side-effect is fatigue, with other adverse events including non-target deposition of microspheres (possibly leading to ulcer formation), fibrosis/scarring of the liver parenchyma and cholecystitis. Radiation-induced liver disease is rare when proper patient selection criteria are applied38, 50.
ASSESSING RESPONSE FOLLOWING EMBOLOTHERAPY
Assessing response to locoregional therapies can be complex. In contradistinction to systemic treatments where all tumors are simultaneously exposed to the agent, this is not the case with embolotherapy. During the course of treatment with embolization, lesions are treated at different times at staged 4–6 week intervals. Hence, response is often assessed in the treated lesion, while untreated lesions are only incorporated once the entire treatment field has been completed.51 Although size criteria are the most common reporting standards, methods using necrosis have been implemented in order to incorporate the mechanism of action of embolization. However, given the lack of standardization of these methods, overall tumor size reduction is still considered the gold standard. In 1979, the World Health Organization (bi-dimensional measurements) published guidance on the anatomical assessment of tumor response to therapy.52http://jama.ama-assn.org/cgi/content/full/303/11/1062-REF-JOC05021-8 This further evolved to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (uni-dimensional measurements).53 The European Association for Study of the Liver guideline was based on percent change in amount of enhancing tumoral tissue (necrosis).5, 53, 54 Recently, the modified RECIST assessment (mRECIST) were published, formally recommending the concept of viable tumor tissue (arterial phase of contrast-enhanced imaging) and aimed to provide a common framework for the imaging response of clinical trials in HCC.55 The field of response assessment following local therapies is dynamic, with studies investigating the optimal number of target lesions, pathology correlates and scoring systems, patterns of disease progression, surrogates of survival and biomarkers51, 56–62.
ONGOING TRIALS
Research in embolotherapy continues to be a very active. Given that cTACE and Sorafenib have both shown to provide a survival advantage, studies with radioembolization have been proposed to either compete against or combine with these other standards of care. Recently, the equivocal findings from a trial using DEB-TACE +/− Sorafenib were announced.63 This study was unable to definitively confirm a role for Sorafenib in combination with embolization. Cooperative groups are also carrying out similar studies in order to further investigate the role of Sorafenib in HCC patients undergoing embolization. There are also several ongoing trials comparing cTACE and radioembolization in randomized designs with TTP as the endpoint (incorporating quality-of-life, econometrics). Finally, there are other studies looking at the role of radioembolization in combination with or comparison to Sorafenib in the advanced patient population. These trials are robust in their rationale, statistical design (international, multicenter, randomized phase III) and primary endpoints (survival)64. Final results of these seminal studies are expected within the next 3–5 years.
CONCLUSION
Chemoembolization and radioembolization are trans-arterial locoregional therapies that have gained widespread recognition for the treatment of HCC. Although a randomized trial comparing cTACE and DEB-TACE did not reach its endpoint, there appears to be better tolerability in more advanced patients with DEB-TACE. Similarly, while a comparative effectiveness study of cTACE and radioembolization did not show a survival advantage, Y90 patients did exhibit lower toxicities and longer TTP. Currently enrolling studies combining these arterial locoregional therapies with targeted systemic therapies are underway. As the results of randomized studies of embolotherapy combined with systemic agents mature, the clinical indications and specific patients ideally suited for these palliative interventions will continue to be refined.
Acknowledgments
Role of Funding: There was no funding provided for this review. RS is funded in part by NIH grant CA126809.
Abbreviations
- BCLC
Barcelona Clinic Liver Cancer
- cTACE
Conventional trans-arterial chemoembolization
- DEB-TACE
Drug-eluting bead trans-arterial chemoembolization
- EASL
European Association for the Study of the Liver
- HCC
Hepatocellular Carcinoma
- PVT
Portal venous thrombosis
- TACE
Trans-arterial chemoembolization
- TTP
time-to-progression
- 90Y
Yttrium-90 radioembolization
Footnotes
Conflict of Interest: RS is an advisor to BTG, Bayer/Onyx, Merit, Nordion and Sirtex.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DMBF, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Geschwind JF, Salem R, Carr BI, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194–205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 6.Brown DB, Gould JE, Gervais DA, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S425–34. doi: 10.1016/j.jvir.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Ensminger W. Hepatic arterial chemotherapy for primary and metastatic liver cancers. Cancer Chemother Pharmacol. 1989;23 (Suppl):S68–73. doi: 10.1007/BF00647244. [DOI] [PubMed] [Google Scholar]
- 8.Eksborg S, Cedermark BJ, Strandler HS. Intrahepatic and intravenous administration of adriamycin--a comparative pharmacokinetic study in patients with malignant liver tumours. Med Oncol Tumor Pharmacother. 1985;2:47–54. doi: 10.1007/BF02934780. [DOI] [PubMed] [Google Scholar]
- 9.Solomon B, Soulen MC, Baum RA, et al. Chemoembolization of hepatocellular carcinoma with cisplatin, doxorubicin, mitomycin-C, ethiodol, and polyvinyl alcohol: prospective evaluation of response and survival in a U.S. population. J Vasc Interv Radiol. 1999;10:793–8. doi: 10.1016/s1051-0443(99)70117-x. [DOI] [PubMed] [Google Scholar]
- 10.Liapi E, Geschwind JF. Transcatheter arterial chemoembolization for liver cancer: is it time to distinguish conventional from drug-eluting chemoembolization? Cardiovasc Intervent Radiol. 2011;34:37–49. doi: 10.1007/s00270-010-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown DB, Nikolic B, Covey AM, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2012;23:287–94. doi: 10.1016/j.jvir.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 12.Coldwell DM, Stokes KR, Yakes WF. Embolotherapy: agents, clinical applications, and techniques. Radiographics. 1994;14:623–43. doi: 10.1148/radiographics.14.3.8066276. quiz 645–6. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 14.Lo C-M, Ngan H, Tso W-K, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 15.Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 17.Lewandowski RJ, Mulcahy MF, Kulik LM, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010;255:955–65. doi: 10.1148/radiol.10091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol. 2012;56:886–92. doi: 10.1016/j.jhep.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Xia J, Ren Z, Ye S, et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol. 2006;59:407–12. doi: 10.1016/j.ejrad.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Kim HK, Chung YH, Song BC, et al. Ischemic bile duct injury as a serious complication after transarterial chemoembolization in patients with hepatocellular carcinoma. J Clin Gastroenterol. 2001;32:423–7. doi: 10.1097/00004836-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Belli L, Magistretti G, Puricelli GP, et al. Arteritis following intra-arterial chemotherapy for liver tumors. Eur Radiol. 1997;7:323–6. doi: 10.1007/s003300050159. [DOI] [PubMed] [Google Scholar]
- 22.Lencioni R, Crocetti L, Petruzzi P, et al. Doxorubicin-eluting bead-enhanced radiofrequency ablation of hepatocellular carcinoma: a pilot clinical study. J Hepatol. 2008;49:217–22. doi: 10.1016/j.jhep.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Liu DM, Kos S, Buczkowski A, et al. Optimization of doxorubicin loading for superabsorbent polymer microspheres: in vitro analysis. Cardiovasc Intervent Radiol. 2012;35:391–8. doi: 10.1007/s00270-011-0168-0. [DOI] [PubMed] [Google Scholar]
- 24.Lencioni R, de Baere T, Burrel M, et al. Transcatheter Treatment of Hepatocellular Carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): Technical Recommendations. Cardiovasc Intervent Radiol. 2012;35:980–5. doi: 10.1007/s00270-011-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Constantin M, Fundueanu G, Bortolotti F, et al. Preparation and characterisation of poly(vinyl alcohol)/cyclodextrin microspheres as matrix for inclusion and separation of drugs. Int J Pharm. 2004;285:87–96. doi: 10.1016/j.ijpharm.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez MV, Tang Y, Phillips GJ, et al. Doxorubicin eluting beads-2: methods for evaluating drug elution and in-vitro:in-vivo correlation. J Mater Sci Mater Med. 2008;19:767–75. doi: 10.1007/s10856-006-0040-y. [DOI] [PubMed] [Google Scholar]
- 27.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 28.Lammer J, Malagari K, Vogl T, et al. Prospective Randomized Study of Doxorubicin-Eluting-Bead Embolization in the Treatment of Hepatocellular Carcinoma: Results of the PRECISION V Study. CardioVascular and Interventional Radiology. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhanasekaran RM. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC) J Surg Oncol. 2010;101:476–480. doi: 10.1002/jso.21522. [DOI] [PubMed] [Google Scholar]
- 30.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–81. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–5. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Pawlik TM, Reyes DK, Cosgrove D, et al. Phase II Trial of Sorafenib Combined With Concurrent Transarterial Chemoembolization With Drug-Eluting Beads for Hepatocellular Carcinoma. J Clin Oncol. 2011;29:3960–7. doi: 10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541–51. doi: 10.1007/s00270-009-9750-0. [DOI] [PubMed] [Google Scholar]
- 34.Malagari K, Pomoni M, Moschouris H, et al. Chemoembolization with Doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012;35:1119–28. doi: 10.1007/s00270-012-0394-0. [DOI] [PubMed] [Google Scholar]
- 35.Malagari K, Pomoni M, Spyridopoulos T, et al. Safety Profile of Sequential Transcatheter Chemoembolization with DC Bead: Results of 237 Hepatocellular Carcinoma (HCC) Patients. CardioVascular and Interventional Radiology. 2011;34:774–785. doi: 10.1007/s00270-010-0044-3. [DOI] [PubMed] [Google Scholar]
- 36.Vogl TJ, Lammer J, Lencioni R, et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197:W562–70. doi: 10.2214/AJR.10.4379. [DOI] [PubMed] [Google Scholar]
- 37.Ingold JA, Reed GB, Kaplan HS, et al. Radiation Hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200–8. [PubMed] [Google Scholar]
- 38.Gil-Alzugaray B, Chopitea A, Inarrairaegui M, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology. 2012 doi: 10.1002/hep.26191. [DOI] [PubMed] [Google Scholar]
- 39.Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol. 2006;29:522–9. doi: 10.1007/s00270-005-0171-4. [DOI] [PubMed] [Google Scholar]
- 40.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization Results in Longer Time-to-Progression and Reduced Toxicity Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2011;140:497–507. e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goin JE. Comparison of Post-embolization Syndrome in the Treatment of Patients with Unresectable Hepatocellular Carcinoma: Trans-catheter Arterial Chemo-embolization versus Yttrium-90 Glass Microspheres. World J Nucl Med. 2004 [Google Scholar]
- 42.Gilbertsen P, Coffey S, Gonda E, et al. Abstract No 182: Quality of life assessment of patients treated with Yttrium-90 or transarterial chemoembolization: A comparative study using the fact-hep. Journal of vascular and interventional radiology : JVIR. 2011;22:S79. [Google Scholar]
- 43.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of (90)Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2007;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 44.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–9. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 46.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 47.Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium(90) radioembolization for intermediate-advanced hepatocarcinoma: A phase II study. Hepatology. 2012 doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 48.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–8. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 49.Memon K, Kulik L, Lewandowski RJ, et al. Radioembolization for Hepatocellular Carcinoma with Portal Vein Thrombosis: Impact of Liver Function on Systemic Treatment Options at Disease Progression. J Hepatol. 2012 doi: 10.1016/j.jhep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riaz A, Lewandowski RJ, Kulik LM, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20:1121–30. doi: 10.1016/j.jvir.2009.05.030. quiz 1131. [DOI] [PubMed] [Google Scholar]
- 51.Riaz A, Miller FH, Kulik LM, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA. 2010;303:1062–9. doi: 10.1001/jama.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.WHO. WHO offset publication. Geneva, Switzerland: WHO; 1979. WHO Handbook for Reporting Results of Cancer Treatment; p. 48. [Google Scholar]
- 53.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 54.Forner A, Ayuso C, Varela M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–23. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 55.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 56.Riaz A, Ryu RK, Kulik LM, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734–42. doi: 10.1200/JCO.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 57.Riaz A, Memon K, Miller FH, et al. Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: Radiologic–pathologic correlation. Journal of Hepatology. 2011;54:695–704. doi: 10.1016/j.jhep.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Memon K, Kulik L, Lewandowski RJ, et al. Radiographic Response to Locoregional Therapy in Hepatocellular Carcinoma Predicts Patient Survival Times. Gastroenterology. 2011;141:526–535.e2. doi: 10.1053/j.gastro.2011.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faivre S, Zappa M, Vilgrain V, et al. Changes in tumor density in patients with advanced hepatocellular carcinoma treated with sunitinib. Clin Cancer Res. 2011;17:4504–12. doi: 10.1158/1078-0432.CCR-10-1708. [DOI] [PubMed] [Google Scholar]
- 60.Edeline J, Boucher E, Rolland Y, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118:147–56. doi: 10.1002/cncr.26255. [DOI] [PubMed] [Google Scholar]
- 61.Memon K, Kulik L, Lewandowski RJ, et al. Alpha-fetoprotein response correlates with EASL response and survival in solitary hepatocellular carcinoma treated with transarterial therapies: a subgroup analysis. J Hepatol. 2012;56:1112–20. doi: 10.1016/j.jhep.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Senthilnathan S, Memon K, Lewandowski RJ, et al. Extrahepatic metastases occur in a minority of hepatocellular carcinoma patients treated with locoregional therapies: analyzing patterns of progression in 285 patients. Hepatology. 2012;55:1432–42. doi: 10.1002/hep.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): Phase II, randomized, double-blind SPACE trial. ASCO Meeting Abstracts; 2012. p. LBA154. [Google Scholar]
- 64.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]